Abstract
Purpose
Stress-induced cardiovascular reactivity is associated with the pathogenesis of cardiovascular disease. This study tested whether a simulated active commute to school dampened cardiovascular reactivity to a cognitive stressor typical to what children might experience during school.
Methods
Forty children (20 girls and 20 boys) ages 10 to 14 y were randomly assigned to simulated sedentary drive to school or active commute (walking) groups. The walking group completed a self-paced 1.6 km walk on a treadmill while images from a real 1.6 km walk through a pleasant neighborhood that finished at a school were projected in front of them. The drive to school group sat in a chair and watched the same slideshow of images of the neighborhood environment. Standardized residualized gain scores of cardiovascular reactivity during a cognitive stressor, the Stroop task, were calculated and used as dependent variables.
Results
Children in the walking group self-selected a walking intensity of 60.6 ± 1.6 %HR max and covered the 1.6 km distance in 21.5 ± 0.5 min. Children in the walking group had lower HR (2 ± 1 vs 11 ± 1 beats˙min−1, P < 0.001), systolic BP (4 ± 1 vs 12 ± 1 mmHg, P < 0.001), pulse pressure (−4 ± 1 vs 6 ± 1 mmHg, P < 0.001), and perceived stress (1.4 ± 0.1 vs 3.0 ± 0.1 cm, P < 0.001) reactivities to cognitive stress than the control group.
Conclusion
Active commuting to school may dampen cardiovascular reactivity and perceived stress when confronted with stressful cognitive challenges during the school day. This may help reduce the risk for cardiovascular disease later in life.
Keywords: walking exercise, Stroop test, cognitive stress, cardiovascular disease, children
INTRODUCTION
A physically active lifestyle in youth is associated with many health benefits (9) including buffering the effects of stress on the antecedents of cardiovascular disease (12). Cardiovascular reactivity to psychological stress is associated with the pathogenesis of cardiovascular disease in children (24) and atherosclerosis in adults (14). An acute bout of exercise dampens the potentially atherogenic increases in heart rate (HR) and blood pressure (BP) during mental stress in children (23). Thus, one way that an active lifestyle may reduce risk for cardiovascular disease is by reducing HR and BP reactivity to daily psychological stressors.
Children must cope with many social and cognitive stressors during a school day including peer relationships, making friends, teasing, and completion of coursework and homework (5). Active commuting (i.e., walking or bicycling) to school is one way to promote daily physical activity in youth (17) while perhaps at the same time dampening cardiovascular responses to school-related stressors.
To date, only the protective effect of an acute bout of higher-intensity interval exercise on the stress-induced cardiovascular reactivity of children has been studied (23). Whether a moderate intensity walk, that simulates a walk to school, would also dampen increases in cardiovascular responses to a cognitive stressor typical to what children might experience during a school day, has not yet been studied. Thus, the aim of this study was to test whether children who complete a self-paced walk that simulated an active commute to school would have dampened HR and BP reactivity and smaller increases in perceived stress to a cognitive stressor compared to a sedentary control group.
METHODS
Subjects
Forty (20 girls and 20 boys) Caucasian 10 to 14 year-old children served as subjects. Children were recruited through flyers and through phone calls to families who had inquired about previous studies conducted by the Division of Behavioral Medicine at the University at Buffalo and had provided consent to be contacted again for future studies. Inclusion criteria included no conditions or diseases that would affect physical activity, no history of diagnosed psychiatric disorder, no current illness, and no current use of medications that would alter baseline stress or stress reactivity. Children were below the 85th percentile for body mass index (BMI) and did not walk to school more than 1 day per week. Socioeconomic status (SES) was assessed by the parent completing a standard questionnaire (11). Parents provided written informed consent for their child to participate. Children gave their written assent to participate. The study was approved by the University at Buffalo Children and Youth Institutional Review Board. An investigator debriefed children and their parents after finishing the experiment.
Children were randomly assigned to either a sedentary control group designed to simulate being driven to school in a suburban neighborhood environment or a walking group that simulated an active commute through the same neighborhood and to the same suburban school. The environment was simulated by taking digital photographs every 50 m of an actual 1.6 km (1 mile) walk to a suburban school. The final 4 images included the school in the frame and were taken successively closer to the school to indicate arriving at the school. The neighborhood is highly walkable and characterized by low traffic, wide sidewalks separated from the road by a tree lawn, and well groomed lawns, shrubs and trees.
