Abstract
Hyperglycemia is considered as one of the major determinants in the development of diabetic retinopathy, but the progression of retinopathy resists arrest after hyperglycemia is terminated, suggesting a metabolic memory phenomenon. Diabetes alters the expression of retinal genes, and this continues even after good glycemic control is re-instituted. Since the expression of genes is affected by chromatin structure that is modulated by post-translational modifications of histones, our objective is to investigate the role of histone acetylation in the development of diabetic retinopathy, and in the metabolic memory phenomenon. Streptozotocin-induced rats were maintained either in poor glycemic control (PC, glycated hemoglobin, GHb >11%) or good glycemic control (GC, GHb <6%) for 12 months, or allowed to be in PC for 6 months followed by in GC for 6 months (PC-GC). On a cellular level, retinal endothelial cells, the target of histopathology of diabetic retinopathy, were incubated in 5mM or 20mM glucose for 4 days. Activities of histone deacetylase (HDAC) and histone acetyltransferase (HAT), and histone acetylation were quantified. Hyperglycemia activated HDAC and increased HDAC1, 2 and 8 gene expressions in the retina and its capillary cells. The activity HAT was compromised and the acetylation of histone H3 was decreased. Termination of hyperglycemia failed to provide any benefits to diabetes-induced changes in retinal HDAC and HAT, and histone H3 remained subnormal. This suggests ‘in principle’ the role of global acetylation of retinal histone H3 in the development of diabetic retinopathy and in the metabolic memory phenomenon associated with its continued progression.
Keywords: Diabetic retinopathy, Histone acetylation, Histone deacetylase, Metabolic memory
Introduction
Diabetic retinopathy, a microvascular complication, is the leading cause of acquired blindness in young adults. Many biochemical and molecular sequelae of hyperglycemia have been implicated in its pathogenesis [Kowluru, 2005a; Kowluru and Chan, 2007; Madsen-Bouterse and Kowluru, 2008], but the exact mechanism has still remained elusive. Intensive glycemic control can prevent/inhibit the development and progression of retinopathy in patients with diabetes [Diabetes Control and Complications Trial Research Group, 1993], and the benefits of intensive control persist for some time even after it is terminated suggesting a ‘metabolic memory’ phenomenon [Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, 2000; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, 2008]. We have shown that the retina continues to experience increased oxidative damage even when hyperglycemic insult is terminated in rats, and proteins continue to be oxidatively modified [Kowluru, 2003; Kowluru et al., 2007]. However, the mechanism responsible for this metabolic memory remains unclear.
The expressions of various genes, which are important in retinal dysmetabolism in diabetes, including manganese superoxide dismutase (MnSOD), interleukin-1B and connexin etc are altered in the retina in diabetes [Joussen et al., 2001; Kowluru et al., 2003; Kowluru et al., 2004b; Navaratna et al., 2007; Kowluru and Kanwar, 2009; Li and Roy, 2009; Kowluru 2010]. Epigenetic modulation of chromatin, including histone acetylation, methylation, phosphorylation, are important mechanisms in regulating gene transcription [Vaissière et al., 2008; Delcuve et al., 2009]. Acetylation/deacetylation of histones allows access of the transcription factors for DNA binding and gene transcription, and the steady-state levels of histone acetylation are maintained by a balance between the opposing activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs). Deregulation of histone acetylation/deacetylation and other epigenetic modulations have been implicated in many pathological conditions including cancer and multiple sclerosis [Yang, 2004; Bonnefil et al., 2008; Hitchler et al., 2008]. How they contribute to the development of diabetic retinopathy remains to be explored.
Recent studies have shown that high glucose exposure of aortic endothelial cells induces epigenetic changes in the promoter of NF-kB subunit p65 [El-Osta et al., 2008], and modification of histone H3 at lysine 9 (H3K9) at the proximal Cox2 promoter bearing the NF-kB binding site is shown to mediate hyperglycemia-induced thioredoxin interacting protein-mediated inflammation in retinal capillary endothelial cells [Perrone et al., 2009]. Furthermore, epigenetic modifications of histones are considered to play a major underlying role in the “metabolic memory” phenomenon [Villeneuve et al., 2008]. Our recent studies have shown that diabetes-induced alterations in retinal genes (e.g. upregulation or suppression) do not reverse and apoptosis continues after hyperglycemia is terminated [Kowluru et al., 2006; Kowluru et al., 2007; Kowluru et al., 2010; Kowluru and Chan, 2010]. The objective of this study is to examine the role of histone modification in the development of diabetic retinopathy and in the resistance of retinopathy to arrest after termination of hyperglycemia. Using rat model of diabetic retinopathy and metabolic memory, we have investigated histone acetylation in the retina of rats diabetic for 12 months, or in poor glycemic control for 6 months followed by good glycemic control for 6 additional months. Since the retina is a complex tissue; key findings were confirmed in in vitro system using isolated retinal endothelial cells, the cells that are the target of histopathology characteristic of diabetic retinopathy.
