Abstract
Background
Elderly patients are more sensitive to myocardial ischemia, which results in higher mortality. We investigated how aging impacts the cardioprotective AMP-activated protein kinase (AMPK) signaling pathway.
Methods and Results
Ischemic AMPK activation was impaired in aged compared to young murine hearts. The expression and secretion of the AMPK upstream regulator, macrophage migration inhibitory factor (MIF), were lower in aged compared to young adult hearts. Additionally, the levels of hypoxia-inducible factor 1α (HIF-1α), a known transcriptional activator of MIF, were reduced in aged compared to young hearts. Ischemia-induced AMPK activation in MIF knock-out (MIF KO) mice was blunted, leading to greater contractile dysfunction in MIF-deficient than in wild type (WT) hearts. Furthermore, intra-myocardial injection of adenovirus encoding MIF (Adv-MIF) in aged mice increased MIF expression and ischemic AMPK activation, and reduced infarct size.
Conclusions
An impaired MIF-AMPK activation response in senescence thus may be attributed to an aging-associated defect in the transcription factor for MIF, HIF-1α. In the clinical setting, impaired cardiac HIF-1α activation and consequent reduced MIF expression may play an important role in the increased susceptibility to myocardial ischemia observed in older cardiac patients.
Keywords: aging, AMPK, MIF, HIF-1α, myocardial infarct
Introduction
The most common cause of damage to the myocardium is ischemic injury resulting from occlusion of the coronary arteries.1 Numerous investigators have observed a decreased ability of the aged myocardium to tolerate an ischemic or hypoxic stress in both animal models and in human subjects.2, 3 In addition, aging decreases myocardial tolerance to specific components of ischemic injury, including oxidative stress.4 It is widely accepted that aging is accompanied by a general decline in stress resistance,5 and clinical trials have demonstrated that the mortality after myocardial infarction, coronary angioplasty, and cardiac surgery in patients 70 years or older is higher than that of younger age groups.6, 7 Although several clinical factors contribute to the poor prognosis for elderly patients with ischemic heart disease,8, 9 evidence from experimental animal studies10 and in humans3, 11 suggest that this effect may be related to a decline in intrinsic myocardial resistance to injury. Nevertheless, the mechanisms responsible for ischemic intolerance are incompletely understood and the signaling pathways that regulate cellular responses to ischemia/reperfusion (I/R) remain largely unknown.
The AMPK signaling pathway is activated in the heart by glucose deprivation, ischemia, hypoxia, oxidative and hyperosmotic stress.12 AMPK regulates many pathways in the heart that control glucose and lipid uptake, storage, and utilization,12, 13 and it modulates metabolic enzymes, ion channels, as well as gene expression. The activity of AMPK may be reduced with age,14, 15 suggesting that it may contribute to the decline in stress tolerance observed with aging.16, 17 Our earlier studies demonstrated that AMPK regulates myocardial metabolism during low-flow I/R and limits ischemic injury and apoptosis during post-ischemic reperfusion.18 We also reported that macrophage migration inhibitory factor (MIF) modulates the activation of AMPK during ischemia, and we suggested that genetic variation in the expression of MIF, which is encoded in a functionally polymorphic locus,19 may impact the responsiveness of the human heart to ischemia via the AMPK pathway.20 In the present report, we sought to examine if AMPK activity is reduced in the senescent heart and whether such a reduction contributes to increased ischemia injury with aging.
Methods
In vivo Regional Ischemia and Experimental Myocardial Infarction
Mice were anaesthetized, placed on a ventilator (Harvard Rodent Ventilator, Harvard)20, and core temperature was maintained at 37°C with a heating pad. After left lateral thoracotomy, the left anterior descending artery (LAD) was occluded for different time period. The hearts were then rapidly excised, and the ischemic region of the left ventricle was freeze clamped in liquid nitrogen21 for biochemical analysis. The LAD was occluded for 20 min with an 8–0 nylon suture and polyethylene tubing to prevent arterial injury, and then reperfused for 4 hr. Electrocardiograms confirmed ischemic repolarization changes (ST-segment elevation) during coronary occlusion (AD Instruments). The hearts were then excised and perfusion-fixed and stained to delineate the extent of myocardial necrosis as a percent of non-perfused ischemic area at risk (AAR).20 Viable tissue in the ischemic region was stained red by 2, 3, 5-triphenyltetrazolium (TTC) and the non-ischemic region was stained blue with Evan's blue dye. Hearts were fixed, sectioned, and photographed using a Leica microscope and analyzed using NIH Image software.
Supplemental Methodology
For a detailed explanation of methods relative to echocardiographic assessment, AMPK activity analysis,20 heart perfusion and cardiac functions,18 immunoblotting,21 real-time reverse-transcriptase polymerase chain reaction (RT-PCR),22 high-energy phosphate and glycogen measurement,23, 24 MIF secretion analysis,20 measurement of isolated cardiomyocytes contractile function23 and MIF adenovirus delivery,25 please see the Method section of the online-only Supplemental Material.
Statistical Analysis
Data were expressed as means ± SEM. A variety of statistical tests employing SAS software (version 9.2; SAS Institute Inc, Cary, NC) was used based on the design required for the specific question being asked (number of age groups × number of treatments). This meant employing t-tests, repeated and non-repeated measures 1-way and 2-way ANOVA. For the single- and multifactorial analyses, where a significant overall F value was obtained, indicative of significant main and/or interaction effects, the appropriate post-hoc test(s) were performed to measure individual group differences of interest. A P value of less than 0.05 was considered statistically significant.
Results
Cardiac Phenotype of Young and Aged Mice
Echocardiographic studies were performed to examine hearts with respect to in vivo left ventricle (LV) function. The heart rate (HR), LV end-systolic and end-diastolic dimensions and percent fractional shortening (FS %) were similar and in the normal range for both young and aged mice under a basal physiological state (Suppl Table 1).