Procedures
Each child came to the Behavioral Medicine Research Laboratory for 1 visit. All visits took place in the morning to model a typical walk to school. Children were instructed not to eat anything 1 h prior to their appointment time and not to participate in any intense exercise the day before their visit. Height and weight of the child were taken at the beginning of the visit. Children completed a 7-day physical activity recall to assess usual activity levels. Children were fitted with a chest strap transmitter to record HR (Polar Vantage XL, Port Washington, NY) and to an automated BP monitor (Suntech 4240, Morrisville, North Carolina).
Children in both groups completed a 10 minute baseline period of quiet sitting while reading age-appropriate magazines. Children assigned to the sedentary control group then continued reading magazines for an additional 10 min and then moved to a comfortable chair and watched a 10 min slideshow of the images taken from the pleasant neighborhood environment. This was used to simulate riding to school in a motorized vehicle. Children assigned to the active commute group performed a 1.6 km walk on a treadmill at a self-selected pace. Children wore a book bag containing10% of the child’s body weight in books and walked while images of the neighborhood environment were projected on a screen in front of the child to simulate a walk to school. Children covered the 1.6 km distance in 21.5 ± 0.5 min or at a speed of 4.51 km/h (2.8 mph).
Children in both groups then completed a 20 minute recovery period, during which time they rested while reading magazines. This reading recovery period was included to control for time between experimental conditions as a recovery period was necessary in the walking condition to allow the cardiovascular measures to return to baseline after the exercise treatment and before beginning the stress paradigm. Children were then given instructions for completing a computerized Stroop color-word test. A series of names of colors written in a different colored font from the color specified were displayed on a computer monitor and children were asked to identify the color of the font, and not read the word. Children were instructed to say the correct color out loud, and that they had 3 min to provide as many correct responses as possible. Children were told that they would be evaluated on both quickness and accuracy of responses.
Apparatus and Physiological Measurement
Anthropometrics
Weight was measured to the nearest 0.1 kg using an electronic scale (Tanita BF-350, Arlington Heights, Illinois) with children wearing light clothing. Height was measured to the nearest 0.1 cm with a calibrated stadiometer (Digi-Kit, North Bend, WA). Body mass index (BMI) was calculated according to the following formula: (BMI = kg/m2). BMI percentile was calculated in relationship to 50th BMI percentile for children based on their sex and age (16).
Stress Reactivity
BP was measured with a Suntech 4240 monitor (Morrisville, North Carolina). The Suntech 4240 uses the auscultatory method aided by electrocardiographic R-wave gating, and an oscillometric transducer to determine SBP and DBP and is a valid and reliable measure of BP during rest and exercise (32). Measurement of BP followed published guidelines (27) regarding cuff length and width, placement of the cuff around the arm 2.5 cm above the antecubital space, and seating of the cuff of the arm by inflating and deflating the cuff before taking any measurements. BP was measured twice during the last 3 min of each protocol period (baseline, walking/sedentary control manipulation, recovery, Stroop task) and averaged, with the two measurements separated by 1 to 1.5 min. Pulse pressure (PP) was calculated as the difference between SBP and DBP. HR was measured continuously using a Polar Vantage XL (Port Washington, NY) HR monitor and averaged over the last 3 min of each protocol period. Perceived stress measurements were taken immediately after each protocol period as an indication of the child’s psychological stress for that time period. Perceived stress was measured by having subjects assess their current degree of psychological stress using a 10 point Likert scale, with anchors of no stress/very stressed. Likert scales are widely used in pediatric research (34) and to assess rapid changes in psychological stress (e.g., (25)). Children prefer Likert scales over VAS measurements and are recommended for use with children (34) with test-retest reliability of 0.95 to 0.99 (22).
Seven–Day Physical Activity Recall
The time spent in physical activity at different intensities was assessed using a standard interview (26). Children were interviewed to recall the time spent in sleep, and in moderate (as strenuous as walking), hard (an intensity between walking and running) and very hard (as strenuous as running) activities. The greatest amount of time each day is spent in light activities (less strenuous than walking, not including sleep) and was determined by subtraction. Total physical activity time was calculated as the sum of daily hours spent in moderate, hard, and very hard physical activity. Children were given instructions on how to properly answer the interviewer’s questions. The interview began with the previous day and worked back the next six days. To aid recall, the subjects were prompted for activities in the morning, noon, and evening of each day. The 7-day physical activity recall is a valid and reliable measure of activity in children (26).