Methods
Rats
Wistar rats (male, 200g) were randomly assigned to normal or diabetic groups and diabetes was induced with streptozotocin (55 mg/kg body weight). Diabetic rats were either allowed to remain in poor metabolic control for 12 months (PC), or maintained in good metabolic control for the entire duration of 12 months (GC), or were maintained in poor metabolic control for 6 months, followed by good metabolic control for 6 additional months (PC-GC). The rats in which poor metabolic control was intended received 1–2 IU insulin 4-5 times a week to prevent ketosis and weight loss, and the rats in which good metabolic control was intended received insulin twice daily (5-7 IU total) to maintain a steady gain in body weight and urine glucose values below 150 mg/24 hours [Kanwar and Kowluru, 2009]. Each group had 10 or more rats. At the end of the desired duration of metabolic control, the animals were sacrificed, and retina were immediately isolated. Treatment of animals conformed to the Association for Research in Vision and Ophthalmology's Resolution on Treatment of Animals in Research (National Institutes of Health) and the institutional guidelines.
Isolated retinal endothelial cells
Retinal endothelial cells were prepared from bovine retina, and were cultured to 80% confluence on plates coated with 0.1% gelatin. The culture medium consisted of Dulbecco's Modified Eagle Medium containing 15% fetal calf serum (heat inactivated), 5% replacement serum (Nu-serum; BD Bioscience, San Jose, CA), heparin (50μg/ml), endothelial growth supplement (25μg/ml), and antibiotic/antimycotic in an environment of 95% O2 and 5% CO2 [Kowluru et al., 2006]. Cells from passage 5th-6th were incubated in 5mM glucose or 20mM glucose media for 4 days, and at the end of the incubation, cells were trypsinized and nuclear fraction was prepared by differential centrifugation, as given below.
Nuclear fraction
Nuclear extract was prepared using the Nuclear Extract Kit (Active Motif, Carlsbad, CA) following manufacturer's protocol. In brief, retina or cells were homogenized in hypotonic buffer, and was centrifuged at 8000 rpm for 10 minutes. The pellet was suspended in hypotonic buffer and centrifuged at 13000 rpm for 30 seconds. The pellets were re-suspended in complete lysis buffer, incubated at 40C for 30 minutes, and centrifuged at 13000 rpm for 10 minutes. The supernatant was used as the ‘nuclear’ fraction.
Activity of HDAC and HAT
HDAC activity was measured by a non-isotopic assay that uses a fluorescent derivative of epsilon-acetyl lysine (HDAC Fluorescent Activity Assay Kit from Upstate, Lake Placid, NY). Briefly, in a total reaction mixture of 30μl, nuclear protein (1-25μg) was mixed with HDAC assay substrate, and incubated at 37°C for 30 minutes, followed by mixing with the activator solution. The fluorescence was read in SpectraMax M2 Microplate Reader (Molecular Devices, Sunnyvale, CA) with excitation of 360nm and emission of 460nm.
Activity of HAT was quantified in the nuclear fraction by non-radioactive indirect ELISA kit from Millipore (Lack Placid, NY). The plate was coated with biotinylated histone H3 or H4 peptide, rinsed and blocked with 3% BSA for 30 minutes. After washing the wells, 0-10μg of nuclear protein was incubated with acetyl-CoA and Na butyrate (HDAC inhibitor). The wells were washed and the samples were incubated with anti-acetyl-lysine antibody. This was followed by washing and incubation with anti-rabbit IgG conjugated with horseradish peroxidase. The final incubation was with tetramethyl-benzidine substrate mixture for 5-10 minutes, and the reaction was stopped by sulfuric acid. The readings were monitored at 450 nm with a reference wavelength of 570 nm. For acetylated histone H3, the linear range was 10-75ng, and for acetylated histone H4, the range was 1-7.5ng.
