Abstract
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are created in normal hepatocytes and are critical for normal physiological processes including oxidative respiration, growth, regeneration, apoptosis, and microsomal defense. When the levels of oxidation products exceed the capacity of normal antioxidant systems, oxidative stress occurs. This type of stress, in the form of ROS and RNS, can be damaging to all liver cells, including hepatocytes, Kupffer cells, stellate cells, and endothelial cells, through induction of inflammation, ischemia, fibrosis, necrosis, apoptosis, or through malignant transformation by damaging lipids, proteins, and/or DNA. In part I of this review, we will discuss basic redox biology in the liver, including a review of ROS, RNS, and antioxidants, with a focus on nitric oxide as a common source of RNS. We will then review the evidence for oxidative stress as a mechanism of liver injury in hepatitis (alcoholic, viral, non-alcoholic). In part II of this review, we will review oxidative stress in common pathophysiological conditions including ischemia/reperfusion injury, fibrosis, hepatocellular carcinoma, iron overload, Wilson’s disease, sepsis and acetaminophen overdose. Finally, biomarkers, proteomic, and antioxidant therapies will be discussed as areas for future therapeutic interventions.
Keywords: nitric oxide, hepatocytes, oxidative stress, reactive oxygen species, hepatitis, ethanol induced hepatitis
Introduction
Part I of this review discussed the role of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in normal physiological function of hepatocytes including oxidative respiration, cell signaling, and protein modification required for normal cellular growth, regeneration, apoptosis, and microsomal defense. However, ROS and RNS can damage any cells in the liver causing inflammation, ischemia, fibrosis, necrosis, apoptosis, or malignant transformation. Previously, we discussed the pathology of hepatitis as it relates to redox biology in the liver. In Part II of this review, we will discuss the pathology of ischemia/reperfusion injury, fibrosis, iron overload, Wilson’s disease, sepsis, and acetaminophen overdose as it relates to redox biology. We will also discuss redox proteomics and the potential of antioxidant therapy in the attenuation of disease progression.
Redox in Pathologic Hepatocytes
Ischemia/Reperfusion in Transplantation
Hepatic ischemia/reperfusion injury occurs in two main settings: after liver resection or transplantation due to anoxia or ischemia of the liver itself or due to systemic hypoxia or low flow states associated with sepsis or shock. Warm ischemia/reperfusion is associated with increased oxidative stress and mitochondrial dysfunction resulting in liver failure and/or multi-system organ failure and the extent of injury is related to preexisting conditions of the liver and the duration of insult. Cold ischemia, seen only in transplantation, is associated with reduced oxidative phosphorylation, decreased ATP, and increased glycolysis [19]. Interventions such as preoperative chemotherapy or embolization may make the liver more susceptible to ischemic stress, [137] while preexisting condition such as cirrhosis and steatosis predispose to poor liver function. In the area of liver transplantation, ischemia reperfusion injury is a common cause of primary graft dysfunction and increased mortality and morbidity [33].
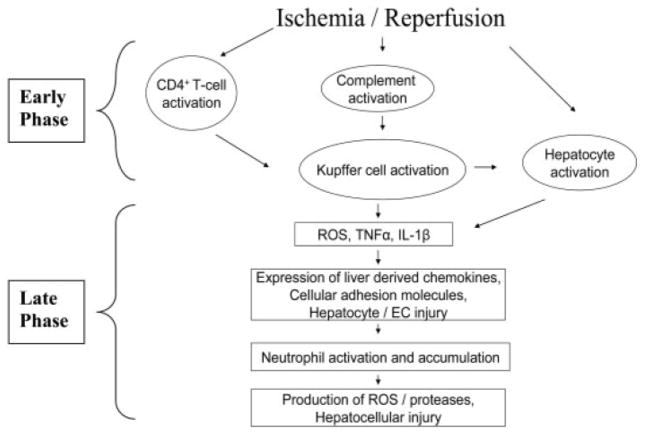
The injury seen with ischemia and reperfusion can, at least in part, be attributed to ROS and RNS because there is an increased level of ROS and RNS produced and a consumption of antioxidants with apoptosis and cell death is noted. (Figure 1) During periods of hypoxia, ROS and RNS species are generated, which then cause increased cellular damage [100]. Initially, the mitochondria also become reduced because of alterations in the respiratory chain, as a secondary reaction to hypoxia. This results in reduction of adenosine triphosphate (ATP), causing membrane ion disturbances, including sodium influx due to the inhibition of the ATP-dependent sodium/potassium ATPase. The subsequent influx of sodium can then cause the cell to swell and rupture [10]. Accumulation of intracellular calcium causes activation of cell membrane phospholipase, which causes phospholipid degradation and membrane damage [24, 32]. Mitochondria become more permeable, lysosomes are disrupted, membranes are disrupted causing cell leakage, and cells themselves swell [77, 180].
Figure 1.
During ischemic/reperfusion injury, there is initial complement and t-cell activation, which leads to reactive oxygen species, TNFα, and IL-1β production. During the late phase, there are chemokines, neutrophil activation, more reactive oxygen species, and hepatocellular injury. Reprinted by permission from Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 11:1031–1047,2005. [40] Copyright 2008 American Association for the Study of Liver Diseases. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
During reperfusion, there are two phases of injury: the early/initial phase (usually the first two hours) and late phase. Initially, damage appears to be related to free radical formation by Kupffer cells and is activated by complement and CD4+T lymphocytes followed by cytokine production and PMN infiltration [31, 70]. Specifically, xanthine dehydrogenase is metabolized to xanthine oxidase during hypoxia. Upon reperfusion, oxygen reacts with xanthine oxidase to produce ROS [29, 100]. This ischemia/reperfusion injury involves APE/Ref-1 and NF-κB, specifically overexpression of APE/Ref-1 that results in decreased oxidative stress, as seen in mice [129]. Kupffer cells also produce cytokines, including interleukin (IL-1) and tumor necrosis factor–α(TNF-α). These cytokines, along with ROS and RNS, are already deleterious to the hepatocytes. TNF-α is of particular importance in ischemia reperfusion injury as it induces expression of adhesion molecules on vascular endothelial cells, stimulates chemokines, and thus recruits neutrophils, which release further ROS and proteases and create further injury [176].
The late phase of ischemia/reperfusion injury is caused by neutrophil activation, ROS, TNF-α, and IL-1B and results in more substantial injury than initially caused by the Kupffer cells. CD4 T-lymphocytes, mediated by TNF-α and IL-1, adhere to hepatic sinusoids and can increase Kupffer cell activation and general cellular recruitment via granulocyte colony-stimulating factor and interferon gamma (INFγ) [183]. There is also upregulation and expression of iNOS, creating large quantities of NO that results in further creation of RNS [66].
Nitric oxide is an important mediator of inflammation, specifically in the hepatocytes themselves during warm ischemia/reperfusion injury [68, 69]. iNOS RNA expression is increased approximately one hour after reperfusion, with increased activity noted at five hours after reperfusion [66]. Whether iNOS is protective or deleterious in the setting of ischemia/reperfusion is controversial. For example, iNOS knockout mice develop less warm ischemia/reperfusion injury [91], but the use of non-specific NOS inhibitors, including L-NNA and L-NAME actually increases liver damage [81, 174]. It is proposed that ONOO−, the product of superoxide anion and NO by iNOS, may be the cause of the damage [103]. Peroxynitrite can cause lipid peroxidation, inhibition of the mitochondrial respiratory chain, inhibition of Na+/K+ ATPase, and/or oxidative protein modifications [25, 159]. This difference between the protective and destructive influences may be dependent on the amount and duration of NO exposure, as well as the pre-insult condition of the liver and the level of antioxidants available to consume the ROS and RNS.
eNOS appears to play a role in liver ischemia/reperfusion injury. Basally, eNOS induces vasodilation at the level of the pre-sinusoids and sinusoids [101, 112] and also prevents platelet adhesion, thrombosis, and PMN accumulation [37, 84, 114, 141]. During the initial phase of ischemia/reperfusion injury, there is a decreased release of NO from eNOS and a relative vasoconstriction of the microvascular bed. Animal studies have demonstrated that inhibition of NO production from eNOS may contribute to ischemia/reperfusion injury [74]. eNOS knockout mice have increased hepatic damage in ischemia/reperfusion, suggesting a protective role of eNOS in this injury [60]. Genetic overexpression of eNOS also attenuates hepatic ischemia/reperfusion injury as compared to control mice [26, 72]. This effect was noted to be ODQ dependent, thus cGC dependent, and HO-1 independent [26]. On the other hand, eNOS knockout mice and eNOS adenovirus treatment resulted in increased ischemia-reperfusion injury in diabetic mice, suggesting that hepatic eNOS is dysfunctional in the setting of diabetes and may lead to increased production of peroxynitrite [28].
In the clinical setting, decreased hepatic eNOS production after orthotopic liver transplantation (OLT) was associated with ischemia/reperfusion injury [169]. Use of inhaled NO in patients undergoing OLT was associated with decreased hospital stay and improved liver function lab tests. Although no changes in inflammatory markers were noted, there was decreased hepatic apoptosis [87]. Use of a NO donor has shown some promise in animal models. Administration of L-arginine, a NO precursor, prior to ischemia/reperfusion injury, was associated with reduced injury and intact microcirculation [166, 167]. In mice, intravenous delivery of NO in the form of polyethylene glycol-conjugated bovine serum albumin (PEG-poly SNO-BSA) during ischemia/reperfusion injury resulted in decreased injury and decreased neutrophil infiltration [75].
In organ transplantation, cold ischemia/reperfusion leads to damage of the endothelial cells [68]. In this setting, the use of L-NAME (a nonspecific NOS inhibitor) demonstrated increased hepatic damage while L-NIL (an iNOS inhibitor) did not [38]. Inhibition of iNOS actually leads to increased apoptosis while subsequent infusion of L-arginine led to decreased injury [38]. Use of NO donors has also been associated decreased portal venous pressure and decrease hepatic injury [145]. All of these studies suggest that, in periods of cold ischemia, both iNOS and eNOS may be protective.
Hemorrhagic Shock
Hemorrhagic shock is similar to ischemia/reperfusion injury. In hemorrhagic shock, there is an initial shock phase followed by a reperfusion phase. Initially, due to shock, there is an activation of JNK and an upregulation of cyclooxygenase (COX)-2, CD14, and HIF-1 [53, 55]. COX-2 is upregulated by oxidative stress and produces large amounts of prostaglandins. HIF-1 and iNOS are activated by hypoxia and regulate cellular adaptation to hypoxia by binding to erythropoietin (EPO), vascular endothelial growth factor (VEGF), heme oxygenase (HO-1) and iNOS, resulting in increased erythropoiesis, angiogenesis, vasodilation, and anaerobic metabolism [44]. There is also systemic upregulation of AP-1, NF-κB, p53, release of prostaglandins, and the release of gut-derived products in response to hypoxia [44].
This shock phase is then followed by the reperfusion phase after resuscitation; this phase is similar to that seen in ischemia/reperfusion injury in organ transplantation. During resuscitative, but not unresuscitative, shock, upregulation of G-CSF was noted in rat lungs and liver [58]. Granulocyte colony-stimulating factor (G-CSF) is the cytokine involved in PMN production and activation. Increased product of the cytokines IL-6 and activation of Stat3 was also noted in resuscitative, but not unresuscitated, rats [57, 58]. This activation of IL-6 may then lead to increased PMN infiltration, and thus, further release of ROS. The activation of these cytokines during the resuscitative phase, as opposed to the shock phase, suggests one possible area of therapeutic intervention during resuscitations [57].
Animal models of shock have results similar to those of warm ischemia/reperfusion injury, where eNOS appears to be protective while iNOS appear to be detrimental, but the studies available are contradictory. Within 1–2 hours of hemorrhagic shock, mice have increased levels of iNOS [54]. This increase in iNOS is associated with increased cytokine mRNA levels and increased NF-κB and Stat3 activation [56]. This increase in oxidative stress is also with a decrease in the levels of antioxidants, including vitamin E, ascorbic acid, and α-tocopherol and also with an impairment of antioxidant capacity, as noted by decreases in superoxide dismutase, catalase, and glutathione peroxidase [53].
In animal models, mice given iNOS inhibitors (L-NIL) or iNOS knockout mice had decreased NF-κB, cytokine production, and infiltration of PMN after resuscitation, [56] possibly due to the decreased production of ONOO− [53]. Rats given NOS inhibitors had improved survival, which decreased with the addition of L-arginine [64, 102, 107]. On the other hand, other studies demonstrated that the administration of L-arginine during hemorrhagic shock was associated with a survival benefit [22]. Rodents given the NO scavenger, NOX, had decreased pulmonary and hepatic injury, decreased inflammation as measured by PMN infiltration, and improved survival [55, 59, 106]. However, the use of an NO donor has also been shown to improve hepatic microcirculatory perfusion and decreased hepatic inflammation in a hemorrhagic shock model [7]. These contradictory results may be due to different experimental designs with different concentration, routes, and timing of pharmacological interventions and various outcome measures. Even though the results of these studies do not clearly elucidate the mechanism by which NO is related to hemorrhagic shock and resuscitation, it is clear that NO does play a role of some sort in the oxidative stress created and thus serves as a potential therapeutic target.
