Abstract
Background
Parental alcoholism substantially raises risk for offspring alcoholism, an effect thought to be mediated by a dysregulation in impulse control. Adult alcoholics have alterations in the frontostriatal system involved in regulating impulsive responses. However, it remains controversial whether these alterations reflect pre-existing traits predisposing to problem alcohol use, or are secondary to alcohol involvement.
Methods
Sixty-one 16 to 22 year olds were tested using a go/no-go task during functional MRI. Forty-one had at least one parent with a diagnosis of alcohol use disorder (AUD; FH+) and 20 had no parent with AUD (FH−). Two FH+ subgroups were created to disentangle alcohol involvement from preexisting risk: the FH+ control (n=20) group had low alcohol problems, differing from the FH− group only by family history. The FH+ problem group (n=21) had high alcohol problems.
Results
The ventral caudate deactivated during successful inhibition in the FH− but not the FH+ groups, regardless of problem alcohol involvement. Regression analyses showed that ventral caudate deactivation was related to fewer externalizing problems as well as family history. Orbital and left medial prefrontal regions were deactivated in both the FH− and FH+ control groups but not the FH+ problem group. Activation in these regions was associated with alcohol and other drug use.
Conclusions
These findings suggest a preexisting abnormality in ventral striatal function in youth at risk for AUD, which may lead to inappropriate motivational responding; and suggest that with alcohol use, the prefrontal “control” mechanism loses efficiency, further dysregulating the frontostriatal motivational circuitry.
Keywords: Adolescent, Medial Prefrontal, Ventral Striatum, Caudate, Response Inhibition, Alcoholism, Vulnerability
Parental alcoholism significantly raises risk for offspring alcoholism (1, 2) and some of this risk is mediated through intermediary behavioral traits (3–6). One of the core traits predicting alcohol use disorder (AUD) from early childhood onward is behavioral undercontrol (7, 8), including externalizing behavior (aggression and delinquency), impulsivity, and sensation-seeking. These variables share the characteristic of behavioral disinhibition, involving the inability, unwillingness, or failure to inhibit behavioral impulses even in the face of negative consequences (9). Weakness in response inhibition specifically has been found to be a general liability factor for a range of externalizing and substance use problems (10). A primarily right-hemisphere network including the prefrontal cortex, parietal cortex and striatum is critical to response inhibition and the control of behavior more generally (e.g., 11, 12, 13).
Few studies have investigated these neural systems directly in children of alcoholics (COA). One study found less activation in the left middle frontal gyrus during response inhibition in 12–14 year old COA compared with nonCOA, despite similar performance (14). Similarly, a dysregulation index of risk was negatively correlated with left frontal eye field activation during inhibition of eye movement, but not with performance in 12–19 year olds (15). These findings suggest that a weakness in frontal response inhibition circuitry may be related to risk.
Other studies have examined the neural correlates of familial risk using cognitive or emotional paradigms that do not specifically probe impulse control, but recruit neural systems involved in behavioral regulation (16–18). A recent study of 12–14 year olds found family history was related to a failure of deactivation in medial prefrontal regions involved in the default mode network (DMN) (19) during spatial working memory compared to vigilance (18). This suggests familial risk may be related to less inhibition of task-irrelevant processing. Using a reward task known to activate the ventral striatum (20), familial risk was investigated in 12 −16 year old COA and nonCOA (16). Although ventral striatal activation did not differ between the groups in this sample, it correlated positively with a personality measure of impulsivity across groups, suggesting a possible relationship with risk.
One limitation of these studies is that the participants showed little evidence of behavior problems typically considered to lie on a developmental spectrum with AUD. Most were non-drinking youth with no behavioral or mood disorders which may result in a diluted representation of risk. Further, it is important to consider these neural systems in conjunction with the developmental transition from late adolescence to early adulthood, when there is major build up of alcohol use and alcohol use-related problem behavior (21).