Impaired Ischemic Activation of AMPK in Aged Heart
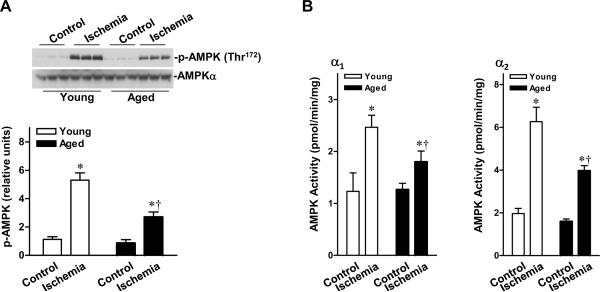
We compared AMPK signaling in hearts from young adult (4–6 months) and aged (24–26 months) mice during in vivo regional ischemia. Ischemia stimulated the phosphorylation of AMPK at Thr172 of the catalytic α subunit (Figure 1A), and the activity of AMPKα1 and α2 (Figure 1B) was decreased in aged hearts compared with their younger counterparts. These results suggest that the AMPK responsiveness to ischemia is reduced in the aged heart.
Figure 1.
Impaired ischemic AMPK activation in aged hearts. A, In vivo regional ischemia (20 min) stimulates differential phosphorylation of AMPK in ischemic area of the hearts, as assessed by immunobloting. B, Differential activation of AMPKα1 and AMPKα2 in the ischemic area of hearts, as assessed by kinase assay. Values are means ± SEM, n=6 per group. *P<0.01 vs. control, respectively; †P<0.01 vs. young ischemia, respectively.
Aged Hearts Demonstrate Intolerance to Ischemic Injury
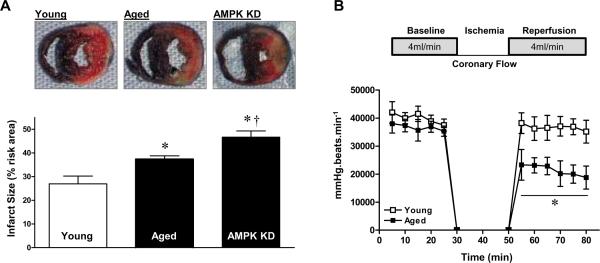
Mounting evidence supports a beneficial effect of AMPK in limiting cardiac damage during I/R.18, 26, 27 We next compared myocardial infarct size in response to in vivo regional I/R in young and aged hearts. After 20 minutes of coronary artery ligation and 4 hours of reperfusion, the myocardial infarct size in aged hearts was significantly larger than in young hearts (Figure 2A). To confirm that reduced AMPK activation was a factor associated with intolerance to ischemic injury during aging, we compared the response to ischemic stress of young AMPK-kinase dead (KD)18 transgenic mouse hearts with that of WT littermates. Notably, the myocardial infarct size was significantly greater in AMPK KD hearts than in WT littermate hearts (Figure 2A). However, there was a significant difference in infarct size between the aged WT and young AMPK-KD hearts (Figure 2A). Aged hearts also demonstrated impaired recovery of post-ischemic LV rate-pressure product in the setting of ischemia and reperfusion while this contractility index was similar to young hearts at baseline (Figure 2B). After 20 minutes of ischemia and 30 minutes post-ischemic reperfusion, the recovery of function in the aged hearts was more noticeably impaired during post-ischemic reperfusion, as evidenced by the reduced heart rate-left ventricular pressure product (LVDP), indicating diminished LV contractility during reperfusion in aged versus young hearts (Figure 2B).
Figure 2.
Intolerance of aged hearts during ischemia/reperfusion. A, Hearts were subjected to in vivo regional ischemia (20 min)/reperfusion (4 hr), and dual staining to assess the extent of myocardial necrosis (upper panel). Bars represent the per cent of ischemic area at risk in young, aged and young AMPK KD hearts (lower panel). Values are means ± SEM, n=4–5 per group. *P<0.05 vs. young; †P=0.02 vs. aged. B, Young and aged hearts were subjected to ex vivo ischemia (20 min)/reperfusion (30 min), and heart rate-left ventricular pressure product was assessed. Values are means ± SEM, n=4 per group. *P=0.006 vs. young.
Resveratrol Activation of AMPK Attenuates Ischemic Injury in Aged Heart
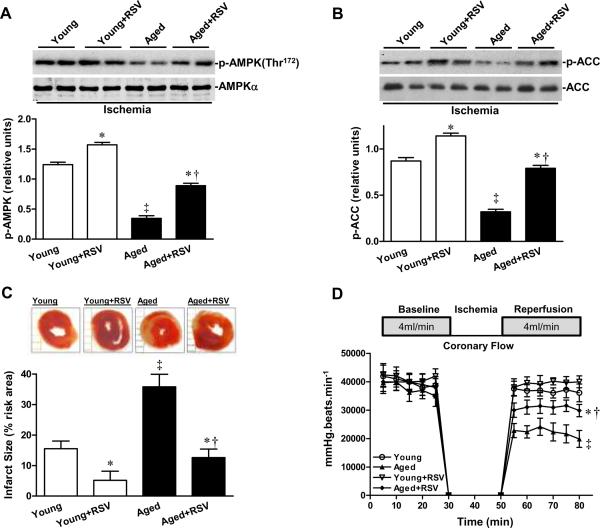
We next compared the effect of an AMPK activator, resveratrol,28, 29 on both young and aged hearts during ischemia. Pretreatment with 20 minutes of resveratrol (10μmol/L) followed by 20 minutes of global ischemia increased ischemia-stimulated AMPK and acetyl-CoA carboxylase (ACC) phosphorylation in both young and aged hearts (Figures 3A and 3B). Resveratrol treatment decreased myocardial infarct size in aged hearts (Figure 3C) and improved recovery of function in aged hearts during the post-ischemic reperfusion period, as evidenced by the significantly elevated heart rate-LVDP (Figure 3D). No differences in ex vivo heart rate were noted between the two age groups (data not shown).