Sepsis
Sepsis is a clinical syndrome characterized by fever, tachycardia, tachypnea, and leucocytes increases as a result of a systemic inflammatory response secondary to a blood stream infection. Infectious organisms including bacteria, viruses, and parasites are known to induce ROS/RNS including NO. Sepsis involves a diffuse inflammatory response with effects on various organ systems, depending on the virulence organism and the inflammatory response of the host. This discussion will focus on the role of NO on hepatocytes in sepsis.
NO in the liver is protective against these microbial invaders in many ways. In sepsis, NO is able to invade these organisms and cause damage to lipids, proteins, enzymes, and DNA [161]. Nitric oxide ameliorates the oxidative stress from xenobiotics such as hypochlorite (ClO−) and carbon tetrachloride (CCL4) by decreasing lipid peroxidation and collagen deposition [118]. Treatment with L-arginine was found to attenuate collagen formation, increase in liver enzymes, and glycogen depletion, but had no effect on lipid peroxidation [118]. IFN-γ is able to decrease intracellular plasmodial parasites in malaria-infected hepatocytes [105], while the addition of arginase and L-NMMA ameliorates this effect [105, 124].
In septic shock, NO has been shown to be protective. Specifically, nonspecific NOS inhibition (L-NMMA and L-NAME) results in increased hepatic damage, while NO donors ameliorate this damage [8, 47, 48, 65, 73]. Although the results are not exactly clear, inhibition of iNOS has been noted to increase hepatic apoptosis [128] and limit PMN accumulation [52]. iNOS expression is necessary for immune-mediated liver injury in mice [148] and NO scavengers (NOX) reduced LPS-induced liver damage [119]. It is, again, likely to be related to the source, amount, and duration of NO exposure, as are the beneficial versus detrimental effects of NO in sepsis. Endothelial NO appears important to the maintenance of vascular homeostasis and the prevention of WBC and platelet aggregation. NO from Kupffer cells, on the other hand, is associated with the increased production of TNF-α. Specifically TNF-α/D-Gal itself can lead to hepatic damage [148].
Initially it was thought that inhibitors of iNOS may improve outcomes in shock, specifically in septic shock, by decreasing the activation of downstream cytokines. Unfortunately, the results were not as expected. While smaller studies had suggested a potential benefit of NOS inhibitors in cardiogenic shock, [20] these results were not seen in larger studies. In a study of septic shock, L-NMMA was noted to cause an increase in vascular tone and blood pressure; however, it also caused a reduction in cardiac output, which is of concern for overall tissue perfusion [133]. Nonselective NOS inhibition was examined again in septic patients and was associated with an increase in mortality [95]. Most recently, L-NMMA was used to treat patients with refractory cardiogenic shock, but the study was terminated because of futility [1]. Therefore, while iNOS is associated with increased inflammatory response, inhibition of NO in the clinical setting does not seem to improve outcomes. Better understanding of the roles of NO and the signaling pathways involved in shock are necessary in order to develop more effective treatment options.
Fibrosis
Hepatic fibrosis is a condition associated with increased extracellular matrix (ECM) proteins, including collagen type I and III, proteoglycans, fibronectin, and laminin. Under normal conditions, matrix metalloproteinases (MMPs), produced by stellate cells, regulate the production of the ECM. In pathologic conditions with noted fibrosis, the deposition of collagen exceeds the ability of MMPs to absorb it and thus, extra collagen deposits and scars form. Fibrosis has been well documented in many chronic liver diseases, including hepatitis, cholestatic disease, hemochromatosis, steatohepatitis, and chronic venous outflow obstructions. These conditions usually begin with an inflammatory phase, which progresses to fibrosis after chronic oxidative stress [85].
During the inflammatory phase, cytokines, chemokines, and ROS are then followed by PMN infiltration and iNOS upregulation. Lymphocytes are also recruited via the portal tract, sinusoids, and hepatic vein. Leukocytes and Kupffer cells are able to produce large amounts of NO and cytokines, especially TGF-β and TNF-α. The influx of Kupffer cells coincides with the activation of stellate cells. Activation leads to increased proliferation, motility, contractility, and synthesis of ECM.
Oxidative stress can activate stellate cells, which are collagen-producing cells in the liver. Specifically, 4-hydroxynonenal is a profibrotic stimulus, which upregulates procollagen and tissue inhibitor metalloproteinase-1 (TIMP1) gene expression [181]. Stellate cells have also been implicated in the regulation of blood flow and tone in the liver. Capillarization of the sinusoids is a condition in which ECM proteins deposit in the sinusoids of the liver. This deposition is a result of stellate cells and has been is associated with portal hypertension and fibrosis [27]. In fibrosis, it appears this loss of parenchyma precedes the onset of venous obstruction, so therapeutic interventions aimed at the prevention or treatment of fibrosis requires an understanding of the activation and action of stellate cells.
The exact mechanism of stellate cell activation is unclear, but associated proteins have been identified. It appears that a zinc finger molecule, KLF6, is involved in activation of many cells, including stellate cells [142]. DDR2 has also been implicated in the upregulation of MMP expression and downstream activation of stellate cells [127]. Stellate cells themselves are also able to express immune proteins (HLA-II), to internalize macromolecules, and to affect T cell activation. In cirrhosis patients, in particular, HLA-II and CD40 expression is noted [172]. Stellate cells can process antigens and are implicated in antigen presentation, internalization of macromolecules, and modulation of T-lymphocyte proliferation [172].
Cytokines play a critical role in the formation of fibrosis. TGFβ is produced by the Kupffer and stellate cells. TGFβ mediates the further activation of stellate cells and the influx of PMS, both of which are necessary for fibrosis formation. TGFβ then upregulates collagen gene expression, expression of TIMP-1, and further expression of TGFβ. Activation of TGF receptors lead to phosphorylation of SMAD proteins. TGF β may be stimulated by IL-6 and ROS in cirrhotic patients, which suggests that therapeutic intervention involving the production of TGFβ or the stimuli of TGFβ may prove beneficial in patients with hepatic fibrosis.
The role of NO in fibrosis formation is not as clearly understood. In patients with cirrhosis, increased resistance of portal blood flow has been associated with impaired NO bioavailability. In animal models, shear-induced NO production from eNOS was attenuated compared to that of control mice. Decreased NOS activity, with increased eNOS-caveolin binding, was also noted although eNOS protein levels were the same as controls [154]. This suggests a decrease in the function of eNOS, resulting in a relative state of vasoconstriction. ROS are also implicated in reduced NO bioavailability in cirrhotic livers [43]. Koreuk et al. noted a decreased SOD level and increased serum NO level in decompensated cirrhosis patients, compared to compensated cirrhosis patients and healthy controls, indicating an antioxidant dysregulation [83]. This implies that reduction of ROS by inhibition of oxidative stress may be clinically beneficial in the prevention of further fibrosis. Continued research is needed to thoroughly understand the process of fibrosis; the roles played by cytokines and NO need to be established before meaningful therapeutic targets can be identified.
Iron overload
Excessive accumulation of iron can result from multiple conditions, including hemochromatosis, hereditary sideroblastic anemias, severe α and beta thalassemia, myelodysplastic syndrome (MDS), and chronic liver diseases. The excess accumulation of iron disturbs the redox equilibrium in the liver and results in oxidative stress. Specifically, accumulation of iron can cause increased oxidation of membrane lipids and subcellular organelles, such as the mitochondria, leading to structural and/or functional impairment and cellular injury [5]. Iron is a transition metal that, when it reacts with oxygen via the Haber-Weiss reaction, is able to create reactive oxygen species. (Table 1) The catalytic properties of iron may also lead to exacerbation of the cytotoxic effects of other ROS-generators, such as chemicals including alcohol, viruses, or other toxins. For example, Tsukamoto et al. noted that animals with alcohol infusions and iron supplementation had increased hepatocyte damage, liver fibrogenesis, and cirrhosis, suggesting a significant interaction between iron and iron-catalyzed oxidant stress noted from lipid peroxidation, increases in procollagen α1, and TGFβ1 [165].
Table 1.
Chemical equations relevant to reactive oxygen and reactive nitrogen species generation.
| Reactive oxygen species generation |
| O2 + e− → O2−• (superoxide anion) |
| O2 −•+ H2O → HO2• (hydroperoxyl radical) |
| HO2• + e− + H → H2O2 (hydrogen peroxide) |
| H2O2 + e− → OH− + •OH (hydroxyl radical) |
| Reactive nitrogen species generation |
| L-arginine + O2 → •NO (nitric oxide) + L-citrulline |
| O2− • + •NO → ONOO− (peroxynitrite) |
| ONOO− + CO2 → ONOOCO2− (nitrosoperoxy carbonate) |
| ONOOCO2− → •NO2 (nitrogen dioxide) + CO3− • (carbonate anion radical) |
| Fenton reaction (catalyzed by transition metals) |
| H2O2 + Fe2+ → OH− + •OH + Fe3+ |
| Haber-Weiss Reaction (catalyzed by transition metals) |
| H2O2 + O2− • → O2+ •OH + OH− |
Reprinted with permission from Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 44:239–267,2004. [79]
The reactive oxygen species created from the Haber-Weiss reaction can then cause lipid peroxidation and the formation of lipid hydroperoxides forms peroxyl-(ROO·) and alkoxyl-(RO·) free radicals. In cases of iron overload, relatively high levels of malonaldehyde and low levels of other toxic hydroxyalkenals are noted [123, 138]. High level of etheno-DNA adducts, a measure of lipid peroxidation, are also noted in these patients. Some patients with hemochromatosis also have increased iNOS expression in the liver, suggesting another source of RNS, although there are limited data on the role of NO in the hemochromatosis [67]. In patients with hemochromatosis, high HNE-protein and MDA-proteins levels are also noted [126]. Chronic accumulation of iron is associated with low cytolysis, progressive fibrosclerosis, cirrhosis, and hepatocellular carcinoma. In animal models, increased iron deposition is associated with increased lipid peroxidation and collagen type 1 deposition [6]. This pattern of iron deposition, occurring mainly in the periportal hepatocytes in rats, closely approximates that of hereditary hemochromatosis seen in humans [131, 136].
Hemochromatosis is associated not only with increased markers of lipid peroxidation but also with decreased levels of antioxidants, including ascorbate, retinol, and α-tocopherol [179]. This iron-mediated toxicity and fibrosis seen in the animal model may be attenuated by antioxidant treatments, including vitamin E or flavonoid silibin [135]. In animal models of iron overload, enhanced TGF-β1 expression was also attenuated by antioxidant treatment [63, 135].
The excess iron may also result in DNA damage from oxidative stress leading to an increased incidence of cancer. The incidence of hepatocellular carcinoma is increased 200-fold in patients with hemochromatosis [122]. Increased p53 mutations in hepatic tissue from hemochromatosis patients strongly suggest a correlation between oxidative damage from iron overload and cancer formation, but further research is necessary to fully understand the mechanism by which this occurs [67, 96]. Morrogi et al. [67] noted various p53 mutations such as G:C to T:A transversions at codon 249 and C:G to A:T and C:G to T:A changes at codon 250 in patients with hemochromatosis or Wilson’s disease. While the relationship between p53 mutation and oxidative stress is compelling, a more complete understanding of the role of oxidative stress and carcinogenesis is needed.
Wilson’s Disease
Wilson’s disease is an autosomal recessive condition characterized by impaired biliary excretion, and thus, excessive hepatic deposition of copper. It is believed that this accumulation of copper results in excessive oxidation and free radical generation, resulting in hepatic damage. As the hepatocytes are damaged, copper spills into the blood and deposits in other organs including the brain, heart and kidney, resulting in subsequent end-organ impairment. As copper enters the blood, ceruloplasmin levels decrease as copper is bound. It is believed that excess copper leads to increased free radical generation, but this is still only theory. Sixty percent of patients with Wilson’s disease were also noted to have increased iNOS expression, another possible source of oxidative stress other than the copper itself [67]. These patients also had increased levels of allantoin, an oxidation product of uric acid, and decreased levels of antioxidants including ascorbate and urate [125]. Increased lipid peroxidation and copper content have been documented and the mitochondrial copper content in these samples correlated with the severity of mitochondrial lipid peroxidation. Hepatic α-tocopherol, an antioxidant, was decreased significantly in hepatocytes of Wilson’s disease patients [155]. Hussain et al. also noted increased p53 mutation in nontumorous hepatocytes in Wilson’s patients [67]. Nair et al. showed a correlation between etheno adducts, DNA damage, and copper content in patients with Wilson’s disease. Limited data are available on this small group of patients, but it appears that, due to excess copper accumulation, they are under increased oxidative stress, as seen by measures of lipid peroxidation and DNA damage; this stress may then lead to increased p53 mutation and possibly cancer formation.