A recent study investigated emotional processing during this developmental period in a design which accounted for both familial risk and behavioral risk (17). This study revealed increased dorsomedial prefrontal activation and decreased striatal activation to emotional versus neutral stimuli in COA showing risky drinking behavior compared to a control (non-risky behavior) COA group and nonCOA. These findings suggest different neural activation in COA on a risky trajectory versus those who are not, highlighting the importance of capturing a range of behavior problems in studies designed to investigate risk. To date, these neural systems have not been studied during response inhibition in the context of familial risk during late adolescence/early adulthood.
Another important consideration is that drug and alcohol use undoubtedly alters the brain maturation processes (reviewed in 22). A recent fMRI study of impulse control found that adult abstinent alcoholics had altered neural processing, including decreases in left dorsolateral prefrontal cortex and increases in bilateral middle frontal gyrus (23). Evidence for altered functional networks has also been found during spatial working memory in adult (24, 25) and adolescent (26) alcoholics.
The present study was designed to build on prior work by investigating frontostriatal functioning during response inhibition during late adolescence/early adulthood and relate it to both familial risk and problem alcohol involvement. A go/no-go task (27, 28) was used during fMRI acquisition. FH+ participants were divided into those with low alcohol problems (FH+ control) matched to a control group (FH−) and those with high alcohol problems (FH+ problem). This allowed the following comparisons: 1) FH− vs. FH+ control isolates effects due to family history; and 2) FH+ control vs. FH+ problem isolates effects of problem alcohol involvement. Based on prior work we expect familial risk to be reflected in a weakness in frontal response inhibition circuitry. Evidence also exists for striatal differences based on risk; however the direction this might take during impulse control is not clear considering there are reports of both striatal activation (e.g., 29) and deactivation (30, 31) during response suppression. We further expected evidence of additional recruitment of frontal regions as a result of problem alcohol involvement due to altered functional networks.
Given that alcohol problems can encompass both externalizing problem behavior (which includes both aggressive and conduct/antisocial problems) and level of alcohol consumption, we additionally conducted a series of supplemental analyses to take into account the contribution of each of these variables to the main findings. Other drug use was also considered as it tends to co-occur with externalizing problems and alcohol consumption and could contribute to differences in BOLD activation. Marijuana use in particular has been shown to increase prefrontal activation during inhibitory processing (32, 33).
Methods and Materials
Participants
Sixty-one (35 males) right-handed participants aged 16 – 22 (mean 19.1 ± 1.6) were recruited from the Michigan Longitudinal Study (MLS), an ongoing, prospective community study of families with high levels of parental AUD and a contrast sample of nonalcoholic families drawn from the same neighborhoods (34). Families in which the target child displayed evidence of fetal alcohol effects were excluded from the original ascertainment. Handedness was determined with the Edinburgh Handedness Inventory (35).
The FH− group (n=20) had no parent history of AUD and low alcohol problems (operationalized below). The FH+ Control group (n=20) had at least one parent with an AUD diagnosis and low alcohol problems. The FH+ Problem group (n=21) had at least one parent with an AUD diagnosis (FH+) and high alcohol problems. Parent diagnosis was based on DSM-IV criteria and established via multiple face-to-face assessments. Characteristics of these groups are summarized in Table 1.
Table 1.
Subject Characteristics and Task Performance.