Analytic Plan
Differences in physical characteristics, physical activity, and Stoop task performance were assessed using separate two-way ANOVA with group (control, walk) and sex (male/female) as between subject factors. A manipulation check was completed to determine the ability of the exercise treatment to increase cardiovascular measures compared to the control treatment. For the manipulation check, HR changes during the sedentary control and walk treatments were determined as the mean value during each treatment minus the mean baseline value. To adjust for individual differences in baseline HR, standardized residual gain scores for responses to the manipulations were calculated by adjusting the manipulation change score for the baseline HR value using regression. Group differences in standardized residual gain scores of HR from the manipulations were then assessed using two-way ANOVA with group and sex as between subject factors. To test whether the cardiovascular and perceived stress data had returned to baseline after the walking or sedentary treatments, differences in baseline and recovery values of the cardiovascular variables and perceived stress were assessed using separate mixed three-way ANOVA with group and sex as between subject factors and protocol period (baseline, recovery) as a within subjects factor.
To assess group differences in stress-induced cardiovascular reactivity and perceived stress, a second change score was calculated as the mean value during the Stroop task minus the mean recovery value. To adjust for individual differences in baseline and recovery levels of the variables, which may have influenced the magnitude of stress reactivity; standardized residual gain scores for stress reactivity were calculated by adjusting for both the baseline and recovery values using regression. Standardized residualized gain scores of the cardiovascular and perceived stress measures during the Stroop task were then used as dependent variables in two-way ANCOVA to test group and sex differences in cardiovascular reactivity during the Stroop task. SES was included as a covariate because lower SES is associated with greater threat interpretations during ambiguous, non-negative situations and with greater cardiovascular reactivity to stress (3, 35).
The standardized residual gain scores for HR, SBP, and DBP reactivity during the Stroop test were also used as dependent variables in separate multiple linear regression models to determine the association of physical activity with cardiovascular reactivity measures. Total physical activity was used as an independent variable in these models to predict HR and BP reactivity. Sex, BMI percentile, and SES were included as demographic covariates in the regression analyses.
RESULTS
As shown in Table 1, there were no group (P ≥ 0.89) or sex (P ≥ 0.99) differences for any of the physical characteristics. Physical activity variables did not differ between groups (P ≥ 0.65) or the boys and girls (P ≥ 0.13). There were also no group (P ≥ 0.51) or sex (P ≥ 0.99) differences in Stroop task performance.
Table 1.
Physical characteristics, physical activity, and Stroop task performance of the boys and girls in the sedentary control and walk groups
| Control group | Walk group | |||
|---|---|---|---|---|
| Boys n = 10 |
Girls n = 10 |
Boys n = 10 |
Girls n = 10 |
|
| Age (y) | 12.8 ± 1.4 | 13.0 ± 0.8 | 12.5 ± 0.9 | 12.0 ± 1.2 |
| SES* | 48.6 ± 10.7 | 48.7 ± 9.4 | 48.3 ± 9.7 | 48.0 ± 10.9 |
| Height (cm) | 160.1± 10.2 | 163.6 ± 6.4 | 159.3 ± 13.0 | 158.0 ± 7.1 |
| Weight (kg) | 46.6 ± 10.1 | 52.5 ± 6.1 | 46.9 ± 2.1 | 45.2 ± 2.1 |
| BMI percentile | 33.9 ± 17.8 | 54.4 ± 22.3 | 42.6 ± 11.3 | 42.5 ± 8.3 |
| Light PA (h˙day−1)** | 13.8 ± 1.3 | 13.1 ± 2.1 | 13.9 ± 11.3 | 13.2 ± 1.9 |
| Moderate PA (h˙day−1) | 0.4 ± 0.4 | 0.8 ± 1.4 | 0.2 ± 0.2 | 0.7 ± 1.3 |
| Hard PA (h˙day−1) | 0.5 ± 0.5 | 0.6 ± 0.8 | 0.5 ± 0.6 | 0.7 ± 0.5 |
| Very hard PA (h˙day−1) | 0.2 ± 0.3 | 0.2 ± 0.4 | 0.2 ± 0.2 | 0.1 ± 0.2 |
| Stroop performance (correct words) | 126 ± 20 | 136 ± 18 | 131 ± 28 | 121 ± 20 |
Data are mean ± SD
Socioeconomic status of 50 is equivalent to owner of medium-sized business, minor professions and technical personnel.
PA: physical activity
There were no significant group or sex differences for any of the variables in Table 1.