Acetaminophen-induced Liver Damage
Acetaminophen (paracetamol) overdose is an uncommon, but potentially devastating, condition characterized by centrilobular hepatic necrosis after excessive consumption of acetaminophen [9, 45]. Acetaminophen is metabolized by sulfation and glucuronidation and is a substrate for CYP2E1. Acetaminophen reacts with CYP2E1 creating quinoneimine, which binds to −SH groups, especially those on glutathione. As glutathione levels decrease, quinoneimine then binds other plasma membrane proteins, mitochondrial proteins, and other protein complexes and this leads to increased intracellular calcium and cell death [46, 140].
NO produced from macrophages, cytokines, superoxide, and peroxynitrite are all implicated in this damage [61, 62, 88, 89]. The destruction of Kupffer cells by gadolinium chloride ameliorates this damage [109]. By the depletion of GSH, the disruption of the mitochondria, and the formation of ONOO−, acetaminophen is able to cause quite an oxidative stress on hepatocytes [80]. iNOS knockout mice and aminoguanidine treated rats showed decreased hepatic damage, [35, 36] although complete inhibition of NOS by L-NMMA demonstrated increased liver damage. Treatment with a liver–selective NO donor (V-PYRRO/NO) also demonstrated decreased lipid peroxidation and hepatic damage [94], which leaves the exact role of NO in acetaminophen induced liver damage still in question.
Acetaminophen overdose is treated with N-acetylcysteine (NAC), a precursor of glutathione. NAC increases glutathione stores, limits the formation of NAPQI by direct binding and increases sulfate conjugations [12, 92, 111]. NAC also functions as an anti-inflammatory and antioxidant by modulating cytokines and scavenging free radicals [49, 76]. NAC is also able to improve hemodynamics and oxygen transport in patients with fulminant hepatic failure [50]. The key to treatment is prompt diagnosis and treatment with NAC. Without immediate intervention, substantial hepatic necrosis may develop, necessitating a liver transplant.
Implication for future interventions
Biomarkers/Proteomics
In order to identify and treat hepatic conditions associated with increased oxidative stress, it is important to identify the genes/proteins whose expression/function serves as markers of oxidative stress. The lack of thorough understanding of the mechanism by which oxidative stress results in hepatic injury limits the identification of unique therapeutic targets. Nonetheless, current proteomic strategies, especially in the emerging area of redox proteomics, promise further understanding of possible molecular markers of disease and future therapeutic targets.
Biomarkers of oxidative stress (lipid peroxidation, DNA damage, certain protein isolation/expression) are helpful in identifying a physiologic state over time. The measurement of biomarkers allows elucidation of the presence of oxidative stress but not necessarily the etiology of that stress. The presence of oxidative stress may be suggestive of causation but, by using biomarkers alone, only a correlation, not causation, can be drawn. Many of the studies previously mentioned in this review of liver pathology noted only the correlation between markers of oxidative stress and liver pathology so, while causation can be inferred, it cannot be accepted as proven fact.
In addition to biomarkers, one can also measure gene expression in relation to oxidative stress. Current research is focusing on the specific gene modifications that are known to be associated with redox biology, such as c-jun [21] and NF-κB [160]. This approach allows close examination of one gene during various periods of stress, but limits the researcher to the gene already identified as being of interest [116]. Rigorous examination of individual genes is necessary for systematic knowledge of specific interactions, but the complexity of convergent signaling cascades is lost. Identification of new genes not previously known to be associated with oxidative stress is also difficult.
In order to obtain a more complete understanding of gene regulation as it relates to oxidative stress, one may utilize DNA microarrays or proteomics in order to see the effects of a particular oxidative stress on the expression of thousands of genes or proteins. The use of proteomics for the study of molecular modifications secondary to oxidative stress may be termed redox proteomics. One of the first studies that focused on redox proteomics examined the effects of oxidative stress on human epithelial lens cells and noted that several cytoskeletal proteins and enzymes that showed differential expression profiles as a result of H2O2 oxidation [132]. Subsequently, Vascotto et al. performed a proteomic analysis of liver tissue subjected to early ischemia/reperfusion injury during human liver transplantation and found 36 proteins that were significantly altered upon injury [171]. These proteins tended to be those involved in lipid and energy metabolism, in different metabolic pathways, in redox signaling, and in oxidative-stress response [171] (Table 2). Some of these proteins were previously known to have been associated with ischemia/reperfusion injury while others were not. Identification of these proteins allows for further understanding of the early response of the liver to reperfusion and identification of future therapeutic targets of early damage from oxidative stress.
Table 2.
Identification of various proteins upregulated by redox stress in hepatocytes via redox proteomics. Reprinted with permission from Vascotto C, Cesaratto L, D’Ambrosio C, Scaloni A, Avellini C, Paron I, Baccarani U, Adani GL, Tiribelli C, Quadrifoglio F, Tell G. Proteomic analysis of liver tissues subjected to early ischemia/reperfusion injury during human orthotopic liver transplantation. Proteomics. 6:3455–3465,2006. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. [171]
| Protein Number | Protein identity | Swiss-Prot entry | MW | P/ | % Seguence coverage | T2 vs T1, ratio | #of patients/9 | Function | Subcellular localization |
|---|---|---|---|---|---|---|---|---|---|
| a) Lipid metabolism | |||||||||
| 1 | 3,2 trans enoyl CoA isomerase | P42126 | 35 (33) | 5.8 (8.8) | 39 | 4.79 | 2 | Lipid metabolism | Mitochondrial |
| 2 | Apolipoprotein A1 | P02647 | 30 (31) | 5.0 (5.6) | 38 | 14.45 | 2 | Lipid metabolism | Plasma |
| 3 | ACADV very long chain | P49748 | 70 (70) | 7.4 (8.9) | 19 | 1.85 | 1 | Lipid metabolism | Mitochondrial |
| 4 | HCD2 | Q99714 | 30 (27) | 6.9 (7.7) | 34 | 2.09 | 1 | Lipid metabolism | Membrane |
| 5 | Long-chain-fatty acid CoA ligase 1 | P33121 | 80 (78) | 6.4 (6.8) | 32 | 0.42 | 1 | Lipid metabolism | Membrane |
| b) Energy metabolism | |||||||||
| 6 | Fructose-biphosphate aldolase B | P05062 | 40 (39) | 5.9 (8.1) | 49 | 5.68 | 3 | Glycolysis | Cytoplasmic |
| 7 | G3P2 | P04406 | 16 (36) | 5.9 (8.6) | 29 | Q | 1 | Glycolysis | Cytoplasmic |
| 8 | Methyl malonate semialdehyde dehydrogenase | Q02252 | 30 (58) | 6.2 (8.7) | 13 | Q | 1 | Metabolic pathways | Mitochondrial |
| 9 | ODPB component beta subunit | P11177 | 40 (39) | 5.3 (6.2) | 26 | 4.37 | 1 | Tricarboxylic acid cycle | Mitochondrial |
| 10 | KHK isoform C | P50053 | 35 (33) | 5.6(5.6) | 39 | 4.07 | 1 | Dietary fructose metabolism | Cytoplasmic |
| 11 | Phosphoglucomutase | P36871 | 85 (61) | 6.0 (6.3) | 22 | 0.24 | 1 | Glucose metabolism | Cytoplasmic |
| 12 | ETFB beta subunit | P38117 | 30 (28) | 8.0 (8.2) | 35 | 3.61 | 1 | Electron transport | Mitochondrial |
| 13 | CYB5 | P00167 | 16 (15) | 4.5 (4.9) | 49 | Q | 1 | Electron transport | ER |
| c) Redox | |||||||||
| 14 | GST A1 | P08263 | 18 (25) | 7.4 (8.9) | 35 | 9.88 | 3 | Conjugation of reduced glutathione | Cytoplasmic |
| 15 | PRDX5 | P30044 | 20 (22) | 6.6 (8.8) | 36 | 1.92 | 1 | Peroxidase activity | Mitochondrial, peroxisomal an Cytoplasmic |
| 16 | GST A2 | P09210 | 30 (26) | 8.5 (8.5) | 36 | 2.14 | 1 | Conjugation of reduced glutathione | Cytoplasmic |
| 17 | BLVRB | P30043 | 25 (22) | 7.1 (7.3) | 62 | 1.87 | 1 | Biliverdin reductase activity | Cytoplasmic |
| 18 | ADHA alpha chain | P07327 | 25 (40) | 8.0 (8.6) | 20 | 9.61 | 5 | Alcohol oxidation | Cytoplasmic |
| 19 | ADH4 | P08319 | 45 (42) | 7.2 (8.2) | 16 | 4.27 | 1 | Alcohol oxidation | Cytoplasmic |
| d) Protein and aminoacid metabolism | |||||||||
| 20 | SPYA | P21549 | 30 (43) | 5.9 (8.6) | 24 | 2.75 | 2 | Aminotransferase activity | Peroxisomal |
| 21 | 4-Hydroxyphenylpiruvate dioxygenase | P32754 | 50 (45) | 6.1 (6.5) | 32 | 0.46 | 2 | Tyr, Phe catabolism | Cytoplasmic |
| 22 | Ornithine carbamoyl transferase | P00480 | 45 (40) | 7.0 (8.8) | 20 | Q | 1 | Urea cycle, Arg biosynthesis | Mitochondrial |
| 23 | 3-Phosphoglycerate dehydrogenase | Q43175 | 65 (57) | 6.0 (6.3) | 28 | 0.34 | 1 | Ser biosynthesis | Cytoplasmic |
| e) Molecular chaperone | |||||||||
| 24 | Peptidyl-prolyl cis-trans iso- merase A (Cyclophilin A) | P05092 | 20 (18) | 7.0 (7.8) | 29 | 1.74 | 1 | Protein folding | Cytoplasmic |
| 25 | Heat shock cognate 71 kDa protein | P11142 | 20 (71) | 6.0 (5.4) | 13 | Q | 1 | Molecular chaperone | Cytoplasmic-nuclear |
| f) Metabolic pathway | |||||||||
| 26 | Liver carboxyl esterase | P23141 | 65 (63) | 5.9 (6.1) | 23 | 7.64 | 2 | Metabolic pathways | EB |
| 27 | CAH1 | P00915 | 35 (29) | 6.5 (6.6) | 43 | 3.47 | 2 | Carbonate dehy dratase activity | Cytoplasmic |
| 28 | C-1- tetrahydrofolate synthase | P11586 | 115 (101) | 6.9 (6.9) | 20 | 2.00 | 1 | Biosynthetic pathways | Cytoplasmic |
| 29 | G6PE | Q95479 | 100 (89) | 6.9 (6.8) | 25 | 1.71 | 1 | Metabolic pathways | ER |
| 30 | Carbonyl reductase | Q9UHY9 | 30 (26) | 7.8 (8.3) | 39 | 1.99 | 1 | Metabolic pathways | Membrane |
| 31 | CAH2 | P00918 | 28 (29) | 6.1 (6.9) | 40 | 6.26 | 1 | Carbonate dehydratase activity | Cytoplasmic |
| 32 | FTHFD | Q75891 | 115 (99) | 5.6 (5.6) | 41 | 0.41 | 1 | Metabolic pathways | Cytoplasmic |
| 33 | Aldo-keto reductase family 1 member C1 | Q04828 | 35:371 | 6.1 (8.0) | 22 | Q | 1 | Metabolic pathways | Cytoplasmic |
| g) Secreted proteins | |||||||||
| 34 | HSA | P02768 | 85 (69) | 5.6 (5.9) | 43 | 9.15 | 3 | Transporter activity | Plasma |
| h) Inflammatory response | |||||||||
| 35 | S10AS | P05109 | 14 (11) | 6.5 (6.5) | 33 | 7.59 | 1 | Calcium ion binding, inflammatory response | Cytoplasm of macrophages |
| i) unknown | |||||||||
| 36 | ES1 protein homologue | P30042 | 30 (28) | 6.6 (8.5) | 55 | 2.08 | 1 | Unknown | Mitochondrial |
Avellini et al. also examined the effects of oxidative stress on protein profiles of hepatic tissue and noted modification in multiple peroxiredoxin proteins including PrxI, PrxII, PrxVI [4]. These proteins function as hydrogen peroxide scavengers, so it is not surprising to see modifications after hydrogen peroxide treatment. Further studies that more closely approximate warm and cold ischemia are needed to more closely simulate the actual oxidative stress experienced by the cells and to make further inferences as to the implications for protein and gene modifications.