Quantification of acetylated histone H3 by ELISA
Acetylated hstone H3 level was quantified d in the nuclear fraction by solid phase sandwich ELISA using PathScan® Acetylated histone H3 Sandwich ELISA kit (Cell Signaling, Beverly, MA). Briefly, 0-2.0μg nuclear extract was incubated for 2 hours in hstone H3 antibody-coated microplates. After rinsing, the samples were incubated with acetylated-lysine mouse monoclonal antibody. The unbound antibody was washed, and this was followed by incubation with enzyme-linked (HRP) anti-mouse IgG. After incubating the samples with the HRP substrate for 5 minutes, the reaction was terminated by the stop solution. The intensity of the color was measured at 450 nm, within 30 minutes of termination. The experiment was performed in duplicate to ensure reproducibility.
Gene expression
Total RNA was extracted from the nuclear fraction with Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized by High Capacity cDNA Reverse Transcription Kit (Applied Biosystem, Foster City, CA) and amplified by PCR. The PCR primers for rat were: HDAC1 forward: 5′-GTT CTT GCG TTC TAT TCG CC-3′ and reverse: 5′-TGT CCG TCT GCT GCT TAT TG-3′; HDAC2 forward: 5′-TCA ACT TTC CCA TGA GGG AC-3′and reverse: 5′-GTT AGG TTG AAG CAG CCC AG-3′; HDAC8 forward: 5′-CGC TAC CCC CGG TTT ATA TT-3′ and reverse: 5′-CCT TCT TGG CTG ACC TTC TG-3′; and β-actin forward: 5′-CCT CTA TGC CAA CAC AGT GC-3′ and reverse: 5′-CAT CGT ACT CCT GCT TGC TG-3′. Bovine primers used were: HDAC1 forward: GCC ATC CCC GAG GAC GCC AT and reverse: TTC TTG CGC CCG CCT TCT CC; HDAC2 forward: TCC GCC TTC ACC CCA GCC TT and reverse: GCC GCC TCC TTG ACT GTA CGC; HDAC8 forward: GGA GCA GGA ACC CGA GCC CA and reverse: GGC CAG GGA GTC GCA CAC G. PCR conditions included initial denaturation at 95°C for 2 minutes followed by 35 cycles at 92°C for 1 minute, annealing at 50°C for 2 minutes, extension at 72°C for 1 minute, and a final extension at 72°C for 7minutes. PCR products were electrophoresed on 1.2% agarose gel and imaged by Benchtop 3UV Transilluminator (UVP Inc., Upland, CA). Quantitative analysis was achieved by measuring the integrated density value of PCR products in gel photographs using Un-Scan-It Gel digitizing software [Madsen-Bouterse et al., 2009].
Gene expression of HDACs was also confirmed using quantitative real-time PCR (RT-PCR). RT-PCR was performed using 90ng cDNA template in 96-well plates using the ABI-7500 sequence detection system and TaqMan primers using β-actin was used as a housekeeping control. Genbank accession numbers of TaqMan primers for rat HDAC1, rat HDAC2, rat HDAC8, rat β-actin, bovine HDAC1, bovine HDAC8 and 18S rRNA are NM_001025409.1, NM_053447.1, NM_001126373.1, NM_031144.2,NM_001037444.1, NM_001075146.1, and X03205.1, respectively. Each sample was measured in triplicate. Fold-change in mRNA abundance was calculated with the ddCt method as routinely used by us [Madsen-Bouterse et al., 2009; Kowluru and Chan, 2010].
Western blotting
For quantifying acetylation of histones 15-30μg of retinal or cell homogenate was analyzed by western blot using primary antibodies against acetylated histone H3 and acetylated histone H3K9 (Abcam, Cambridge, MA) and total histone H3 (Cell Signaling, Beverly, MA). The band intensity was quantified using Un-Scan-It Gel digitizing software (Silk Scientific Inc, Orem, UT).
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analysis was performed using the nonparametric Kruskal-Wallis test followed by Mann-Whitney U test. P<0.05 was considered statistically significant.
Results
The average body weight of the rats in poor glycemic control was significantly lower (PC= 290g and normal=550g), and their GHb values were over two fold higher (>11.5% and 5.5%) compared to the age-matched normal control rats. The rats that were maintained in good glycemic control for the entire duration (GC group) had similar body weight (520g) and GHb values (5.7%) as their age-matched normal rats (p>0.05). However, the rats in PC-GC group had their body weight and GHb values before initiation of good glycemic control (310g and 12%) that were not different from the rats in PC group, but these values became similar to those in normal group after initiation of good glycemic control.