Figure 3.
Resveratrol (RSV) activation of AMPK suppresses ischemic injury in aged hearts. The phosphorylation of A, AMPK and B, ACC of isolated young and aged heart during ex vivo global ischemia with or without RSV (10 μmol/L) treatment, n=4 per group, *P<0.05 vs. young or aged, respectively; †P=0.03 vs. young+RSV; ‡P=0.01 vs. young. C, The percent of infarct size of isolated young and aged heart with or without RSV treatment. Values are means ± SEM, n=5 per group. *P<0.05 vs. young or aged, respectively; †P=0.03 vs. young+RSV; ‡P=0.01 vs. young. D, Heart rate-left ventricular pressure product of isolated young and aged heart with or without RSV treatment. Values are means ± SEM, n=5 per group. *P=0.02 vs. aged; †P=0.03 vs. young+RSV; ‡P=0.01 vs. young.
Down-regulation of MIF Expression and Secretion in Aged Heart
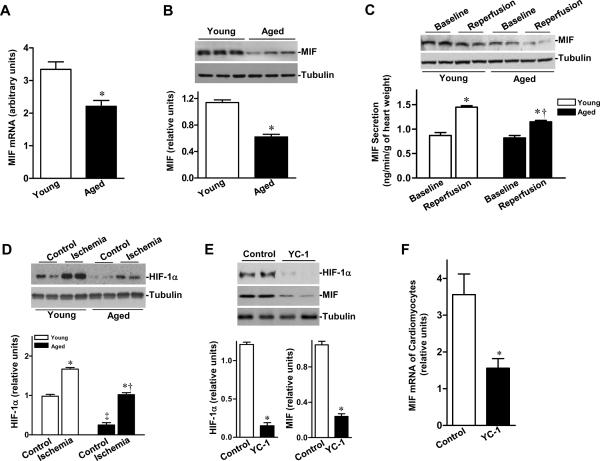
MIF modulates the activation of cardiac AMPK, which plays an important role in mitigating cardiac damage caused by I/R.20 To determine whether a blunted ischemic AMPK activation was due to MIF deficiency in senescence, we examined the expression levels of MIF in both young and aged hearts. The results demonstrated that both mRNA and protein expression of cardiac MIF were markedly decreased in the aged compared to young non-perfused hearts (Figures 4A and 4B), supporting our hypothesis of an aging-associated reduction of MIF, an upstream factor in ischemia-induced AMPK activation, in the heart. We further examined whether cardiac MIF secretion is decreased in aged heart. We studied the isolated mouse heart perfused with crystalloid buffer, thereby eliminating the potential contribution of MIF from circulating cells. Ischemia triggered cardiac MIF release was attenuated in senescence (Figure 4C). Furthermore, we observed no change in activities of the upstream AMPK activating kinases, LKB1 and Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ), in young and aged hearts (Suppl Figure 1).
Figure 4.
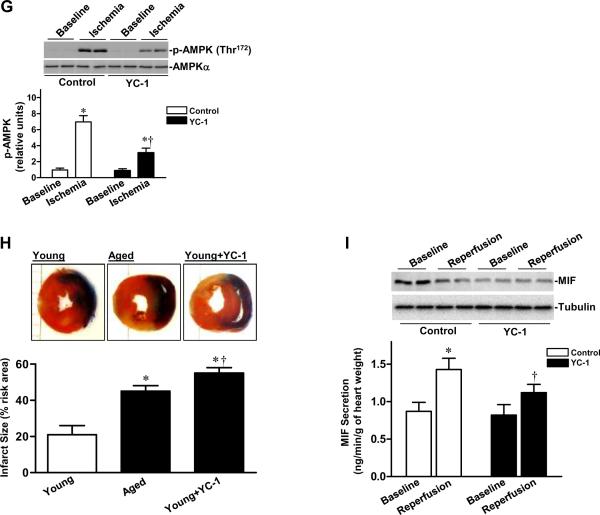
Cardiac MIF-AMPK axis in young and aged hearts. A, Quantitative PCR for MIF expression of non-perfused heart as described in the Supplemental Methods, n=4 per group, *P=0.01 vs. young. B, The relative levels of MIF of non-perfused hearts after normalization to β-tubulin, n=5 per group, *P=0.001 vs. young. C, MIF content in heart homogenates from young and aged hearts after control perfusion (baseline) or after ischemia and reperfusion (reperfusion) (upper panel), bars show the rates of coronary effluent MIF production from young and aged hearts during normal perfusion or washed out following 20 min of ischemia, MIF concentration was measured by ELISA and multiplied by the coronary flow rate to calculate the production rate, n=5 per group. *P<0.05 vs. baseline, respectively; †P=0.03 vs. young reperfusion. D, Levels of HIF-1α in ischemic area of young vs. aged hearts during sham control or in vivo regional ischemia (20 min), n=4 per group. *P<0.05 vs. control, respectively; ‡P=0.01 vs. young control; †P=0.02 vs. young ischemia. E, The relative levels of HIF-1α and MIF proteins from young control or HIF-1α inhibitor (YC-1) treated hearts, n=3–4 per group, *P<0.01 vs. control, respectively. F, Quantitative PCR for MIF mRNA in isolated cardiomyocytes from young control or YC-1 treated hearts, n= 4 per group *P=0.01 vs. control. G, Phosphorylation of AMPK from young control or YC-1 treated hearts during ex vivo ischemia (20 min), n=6 per group, *P<0.05 vs. baseline, respectively; †P=0.01 vs. control ischemia. H, Hearts were subjected to in vivo regional ischemia (20 min)/reperfusion (4 hr), and dual staining to assess the extent of myocardial necrosis (upper panel). Bars represent the percent of infarct size to area-at-risk in young, aged and young YC-1 treated hearts (lower panel), n=4 per group, *P<0.05 vs. young, respectively; †P=0.03 vs. aged. I, Immunoblots of MIF content in heart homogenates from young control and YC-1 treated hearts after control perfusion (baseline) or after ischemia and reperfusion (reperfusion) (upper panel), bars show the rates of coronary effluent MIF production from young control or YC-1 treated hearts during normal perfusion or washed out following 20 minutes of ischemia, MIF concentration was measured by ELISA and multiplied by the coronary flow rate to calculate the production rate, n=4 per group, *P=0.01 vs. baseline; †P=0.03 vs. control reperfusion.