Further studies are needed to elucidate the role of the above noted proteins in ischemia/reperfusion injury and the interaction between these proteins and the known signaling pathways involved in oxidative stress. Future studies in redox proteomics are also needed in other liver pathologies, such as alcohol induce liver injury, viral hepatitis, and hepatocellular carcinoma.
Antioxidants
While endogenous antioxidants are critical for the regulation of hepatic homeostasis, exogenous antioxidants have been of limited usefulness in the treatment of hepatic injury. Studies, as outlined below, have used antioxidants to prevent and/or treat liver injury related to ROS but have had mixed results. [40] The data are difficult to interpret due to mixed and/or varied patient populations, different types and amounts of antioxidant therapy, and differing outcome measures, but some of these studies show the promise of antioxidants for amelioration of hepatic injury arising from oxidative stress.
Alcohol-Induced Liver Disease
To treat alcohol-induced liver disease, some researchers have focused on the administration of a single antioxidant, while others have advocated for combination antioxidant therapy. The type of antioxidant is usually driven by previous studies, indicating a relative antioxidant deficiency in patients with this condition.
In a promising study, Wenzel et al. treated patients suffering from acute alcohol-induced hepatitis with D-α tocopherol, selenium, and zinc. Compared to controls, the patients receiving adjuvant antioxidant therapy had decreased mortality from 40% to 6.5% [177]. Results from other studies investigating the potential of combined antioxidant therapy on progression of alcohol-induced liver disease were not as promising. Treatment of severe alcoholic hepatitis with a combination of antioxidants versus corticosteroids noted a decreased short term (1 month) but not a longer term (1 year) mortality and sepsis in the corticosteroid treated patients [134]. This study did not have a steroid and antioxidant group; thus, an appropriate control group was lacking, until Stewart et al. examined the effects of antioxidant therapy alone or with corticosteroids in severe alcoholic hepatitis [157]. The antioxidant therapy included N-acetylcysteine, vitamin A-E, biotin, selenium, zinc, manganese, copper, magnesium, folic acid, and coenzyme Q, none of which improved 6-month survival when given alone or in combination with corticosteroids.
Vitamin E supplementation for alcohol-induced liver disease does not look promising as a therapeutic intervention. de la Maza conducted a study treating decompensated ambulatoryalcoholic cirrhotic with vitamin E supplementation and found no change in hepatic laboratory values, mortality, or hospitalization rates in these patients [23]. Treatment of mild to moderate alcoholic hepatitis with vitamin E was found to decrease hyaluronic acid level but showed no other improvement in liver function test [108]. A meta-analyses by Miller et al. noted an increase in all-cause mortality after high-dose vitamin E supplementation in patients with a variety of medical conditions, but the significance of this finding is unclear with such a large heterogeneity of patients [110].
S-adenosylmethionine (SAMe) is an antioxidant that functions as a methylating agent, a precursor for glutathione, and a modulator of cytokine metabolism. Patients with alcohol-induced liver disease treated with SAMe were noted to have improved mortality and decreased need for transplantation [99]. Specifically, when patients with severe liver disease (Child class C) were excluded, overall mortality/liver transplantation was significantly greater in the placebo group (29%) verses the SAMe group (12%) and the differences lasted through 2 year follow-up [99].
These studies, taken in combination, suggest that treatment of alcoholic hepatitis is probably not improved with vitamin E therapy but may be improved with S-adenosylmethionine (SAMe) or a combination of antioxidant therapy but, due to the variability of the studies, firm conclusions cannot be drawn as yet.
NAFLD
In patients with NAFLD, antioxidant therapy has also been used in varying degrees to improve biochemical or clinical evidence of hepatitis. Pamuk & Sonsoz treated NAFLD patientswith N-acetylcysteine and noted an equivocal biochemical responses [130]. Other studies noted that patients treated vitamin E were able to normalized their serum aminotransferase [90], while vitamin E and C in combination were able to decrease fibrosis scores at 6 months [51]. Sanyal et al. evaluated Vitamin E versus vitamin E plus pioglitazone for NAFLD and noted decreased steatosis in both groups, but noted an improved pericellular fibrosis, Mallory’s hyalin, and glucose clearance only in the combination group [147]. Vitamin E and/or adherence to a low-calorie diet was also associated with normalization of transaminases in children with obesity-related NAFLD [168], although a study examining metformin versus Vitamin E or a prescriptive diet in NAFLD patients noted an improvement in the metformin arm, with no benefits noted with vitamin E supplementation. A Cochrane review noted that, due to the small number of trials and to the variability in the methods, assessments, treatments and outcomes, no conclusions could be drawn on the use of antioxidants for the treatment of non-alcoholic fatty liver disease [93].
Viral Hepatitis
For patients with Hepatitis C, ROS/RNS have been strongly implicated in the onset and progression of disease and its malignant transformation, but trials of antioxidants in animals and patients to attenuate HCV infection have had mixed results. von Herbay noted that vitamin E improved aminotransferase status of patients, although no long term follow-up is available [173]. Look et al. found that treatment of HCV infected patient with IFN-α plus vitamin E versus IFN-α alone resulted in a trend toward more complete recovery and decreased viral load in the IFNα plus vitamin E patients. These studies are limited by their size and significance but suggest interesting areas for further study.
The most substantive human trials of antioxidants for chronic HCV infection used a combination of oral antioxidants (glycyrrhizin, schisandra, silymarin, ascorbic acid, lipoic acid, L-glutathione, α-tocopherol) in chronic HCV patients and noted improvement in liver enzymes (44%), decreased viral load (25%), histologic improvement (36%), and improved quality of life [104]. Due to the success of this trial, a phase 2 randomized, double-blinded placebo controlled trial examined the use of the IV and/or oral antioxidants versus placebo in chronic HCV patients. An improvement in liver enzymes, improvement in histology, and a decrease in viral load was found in patients receiving combined oral and intravenous antioxidant therapy but not in patients receiving oral therapy alone [34]. While the route of administration is at issue between these studies, these results do suggest a beneficial effect of antioxidant therapy. Further studies are needed to properly elucidate the types, amounts, and routes of antioxidant drug administration that are most appropriate.
Ischemia/Reperfusion Injury
There have been many antioxidants that have been shown beneficial effects in ischemia reperfusion injury in animals, although few of these have been properly studied in humans. Vitamin E, often used interchangeably with α-tocopherol, is a nutritional term referring to eight different substances with similar fat-soluble properties and biochemical properties. The most effective form of vitamin E is RRR-α-tocopherol (alpha-tocopherol), which is the most biologically active form. Alpha-tocopherol is an antioxidant that functions by scavenging free radicals, increasing GSH, inhibiting protein kinase C, and attenuating lipid peroxidation [11, 14, 98, 143]. Alpha-tocopherol is decreased after reperfusion and pretreatment with α-tocopherol improves ATP levels, prevents lipid peroxidation product formation, attenuates glutathione loss, and increases survival [42, 97, 156]. Alpha-tocopherol analogs also show beneficial effects, as does the combination of α-tocopherol with pentoxyphylline [30, 170] (Table 3).
Table 3.
List of Antioxidants Treatments with beneficial effects in liver ischemia/reperfusion injury.
| List of Antioxidant Treatments With Beneficial Effects in Liver Ischemia/Reperfusion Injury | |||||||
|---|---|---|---|---|---|---|---|
| Antioxidant | Category | Species | Injury Type | Mode of Adm A | Dose | Main Protective Effect | Author |
| α-Tocopherol | Vitamin-diet | Rat | WIB | Im | 30 and 300 mg/kg | Survival, histology | Giacoustidis[39] |
| α-Tocopherol | Vitamin-diet | Rat | CIC/WIB | iv | 50 IU/Kg | Histology | Gondolesi[42] |
| α-Tocopherol/Ascorbate | Vitamins-diet | Clinical | WIB | iv | 2 mg/1,000 mg | Better PT ↓Postop. complications | Cerwenka[15] |
| Ascorbate | Vitamin-diet | Rat | WIB | iv | 30 and 100 mg/kg | ↓Lipid peroxidation | Seo[153] |
| Coenzyme Q/Pentoxyfylline | In vivo LMMD agent | Rat | WIB | in/ip | 10 mg/kg/50 mg/kg | ↓Lipid peroxidation | Portakal[139] |
| Idebenone | Coenzyme Q derivative | Pig | CIC | ↑GSH levels | Schutz[151] | ||
| Lipoic acid | In vivo LMM D agent | Rat | CIC | iv | 500μM | Histology | Muller[117] |
| Deferrioxamine | Iron chelator | Dog | CIC/WIB | iv | 20 mg/kg | ↓AST activity | Park[131] |
| Trimetazidine | Metal chelator | Rat | WIB | iv/ip | 2.5 mg/kg | Histology | Tsimoyiannis[163] |
| Quercetin | Plant phenol | Rat | WIB | po | 0.13 mmol/kg | ↓ALT, AST | Su[158] |
| Cyanidin | Plant phenol | Rat | WIB | po | 0.9 mmol/kg | ↓Lipid peroxidation | Tsuda[164] |
| Green Tea Extracts | Plant extracts (catechines) | Rat | WIB | po | 0.1% | Histology | Zhong[182] |
| Magnifera indica | Plant extract | Rat | WI B | po | 250 mg/kg | ↓AST, ALT ↓ Lipid peroxidation | Sanchez[146] |
| GSH | In vivo LMM D agent | Rat | WIB | iv | 100μM/h/kg | ↓ALT↑ Survival | Schauer[149] |
| GSH | In vivo LMM D agent | Rat | CIC/WIB | iv | 100μM/h/kg | ↓ ALT↑ Bile flow | Schauer[150] |
| N-acetylcysteine | Thiol compound GSH precursor | Rabbit | WIB | iv | 150 mg/kg | ↓ ALT↑ Microcirculation | Glantzounis[40] |
| N-acetylcysteine/Melatonin | Thiol compound | Rat | WIB | ip | 150 mg/kg/10 mg/kg | ↓ AST, ALT↓ Lipid peroxidation | Sener[152] |
| N-acetylcysteine | Thiol compound | Clinical | CIC/WIB | iv | ↓ ICAM,E↓ α-GSTF | Weigand[175] | |
| Bucillamine | Thiol compound | Rat | CIC/WIB | iv | Survival | Amersi[2] | |
| SOD derivatives | Intracellular enzyme | Rat | WIB | iv | 5,000 IU/Kg | ↓Lipid peroxidation | Nguyen[121] |
| CAT derivatives | Intracellular enzyme | Rat | WIB | iv | 0.1 mg/kg | ↓ ALT, AST | Yabe[178] |
| Allopurinol | XOG Inhibitor | Rat | WIB | ip | 50 mg/kg | ↓ AST | Jeon[71] |
| Aminoguanidine | iNOS inhibitor | Pig | CIC/WIB | iv | 10 mg/kg | Survival, histology | Kimura[78] |
Adm., administration;
WI, warm ischemia;
CI, cold ischemia;
LMM, low molecular molecule;
ICAM-1, intercellular adhesion molecule-1;
α-GST, α-glutathione S-transferase;
XO, xanthine oxidase;
Reprinted with permission from Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. [40]
Vitamin C supplementation has had mixed results in the prevention of ischemia/reperfusion injury. Vitamin C, also known as ascorbic acid, is synthesized from l-gulono-γ-lactone and oxygen thus creating l-ascorbate and hydrogen peroxide. This reaction occurs in plants and some animals, but humans are not able to synthesize ascorbate so intake is diet-dependent. In addition to regular dietary intake, low doses of vitamin C supplementation have been shown to decrease liver peroxidase activity while high dose vitamin C has been shown to increase ischemia/reperfusion injury, perhaps from the excess reduction of iron [153]. One clinical study demonstrated improved PT time and decreased complication in patients supplemented with an antioxidant vitamin infusion containing α-tocopherol acetate and ascorbate after liver resection [15] (Table 3). Currently, studies on vitamin C supplementation are preliminary with mixed results. Further systemic evaluation is necessary before conclusions can be drawn on the potential benefits of supplementation.
Melatonin is another antioxidant that is normally produced in the pineal gland and is known to regulate circadian rhythms. The precursor is serotonin, which, once methylated and acetylated, becomes melatonin. It has mild antioxidant effects through inhibitory action on lipid peroxidation. Melatonin was noted to inhibit iNOS, reduce TNF-α, and preserve functional status in mice [144]. When melatonin was given to a small cohort of patients after liver resection, there was enhanced neutrophil apoptosis and neutrophil responsiveness[17]. Again, these studies are small and need to be followed in larger clinical trials with measures of clinical outcomes beforeconclusions can be drawn.