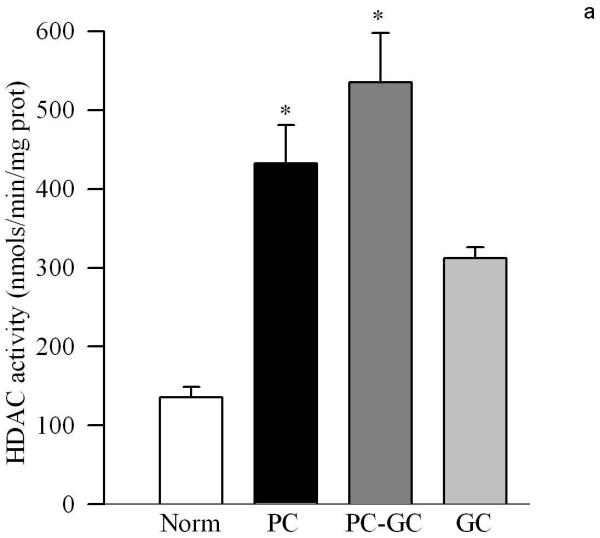
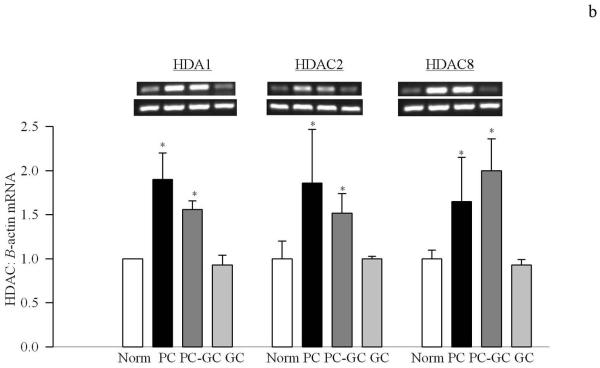
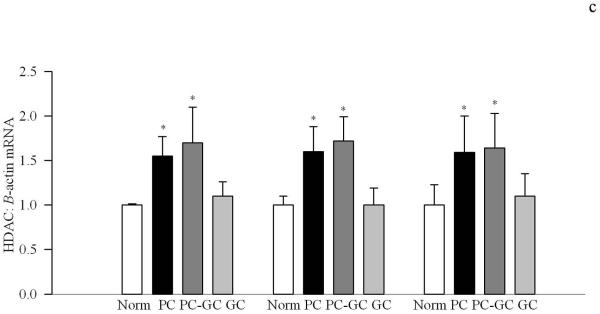
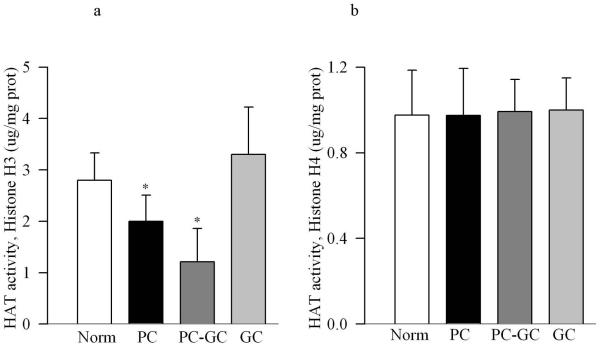
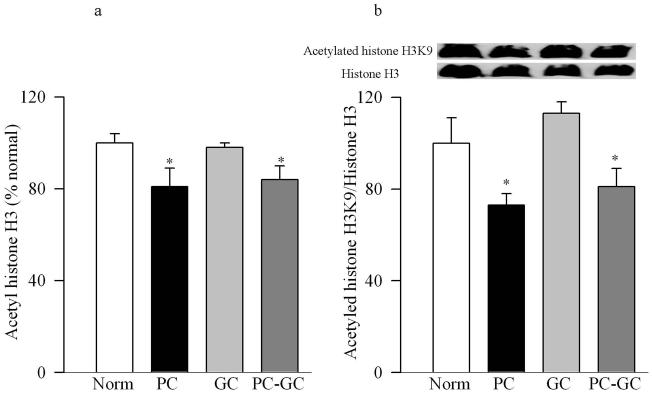
Poor glycemic control for 12 months activated HDAC by over 3 fold in the retinal nuclear fraction (Figure 1a), and the gene expressions of HDAC1, HDAC2 and HDAC 8 were elevated by 70-90% compared to the values obtained from age-matched normal rats (Figure 1b&c). In the same diabetic rats, the activity of the enzyme responsible for acetylating histones H3 was decreased by 30% in the nuclear fraction, but the enzyme responsible for acetylation of histone H4 remained unchanged (Figure 2a&b). The amount of total acetylated histone H3, as determined by solid phase ELISA (Figure 3a) or by western blot technique (Figure 3b), was decreased by about 20-30% in diabetes compared to the values obtained from normal rats (p<0.05 vs normal).