To ascertain potential mechanisms for the down-regulation of MIF in the aged hearts, we examined the transcriptional factor, HIF-1α, which regulates MIF expression.30 As shown in Figure 4D, HIF-1α protein levels were significantly reduced in aged hearts compared to young hearts. Although HIF-1α levels were up-regulated by ischemia in both groups, the magnitude of HIF-1α induced by ischemia in aged hearts was significantly lower than in young hearts. The HIF-1α inhibitor, YC-131 (10 mg/kg/day, i.p. for 3 days), depressed cardiac HIF-1α levels, decreased MIF expression in young adult hearts (Figure 4E), and down-regulated the expression of MIF mRNA in cardiomyocytes (Figure 4F). The treatment of young hearts with YC-1 also blunted ischemic AMPK activation (Figure 4G) and resulted in larger myocardial infarct sizes than in untreated controls (Figure 4H), and the infracted area was even larger than that seen in aged control hearts. Notably, YC-1 treatment reduced ischemia-triggered MIF secretion of young hearts but it did not affect the baseline MIF secretion (Figure 4I).
Impaired AMPK Activation of MIF KO Hearts/Cardiomyocytes in Response to Ischemia/Hypoxia
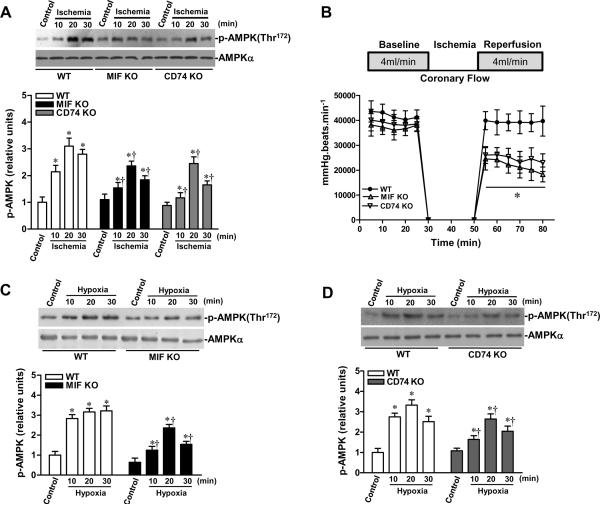
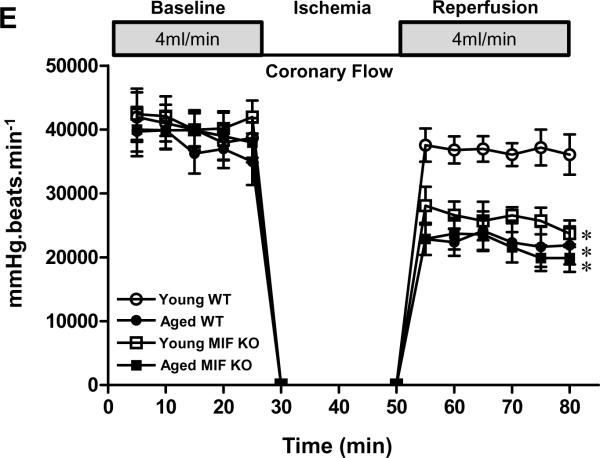
To verify the permissive role of MIF as a mediator of ischemic AMPK activation, in vivo regional ischemia was performed by LAD occlusion (10, 20 or 30 min) in MIF KO and WT mice. The results demonstrated that AMPK activation was markedly reduced in MIF KO versus WT hearts (Figure 5A). Moreover, ischemic activation of AMPK also was reduced in the MIF receptor, CD74-deficient heart (Figure 5A). The MIF KO and CD74 KO hearts also demonstrated significantly impaired recovery of post-ischemic LV contractile function in the setting of ischemia and reperfusion (Figure 5B). We next measured the response of isolated cardiomyocytes from WT, MIF KO or CD74 KO hearts to 10, 20 or 30 min of hypoxia treatment; the data showed that hypoxia stimulated AMPK phosphorylation of cardiomyocytes in a time-dependent manner and that the hypoxic AMPK activation of MIF KO and CD74 KO cardiomyocytes was significantly impaired when compared to WT cardiomyocytes (Figures 5C and 5D). MIF KO20 and CD74 KO mice nevertheless demonstrated a normal baseline cardiac phenotype with respect to left ventricular size and function (Suppl Table 2). Notably, there is no significant difference in ischemic tolerance between young and aged MIF KO hearts (Figure 5E), indicating that aging-associated decline in MIF expression is an important factor for ischemic intolerance in aged heart.
Figure 5.
Impaired AMPK signaling in MIF KO and MIF receptor (CD74) KO hearts. A, MIF KO, CD74 KO and WT mice were subjected to in vivo regional ischemia by LAD occlusion for either10, 20, or 30 min to determine the degree of ischemic AMPK activation (upper panel). Bars represent the relative levels of p-AMPK (lower panel). n=6 per group, *P<0.01 vs. control, respectively; †P<0.05 vs. WT ischemia, respectively. B, Heart rate-left ventricular pressure product of isolated WT, MIF KO and CD74 KO hearts, n=4 per group, *P<0.05 (both MIF KO and CD74 KO) vs. WT. C and D, The kinetics of AMPK phosphorylation induced by hypoxia in WT, MIF KO and CD74 KO cardiomyocytes, n=6 per group, *P<0.05 vs. control, respectively; †P<0.05 vs. WT hypoxia, respectively. E, Heart rate-left ventricular pressure product of isolated young and aged WT hearts, young and aged MIF KO hearts, n=4 per group. *P<0.05 vs. young WT, respectively.