Glutathione is a ubiquitous tripeptide important in many metabolic processes, including ascorbate metabolism, gap junction communication, and antioxidant protection of thiol-protein groups. Glutathione can react with hydroxyl radicals, hypochlorous acid, peroxynitrate, and carbon radicals, but not with superoxide radicals. Because glutathione reacts with so many reactive oxygen species and reactive nitrogen species, it is an effective antioxidant and its measurement can be a useful assessment of redox biology. Glutathione peroxidase is a major antioxidant enzyme in the cell and thus the administration of GSH would be expected to produce beneficial antioxidant effects. Unfortunately, initial studies have not shown any protective effects in the setting of warm ischemia[18], which may be due to limited cellular uptake of the large molecule. On the other hand, recent studies have noted a protective effect of intravenous large doses of glutathione in the prevention of warm and cold ischemic injury [149, 150]. N-acetylcysteine is the precursor to glutathione and can be administered with effective uptake, but the effects on ischemia-reperfusion injury in the liver are unclear [16, 82]. NAC acts by allowing GSH synthesis, cysteine metabolism, and the scavenging of free radicals [115]; thus, it is used to treat a variety of conditions including cystic fibrosis and acetaminophen overdose. In rabbit models, intravenous NAC administration improved tissue oxygenation, microcirculation, alanine aminotransferase activity, and indocyanine green clearance [41]. Clinical trials of intravenous NAC have also had equivocal results, but three studies did document improved liver function, decreased liver injury, less graft dysfunction, less reject, and/or decreased hospital stay [13, 162, 175] (Table 3).
SOD treatment has also shown mixed results, probably again due to the difficulty of getting SOD into the cells. SOD is not known to cross cell membranes but in order for it to consume superoxide, it would have to enter the cell or superoxide would have to leave the cell. Of the four animal studies conducted, two showed beneficial effects [3, 120] while two showed no effect [86, 113].
Inhibitors of iNOS have shown promising results in animal models of ischemia reperfusion injury [69, 78]. In a pig model of warm ischemia, iNOS inhibition decreased aspartate aminotransferase levels after reperfusion, inhibited nitrotyrosine expression, and attenuated hepatic damage [69]. In a pig model of cold ischemia, iNOS inhibition resulted in decreased serum nitrate/nitrite and AST. There were also decreased hepatic damage, thrombi, and iNOS staining compared with control animals [78]. The theory is that increased expression of iNOS leads to increased peroxynitrite (ONOO−) production that is out of proportion to the antioxidant capacity of the liver and that leads to damaging peroxynitrite production and cellular damage. Targeting peroxynitrite production through superoxide dismutase, iNOS, or peroxynitrite scavengers may prove beneficial.
Other antioxidant agents that have been shown to have beneficial effects in liver ischemia reperfusion injury include coenzyme Q/pentoxyfylline [139], idebenone [151], lipoic acid [117], desferrioxamine [131], trimetaxidine [163], quercetin [158], cyanidin [164], plant extracts [164, 182], bucillamine [2], SOD derivatives [121], CAT derivatives [178], allopurinol [71], and aminoguanidine [78]. (Table 3) Allopurinol at high [71], but not low doses [70], have shown some beneficial effects in preventing liver damage, but again, the results are preliminary, with more systematic in depth evaluation needed.
Conclusion
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are clearly important for normal physiological function of hepatocytes and are involved in physiological metabolism including oxidative respiration, cell signaling, and protein modification required for normal cellular growth, regeneration, apoptosis, and microsomal defense. However, when production of ROS/RNS overwhelms consumption, oxidative stress develops. In the form of ROS and RNS, this stress can damage all of the cells present in the liver, including hepatocytes, Kupffer cells, stellate cells, and endothelial cells, by induction of inflammation, ischemia, fibrosis, necrosis, apoptosis, or malignant transformation through damage to lipids, proteins, and/or DNA. This is seen in the setting of hepatitis, ischemia/reperfusion injury, fibrosis, iron overload, Wilson’s disease, sepsis, and acetaminophen overdose, although the exact mechanism(s) by which ROS/RNS results in the clinical syndromes seen is unclear. Increased protein modification/degradation is seen, but the functional roles of these modifications and their role in signal transduction is poorly understood. Upregulation of cytokines and chemokines both induce and are induced by ROS/RNS, although again the mechanisms are unclear. Further thoughtful, systematic investigation is needed to better understand the role of ROS/RNS in liver disease. In order to better appreciate the role of redox biology in protein and gene expression, redox proteomic and micoarray gene analysis may be able to clarify the complex role of oxidative stress. Continued research is also needed in the areas of antioxidant therapy, in order to appreciate the impact of oxidative stress on disease, and thus, the potential of antioxidant therapy in the attenuation of disease progression.
Abbreviations
- AP
apurinic/apyrimidinic
- AP-1
activator protein-1
- APE/Ref-1
apurinic/apyrimidinic endonuclease/redox factor 1
- ATP
adenosine triphosphate
- ALT
aminotransferase
- Bcl-2
B-cell lymphoma-2
- BER
base excision repair
- BH4
tetrahydrobiopterin
- Bid
A BH3 domain-only death agonist protein
- CaM
calmodulin
- CAT
catalase
- cGMP
cyclic guanosine monophosphate
- COX-2
cyclooxygenase-2
- CSF
colony stimulating factor
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- EPO
erythropoietin
- eNOS
endothelial nitric oxide synthase
- ESR
electron spin resonance
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- γ-IRE
γ-interferon response element
- G-CSF
granulocyte colony stimulating factor
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIF
hypoxia-inducible factor
- HO-1
heme oxygenase
- HNE
4-hydroxynonenal
- HSP70
heat shock protein 70
- IL
interleukin
- INFγ
interferon gamma
- im
intramuscular
- iNOS
inducible nitric oxide synthase
- iv
intravenous
- JNK
c-Jun NH2-terminal kinase
- KLF6
a zinc finger molecule
- L-NIL
N-iminoethyl-L-lysine
- L-NMMA
L-N(G)-monomethyl arginine citrate
- L-NNA
L-Nω-nitro-L-arginine
- LPS
lipopolysaccharides
- MAP kinase
mitogen-activated protein kinase
- MAT
methionine adenosyltransferase
- MDA
malondialdehyde
- MDS
myelodysplastic syndrome
- MEOS
microsomal ethanol-oxidizing system
- MMP
matrix metalloproteinases
- MnSOD
manganese-containing superoxide dismutase
- MS
methionine synthase
- NAC
N-acetylcysteine
- NADQI
N-acetyl-p-benzoquinone imine NAFLD, non-alcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NOX
nitric oxide scavenger
- nNOS
neuronal nitric oxide synthase
- NADH
reduced nicotinamide adenine dinucleotide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide; reactive halogen species
- NS5A
non-structural 5A protein
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- OLT
orthotopic liver transplant
- PEG-poly SNO-BSA
polyethylene glycol-conjugated bovine serum albumin
- PMN
polymorphonuclear leukocytes
- po
oral
- PT
prothrombin time
- RHS
reactive hydrogen species
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SAMe
S-adenosylmethionine
- sGC
soluble guanylate cyclase
- SMAD
mothers against decapentaplegic
- SOD
superoxide dismutase
- TGFα/β
transforming growth factor α/β
- TIMP1
tissue inhibitor metalloproteinase-1
- TNFα/β
tumor necrosis factor α/β
- TRAIL
TNF related apoptosis inducing ligand
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
- 1400W
N-(3-aminomethyl)benzyl-acetamindine
- 8-OH-dG
8-hydroxydeoxyguanosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander JH, Reynolds HR, Stebbins AL, Dzavik V, Harrington RA, Van de Werf F, Hochman JS. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657–1666. doi: 10.1001/jama.297.15.joc70035. [DOI] [PubMed] [Google Scholar]
- 2.Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, Horwitz LD, Busuttil RW, Horwitz MA. Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci U S A. 2002;99:8915–8920. doi: 10.1073/pnas.132026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atalla SL, Toledo-Pereyra LH, MacKenzie GH, Cederna JP. Influence of oxygen-derived free radical scavengers on ischemic livers. Transplantation. 1985;40:584–590. doi: 10.1097/00007890-198512000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Avellini C, Baccarani U, Trevisan G, Cesaratto L, Vascotto C, D’Aurizio F, Pandolfi M, Adani GL, Tell G. Redox proteomics and immunohistology to study molecular events during ischemia-reperfusion in human liver. Transplant Proc. 2007;39:1755–1760. doi: 10.1016/j.transproceed.2007.05.082. [DOI] [PubMed] [Google Scholar]
- 5.Bacon BR, Britton RS. The pathology of hepatic iron overload: a free radical--mediated process? Hepatology. 1990;11:127–137. doi: 10.1002/hep.1840110122. [DOI] [PubMed] [Google Scholar]
- 6.Bacon BR, O’Neill R, Britton RS. Hepatic mitochondrial energy production in rats with chronic iron overload. Gastroenterology. 1993;105:1134–1140. doi: 10.1016/0016-5085(93)90959-g. [DOI] [PubMed] [Google Scholar]
- 7.Bauer C, Kuntz W, Ohnsmann F, Gasser H, Weber C, Redl H, Marzi I. The attenuation of hepatic microcirculatory alterations by exogenous substitution of nitric oxide by s-nitroso-human albumin after hemorrhagic shock in the rat. Shock. 2004;21:165–169. doi: 10.1097/01.shk.0000107442.26299.fb. [DOI] [PubMed] [Google Scholar]
- 8.Billiar TR, Curran RD, Harbrecht BG, Stuehr DJ, Demetris AJ, Simmons RL. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990;48:565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- 9.Black M. Acetaminophen hepatotoxicity. Annu Rev Med. 1984;35:577–593. doi: 10.1146/annurev.me.35.020184.003045. [DOI] [PubMed] [Google Scholar]
- 10.Blum H, Osbakken MD, Johnson RG., Jr Sodium flux and bioenergetics in the ischemic rat liver. Magn Reson Med. 1991;18:348–357. doi: 10.1002/mrm.1910180209. [DOI] [PubMed] [Google Scholar]
- 11.Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76:703–716. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- 12.Buckpitt AR, Rollins DE, Mitchell JR. Varying effects of sulfhydryl nucleophiles on acetaminophen oxidation and sulfhydryl adduct formation. Biochem Pharmacol. 1979;28:2941–2946. doi: 10.1016/0006-2952(79)90590-2. [DOI] [PubMed] [Google Scholar]
- 13.Bucuvalas JC, Ryckman FC, Krug S, Alonso MH, Balistreri WF, Kotagal U. Effect of treatment with prostaglandin E1 and N-acetylcysteine on pediatric liver transplant recipients: a single-center study. Pediatr Transplant. 2001;5:274–278. doi: 10.1034/j.1399-3046.2001.005004274.x. [DOI] [PubMed] [Google Scholar]
- 14.Calfee-Mason KG, Spear BT, Glauert HP. Vitamin E inhibits hepatic NF-kappaB activation in rats administered the hepatic tumor promoter, phenobarbital. J Nutr. 2002;132:3178–3185. doi: 10.1093/jn/131.10.3178. [DOI] [PubMed] [Google Scholar]
- 15.Cerwenka H, Khoschsorur G, Bacher H, Werkgartner G, El-Shabrawi A, Quehenberger F, Rabl H, Mischinger HJ. Normothermic liver ischemia and antioxidant treatment during hepatic resections. Free Radic Res. 1999;30:463–469. doi: 10.1080/10715769900300501. [DOI] [PubMed] [Google Scholar]
- 16.Chavez-Cartaya R, Jamieson NV, Ramirez P, Marin J, Pino-Chavez G. Free radical scavengers to prevent reperfusion injury following experimental warm liver ischaemia. Is there a real physiological benefit? Transpl Int. 1999;12:213–221. doi: 10.1007/s001470050213. [DOI] [PubMed] [Google Scholar]