Figure 1.
Diabetes activates HDAC in the retina and elevates its gene expression. (a) The activity of retinal HDAC was measured using HDAC Fluorescent Activity Assay Kit, and the gene expression of HDAC1, HDAC2 and HDAC 8 was quantified by (a) conventional PCR using the primers provided in the method section, or (c) real time quantitative PCR using TaqMan primers. β-actin was used as a housekeeping control. Figure represents expression of the HDAC adjusted to that of β-actin in the same sample. Each measurement was made in duplicate or triplicate. The values are represented as mean ±SD of 5-7 rats in each of the four groups. Norm=normal, PC=poor control for 12 months, PC-GC= poor control for 6 months followed by good control for 6 additional months, GC= good control for entire 12 months. *P<0.05 compared to normal
Figure 2.
Diabetes inhibits retinal HAT activity. The activity of HAT was quantified in the nuclear fraction by an ELISA method using either acetylated histone H3 or histone H4. Each measurement was made in duplicate in 5-6 rats in each group.
Figure 3.
Acetylated histone H3 levels are attenuated in the retina in diabetes. (a)Total acetylated histone H3 levels were measured in the retinal nuclear fraction by solid phase sandwich ELISA busing 0-2.0μg nuclear protein. Each sample was measured in duplicate using 5 or more rats in each group. (b) The expression of acetylated histone H3K9 was quantified by western blot technique, and was adjusted to the expression of total histone H3 in each sample. *P<0.05 compared to age-matched normal rats
Re-institution of good glycemic control after 6 months of poor glycemic control (PC-GC group) failed to protect retinal nucleus from increases in the activity of HDAC and gene expressions of HDAC1, HDAC2 and HDAC 8 (Figure 1). In addition, the activity of HAT also remained compromised with reduced levels of acetylated histone H3 (Figures 2 & 3). Although the extent of alterations in HDAC and HAT activities in the PC-GC group is higher than compared to the values obtained in PC group, these values did not achieve any statistical significance.
However, when good glycemic control was initiated soon after induction of diabetes (GC group), activation of HDAC and inhibition of HAT was prevented (Figures 1-3) suggesting that the effect of good glycemic control on various parameters in PC-GC group animals is not influenced by the high insulin dose administered to maintain the glycemic control, and further strengthen the importance of early and sustained good glycemic control for diabetic patients.
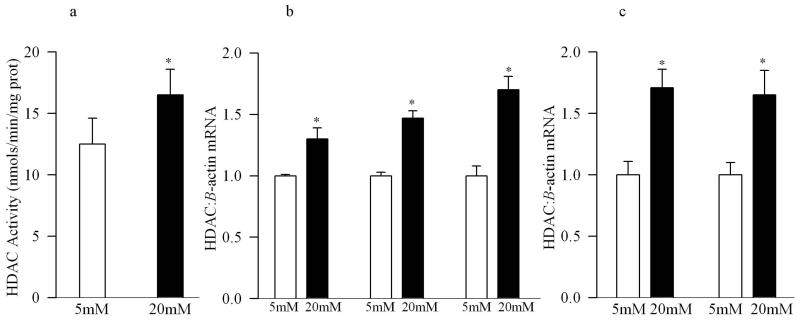
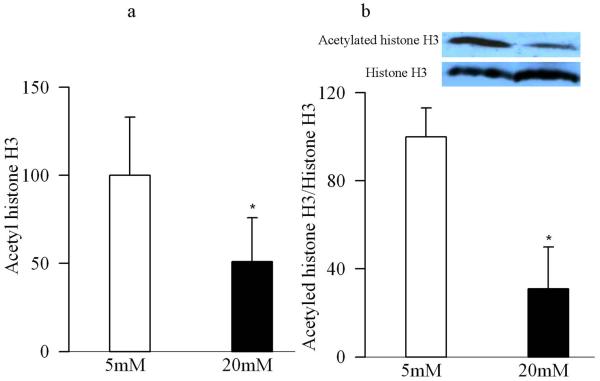
Since the retina is a multi-layered sensory tissue that has many cell types, in order to specifically understand the role of histone acetylation in the development of vascular pathology of diabetic retinopathy, key experiments were performed in endothelial cells isolated from the retina. As shown in figure 4a, the activity of HDAC was increased by over 40% in the nuclear fractions of the cells incubated in high glucose compared to normal glucose, with concomitant increases in the gene expressions of HDAC1, HDAC2 and HDAC8 as observed by both conventional and quantitative PCR measurements (Figure 4b&c). In the same cell preparations, high glucose exposure also reduced the amount of total acetyl histone H3 as quantified by ELISA (Figure 5a), or by its protein expression (Figure 5b).