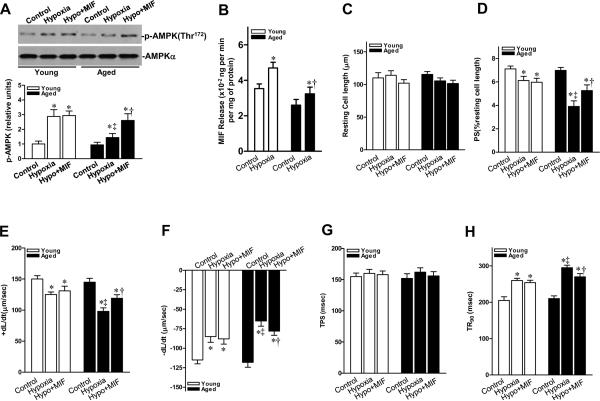
We next compared the response to hypoxia of isolated cardiomyocytes from aged hearts with those from young adult hearts. Hypoxia exposure resulted in depressed contractile function in both young and aged cardiomyocytes, i.e. reduced peak shortening (PS) (Figure 6D) and maximal velocity of shortening/relengthening (±dL/dt) (Figures 6E and 6F), and prolonged time-to-90% re-lengthening (TR90) (Figure 6H). Nonetheless, the extent of hypoxic dysfunction was significantly accentuated in aged versus young cardiomyocytes. Additionally, hypoxia-triggered MIF release from young cardiomyocytes was significantly more than that from aged cardiomyocytes (Figure 6B). To determine whether relative MIF deficiency in the aged heart was responsible for compromised cardiomyocyte mechanical function and AMPK activation during hypoxic stress, recombinant murine MIF (10 ng/mL) was added to the media during hypoxic incubation. Exogenous MIF restored hypoxia-stimulated AMPK activation in aged cardiomyocytes (Figure 6A) and partially restored contractile function in response to hypoxia (Figures 6 C–H). In contrast, MIF had no effect on contractility indices and AMPK activation in young cardiomyocytes. These data indicate that endogenous MIF maximally induces AMPK phosphorylation and contractility during hypoxia in young cardiomyocytes. However, in the aged and relatively MIF-deficient cardiomyocytes, exogenous MIF augmented contractility and AMPK activation during hypoxia.
Figure 6.
Recombinant MIF restores the contractility of aged cardiomyocytes. A, Immunoblots of p-AMPK of young and aged cardiomyocyte with or without MIF (10 ng/mL) treatment (upper panel). Bars represent the relative levels of p-AMPK (lower panel), n=4 per group, *P<0.05 vs. control, respectively; ‡P=0.03 vs. young hypoxia, †P=0.02 vs. aged hypoxia. B, MIF release from young and aged cardiomyocytes in response to hypoxia treatment, n=4 per group, *P<0.05 vs. control, respectively; †P=0.04 vs. young hypoxia. C, Resting cell length; D, Peak shortening (PS, normalized to cell length); E, Maximal velocity of shortening (+dL/dt) and F, re-lengthening (−dL/dt); G, Time-to-PS (TPS) and H, Time-to-90% relengthening (TR90). For C-H, n = 60–90 cells per group, *P<0.05 vs. control, respectively; ‡P<0.05 vs. young hypoxia, respectively; †P<0.05 vs. aged hypoxia, respectively.
MIF Increases AMPK Activity and Suppresses Ischemic Injury in Aged Heart
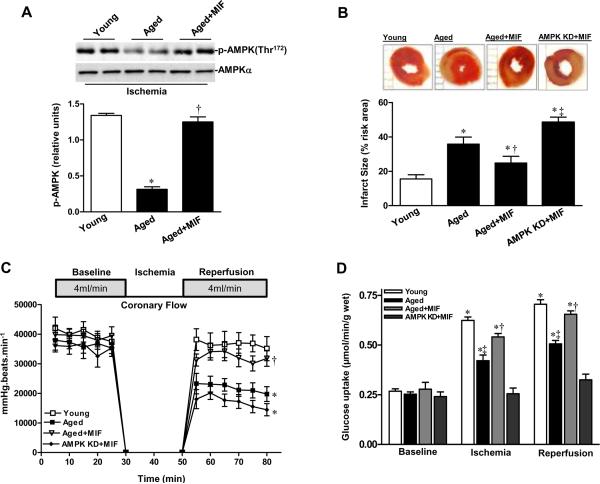
To assess whether MIF supplementation decreases ischemic injury in the isolated perfused senescent heart, pretreatment for 20 minutes with MIF (10 ng/mL) followed by 20 minutes global ischemia significantly increased ischemia-stimulated AMPK phosphorylation (Figure 7A). Furthermore, recombinant MIF treatment decreased myocardial infarct size in these aged hearts (Figure 7B) and markedly increased recovery-of-function during the post-ischemic reperfusion period, as evidenced by the significantly improved heart rate-LVDP (Figure 7C). However, recombinant MIF did not affect myocardial infarct size and recovery-of-function during the post-ischemic reperfusion period for AMPK KD hearts (Figures 7B and 7C). Moreover, recombinant MIF enhanced glucose uptake during ischemia or reperfusion in aged hearts but failed to be effective in AMPK KD hearts (Figure 7D).
Figure 7.