| FH− | FH+ Control | FH+ Problem | |
|---|---|---|---|
| N | 20 | 20 | 21 |
| Males: Females | 11:9 | 12:8 | 12:9 |
| Age (years) | 19.2 (1.9) | 19.0 (1.6) | 19.3 (1.3) |
| IQ (WISC-III)a | 112 (9) | 109 (13) | 110 (10) |
| Alcohol Abuse or Dependence | 0 | 0 | 6 |
| Mj Abuse or Dependence | 0 | 0 | 5 |
| Other Drug Abuse or Dependence | 0 | 0 | 2 |
| Any Substance Use Disorder Dxb | 0 | 0 | 8 |
| Conduct Disorder Dx | 0 | 0 | 4 |
| Attention Deficit Disorder Dx | 0 | 0 | 1 |
| Any Dxc | 0 | 0 | 8 |
| # Alcohol Problems from DDHx | 0.9 (1.4) | 1.6 (1.7) | 11.4 (4.8) * |
| Drinking Volumed from DDHx | 19.0(43.5) | 26.7(59.4) | 55.2(77.8)* |
| Mj Use – past 12 months from DDHx | 1.1(2.4) | .8(1.8) | 3.3(2.8) * |
| # Illicit drugs ever used from DDHx | .45(.83) | .55(.89) | 2.67(2.4) * |
| Mother/Father/Both Dependence | 0/0/0 | 2/9/7 | 3/10/7 |
| Mother/Father/Both Abuse | 0/0/0 | 2/4/0 | 1/1/0 |
| Mother/Father/Both Dependence or Abuse | 0/0/0 | 0/9/11 | 3/10/8 |
| Mother/Father/Both Abused other Drugs | 0/3/0 | 1/6/2 | 2/11/3 |
| Youth Self Report (YSR) Form – T scores | |||
| Total Internalizing | 44(12.8) | 43(12.1) | 49(7.8) |
| Total Externalizing | 46(8.7) | 49(12.6) | 57(9.7) * |
| Go-no-go task performance | |||
| RTs (msecs) | 420 (55) | 404 (52) | 430 (44) |
| False alarm rate | .24 (.19) | .25 (.13) | .20 (.10) |
| Total error rate | .26 (.21) | .27 (.14) | .22 (.12) |
Dx, diagnosis; Mj, marijuana; msecs, milliseconds; DDHx, drinking and drug history form.
Wechsler Intelligence Scale for Children – 3rd edition. These data were collected when participants were between the ages of 12 and 14 years as part of the ongoing Michigan Longitudinal Study.
Includes alcohol abuse or dependence, marijuana abuse or dependence and/or other drug abuse or dependence
Includes conduct disorder, attention deficit disorder and/or any substance use disorder.
drinking days over past year X usual # of drinks per day.
Significant differences between groups (described fully in text).
Exclusionary criteria for the present study were: neurological, acute, uncorrected or chronic medical illness; current or recent (within six months) treatment with centrally active medications; and history of psychosis or schizophrenia in first-degree relatives. In addition, participants were given a multi-drug 5-panel urine screen before scanning and those with a positive drug screen were not included in this study. The presence of most Axis I psychiatric or developmental disorders was exclusionary, with the exception of conduct disorder, attention deficit/ hyperactivity disorder (ADHD), or prior substance use disorder (SUD). These disorders were allowed because they are believed to lie on an externalizing developmental spectrum with AUD risk (36) and their exclusion would preferentially eliminate part of the phenomena of interest. Diagnosis was determined using the Diagnostic Interview Schedule-Child (37) for participants under the age of 18 and the Diagnostic Interview Schedule – Version IV for participants 18 and older (38).
All participants gave written informed consent after explanation of the experimental protocol, as approved by the local Institutional Review Board. Subjects under the age of 18 signed their assent and at least one parent gave written informed consent.
Measures
fMRI Task
A go-no-go task (27) was used to probe response inhibition. Participants were instructed to respond to target stimuli (letters other than “X”) by pressing a button (go trials) but make no response to infrequent non-target stimuli (“X”: no-go trials). Stimulus duration was 500 milliseconds, followed by 3500 milliseconds of fixation. There were 5 runs of 49 trials, each lasting 3 minutes, 24 seconds and containing 11, 12 or 13 no-go trials for a total of 60 no-go trials out of 245 total trials. Before scanning, all participants had a practice session of 49 trials on a desktop computer. False alarm rate and reaction times (RTs) to hits were calculated as performance measures.