Figure 4.
Exposure of retinal endothelial cells to high glucose activates HDAC activity and increases their gene transcripts. (a) HDAC activity was quantified in the cell incubated in 5mM or 20mM glucose for 4 days by Fluorescent Activity Assay Kit, and the gene expressions for HDAC1, HDAC2 and HDAC8 were quantified by (b) conventional PCR using bovine primers, β-actin was used as the housekeeping gene, or (c) real time quantitative PCR using bovine (made to order) TaqMan primers. 18s was used as a housekeeping control. Figure represents expression of HDACs adjusted to that of the housekeeping protein in the same sample. Each measurement was made in duplicate. The values are represented as mean ±SD obtained from 3 or more different cell preparations. 5mM=5mM glucose, 20mM=20mM glucose; *P<0.05 compared to 5mM glucose.
Figure 5.
High glucose decreases acetylated histone H3 in retinal endothelial cells. (a) Acetylated histone H3 level was measured in the nuclear fractions obtained from the retinal endothelial cells incubated in 5mM glucose or 20mM glucose for 4 days by using a solid phase sandwich ELISA. (b) Western blot technique was used to quantify the expression of acetylated histone H3, and the expression of acetylated histone H3 in each sample was adjusted to the expression of total histone in that sample. The values obtained from cells incubated in 5mM glucose are considered as 100%.
Discussion
This is the first study demonstrating alterations in the global acetylation of retinal histones in diabetes. Activity of HDAC activity and its gene transcripts are increased, and HAT activity is decreased, resulting in the reduced acetylation of histone H3. On a cellular level, high glucose exposure of the cells that are the targets of pathology associated with diabetic retinopathy, the endothelial cells, also show increases in HDAC activity and its transcripts, and decrease in acetylated histone H3 and H3K9, suggesting that histone acetylation has an important role in the development of diabetic retinopathy. Reversal of hyperglycemia for 6 months in diabetic rats, which our previous studies have shown to provide no benefit to retinal capillary cell apoptosis and the development of histopathology [Kowluru et al., 2007; Kowluru and Chan, 2010], also fails to provide any benefit to the retinal histone acetylation and the enzymes responsible for maintaining acetylation homeostasis. This strongly suggests that histone acetylation is an important contributor in the progression of diabetic retinopathy after hyperglycemia is terminated.
Histones are important in the regulation of gene expressions, and post-translational modifications of histones regulate transcription and genome stability [Bhaumik et al., 2007]. Acetylation of histones activates gene transcriptional activity [Vaissière et al., 2008; Delcuve et al., 2009], and HDACs remove acetyl groups potentially inhibiting the expression of genes regulated by that portion of DNA. These covalent modifications, by altering chromatin structure affect DNA regulatory processes including replication, repair and transcription [Haberland et al., 2009]. Here we show that the activity of HDAC is increased in the retina in diabetes, while that of HAT is decreased, this is supported by reports showing diabetes-induced increase HDAC activity in the kidney and in cardiac tissue, the tissues that are associated with micro- and macro-vascular complications respectively [Dong and Ren, 2007; Lee et al., 2007; Noh et al., 2009]. A number of acetyltransferases with different HAT complexes acetylate histones, and regulate different levels/stages of transcription or other nuclear processes [Shahbazian and Grunstein, 2007]. Acetylation of histone H3 is important in cell cycle progression, and that of histone H4 in viability [Howe et al., 2001]. Our results show that the activity of histone H3 acetyltransferase is decreased in diabetes, but that of histone H4 acetyltransferase is not affected; however, the reason for such divergent results is not clear.
Reactive oxygen species (ROS) are shown to increase HDAC activity and decreases HAT activity, and inhibit histone acetylation [Kang et al., 2005; Berthiaume et al., 2006]. In diabetes, retina and its capillary cells experience increased oxidative stress [Kowluru et al., 2001; Kowluru, 2005b], and hyperglycemia-induced superoxide overproduction activates the major pathways that are considered important in the development of diabetic retinopathy [Brownlee, 2005; Kanwar and Kowluru, 2009]. Thus, the plausible mechanism by which diabetes regulates retinal HDAC and HAT could include increased oxidative stress. Histone deacetylase activity is also stimulated by ischemia and hypoxia [Endres et al., 2000; Granger et al., 2008; Ellis et al., 2009]. Hypoxia is considered as the major stimulus for retinal neovascularization observed in diabetes [Frank, 2004], and the contribution of increased retinal hypoxia in diabetes in the activation of retinal HDAC remains a strong possibility.