- 17.Chen JC, Ng CJ, Chiu TF, Chen HM. Altered neutrophil apoptosis activity is reversed by melatonin in liver ischemia-reperfusion. J Pineal Res. 2003;34:260–264. doi: 10.1034/j.1600-079x.2003.t01-1-00031.x. [DOI] [PubMed] [Google Scholar]
- 18.Cho WH, Kim DG, Murase N, Mischinger HJ, Todo S, Starzl TE. Comparison of superoxide dismutase, allopurinol, coenzyme Q10, and glutathione for the prevention of warm ischemic injury. Transplantation. 1990;50:353–355. [PMC free article] [PubMed] [Google Scholar]
- 19.Churchill TA, Cheetham KM, Fuller BJ. Glycolysis and energy metabolism in rat liver during warm and cold ischemia: evidence of an activation of the regulatory enzyme phosphofructokinase. Cryobiology. 1994;31:441–452. doi: 10.1006/cryo.1994.1054. [DOI] [PubMed] [Google Scholar]
- 20.Cotter G, Kaluski E, Milo O, Blatt A, Salah A, Hendler A, Krakover R, Golick A, Vered Z. LINCS: L-NAME (a NO synthase inhibitor) in the treatment of refractory cardiogenic shock: a prospective randomized study. Eur Heart J. 2003;24:1287–1295. doi: 10.1016/s0195-668x(03)00193-3. [DOI] [PubMed] [Google Scholar]
- 21.Czaja MJ, Liu H, Wang Y. Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology. 2003;37:1405–1413. doi: 10.1053/jhep.2003.50233. [DOI] [PubMed] [Google Scholar]
- 22.Daughters K, Waxman K, Nguyen H. Increasing nitric oxide production improves survival in experimental hemorrhagic shock. Resuscitation. 1996;31:141–144. doi: 10.1016/0300-9572(95)00922-1. [DOI] [PubMed] [Google Scholar]
- 23.de la Maza MP, Petermann M, Bunout D, Hirsch S. Effects of long-term vitamin E supplementation in alcoholic cirrhotics. J Am Coll Nutr. 1995;14:192–196. doi: 10.1080/07315724.1995.10718493. [DOI] [PubMed] [Google Scholar]
- 24.Dhar DK, Takemoto Y, Nagasue N, Uchida M, Ono T, Nakamura T. FK506 maintains cellular calcium homeostasis in ischemia-reperfusion injury of the canine liver. J Surg Res. 1996;60:142–146. doi: 10.1006/jsre.1996.0023. [DOI] [PubMed] [Google Scholar]
- 25.Doulias PT, Barbouti A, Galaris D, Ischiropoulos H. SIN-1-induced DNA damage in isolated human peripheral blood lymphocytes as assessed by single cell gel electrophoresis (comet assay) Free Radic Biol Med. 2001;30:679–685. doi: 10.1016/s0891-5849(00)00511-6. [DOI] [PubMed] [Google Scholar]
- 26.Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2980–2986. doi: 10.1152/ajpheart.01173.2005. [DOI] [PubMed] [Google Scholar]
- 27.Ekataksin W, Kaneda K. Liver microvascular architecture: an insight into the pathophysiology of portal hypertension. Semin Liver Dis. 1999;19:359–382. doi: 10.1055/s-2007-1007126. [DOI] [PubMed] [Google Scholar]
- 28.Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, Lefer DJ. eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res. 2006;99:78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- 29.Engerson TD, McKelvey TG, Rhyne DB, Boggio EB, Snyder SJ, Jones HP. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987;79:1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eum HA, Lee SH, Lee SM. Trolox C ameliorates hepatic drug metabolizing dysfunction after ischemia/reperfusion. Arch Pharm Res. 2002;25:940–945. doi: 10.1007/BF02977017. [DOI] [PubMed] [Google Scholar]
- 31.Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77:577–592. doi: 10.1007/s001099900029. [DOI] [PubMed] [Google Scholar]
- 32.Farber JL. The role of calcium in cell death. Life Sci. 1981;29:1289–1295. doi: 10.1016/0024-3205(81)90670-6. [DOI] [PubMed] [Google Scholar]
- 33.Fellstrom B, Akuyrek LM, Backman U, Larsson E, Melin J, Zezina L. Postischemic reperfusion injury and allograft arteriosclerosis. Transplant Proc. 1998;30:4278–4280. doi: 10.1016/s0041-1345(98)01412-2. [DOI] [PubMed] [Google Scholar]
- 34.Gabbay E, Zigmond E, Pappo O, Hemed N, Rowe M, Zabrecky G, Cohen R, Ilan Y. Antioxidant therapy for chronic hepatitis C after failure of interferon: results of phase II randomized, double-blind placebo controlled clinical trial. World J Gastroenterol. 2007;13:5317–5323. doi: 10.3748/wjg.v13.i40.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang XJ, DeGeorge GL, Laskin JD, Laskin DL. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology. 1998;27:748–754. doi: 10.1002/hep.510270316. [DOI] [PubMed] [Google Scholar]
- 36.Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordon MK, Gerecke DR, Zhou P, Laskin DL. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol Appl Pharmacol. 2002;184:27–36. [PubMed] [Google Scholar]
- 37.Gauthier TW, Davenpeck KL, Lefer AM. Nitric oxide attenuates leukocyte-endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am J Physiol. 1994;267:G562–568. doi: 10.1152/ajpgi.1994.267.4.G562. [DOI] [PubMed] [Google Scholar]
- 38.Geller DA, Chia SH, Takahashi Y, Yagnik GP, Tsoulfas G, Murase N. Protective role of the L-arginine-nitric oxide synthase pathway on preservation injury after rat liver transplantation. JPEN J Parenter Enteral Nutr. 2001;25:142–147. doi: 10.1177/0148607101025003142. [DOI] [PubMed] [Google Scholar]
- 39.Giakoustidis D, Papageorgiou G, Iliadis S, Kontos N, Kostopoulou E, Papachrestou A, Tsantilas D, Spyridis C, Takoudas D, Botsoglou N, Dimitriadou A, Giakoustidis E. Intramuscular administration of very high dose of alpha-tocopherol protects liver from severe ischemia/reperfusion injury. World J Surg. 2002;26:872–877. doi: 10.1007/s00268-002-6271-2. [DOI] [PubMed] [Google Scholar]
- 40.Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11:1031–1047. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- 41.Glantzounis GK, Yang W, Koti RS, Mikhailidis DP, Seifalian AM, Davidson BR. Continuous infusion of N-acetylcysteine reduces liver warm ischaemia-reperfusion injury. Br J Surg. 2004;91:1330–1339. doi: 10.1002/bjs.4694. [DOI] [PubMed] [Google Scholar]
- 42.Gondolesi GE, Lausada N, Schinella G, Semplici AM, Vidal MS, Luna GC, Toledo J, de Buschiazzo PM, Raimondi JC. Reduction of ischemia-reperfusion injury in parenchymal and nonparenchymal liver cells by donor treatment with DL-alpha-tocopherol prior to organ harvest. Transplant Proc. 2002;34:1086–1091. doi: 10.1016/s0041-1345(02)02809-9. [DOI] [PubMed] [Google Scholar]
- 43.Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, Garcia-Caldero H, Fernandez M, Bosch J, Garcia-Pagan JC. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008;47:1248–1256. doi: 10.1002/hep.22166. [DOI] [PubMed] [Google Scholar]
- 44.Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 45.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 46.Halliwell B, JMC G. Free Radicals in Biology and Medicine. oxford: Oxford University Press; 2007. [Google Scholar]
- 47.Harbrecht BG, Billiar TR, Stadler J, Demetris AJ, Ochoa J, Curran RD, Simmons RL. Inhibition of nitric oxide synthesis during endotoxemia promotes intrahepatic thrombosis and an oxygen radical-mediated hepatic injury. J Leukoc Biol. 1992;52:390–394. doi: 10.1002/jlb.52.4.390. [DOI] [PubMed] [Google Scholar]
- 48.Harbrecht BG, Billiar TR, Stadler J, Demetris AJ, Ochoa JB, Curran RD, Simmons RL. Nitric oxide synthesis serves to reduce hepatic damage during acute murine endotoxemia. Crit Care Med. 1992;20:1568–1574. doi: 10.1097/00003246-199211000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Harrison PM, Keays R, Bray GP, Alexander GJ, Williams R. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 1990;335:1572–1573. doi: 10.1016/0140-6736(90)91388-q. [DOI] [PubMed] [Google Scholar]
- 50.Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–1857. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 51.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 52.Hickey MJ, Sharkey KA, Sihota EG, Reinhardt PH, Macmicking JD, Nathan C, Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997;11:955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- 53.Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hierholzer C, Harbrecht BG, Billiar TR, Tweardy DJ. Hypoxia-inducible factor-1 activation and cyclo-oxygenase-2 induction are early reperfusion-independent inflammatory events in hemorrhagic shock. Arch Orthop Trauma Surg. 2001;121:219–222. doi: 10.1007/s004020000211. [DOI] [PubMed] [Google Scholar]
- 56.Hierholzer C, Kalff JC, Billiar TR, Bauer A, Tweardy DJ. Activation of STAT proteins following ischemia reperfusion injury demonstrates a distinct IL-6 and G-CSF mediated profile. Transplant Proc. 1998;30:2647. doi: 10.1016/s0041-1345(98)00771-4. [DOI] [PubMed] [Google Scholar]
- 57.Hierholzer C, Kalff JC, Omert L, Tsukada K, Loeffert JE, Watkins SC, Billiar TR, Tweardy DJ. Interleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injury. Am J Physiol. 1998;275:L611–621. doi: 10.1152/ajplung.1998.275.3.L611. [DOI] [PubMed] [Google Scholar]
- 58.Hierholzer C, Kelly E, Billiar TR, Tweardy DJ. Granulocyte colony-stimulating factor (G-CSF) production in hemorrhagic shock requires both the ischemic and resuscitation phase. Arch Orthop Trauma Surg. 1997;116:173–176. doi: 10.1007/BF00426067. [DOI] [PubMed] [Google Scholar]
- 59.Hierholzer C, Menezes JM, Ungeheuer A, Billiar TR, Tweardy DJ, Harbrecht BG. A nitric oxide scavenger protects against pulmonary inflammation following hemorrhagic shock. Shock. 2002;17:98–103. doi: 10.1097/00024382-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Hines IN, Kawachi S, Harada H, Pavlick KP, Hoffman JM, Bharwani S, Wolf RE, Grisham MB. Role of nitric oxide in liver ischemia and reperfusion injury. Mol Cell Biochem. 2002;234–235:229–237. [PubMed] [Google Scholar]
- 61.Hinson JA, Michael SL, Ault SG, Pumford NR. Western blot analysis for nitrotyrosine protein adducts in livers of saline-treated and acetaminophen-treated mice. Toxicol Sci. 2000;53:467–473. doi: 10.1093/toxsci/53.2.467. [DOI] [PubMed] [Google Scholar]
- 62.Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 1998;11:604–607. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- 63.Houglum K, Bedossa P, Chojkier M. TGF-beta and collagen-alpha 1 (I) gene expression are increased in hepatic acinar zone 1 of rats with iron overload. Am J Physiol. 1994;267:G908–913. doi: 10.1152/ajpgi.1994.267.5.G908. [DOI] [PubMed] [Google Scholar]
- 64.Hua TC, Moochhala SM. Influence of L-arginine, aminoguanidine, and NG-nitro-L-arginine methyl ester (L-name) on the survival rate in a rat model of hemorrhagic shock. Shock. 1999;11:51–57. doi: 10.1097/00024382-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Huang TP, Nishida T, Kamike W, Kosaka H, Seiyama A, Morimoto Y, Tanaka S, Obunai S, Takei Y, Shiga T, Matsuda H. Role of nitric oxide in oxygen transport in rat liver sinusoids during endotoxemia. Hepatology. 1997;26:336–342. doi: 10.1002/hep.510260213. [DOI] [PubMed] [Google Scholar]
- 66.Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, Lee JH. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-kappaB. Biochem Biophys Res Commun. 1999;261:917–922. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- 67.Hussain SP, Raja K, Amstad PA, Sawyer M, Trudel LJ, Wogan GN, Hofseth LJ, Shields PG, Billiar TR, Trautwein C, Hohler T, Galle PR, Phillips DH, Markin R, Marrogi AJ, Harris CC. Increased p53 mutation load in nontumorous human liver of wilson disease and hemochromatosis: oxyradical overload diseases. Proc Natl Acad Sci U S A. 2000;97:12770–12775. doi: 10.1073/pnas.220416097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ikeda T, Yanaga K, Kishikawa K, Kakizoe S, Shimada M, Sugimachi K. Ischemic injury in liver transplantation: difference in injury sites between warm and cold ischemia in rats. Hepatology. 1992;16:454–461. doi: 10.1002/hep.1840160226. [DOI] [PubMed] [Google Scholar]
- 69.Isobe M, Katsuramaki T, Hirata K, Kimura H, Nagayama M, Matsuno T. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation. 1999;68:803–813. doi: 10.1097/00007890-199909270-00013. [DOI] [PubMed] [Google Scholar]
- 70.Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115–136. doi: 10.1016/0009-2797(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 71.Jeon BR, Yeom DH, Lee SM. Protective effect of allopurinol on hepatic energy metabolism in ischemic and reperfused rat liver. Shock. 2001;15:112–117. doi: 10.1097/00024382-200115020-00006. [DOI] [PubMed] [Google Scholar]