Drinking and Drug History (DDHx)
The self-report Drinking and Drug History Form (39, 40) was used to determine alcohol problems, drinking volume, frequency of marijuana smoking, and number of illicit drugs used as part of the ongoing MLS. The alcohol problem score was the number of drinking-related problems out of a possible 37 reported by the subject since the age of 11 (total sample mean ± SD: 4.9 ± 5.7). “Problem” alcohol involvement was defined as a score above the mean. Supplement 1 provides more detailed information regarding the DDHx form and specific alcohol problems reported.
Externalizing Behavior Problems
Behavior problems were assessed with the Youth Self-Report (YSR) (41) as part of the ongoing MLS. The YSR yields scores on two broad-band subscales (externalizing and internalizing) and was completed by each participant when they were between 15 and 17 years old. Supplement 1 provides more detail on this measure.
MRI Data Acquisition
Whole-brain blood-oxygen-level-dependent (BOLD) functional images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, WI) using a T2*-weighted single-shot combined spiral in/out sequence (42) with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°; field-of-view (FOV) = 200 mm; matrix size = 64×64; in-plane resolution = 3.12×3.12 mm; and slice thickness = 4 mm. The entire volume of 29 axial slices was acquired every 2 seconds. A high-resolution anatomical T1 scan was obtained for spatial normalization (three-dimensional spoiled gradient-recalled echo [3-DSPGR], TR=25 msec, min TE, FOV=25 cm, 256×256 matrix, slice thickness=1.4mm). Participant motion was minimized with the use of foam pads placed around the head along with a forehead strap. In addition, the importance of keeping as still as possible was emphasized.
Data Analysis
fMRI Data
Functional images were reconstructed using an iterative algorithm (43, 44). Subject head motion and slice-acquisition timing were corrected using FSL 4.0 (Analysis Group, FMRIB, Oxford, UK) (45). Analysis of estimated motion parameters confirmed that overall head motion within each run did not exceed 2 mm translation or 2 degree rotation in any direction. All remaining image processing and statistical analysis were completed using statistical parametric mapping (SPM2; Wellcome Institute of Cognitive Neurology, London, UK). Functional images were spatially normalized to a standard stereotactic space as defined by the Montreal Neurological Institute (MNI). A 6 mm full-width half-maximum Gaussian spatial smoothing kernel was applied to improve signal-to-noise ratio and to account for individual differences in anatomy.
Statistical Analyses
Individual analysis was completed using a general linear model. Three regressors of interest (correct no-go trials, failed no-go, and all go) were convolved with the canonical hemodynamic response function, with event durations of 4 seconds from stimulus presentation. Motion parameters were modeled as nuisance regressors to remove residual motion artifacts. The main contrast of interest was correct no-go trials minus go trials, as described in previous studies (e.g.,28, 46, 47), and was calculated for second-level group analyses by linearly combining parameter estimates over all 5 runs of the task.
A random effects model was used for group-level analyses. Task effect was first determined with one-sample t-tests for each group. Areas of activation (no-go>go) and deactivation (go>no-go) were deemed significant if they reached a threshold of p<.05, corrected for spatial extent and multiple comparisons (48). Group effects (FH−, FH+ control, FH+ problem) were modeled with a one-way ANOVA. Differences within the a-priori hypothesized regions of the prefrontal cortex and striatum were deemed significant if they reached a statistical threshold of p<0.0001, uncorrected for multiple comparisons and a cluster extent of 10. Five regions were found to have a main effect of group. Region-of-interest (ROI) masks for these clusters were generated using MarsBaR region of interest toolbox (49). Effect sizes for no-go minus go activation were extracted from parameter estimates over all 5 runs and linearly combined for post-hoc analyses, described below.
Performance, DDHx measures and brain activation were tested for normality using SPSS. All DDHx variables were found to be right skewed and Kurtotic. Therefore, an inverse transformation [1/(1+the skewed variable)] was performed on these variables for further analyses. One-way ANOVAs with group as the main factor were performed separately on performance, DDHx measures and YSR behavior problems. Tukey’s post-hoc tests were used to determine significant pairwise differences between groups.