Alterations in retinal HDAC and HAT activities in diabetes are accompanied by decreased levels of total acetylated histone H3 and histone H3K9, and this is also observed at duration of diabetes when histopathology of diabetic retinopathy are observed [Mizutani et al., 1996; Kern et al., 2000]. The level of acetylated H3K9 in promoter regions is shown to strongly correlate with gene expression [Suzuki et al. 2008]. This suggests that deacetylation of histone H3 could be contributing to the altered expressions of the genes implicated in the development of diabetic retinopathy. In support, our in vitro data show similar phenomenon in retinal endothelial cells incubated in high glucose. Although hyperglycemia-induced increase in HDAC activity in the whole retina was about 3 fold compared to 40% in isolated endothelial cells, the reason for this could be the whole retina represents contribution from multiple cells and raises the possibility that the extent of increase in HDAC is much larger in non vascular cells than the vascular cells. The expression of many retinal genes, including genes involved in maintaining redox status and apoptosis are altered in diabetes [Joussen et al., 2001; Kowluru et al., 2003; Navaratna et al., 2007; Kowluru and Kanwar, 2009; Li and Roy, 2009; Kowluru 2010]. The global deacetylation of histone H3 may possibly be related to downregulation of these genes, and the reduction of histone acetylation, via repressesing gene transcription, could be contributing to the accelerated retinal cell loss seen in diabetic retinopathy
The retina continues to experience increased oxidative damage, and capillary cell apoptosis and histopathology characteristic of diabetic retinopathy do not halt after hyperglycemic insult is terminated in rats for at least 6 months [Kowluru, 2003; Kowluru and Odenbach, 2004a; Kowluru et al., 2007; Kowluru and Chan, 2010]. Here we show that the termination of poor glycemic control for 6 months does not protect retinal nucleus from alterations in the activities of both HDAC and HAT, and the levels of acetylated histone H3 and H3K9 remain compromised, suggesting that histone modifications are important in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy. In support, epigenetic histone modification is considered as a major underlying mechanism for sustained proinflammatory phenotype of diabetic cells [Villeneuve et al., 2008]. Histone modifications, such as methylation of H3K9 and H3K4, are related with persistent activation of inflammation genes after transient high glucose [El-Osta et al., 2008; Reddy et al., 2008; Villeneuve et al., 2008]. This raises the possibility that the persistent alterations in acetylation of histone may cause continuous changes in the genes that are important in the development and progression of diabetic retinopathy.
Thus, in summary, our study shows ‘in principle’ the role of global acetylation of retinal histone H3 in the development of diabetic retinopathy and in the metabolic memory phenomenon associated with its resistance to reverse after termination of hyperglycemia, and paves path for future studies to explore the mechanism via which this acetylation could contribute to the pathogenesis of diabetic retinopathy.
Acknowledgement
Authors thank Dr. Srirupa Das for her help in the initial work, and Mamta Kanwar and Yakov Shamailov for technical assistance. This work was supported by grants from the National Institutes of Health (R01EY17313), Juvenile Diabetes Research Foundation, The Thomas Foundation and Research to Prevent Blindness.