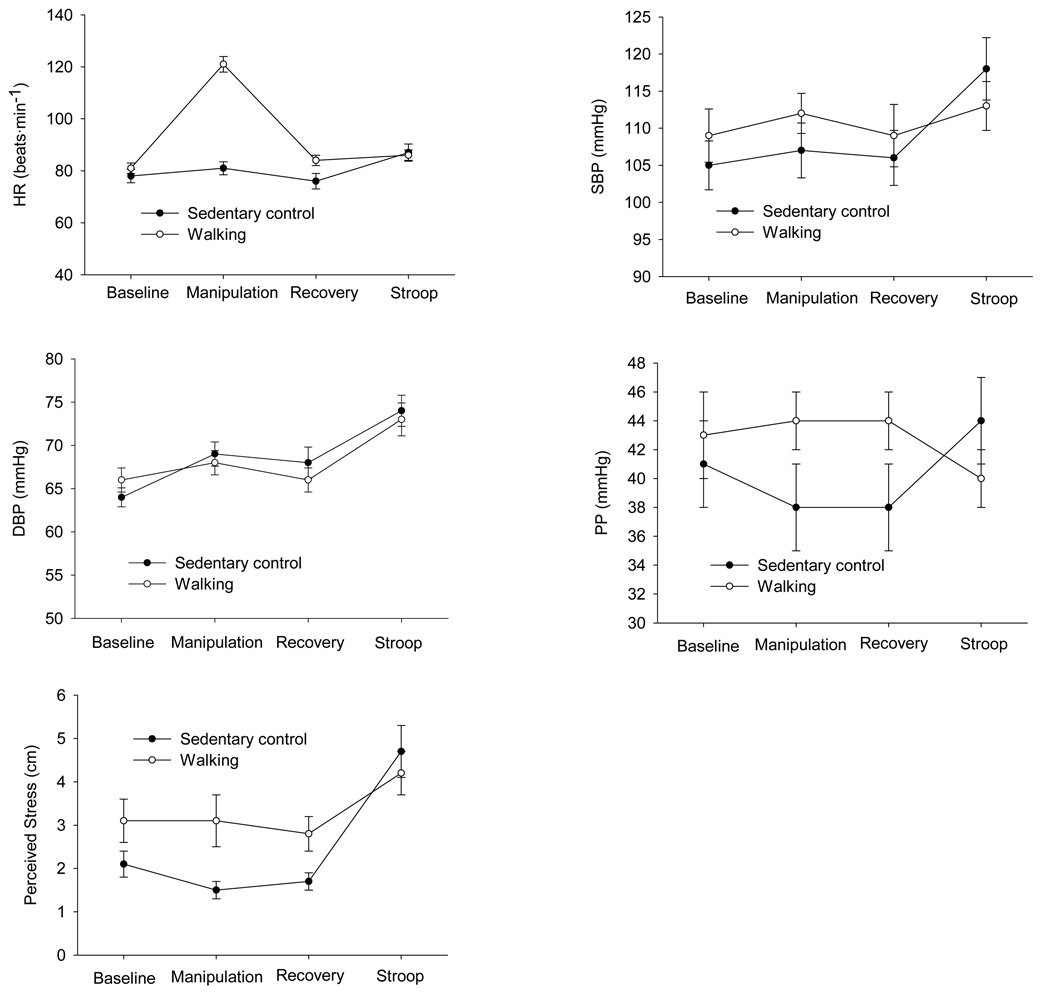
Cardiovascular outcomes measured at the different protocol periods are shown in Figure 1. As expected, results of the manipulation check demonstrated that HR (P < 0.001) increased more in the walking group (40 beat˙min−1) than in the control group (3 beat˙min−1) during the manipulations. The group by sex interaction (P ≥ 0.23) was not significant. Children in the walking group self-selected a walking intensity of 60.6 ± 1.6% HR max while the children in the control group remained at a sedentary heart rate of 40.4 ± 1.3% HR max (P < 0.001). HR was not significantly different (P ≥ 0.19) in the sedentary control group when comparing baseline and recovery values, but was increased (P < 0.02) a slight 3% at recovery in the walking group. SBP (P ≥ 0.63) and PP (P ≥ 0.29) did not differ in either group when baseline and recovery values were compared. Perceived stress was 19.5% lower (P ≥ 0.07) in the control group and only 8%, but significantly (P < 0.01), lower in the walking group between baseline and recovery.
Figure 1.
Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure (PP), and perceived stress outcomes measured at the different protocol periods. Data are mean ± SE of the raw data.