- 72.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, van Haperen R, de Crom R, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 73.Kamiya K, Kono T, Iwamoto J, Yoneda M, Kotani H, Kasai S. The cytoprotective role of lipopolysaccharide-induced nitric oxide against liver damage during early phase of endotoxemia in rats. Shock. 2000;14:229–233. doi: 10.1097/00024382-200014020-00025. [DOI] [PubMed] [Google Scholar]
- 74.Kanwar S, Tepperman BL, Payne D, Sutherland LR, Kubes P. Time course of nitric oxide production and epithelial dysfunction during ischemia/reperfusion of the feline small intestine. Circ Shock. 1994;42:135–140. [PubMed] [Google Scholar]
- 75.Katsumi H, Nishikawa M, Yamashita F, Hashida M. Prevention of hepatic ischemia/reperfusion injury by prolonged delivery of nitric oxide to the circulating blood in mice. Transplantation. 2008;85:264–269. doi: 10.1097/TP.0b013e31815e902b. [DOI] [PubMed] [Google Scholar]
- 76.Keays R, Harrison PM, Wendon JA, Forbes A, Gove C, Alexander GJ, Williams R. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303:1026–1029. doi: 10.1136/bmj.303.6809.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 78.Kimura H, Katsuramaki T, Isobe M, Nagayama M, Meguro M, Kukita K, Nui A, Hirata K. Role of inducible nitric oxide synthase in pig liver transplantation. J Surg Res. 2003;111:28–37. doi: 10.1016/s0022-4804(03)00036-2. [DOI] [PubMed] [Google Scholar]
- 79.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 80.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi H, Nonami T, Kurokawa T, Takeuchi Y, Harada A, Nakao A, Takagi H. Role of endogenous nitric oxide in ischemia-reperfusion injury in rat liver. J Surg Res. 1995;59:772–779. doi: 10.1006/jsre.1995.1238. [DOI] [PubMed] [Google Scholar]
- 82.Koeppel TA, Thies JC, Lehmann T, Gebhard MM, Herfarth C, Otto G, Post S. Improvement of hepatic microhemodynamics by N-acetylcysteine after warm ischemia. Eur Surg Res. 1996;28:270–277. doi: 10.1159/000129466. [DOI] [PubMed] [Google Scholar]
- 83.Koruk M, Aksoy H, Akcay F, Onuk MD. Antioxidant capacity and nitric oxide in patients with hepatic cirrhosis. Ann Clin Lab Sci. 2002;32:252–256. [PubMed] [Google Scholar]
- 84.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurose I, Higuchi H, Miura S, Saito H, Watanabe N, Hokari R, Hirokawa M, Takaishi M, Zeki S, Nakamura T, Ebinuma H, Kato S, Ishii H. Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology. 1997;25:368–378. doi: 10.1053/jhep.1997.v25.pm0009021949. [DOI] [PubMed] [Google Scholar]
- 86.Lambotte L, d’Udekem Y, Amrani M, Taper H. Free radicals and liver ischemia-reperfusion injury. Transplant Proc. 1988;20:977. [PubMed] [Google Scholar]
- 87.Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laskin DL, Pilaro AM. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicol Appl Pharmacol. 1986;86:204–215. doi: 10.1016/0041-008x(86)90051-7. [DOI] [PubMed] [Google Scholar]
- 89.Laskin DL, Pilaro AM, Ji S. Potential role of activated macrophages in acetaminophen hepatotoxicity. II. Mechanism of macrophage accumulation and activation. Toxicol Appl Pharmacol. 1986;86:216–226. doi: 10.1016/0041-008x(86)90052-9. [DOI] [PubMed] [Google Scholar]
- 90.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 91.Lee VG, Johnson ML, Baust J, Laubach VE, Watkins SC, Billiar TR. The roles of iNOS in liver ischemia-reperfusion injury. Shock. 2001;16:355–360. doi: 10.1097/00024382-200116050-00006. [DOI] [PubMed] [Google Scholar]
- 92.Lin JH, Levy G. Sulfate depletion after acetaminophen administration and replenishment by infusion of sodium sulfate or N-acetylcysteine in rats. Biochem Pharmacol. 1981;30:2723–2725. doi: 10.1016/0006-2952(81)90547-5. [DOI] [PubMed] [Google Scholar]
- 93.Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007:CD004996. doi: 10.1002/14651858.CD004996.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J, Li C, Waalkes MP, Clark J, Myers P, Saavedra JE, Keefer LK. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced hepatotoxicity in mice. Hepatology. 2003;37:324–333. doi: 10.1053/jhep.2003.50063. [DOI] [PubMed] [Google Scholar]
- 95.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 96.Marrogi AJ, Khan MA, van Gijssel HE, Welsh JA, Rahim H, Demetris AJ, Kowdley KV, Hussain SP, Nair J, Bartsch H, Okby N, Poirier MC, Ishak KG, Harris CC. Oxidative stress and p53 mutations in the carcinogenesis of iron overload-associated hepatocellular carcinoma. J Natl Cancer Inst. 2001;93:1652–1655. doi: 10.1093/jnci/93.21.1652. [DOI] [PubMed] [Google Scholar]
- 97.Marubayashi S, Dohi K, Yamada K, Kawasaki T. Changes in the levels of endogenous coenzyme Q homologs, alpha-tocopherol, and glutathione in rat liver after hepatic ischemia and reperfusion, and the effect of pretreatment with coenzyme Q10. Biochim Biophys Acta. 1984;797:1–9. [PubMed] [Google Scholar]
- 98.Masaki H, Okano Y, Ochiai Y, Obayashi K, Akamatsu H, Sakurai H. alpha-tocopherol increases the intracellular glutathione level in HaCaT keratinocytes. Free Radic Res. 2002;36:705–709. doi: 10.1080/10715760210873. [DOI] [PubMed] [Google Scholar]
- 99.Mato JM, Camara J, Fernandez de Paz J, Caballeria L, Coll S, Caballero A, Garcia-Buey L, Beltran J, Benita V, Caballeria J, Sola R, Moreno-Otero R, Barrao F, Martin-Duce A, Correa JA, Pares A, Barrao E, Garcia-Magaz I, Puerta JL, Moreno J, Boissard G, Ortiz P, Rodes J. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–1089. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 100.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 101.McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20:3–7. doi: 10.1034/j.1600-0676.2000.020001003.x. [DOI] [PubMed] [Google Scholar]
- 102.McDonald MC, Izumi M, Cuzzocrea S, Thiemermann C. A novel, potent and selective inhibitor of the activity of inducible nitric oxide synthase ( GW274150) reduces the organ injury in hemorrhagic shock. J Physiol Pharmacol. 2002;53:555–569. [PubMed] [Google Scholar]
- 103.Meguro M, Katsuramaki T, Kimura H, Isobe M, Nagayama M, Kukita K, Nui A, Hirata K. Apoptosis and necrosis after warm ischemia-reperfusion injury of the pig liver and their inhibition by ONO-1714. Transplantation. 2003;75:703–710. doi: 10.1097/01.TP.0000053400.42842.5C. [DOI] [PubMed] [Google Scholar]
- 104.Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, Hemed N, Rowe M, Ohana H, Zabrecky G, Cohen R, Ilan Y. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J Clin Gastroenterol. 2005;39:737–742. doi: 10.1097/01.mcg.0000174023.73472.29. [DOI] [PubMed] [Google Scholar]
- 105.Mellouk S, Green SJ, Nacy CA, Hoffman SL. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991;146:3971–3976. [PubMed] [Google Scholar]
- 106.Menezes J, Hierholzer C, Watkins SC, Lyons V, Peitzman AB, Billiar TR, Tweardy DJ, Harbrecht BG. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am J Physiol. 1999;277:G144–151. doi: 10.1152/ajpgi.1999.277.1.G144. [DOI] [PubMed] [Google Scholar]
- 107.Menezes JM, Hierholzer C, Watkins SC, Billiar TR, Peitzman AB, Harbrecht BG. The modulation of hepatic injury and heat shock expression by inhibition of inducible nitric oxide synthase after hemorrhagic shock. Shock. 2002;17:13–18. doi: 10.1097/00024382-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 108.Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40–46. doi: 10.1016/s0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 109.Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- 110.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 111.Miners JO, Drew R, Birkett DJ. Mechanism of action of paracetamol protective agents in mice in vivo. Biochem Pharmacol. 1984;33:2995–3000. doi: 10.1016/0006-2952(84)90599-9. [DOI] [PubMed] [Google Scholar]
- 112.Ming Z, Han C, Lautt WW. Nitric oxide mediates hepatic arterial vascular escape from norepinephrine-induced constriction. Am J Physiol. 1999;277:G1200–1206. doi: 10.1152/ajpgi.1999.277.6.G1200. [DOI] [PubMed] [Google Scholar]
- 113.Minor T, Chung CW, Yamamoto Y, Obara M, Saad S, Isselhard W. Evaluation of antioxidant treatment with superoxide dismutase in rat liver transplantation after warm ischemia. Eur Surg Res. 1992;24:333–338. doi: 10.1159/000129225. [DOI] [PubMed] [Google Scholar]
- 114.Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol. 1994;267:G416–422. doi: 10.1152/ajpgi.1994.267.3.G416. [DOI] [PubMed] [Google Scholar]
- 115.Moldeus P, Cotgreave IA, Berggren M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration. 1986;50 (Suppl 1):31–42. doi: 10.1159/000195086. [DOI] [PubMed] [Google Scholar]
- 116.Morgan KT, Ni H, Brown HR, Yoon L, Qualls CW, Jr, Crosby LM, Reynolds R, Gaskill B, Anderson SP, Kepler TB, Brainard T, Liv N, Easton M, Merrill C, Creech D, Sprenger D, Conner G, Johnson PR, Fox T, Sartor M, Richard E, Kuruvilla S, Casey W, Benavides G. Application of cDNA microarray technology to in vitro toxicology and the selection of genes for a real-time RT-PCR-based screen for oxidative stress in Hep-G2 cells. Toxicol Pathol. 2002;30:435–451. doi: 10.1080/01926230290105613. [DOI] [PubMed] [Google Scholar]
- 117.Muller C, Dunschede F, Koch E, Vollmar AM, Kiemer AK. Alpha-lipoic acid preconditioning reduces ischemia-reperfusion injury of the rat liver via the PI3-kinase/Akt pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G769–778. doi: 10.1152/ajpgi.00009.2003. [DOI] [PubMed] [Google Scholar]
- 118.Muriel P. Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochem Pharmacol. 1998;56:773–779. doi: 10.1016/s0006-2952(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 119.Nadler EP, Dickinson EC, Beer-Stolz D, Alber SM, Watkins SC, Pratt DW, Ford HR. Scavenging nitric oxide reduces hepatocellular injury after endotoxin challenge. Am J Physiol Gastrointest Liver Physiol. 2001;281:G173–181. doi: 10.1152/ajpgi.2001.281.1.G173. [DOI] [PubMed] [Google Scholar]
- 120.Nauta RJ, Tsimoyiannis E, Uribe M, Walsh DB, Miller D, Butterfield A. Oxygen-derived free radicals in hepatic ischemia and reperfusion injury in the rat. Surg Gynecol Obstet. 1990;171:120–125. [PubMed] [Google Scholar]
- 121.Nguyen WD, Kim DH, Alam HB, Provido HS, Kirkpatrick JR. Polyethylene glycol-superoxide dismutase inhibits lipid peroxidation in hepatic ischemia/reperfusion injury. Crit Care. 1999;3:127–130. doi: 10.1186/cc358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 123.Niemela O, Parkkila S, Britton RS, Brunt E, Janney C, Bacon B. Hepatic lipid peroxidation in hereditary hemochromatosis and alcoholic liver injury. J Lab Clin Med. 1999;133:451–460. doi: 10.1016/s0022-2143(99)90022-7. [DOI] [PubMed] [Google Scholar]
- 124.Nussler A, Drapier JC, Renia L, Pied S, Miltgen F, Gentilini M, Mazier D. L-arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991;21:227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- 125.Ogihara H, Ogihara T, Miki M, Yasuda H, Mino M. Plasma copper and antioxidant status in Wilson’s disease. Pediatr Res. 1995;37:219–226. doi: 10.1203/00006450-199502000-00016. [DOI] [PubMed] [Google Scholar]
- 126.Ohhira M, Ohtake T, Matsumoto A, Saito H, Ikuta K, Fujimoto Y, Ono M, Toyokuni S, Kohgo Y. Immunohistochemical detection of 4-hydroxy-2-nonenal-modified-protein adducts in human alcoholic liver diseases. Alcohol Clin Exp Res. 1998;22:145S–149S. doi: 10.1111/acer.1998.22.s3_part1.145s. [DOI] [PubMed] [Google Scholar]
- 127.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–1378. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ou J, Carlos TM, Watkins SC, Saavedra JE, Keefer LK, Kim YM, Harbrecht BG, Billiar TR. Differential effects of nonselective nitric oxide synthase (NOS) and selective inducible NOS inhibition on hepatic necrosis, apoptosis, ICAM-1 expression, and neutrophil accumulation during endotoxemia. Nitric Oxide. 1997;1:404–416. doi: 10.1006/niox.1997.0136. [DOI] [PubMed] [Google Scholar]