Grant Support Contract grant sponsor: National Institutes of Health, Contract grant number: EY017733; Contract grant sponsor: Juvenile Diabetes Research Foundation, Contract grant number: JDRF-1-2008-764; Contract grant sponsor: The Thomas Foundation; Contract grant sponsor: Research to Prevent Blindness
References
- Berthiaume M, Boufaied N, Moisan A, Gaudreau L. High levels of oxidative stress globally inhibit gene transcription and histone acetylation. DNA Cell Biol. 2006;25:124–134. doi: 10.1089/dna.2006.25.124. [DOI] [PubMed] [Google Scholar]
- Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- Bonnefil PC, Pandozy G, Mastronardi F. Evaluating epigenetic landmarks in the brain of multiple sclerosis patients: A contribution to the current debate on disease pathogenesis. Prog Neurobiol. 2008;86:368–378. doi: 10.1016/j.pneurobio.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Delcuve GP, Rastegar M, Davie JR. Epigenetic control. J Cell Physiol. 2009;219:243–250. doi: 10.1002/jcp.21678. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol. 2008;126:1707–1715. doi: 10.1001/archopht.126.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Ren J. Fidarestat improves cardiomyocyte contractile function in db/db diabetic obese mice through a histone deacetylase Sir2-dependent mechanism. J Hypertens. 2007;25:2138–2147. doi: 10.1097/HJH.0b013e32828626d1. [DOI] [PubMed] [Google Scholar]
- Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;250:145–153. doi: 10.1016/j.canlet.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, Lipski A, Jaenisch R, Moskowitz NA, Dirnagl U. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci. 2000;20:3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RN. Diabetic Retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature Reviews Genetics. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchler MJ, Oberley LW, Domann FE. Epigenetic silencing of SOD2 by histone modifications in human breast cancer cells. Free Radl Biol Med. 2008;45:1573–1580. doi: 10.1016/j.freeradbiomed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L, Auston D, Grant P, John S, Cook RG, Workman JL, Pillus L. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 2001;15:3144–3154. doi: 10.1101/gad.931401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Huang S, Poulaki V, Camphausen K, Beecken WD, Kirchhof B, Adamis AP. In vivo retinal gene expression in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:3047–3057. [PubMed] [Google Scholar]
- Kang J, Chen J, Shi Y, Jia J, Zhang Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem Pharmacol. 2005;69:1205–1213. doi: 10.1016/j.bcp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kanwar M, Kowluru R. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58:227–234. doi: 10.2337/db08-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: Comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Research. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Effect of re-institution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52:818–823. doi: 10.2337/diabetes.52.3.818. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Chakrabarti S, Chen S. Re-Institution of good metabolic control in diabetic rats on the activation of caspase-3 and nuclear transcriptional factor (NF-kB) in the retina. Acta Diabetologica. 2004a;44:194–199. doi: 10.1007/s00592-004-0165-8. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Diabetic retinopathy, oxidative stress and antioxidants. Current Topics in Nutraceutical Res. 2005a;3:209–218. [Google Scholar]
- Kowluru RA. Diabetic retinopathy: Mitochondrial dysfunction and retinal capillary cell death. Antioxidants & Redox Signalling. 2005b;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Atasi L, Ho YS. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47:1594–1599. doi: 10.1167/iovs.05-1276. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Kanwar M, Kennedy A. Metabolic memory phenomenon and accumulation of peroxynitrite in retinal capillaries. Exp Diabetes Res. 2007;2007:2196. doi: 10.1155/2007/21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Kanwar M. Oxidative stress and the development of diabetic retinopathy: Contributory role of matrix metalloproteinase-2. Free Rad Biol Med. 2009;46:1677–1685. doi: 10.1016/j.freeradbiomed.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA. Role of matrix metalloproteinase-9 in the development of diabetic retinopathy and its regulation by H-Ras. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-4851. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Chan PS. Metabolic memory in diabetes - from in vitro oddity to in vivo problem: Role of Apoptosis. Brain Res Bull. 2010;81:297–302. doi: 10.1016/j.brainresbull.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HB, Noh H, Seo JY, Yu MR, Ha H. Histone deacetylase inhibitors: a novel class of therapeutic agents in diabetic nephropathy. Kidney Int Suppl. 2007;106:S61–S66. doi: 10.1038/sj.ki.5002388. [DOI] [PubMed] [Google Scholar]
- Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2009;50:1400–1407. doi: 10.1167/iovs.07-1519. [DOI] [PubMed] [Google Scholar]
- Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–327. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- Madsen-Bouterse S, Mohammad G, Kowluru RA. Glyceraldehyde 3 phosphate dehydrogenase in retinal microvasculature: Implications for the development and progression of diabetic retinopathy. Invest Ophthalmol Vis Sci Oct. 2009:29. doi: 10.1167/iovs.09-4171. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380–2387. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H, Lee HB. Histone deacetylase 2 is a key regulator of diabetes and transforming growth factor-{beta}1-induced renal injury. Am J Physiol Renal Physiol. 2009;297:F729–F739. doi: 10.1152/ajprenal.00086.2009. [DOI] [PubMed] [Google Scholar]
- Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Villeneuve LM, Wang M, Lanting L, Natarajan R. Role of the lysine-specific demethylase 1 in the proinflammatory phenotype of vascular smooth muscle cells of diabetic mice. Circ Res. 2008;103:615–623. doi: 10.1161/CIRCRESAHA.108.175190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kondo S, Wakayama T, Cizdziel PE, Hayashizaki Y. Genome-wide analysis of abnormal H3K9 acetylation in cloned mice. PLoS One. 2008;3:e1905. doi: 10.1371/journal.pone.0001905. Genome-wide analysis of abnormal H3K9 acetylation in cloned mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissière T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–48. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acid Res. 2004;32:959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]