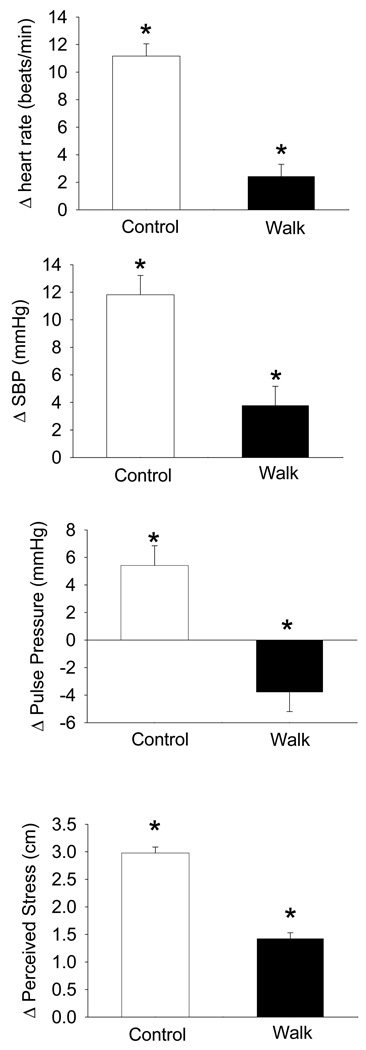
Standardized residual gain scores of the cardiovascular reactivity outcome measures during the Stroop stressor are shown in Figure 2. These scores are adjusted for individual differences in baseline and recovery cardiovascular values so that the reactivity results were not affected by the magnitude of the baseline or recovery value. Children in the walking group had lower HR reactivity (P < 0.001), SBP reactivity (P < 0.001), and PP reactivity (P < 0.001) than the control group. There were no sex differences (P ≥ 0.63) in cardiovascular reactivity. The group by sex interactions for HR (P ≥ 0.85), SBP (P ≥ 0.94), and PP (P ≥ 0.63) reactivity also were not significant. DBP reactivity did not differ (P ≥ 0.20) between the walking (7.6 ± 0.8 mmHg) and control (6.2 ± 0.7 mmHg) groups or between the sexes (P ≥ 0.61). Perceived stress reactivity was lower (P < 0.001) in the walking group than in the control group during the Stroop stressor with no significant main (P ≥ 0.22) or interaction (P ≥ 0.63) effects of sex. Removal of SES as a covariate in the ANOVA models did not change these results.
Figure 2.
Heart rate, systolic blood pressure (SBP), pulse pressure, and perceived stress reactivity during the Stroop cognitive stressor for the control and walk groups. *P < 0.05 for comparison of control and walk group means. Data are mean ± SE of standardized residualized gain scores that adjusted for individual differences in baseline and recovery values of the outcome variables.
Regression analyses demonstrated that greater usual physical activity by the children predicted a reduced DBP response to cognitive stress (Table 2). Usual physical activity did not significantly predict HR (P ≥ 0.93), SBP (P ≥ 0.89), or PP (P ≥ 0.67) reactivity.
Table 2.
Multiple linear regression of usual physical activity predicting diastolic blood pressure reactivity
| B | SE | β | P | |
|---|---|---|---|---|
| Constant | 11.24 | 3.06 | < 0.001 | |
| Sex* | −0.11 | 1.10 | −0.02 | 0.92 |
| BMI percentile | 0.03 | 0.02 | 0.17 | 0.29 |
| SES | −0.09 | 0.05 | −0.24 | 0.13 |
| Physical activity (h˙day−1) | −1.10 | 0.53 | −0.33 | 0.05 |
male = 0, female = 1
DISCUSSION
The main finding of this study was that HR, SBP, and perceived stress reactivity during a cognitive stressor were reduced in children who completed a simulated 1.6 km walk to school compared to a group that received a simulated passive commute to school. Children chose to walk an average of 4.5 km/h, very similar to the average 4.8 km/h self-chosen walking speed of children (20), which supports the external validity of these laboratory results. Roemmich and colleagues (23) also found that higher intensity interval exercise was protective against cardiovascular reactivity to interpersonal stress. The current results extends this research by providing evidence that a moderate-intensity walk, similar to a walk to school, can protect against the stress reactivity to a cognitive stressor typical to what children might experience during the school day.
SBP reactivity was dampened 2.1 fold in the present study after a self-paced walk performed at 61% HR max, which agrees with the 1.7 fold dampening of SBP reactivity observed in previous work using interval exercise performed at an average HR of approximately 78% HR max (23) Given that there is only one published study of the protective effect of exercise on children stress-induced cardiovascular reactivity, the results of the present study are also discussed in reference to data from studies of adults. Although exercise protocols of greater intensity have generally been more effective in reducing BP responses to psychological stress in adults, moderate intensity exercise has also produced dampening effects (8). The walking intensity used in our study was less intense than most of the exercise protocols used in previous studies of adults, though a study in adult smokers showed that a self-paced 15 min walk reduced SBP reactivity to psychological stress (31).
While SBP reactivity was dampened in the present study, walking exercise did not reduce DBP reactivity. Stress-induced DBP reactivity was found to be 63% to 80% reduced after higher intensity interval exercise in previous studies of children (23). Differences in stress-induced BP responses between these studies may have resulted from differences in intensity of the preceding exercise bout (10), but may also be a result of the stressor used to induce cardiovascular reactivity. The previous study of children (23) used an interpersonal speech task while the current study used the Stroop task to model the cognitive stress children experience during school. Both the interpersonal speech and the Stroop tasks require active coping, but the speech task evokes a greater level of social and evaluative threat (1). More socially relevant stressors produce greater and more consistent increases in beta adrenergic responses, including greater increases in DBP reactivity (1).