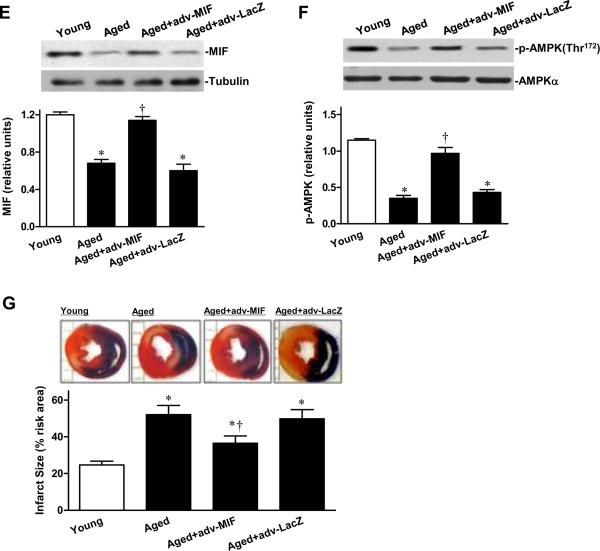
Supplemental MIF restores ischemic AMPK signaling in aged heart. A, Phosphorylation of AMPK in isolated young and aged hearts pretreated with or without MIF (10 ng/mL) during ex vivo global ischemia, n=4 per group, *P=0.01 vs. young; †P=0.01 vs. aged. B, The percent of infarct size to area-at-risk of isolated young, aged and AMPK KD hearts subjected to ex vivo global ischemia (20 min)/reperfusion (2 hr), n=4 per group. *P<0.05 vs. young, respectively; †P=0.03 vs. aged; ‡P=0.01 vs. aged+MIF. C, The heart rate-left ventricular pressure products of isolated young, aged and AMPK KD hearts with or without MIF treatment, n=4 per group, *P<0.05 vs. young, respectively; †P=0.01 vs. aged. D, Glucose uptake under baseline, ischemia and reperfusion in hearts from young, aged and AMPK KD mice supplemented with or without recombinant MIF, n=5 per group. *P<0.05 vs. baseline, respectively; ‡P<0.05 vs. young ischemia or reperfusion, respectively; †P<0.05 vs. aged ischemia or reperfusion, respectively. E, The expression levels of cardiac MIF (upper panel). Bars represent the relative levels of MIF protein (lower panel), n=4 per group, *P<0.05 vs. young, respectively; †P=0.01 vs. aged. F, Phosphorylation of AMPK in ischemic area of young, aged, and aged hearts with intra-myocardial adv-MIF during in vivo regional ischemia (20 min), n=4 per group, *P<0.05 vs. young, respectively; †P=0.01 vs. aged. G, The percent of infarct size to area-at-risk of young, aged and aged hearts with adv-MIF or adv-LacZ treatment, all were subjected to in vivo regional ischemia (20 min)/reperfusion (4 hr) (upper panel). Bars represent the percent of infarct size to area-at-risk (lower panel), n=5 per group, *P<0.05 vs. young, respectively; †P=0.02 vs. aged.
We then used a genetic approach to address whether the up-regulation of MIF in senescent hearts to levels observed in young hearts could likewise activate AMPK and preserve the cardiac response to ischemia. Following intra-myocardial injection of adenoviral encoded MIF (Adv-MIF, 5×109 IFU/ml) into the LV wall of aged hearts, the levels of cardiac MIF expression increased to those observed in young hearts (Figure 7E). Ischemic AMPK activity also was markedly increased in the Adv-MIF treatment group compared to the two control groups (Figure 7F). Following 20 minutes of coronary artery ligation and 4 hours of reperfusion, myocardial infarct size in the Adv-MIF treated aged hearts was reduced compared to aged or control adenoviral (adv-LacZ) injected hearts (Figure 7G). Therefore, an up-regulation of cardiac MIF expression in aged hearts to levels seen in young adult hearts increased both AMPK activation and the tolerance of these hearts to ischemic injury.
Discussion
Elucidation and remediation of the mechanisms of aging-associated deterioration in I/R response may serve to improve clinical outcomes in the aging population. In this study, we demonstrate for the first time that endogenous MIF, an up-stream activator cascade of AMPK,20 is reduced in aged hearts. Specifically, impaired ischemia-induced AMPK activation was associated with an inability to augment glucose uptake during ischemia. Furthermore, impaired MIF-AMPK activation has important functional consequences in the reperfused post-ischemic senescence hearts, including reduced recovery of LV contractile function and larger infarct size. While these observations provide evidence that cardiac MIF down-regulation and a resulting impairment of ischemic AMPK activation plays a causative role in the intolerance of the aged heart to ischemic injury, we cannot fully rule out the possibility that other factors, such as mitochondrial dysfunction, reactive oxygen species (ROS) formation and impaired nitric oxide signaling32 also may contribute to ischemic intolerance during aging.
The data in the present study support the conclusion that AMPK activity is significantly reduced in aged hearts, which leads to a dysregulation of glucose uptake during both ischemia and reperfusion in the aged hearts and that likely accounts for reduced tolerance to ischemic stress in senescence. The observation was that there is no significant difference in the content of AMP and ATP at baseline and at the end of ischemia/reperfusion, only lower ATP levels in the aged versus young hearts following ischemia (Suppl Figure 2B). However, the glycogen levels are different between young and aged hearts during both ischemia and reperfusion (Suppl Figure 2C). Therefore, these findings suggest that impaired ischemic AMPK activation of the aged heart leads to less ATP production and a greater shunting of glucose toward glycogen synthesis and to less glycolysis18, 24 (Suppl Figure 2). Therefore, a loss of ability to activate AMPK in the aged heart may result in impaired energy utilization that contributes directly to post-ischemia contractile dysfunction. To further address these issues, the metabolic effects of impaired AMPK activation in the aged heart require further investigation.