- 129.Ozaki M, Suzuki S, Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J. 2002;16:889–890. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]
- 130.Pamuk GE, Sonsuz A. N-acetylcysteine in the treatment of non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2003;18:1220–1221. doi: 10.1046/j.1440-1746.2003.03156.x. [DOI] [PubMed] [Google Scholar]
- 131.Park K, Chung KY, Sung SH, Kim BR, Kim YS. Protective effect of desferrioxamine during canine liver transplantation: significance of peritransplant liver biopsy. Transplant Proc. 2003;35:117–119. doi: 10.1016/s0041-1345(02)03971-4. [DOI] [PubMed] [Google Scholar]
- 132.Paron I, D’Elia A, D’Ambrosio C, Scaloni A, D’Aurizio F, Prescott A, Damante G, Tell G. A proteomic approach to identify early molecular targets of oxidative stress in human epithelial lens cells. Biochem J. 2004;378:929–937. doi: 10.1042/BJ20031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994;28:34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 134.Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006;44:784–790. doi: 10.1016/j.jhep.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 135.Pietrangelo A, Borella F, Casalgrandi G, Montosi G, Ceccarelli D, Gallesi D, Giovannini F, Gasparetto A, Masini A. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941–1949. doi: 10.1016/0016-5085(95)90762-9. [DOI] [PubMed] [Google Scholar]
- 136.Pietrangelo A, Rocchi E, Schiaffonati L, Ventura E, Cairo G. Liver gene expression during chronic dietary iron overload in rats. Hepatology. 1990;11:798–804. doi: 10.1002/hep.1840110513. [DOI] [PubMed] [Google Scholar]
- 137.Pocard M, Vincent-Salomon A, Girodet J, Salmon RJ. Effects of preoperative chemotherapy on liver function tests after hepatectomy. Hepatogastroenterology. 2001;48:1406–1408. [PubMed] [Google Scholar]
- 138.Poli G, Dianzani MU, Cheeseman KH, Slater TF, Lang J, Esterbauer H. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J. 1985;227:629–638. doi: 10.1042/bj2270629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Portakal O, Inal-Erden M. Effects of pentoxifylline and coenzyme Q10 in hepatic ischemia/reperfusion injury. Clin Biochem. 1999;32:461–466. doi: 10.1016/s0009-9120(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 140.Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940–17953. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- 141.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 142.Ratziu V, Lalazar A, Wong L, Dang Q, Collins C, Shaulian E, Jensen S, Friedman SL. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proc Natl Acad Sci U S A. 1998;95:9500–9505. doi: 10.1073/pnas.95.16.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ricciarelli R, Zingg JM, Azzi A. The 80th anniversary of vitamin E: beyond its antioxidant properties. Biol Chem. 2002;383:457–465. doi: 10.1515/BC.2002.048. [DOI] [PubMed] [Google Scholar]
- 144.Rodriguez-Reynoso S, Leal C, Portilla E, Olivares N, Muniz J. Effect of exogenous melatonin on hepatic energetic status during ischemia/reperfusion: possible role of tumor necrosis factor-alpha and nitric oxide. J Surg Res. 2001;100:141–149. doi: 10.1006/jsre.2001.6185. [DOI] [PubMed] [Google Scholar]
- 145.Rodriguez JV, Guibert EE, Quintana A, Scandizzi A, Almada L. Role of sodium nitroprusside in the improvement of rat liver preservation in University of Wisconsin solution: A study in the isolated perfused liver model. J Surg Res. 1999;87:201–208. doi: 10.1006/jsre.1999.5750. [DOI] [PubMed] [Google Scholar]
- 146.Sanchez GM, Rodriguez HM, Giuliani A, Nunez Selles AJ, Rodriguez NP, Leon Fernandez OS, Re L. Protective effect of Mangifera indica L. extract (Vimang) on the injury associated with hepatic ischaemia reperfusion. Phytother Res. 2003;17:197–201. doi: 10.1002/ptr.921. [DOI] [PubMed] [Google Scholar]
- 147.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 148.Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest. 2001;107:439–447. doi: 10.1172/JCI10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schauer RJ, Gerbes AL, Vonier D, Meissner H, Michl P, Leiderer R, Schildberg FW, Messmer K, Bilzer M. Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg. 2004;239:220–231. doi: 10.1097/01.sla.0000110321.64275.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864–870. doi: 10.3748/wjg.v10.i6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schutz E, Wieland E, Hensel A, Niedmann PD, Dreiss A, Armstrong VW, Schuff-Werner P, Oellerich M. Suppression of leukocyte-enhanced cold ischemia/reperfusion injury of liver endothelium with the benzoquinone antioxidant idebenone. Clin Biochem. 1997;30:619–624. doi: 10.1016/s0009-9120(97)00123-9. [DOI] [PubMed] [Google Scholar]
- 152.Sener G, Tosun O, Sehirli AO, Kacmaz A, Arbak S, Ersoy Y, Ayanoglu-Dulger G. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003;72:2707–2718. doi: 10.1016/s0024-3205(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 153.Seo MY, Lee SM. Protective effect of low dose of ascorbic acid on hepatobiliary function in hepatic ischemia/reperfusion in rats. J Hepatol. 2002;36:72–77. doi: 10.1016/s0168-8278(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 154.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 155.Sokol RJ, Twedt D, McKim JM, Jr, Devereaux MW, Karrer FM, Kam I, von Steigman G, Narkewicz MR, Bacon BR, Britton RS, et al. Oxidant injury to hepatic mitochondria in patients with Wilson’s disease and Bedlington terriers with copper toxicosis. Gastroenterology. 1994;107:1788–1798. doi: 10.1016/0016-5085(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 156.Soltys K, Dikdan G, Koneru B. Oxidative stress in fatty livers of obese Zucker rats: rapid amelioration and improved tolerance to warm ischemia with tocopherol. Hepatology. 2001;34:13–18. doi: 10.1053/jhep.2001.25452. [DOI] [PubMed] [Google Scholar]
- 157.Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, Record C, Day CP. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277–283. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 158.Su JF, Guo CJ, Wei JY, Yang JJ, Jiang YG, Li YF. Protection against hepatic ischemia-reperfusion injury in rats by oral pretreatment with quercetin. Biomed Environ Sci. 2003;16:1–8. [PubMed] [Google Scholar]
- 159.Szabo C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140–141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 160.Tacchini L, Radice L, Pogliaghi G, Bernelli-Zazzera A. Differential activation of heat shock and nuclear factor kappaB transcription factors in postischemic reperfused rat liver. Hepatology. 1997;26:186–191. doi: 10.1053/jhep.1997.v26.pm0009214468. [DOI] [PubMed] [Google Scholar]
- 161.Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc) 1998;63:766–781. [PubMed] [Google Scholar]
- 162.Thies JC, Teklote J, Clauer U, Tox U, Klar E, Hofmann WJ, Herfarth C, Otto G. The efficacy of N-acetylcysteine as a hepatoprotective agent in liver transplantation. Transpl Int. 1998;11 (Suppl 1):S390–392. doi: 10.1007/s001470050505. [DOI] [PubMed] [Google Scholar]
- 163.Tsimoyiannis EC, Moutesidou KJ, Moschos CM, Karayianni M, Karkabounas S, Kotoulas OB. Trimetazidine for prevention of hepatic injury induced by ischaemia and reperfusion in rats. Eur J Surg. 1993;159:89–93. [PubMed] [Google Scholar]
- 164.Tsuda T, Horio F, Kato Y, Osawa T. Cyanidin 3-O-beta-D-glucoside attenuates the hepatic ischemia-reperfusion injury through a decrease in the neutrophil chemoattractant production in rats. J Nutr Sci Vitaminol (Tokyo) 2002;48:134–141. doi: 10.3177/jnsv.48.134. [DOI] [PubMed] [Google Scholar]
- 165.Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–630. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Uhlmann D, Scommotau S, Witzigmann H, Spiegel HU. Exogenous L-arginine protects liver microcirculation from ischemia reperfusion injury. Eur Surg Res. 1998;30:175–184. doi: 10.1159/000008574. [DOI] [PubMed] [Google Scholar]
- 167.Uhlmann D, Uhlmann S, Spiegel HU. Endothelin/nitric oxide balance influences hepatic ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2000;36:S212–214. doi: 10.1097/00005344-200036051-00064. [DOI] [PubMed] [Google Scholar]
- 168.Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, Capuano G, Migliaro F. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr. 2004;38:48–55. doi: 10.1097/00005176-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 169.Varadarajan R, Golden-Mason L, Young L, McLoughlin P, Nolan N, McEntee G, Traynor O, Geoghegan J, Hegarty JE, O’Farrelly C. Nitric oxide in early ischaemia reperfusion injury during human orthotopic liver transplantation. Transplantation. 2004;78:250–256. doi: 10.1097/01.tp.0000128188.45553.8c. [DOI] [PubMed] [Google Scholar]
- 170.Vardareli E, Saricam T, Koken T, Degirmenci I, Aral E, Erenoglu E. The effect of alpha-tocopherol and pentoxyfilline on ischemia-reperfusion induced liver injury in rats. Hepatogastroenterology. 1998;45:1505–1508. [PubMed] [Google Scholar]
- 171.Vascotto C, Cesaratto L, D’Ambrosio C, Scaloni A, Avellini C, Paron I, Baccarani U, Adani GL, Tiribelli C, Quadrifoglio F, Tell G. Proteomic analysis of liver tissues subjected to early ischemia/reperfusion injury during human orthotopic liver transplantation. Proteomics. 2006;6:3455–3465. doi: 10.1002/pmic.200500770. [DOI] [PubMed] [Google Scholar]
- 172.Vinas O, Bataller R, Sancho-Bru P, Gines P, Berenguer C, Enrich C, Nicolas JM, Ercilla G, Gallart T, Vives J, Arroyo V, Rodes J. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 173.von Herbay A, Stahl W, Niederau C, Sies H. Vitamin E improves the aminotransferase status of patients suffering from viral hepatitis C: a randomized, double-blind, placebo-controlled study. Free Radic Res. 1997;27:599–605. doi: 10.3109/10715769709097863. [DOI] [PubMed] [Google Scholar]
- 174.Wang Y, Mathews WR, Guido DM, Farhood A, Jaeschke H. Inhibition of nitric oxide synthesis aggravates reperfusion injury after hepatic ischemia and endotoxemia. Shock. 1995;4:282–288. doi: 10.1097/00024382-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 175.Weigand MA, Plachky J, Thies JC, Spies-Martin D, Otto G, Martin E, Bardenheuer HJ. N-acetylcysteine attenuates the increase in alpha-glutathione S-transferase and circulating ICAM-1 and VCAM-1 after reperfusion in humans undergoing liver transplantation. Transplantation. 2001;72:694–698. doi: 10.1097/00007890-200108270-00023. [DOI] [PubMed] [Google Scholar]
- 176.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 177.Wenzel G, Kuklinski B, Ruhlmann C, Ehrhardt D. Alcohol-induced toxic hepatitis--a “free radical” associated disease. Lowering fatality by adjuvant antioxidant therapy. Z Gesamte Inn Med. 1993;48:490–496. [PubMed] [Google Scholar]
- 178.Yabe Y, Koyama Y, Nishikawa M, Takakura Y, Hashida M. Hepatocyte-specific distribution of catalase and its inhibitory effect on hepatic ischemia/reperfusion injury in mice. Free Radic Res. 1999;30:265–274. doi: 10.1080/10715769900300291. [DOI] [PubMed] [Google Scholar]
- 179.Young IS, Trouton TG, Torney JJ, McMaster D, Callender ME, Trimble ER. Antioxidant status and lipid peroxidation in hereditary haemochromatosis. Free Radic Biol Med. 1994;16:393–397. doi: 10.1016/0891-5849(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 180.Zahrebelski G, Nieminen AL, al-Ghoul K, Qian T, Herman B, Lemasters JJ. Progression of subcellular changes during chemical hypoxia to cultured rat hepatocytes: a laser scanning confocal microscopic study. Hepatology. 1995;21:1361–1372. [PubMed] [Google Scholar]
- 181.Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O, Autelli R, Colombatto S, Dianzani MU, Pinzani M, Parola M. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 182.Zhong Z, Froh M, Connor HD, Li X, Conzelmann LO, Mason RP, Lemasters JJ, Thurman RG. Prevention of hepatic ischemia-reperfusion injury by green tea extract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G957–964. doi: 10.1152/ajpgi.00216.2001. [DOI] [PubMed] [Google Scholar]
- 183.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]