The lower SBP reactivity and similar DBP reactivity of the walking group compared to the sedentary control group resulted in PP reactivity being lower during the Stroop task in the children who performed an active commute. Greater PP reactivity to stress during adolescence is a risk factor for cardiovascular disease in adulthood (21). Attenuating PP responses to mental stress through a brief walk may be a simple yet effective means of slowing or preventing atherosclerosis and the risk for cardiovascular disease later in life.
HR reactivity was also lower in children after an active commute, providing additional evidence that exercise is protective against stress-induced cardiovascular reactivity. This result is consistent with previous work that found an intense bout of interval exercise was protective against HR reactivity in children (23). Results from studies of adults are mixed, with some reporting a reduction in HR reactivity and others finding no protective effect of exercise (8). Studies that did not find a protective effect on stress-induced HR reactivity employed high intensity exercise (8). High intensity exercise in adults promotes extended elevations in HR, which may confound stress-induced HR reactivity data if the recovery period is not of a great enough duration or if the recovery HR is not adjusted for in the analysis (8). Children’s HR returns to baseline more quickly after high intensity exercise than adults (36), which may help to explain the more consistent reductions of HR during mental stress after acute exercise in children.
The mechanisms by which exercise can dampen cardiovascular reactivity to psychological stress are still being explored. Sympathetic stimulation produces acute increases in arterial pressure by increasing heart rate, heart muscle contractility, and increasing resistance to blood flow (19). A reduction in sympathetic activity after exercise is likely to play a key role in the buffering of stress-induced cardiovascular responses. In adults, decreases in blood pressure reactivity to mental stress after 25 minutes of cycling is associated with decreased neuroendocrine responses to stress including a reduction in norepinephrine secretion and increased B2-adrenergic receptor responsivity (2). Furthermore, norepinephrine reactivity was the best predictor of reduced blood pressure reactivity to stress after exercise (2). These alterations promote greater stress-induced vasodilatation and may result in the attenuation of blood pressure responses to subsequent mental stress (2).
Perceived stress reactivity was also dampened by completing a 1-mile walk. One way that walking exercise may dampen stress-induced cardiovascular reactivity is by altering an individual’s evaluation of stressful stimuli such as reducing the perceived threat of the event. An acute bout of moderate intensity exercise improves mood (30) and self-esteem (4) and reduces anxiety (29), which may also play a role in reducing perceived mental stress to a cognitive stress task after exercise. Future studies in children should include measurements of sympathetic activity and psychosocial measures to examine possible mechanisms that may be involved in exercise reducing stress-induced cardiovascular reactivity.
Studies of adults have shown an inverse association between physical activity level and post-exercise stress reactivity (18). Daily or frequent physical activity could attenuate cardiovascular stress reactivity by repeatedly placing an individual in a protective post-exercise window when encountering stress (8). Regression analyses found an inverse association between physical activity and DBP reactivity, but not SBP or HR reactivity in the present study. In contrast, no significant associations were found between physical activity and stress-induced cardiovascular reactivity in a previous study of children (23). It is unclear why the results are not consistent across experiments of children, though there were methodological differences in the assessment (accelerometry versus interview) of physical activity. The data from adults also are not consistent as some studies (7) have not found an association between physical activity level and post-exercise stress reactivity. Importantly, the groups in the present study did not differ in physical activity so this could not account for the group differences in cardiovascular reactivity to the cognitive stressor.
There are several potential weaknesses with this study. The study used a between subjects research design and had a restricted sample size. The walk to school was simulated in the laboratory rather than occurring in the field. It is not known whether the same effects would have been observed if the children actually completed the walk in a neighborhood environment. Similarly, the results may have been different if the stress testing had been completed in the school rather in the laboratory. Frequent stressful experiences at the school could result in associating the school with psychological stress resulting in stress-induced cardiovascular reactivity. The choice to use a simulated walk and measuring stress reactivity in a laboratory setting was motivated by the novel nature of the research and the need to control for biasing or confounding environmental influences such as cue exposure of being in school, pretesting social interactions, and wind, temperature, and humidity on stress reactivity and cardiovascular responses. Given that significant effects were observed in a carefully controlled experiment and environment, the research could now be extended to field testing. Cardiovascular responses to only one type of stressor were tested. Cardiovascular reactivity to other standard stress paradigms that closely model the types of stressors children experience during school, such as mental arithmetic or interpersonal speech tasks, should be included in future studies. Aggregating cardiovascular reactivity data from several tasks may improve reliability of the cardiovascular outcome measures (15). Measurement of sympathetic activity or other potential mechanisms that may explain the blunted cardiovascular reactivity during stress in the exercise group should also be included in future studies. A limitation of this type of research is that blinding is difficult because exercise participation is hard to disguise. It is possible that the children reacted differently after knowing whether they were in the exercise or control condition. Finally, only Caucasian youth were studied. Future research should include minority youth.