We further show by complementary studies with recombinant or adenoviral encoded MIF that increased AMPK activation in aged hearts effectively attenuates the impaired response to ischemic injury. Resveratrol (RSV) reduces ischemic damage by several mechanisms, such as reducing reactive oxygen species (ROS) and activating nitric oxide, SIRT1 and Akt signaling pathways33, but it also activates AMPK as part of its cellular protective actions33–36 and reduces infarct size in aged hearts. Exogenously added MIF also restores the impaired AMPK signaling of aged hearts and modulates the substrate metabolism to adapt the stress conditions. Exogenous MIF did not influence infarct size in young hearts (data not shown), suggesting that endogenous MIF can maximally activate ischemic AMPK signaling in the young heart. There also is no significant difference in MIF secretion between young and aged hearts during non-stress conditions; however, with aging an impairment in MIF secretion occurs leading to reduced AMPK activation and an increase in ischemic damage.
The precise mechanism of reduced MIF secretion in the aged heart is unknown. A recent report has identified the trafficking protein, p115, as mediating MIF secretion25; whether p115 expression or function is reduced in the aged heart would represent one avenue for further investigation. Hypoxia-inducible factor (HIF) plays an important role in the cellular response to hypoxia or ischemia.37 It is also known that HIF-1α delays premature senescence through activation of MIF in murine embryonic fibroblasts.30 An age-dependent decrease in HIF-1α expression was reported in brain, liver and kidney of mice38, which supports the decreased ability of such aged tissues to respond to hypoxic stress. Rohrbach et al have reported an aging-associated increase in PHD expression in mouse and human heart39, which may account for a reduction in the activity of HIF-1α in aged heart. The potentially protective role for HIF-1α in cellular senescence raises the possibility of a functional link between HIF-1α and MIF in explaining the sensitivity of the senescent heart to ischemia/reperfusion stress (Suppl Figure 3).
We conclude that an aging-associated decrease in the activity of the HIF-1α -MIF axis in the heart may explain the impaired AMPK activation response to ischemia in senescent heart. Pharmacologic interventions that restore MIF signaling and AMPK activity in the senescent heart may be a novel means to limit cardiac damage caused by ischemia/reperfusion in older cardiac patients.
CLINICAL PERSPECTIVE.
Cardiovascular disease remains the most frequent single cause of death among persons over 70 years of age. The aged heart is inherently more susceptible to injury during myocardial ischemia. However, the cause(s) of this increased susceptibility remain poorly understood. Based on both in vitro and in vivo observations, AMPK has emerged as an important component of the cardioprotective response against ischemic injury. The present study provides the first evidence that the senescent heart manifests an impaired AMPK activation in response to ischemic stress, which is associated with more severe myocardial damage during ischemia and reperfusion. This study also showed that cardiomyocyte production of an upstream activator of AMPK, macrophage migration inhibitory factor (MIF), is impaired in the aged heart. Importantly, supplementary administration of MIF by pharmacological or genetic approaches restored AMPK function in the aged heart, limited ischemic damage, and improved cardiac function following ischemia and reperfusion. Evidence is also provided that defective hypoxia-inducible factor-1, α subunit (HIF-1α), in the senescent heart may account for the impairment in MIF expression. An aging-associated decrease in the function of the HIF-1α -MIF axis may play a causative role in the intolerance of the senescent heart to ischemic injury. Pharmacologic interventions that restore MIF signaling and AMPK activity in the senescent heart may be a useful means to reduce cardiac damage caused by ischemic injury in older individuals.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by the following grants: NIH/NIA R03AG028163 (to Dr Li), American Heart Association (AHA) National SDG0835168N (to Dr Li), American Federation for Aging Research (AFAR) 08007 (to Dr Li), NIH/NIAID R01AI04230 (to Dr Bucala), The Brookdale Foundation (to Dr Bucala), The Deutsche Forschungsgemeinschaft (DFG)-FOR 809/TP1 (to Dr. Bernhagen), American Diabetes Association (ADA) 7-08-RA-130 (to Dr Ren), American Heart Association (AHA) Postdoctoral Fellowship 09POST2250477 (to Dr Ma).
Footnotes
Disclosures None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubino A, Yellon DM. Ischaemic preconditioning of the vasculature: an overlooked phenomenon for protecting the heart? Trends Pharmacol Sci. 2000;21:225–230. doi: 10.1016/s0165-6147(00)01483-8. [DOI] [PubMed] [Google Scholar]
- 2.Starnes JW, Bowles DK, Seiler KS. Myocardial injury after hypoxia in immature, adult and aged rats. Aging (Milano) 1997;9:268–276. doi: 10.1007/BF03341829. [DOI] [PubMed] [Google Scholar]
- 3.Mariani J, Ou R, Bailey M, Rowland M, Nagley P, Rosenfeldt F, Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120:660–667. doi: 10.1067/mtc.2000.106528. [DOI] [PubMed] [Google Scholar]
- 4.Abete P, Napoli C, Santoro G, Ferrara N, Tritto I, Chiariello M, Rengo F, Ambrosio G. Age-related decrease in cardiac tolerance to oxidative stress. J Mol Cell Cardiol. 1999;31:227–236. doi: 10.1006/jmcc.1998.0862. [DOI] [PubMed] [Google Scholar]