In conclusion, an active commute to school in a pleasant neighborhood may dampen children’s cardiovascular reactivity when confronted with cognitive stressors during the school day. In addition, physical activity behaviors established in youth often track into adulthood (6). Regular walking to school may increase the likelihood of long-term use of walking as a mode of active transportation (28). This may have additional health benefits for children such as increasing the total amount of energy expended during the day (33) and improved cognitive function and academic performance (13, 28). Future research should determine the effects of repeated bouts of walking to school to determine whether children habituate to the protective effects of a brief walk on cardiovascular and perceived stress reactivity or whether such exercise indeed dampens reactivity, while providing additional health benefits. The minimal amount of exercise necessary to produce a protective effect on stress reactivity and the duration of the protective refractory period also should be investigated in future work.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant HD055270 to Dr. Roemmich. The results of this study do not constitute endorsement by ACSM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al'Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34(3):266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 2.Brownley KA, Hinderliter AL, West SG, Girdler SS, Sherwood A, Light KC. Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med Sci Sports Exerc. 2003;35(6):978–986. doi: 10.1249/01.MSS.0000069335.12756.1B. [DOI] [PubMed] [Google Scholar]
- 3.Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Dev. 2004;75(4):1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 4.Ekeland E, Heian F, Hagen KB. Can exercise improve self esteem in children and young people? A systematic review of randomised controlled trials. Br J Sports Med. 2005;39(11):792–798. doi: 10.1136/bjsm.2004.017707. discussion -8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias MJ, Gara M, Ubriaco M. Sources of stress and support in children's transition to middle school: An empirical analysis. J Child Clinical Psychol. 1985;14(2):112–118. [Google Scholar]
- 6.Gordon-Larsen P, Nelson MC, Popkin BM. Longitudinal physical activity and sedentary behavior trends: adolescence to adulthood. Am J Prev Med. 2004;27(4):277–283. doi: 10.1016/j.amepre.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Hamer M, Boutcher Y, Boutcher SH. Cardiovascular and renal responses to mental challenge in highly and moderately active males with a family history of hypertension. J Human Hypertens. 2002;16(5):319–326. doi: 10.1038/sj.jhh.1001396. [DOI] [PubMed] [Google Scholar]
- 8.Hamer M, Taylor A, Steptoe A. The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biol Psychol. 2006;71(2):183–190. doi: 10.1016/j.biopsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Hills AP, King NA, Armstrong TP. The contribution of physical activity and sedentary behaviours to the growth and development of children and adolescents: implications for overweight and obesity. Sports Med. 2007;37(6):533–545. doi: 10.2165/00007256-200737060-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hobson ML, Rejeski WJ. Does the dose of acute exercise mediate psychophysiological responses to mental stress? J Sport Exerc Psychol. 1993;15:77–87. [Google Scholar]
- 11.Hollingshead AB. Four factor index of social status. New Haven, Conn: Yale University; 1975. p. 18. [Google Scholar]
- 12.Holmes ME, Eisenmann JC, Ekkekakis P, Gentile D. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health. 2008;5(2):294–307. doi: 10.1123/jpah.5.2.294. [DOI] [PubMed] [Google Scholar]
- 13.Hulley A, Bentley N, Clough C, et al. Active and passive commuting to school: influences on affect in primary school children. Res Q Exerc Sport. 2008;79(4):525–534. doi: 10.1080/02701367.2008.10599519. [DOI] [PubMed] [Google Scholar]
- 14.Jennings JR, Kamarck TW, Everson-Rose SA, Kaplan GA, Manuck SB, Salonen JT. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110(15):2198–2203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- 15.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosom Med. 2003;65(1):9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 17.Lee MC, Orenstein MR, Richardson MJ. Systematic review of active commuting to school and childrens physical activity and weight. J Phys Act Health. 2008;5(6):930–949. doi: 10.1123/jpah.5.6.930. [DOI] [PubMed] [Google Scholar]
- 18.Light KC, Obrist PA, James SA, Strogatz DS. Cardiovascular responses to stress: II. Relationships to aerobic exercise patterns. Psychophysiology. 1987;24(1):79–86. doi: 10.1111/j.1469-8986.1987.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 19.Lovallo WR. Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. Int J Psychophysiol. 2005;58(2–3):119–132. doi: 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Morgan DW. Economy of locomotion. In: Armstrong N, van Mechelen W, editors. Paediatric Exercise Science and Medicine. Oxford: University Press; 2000. pp. 183–190. [Google Scholar]
- 21.Raitakari OT, Juonala M, Taittonen L, et al. Pulse pressure in youth and carotid intimamedia thickness in adulthood: the cardiovascular risk in young Finns study. Stroke. 2009;40(4):1519–1521. doi: 10.1161/STROKEAHA.108.525253. [DOI] [PubMed] [Google Scholar]
- 22.Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976;31(9):1191–1198. doi: 10.1111/j.1365-2044.1976.tb11971.x. [DOI] [PubMed] [Google Scholar]
- 23.Roemmich JN, Lambiase M, Salvy SJ, Horvath PJ. Protective effect of interval exercise on psychophysiological stress reactivity in children. Psychophysiology. 2009;46(4):852–861. doi: 10.1111/j.1469-8986.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 24.Roemmich JN, Lobarinas CL, Joseph PN, Lambiase MJ, Archer Iii FD, Dorn J. Cardiovascular reactivity to psychological stress and carotid intima-media thickness in children. Psychophysiology. 2009;46(2):293–299. doi: 10.1111/j.1469-8986.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 25.Roemmich JN, Smith JR, Epstein LH, Lambiase M. Stress reactivity and adiposity of youth. Obesity. 2007;15(9):2303–2010. doi: 10.1038/oby.2007.273. [DOI] [PubMed] [Google Scholar]
- 26.Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25(1):99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro D, Jamner LD, Lane JD, et al. Blood pressure publication guidelines.Society for Psychophysical Research. Psychophysiology. 1996;33(1):1–12. doi: 10.1111/j.1469-8986.1996.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 28.Sirard JR, Riner WF, Jr, McIver KL, Pate RR. Physical activity and active commuting to elementary school. Med Sci Sports Exerc. 2005;37(12):2062–2069. doi: 10.1249/01.mss.0000179102.17183.6b. [DOI] [PubMed] [Google Scholar]
- 29.Smith JC, O'Connor PJ, Crabbe JB, Dishman RK. Emotional responsiveness after low-and moderate-intensity exercise and seated rest. Med Sci Sports Exerc. 2002;34(7):1158–1167. doi: 10.1097/00005768-200207000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Steptoe A, Cox S. Acute effects of aerobic exercise on mood. Health Psychol. 1988;7(4):329–340. doi: 10.1037//0278-6133.7.4.329. [DOI] [PubMed] [Google Scholar]
- 31.Taylor A, Katomeri M. Effects of a brisk walk on blood pressure responses to the Stroop, a speech task and a smoking cue among temporarily abstinent smokers. Psychopharmacology (Berl) 2006;184(2):247–253. doi: 10.1007/s00213-005-0275-1. [DOI] [PubMed] [Google Scholar]
- 32.Taylor RS, Gallen I. Evaluation of SunTech 4240 during rest and during exercise:A novel automated blood pressure device. J Cardiopulmonary Rehab. 1994;14:330–334. [Google Scholar]
- 33.Tudor-Locke C, Ainsworth BE, Adair LS, Popkin BM. Objective physical activity of filipino youth stratified for commuting mode to school. Med Sci Sports Exerc. 2003;35(3):465–471. doi: 10.1249/01.MSS.0000053701.30307.A6. [DOI] [PubMed] [Google Scholar]
- 34.van Laerhoven H, van der Zaag-Loonen HJ, Derkx BH. A comparison of Likert scale and visual analogue scales as response options in children's questionnaires. Acta Paediatr. 2004;93(6):830–835. doi: 10.1080/08035250410026572. [DOI] [PubMed] [Google Scholar]
- 35.Williams RB, Marchuk DA, Siegler IC, et al. Childhood socioeconomic status and serotonin transporter gene polymorphism enhance cardiovascular reactivity to mental stress. Psychosom Med. 2008;70(1):32–39. doi: 10.1097/PSY.0b013e31815f66c3. [DOI] [PubMed] [Google Scholar]
- 36.Zafeiridis A, Dalamitros A, Dipla K, Manou V, Galanis N, Kellis S. Recovery during high-intensity intermittent anaerobic exercise in boys, teens, and men. Med Sci Sports Exerc. 2005;37(3):505–512. doi: 10.1249/01.mss.0000155394.76722.01. [DOI] [PubMed] [Google Scholar]