- 5.Jansen-Durr P, Osiewacz HD. Healthy ageing: a question of stress, damage and repair. Meeting on mechanisms of biological ageing. EMBO Rep. 2002;3:1127–1132. doi: 10.1093/embo-reports/kvf247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldarone CA, Krukenkamp IB, Burns PG, Gaudette GR, Schulman J, Levitsky S. Blood cardioplegia in the senescent heart. J Thorac Cardiovasc Surg. 1995;109:269–274. doi: 10.1016/S0022-5223(95)70388-8. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Grines CL, Browne KF, Marco J, Rothbaum D, O'Keefe J, Hartzler GO, Overlie P, Donohue B, Chelliah N, et al. Predictors of in-hospital and 6-month outcome after acute myocardial infarction in the reperfusion era: the Primary Angioplasty in Myocardial Infarction (PAMI) trail. J Am Coll Cardiol. 1995;25:370–377. doi: 10.1016/0735-1097(94)00367-y. [DOI] [PubMed] [Google Scholar]
- 8.Aguirre FV, McMahon RP, Mueller H, Kleiman NS, Kern MJ, Desvigne-Nickens P, Hamilton WP, Chaitman BR. Impact of age on clinical outcome and postlytic management strategies in patients treated with intravenous thrombolytic therapy. Results from the TIMI II Study. TIMI II Investigators. Circulation. 1994;90:78–86. doi: 10.1161/01.cir.90.1.78. [DOI] [PubMed] [Google Scholar]
- 9.Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, Col J, Simoons M, Aylward P, Van de Werf F, Califf RM. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91:1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 10.Tani M, Suganuma Y, Hasegawa H, Shinmura K, Hayashi Y, Guo X, Nakamura Y. Changes in ischemic tolerance and effects of ischemic preconditioning in middle-aged rat hearts. Circulation. 1997;95:2559–2566. doi: 10.1161/01.cir.95.11.2559. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeldt FL, Pepe S, Linnane A, Nagley P, Rowland M, Ou R, Marasco S, Lyon W. The effects of ageing on the response to cardiac surgery: protective strategies for the ageing myocardium. Biogerontology. 2002;3:37–40. doi: 10.1023/a:1015299127969. [DOI] [PubMed] [Google Scholar]
- 12.Young LH, Li J, Baron SJ, Russell RR. AMP-activated protein kinase: a key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 14.Lin SS, Manchester JK, Gordon JI. Sip2, an N-myristoylated beta subunit of Snf1 kinase, regulates aging in Saccharomyces cerevisiae by affecting cellular histone kinase activity, recombination at rDNA loci, and silencing. J Biol Chem. 2003;278:13390–13397. doi: 10.1074/jbc.M212818200. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Yang X, Lopez de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003;278:27016–27023. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- 16.Arad M, Moskowitz IP, Patel VV, Ahmad F, Perez-Atayde AR, Sawyer DB, Walter M, Li GH, Burgon PG, Maguire CT, Stapleton D, Schmitt JP, Guo XX, Pizard A, Kupershmidt S, Roden DM, Berul CI, Seidman CE, Seidman JG. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–2856. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- 17.Andersson L. Identification and characterization of AMPK gamma 3 mutations in the pig. Biochem Soc Trans. 2003;31:232–235. doi: 10.1042/bst0310232. [DOI] [PubMed] [Google Scholar]
- 18.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bucala R. MIF and the Genetic Basis of Macrophage Responsiveness. Current Immunol Revs. 2006;2:217–223. [Google Scholar]
- 20.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, 3rd, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 22.Kevill KA, Bhandari V, Kettunen M, Leng L, Fan J, Mizue Y, Dzuira JD, Reyes-Mugica M, McDonald CL, Baugh JA, O'Connor CL, Aghai ZH, Donnelly SC, Bazzy-Asaad A, Bucala RJ. A role for macrophage migration inhibitory factor in the neonatal respiratory distress syndrome. J Immunol. 2008;180:601–608. doi: 10.4049/jimmunol.180.1.601. [DOI] [PubMed] [Google Scholar]
- 23.Zhao P, Wang J, He L, Ma H, Zhang X, Zhu X, Dolence EK, Ren J, Li J. Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J Cell Mol Med. 2009;13:1513–1525. doi: 10.1111/j.1582-4934.2009.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folmes CD, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD. Suppression of 5'-AMP-activated protein kinase activity does not impair recovery of contractile function during reperfusion of ischemic hearts. Am J Physiol Heart Circ Physiol. 2009;297:H313–321. doi: 10.1152/ajpheart.01298.2008. [DOI] [PubMed] [Google Scholar]
- 25.Merk M, Baugh J, Zierow S, Leng L, Pal U, Lee SJ, Ebert AD, Mizue Y, Trent JO, Mitchell R, Nickel W, Kavathas PB, Bernhagen J, Bucala R. The Golgi-associated protein p115 mediates the secretion of macrophage migration inhibitory factor. J Immunol. 2009;182:6896–6906. doi: 10.4049/jimmunol.0803710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young LH. AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation. 2008;117:832–840. doi: 10.1161/CIRCULATIONAHA.107.713115. [DOI] [PubMed] [Google Scholar]
- 28.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Welford SM, Bedogni B, Gradin K, Poellinger L, Broome Powell M, Giaccia AJ. HIF1alpha delays premature senescence through the activation of MIF. Genes Dev. 2006;20:3366–3371. doi: 10.1101/gad.1471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol. 2007;72:440–449. doi: 10.1124/mol.107.036418. [DOI] [PubMed] [Google Scholar]
- 32.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 33.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 34.Hwang JT, Kwon DY, Park OJ, Kim MS. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008;2:323–326. doi: 10.1007/s12263-007-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 36.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czibik G, Sagave J, Martinov V, Ishaq B, Sohl M, Sefland I, Carlsen H, Farnebo F, Blomhoff R, Valen G. Cardioprotection by hypoxia-inducible factor 1 alpha transfection in skeletal muscle is dependent on haem oxygenase activity in mice. Cardiovasc Res. 2009;82:107–114. doi: 10.1093/cvr/cvp035. [DOI] [PubMed] [Google Scholar]
- 38.Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- 39.Rohrbach S, Simm A, Pregla R, Franke C, Katschinski DM. Age-dependent increase of prolyl-4-hydroxylase domain (PHD) 3 expression in human and mouse heart. Biogerontology. 2005;6:165–171. doi: 10.1007/s10522-005-7950-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.