Activation in ROIs were entered into the following post-hoc analyses: 1) Tukey’s post-hoc t-tests to determine pairwise differences (FH− vs. FH+ control; FH+ control vs. FH+ problem); 2) Pearson correlation with YSR externalizing problems and DDHx variables and exploratory multiple regressions described below; and 3) Pearson correlation with task performance. Bonferroni correction for multiple comparisons was used to determine significance for regression analyses (.05/5 brain regions=.01).
Results
Performance, YSR, DDHx
No differences emerged between groups on performance measures (Table 1). An effect of group was found in YSR externalizing problems (F=6.8; df=57,2; p<.01) - FH+ problem differed from FH− (p=.002) and approached difference with FH+ control (p=.06). FH− and FH+ control did not differ (p>.5). As expected, an effect of group was also found for drinking volume (F=6.7, df=58,2; p<.01), marijuana use (F=6.6; df=58,2, p<.01) and number of illicit drugs used (F=9.4, df=58,2; p<.001), with FH+ problem differing in all 3 use variables from FH+ control and FH− (all p≤.01). There were no differences between FH− and FH+ control (all p>.9).
Task effect
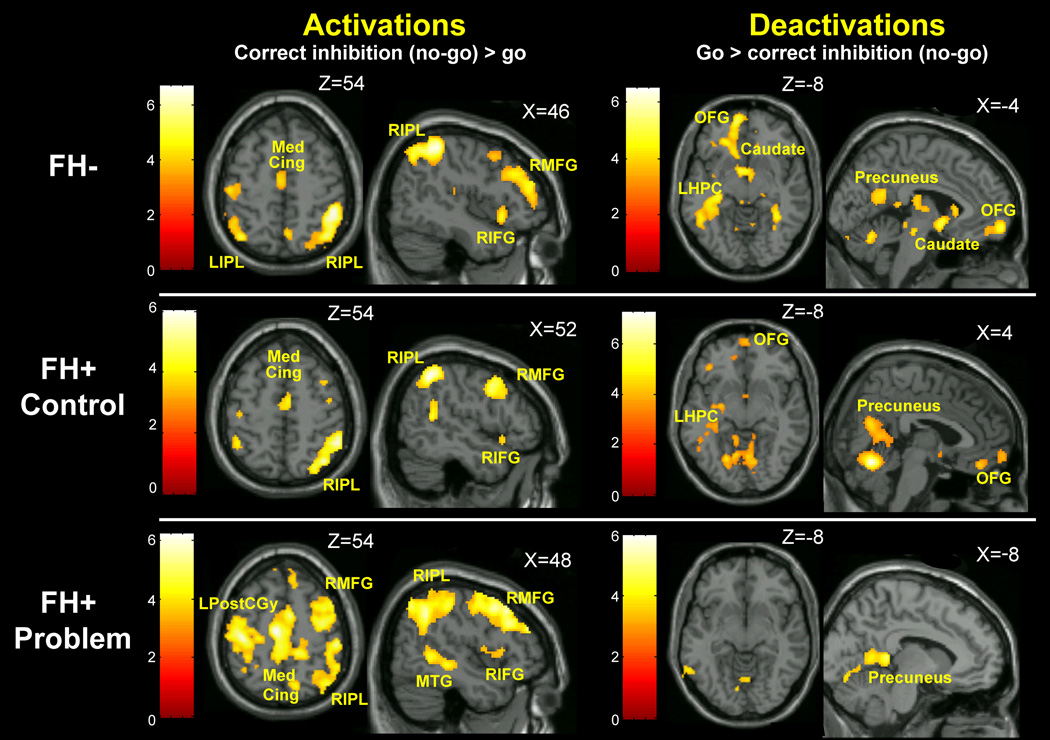
Effect of task for each group is reported in Figure 1 and Table 2. Each group activated a primarily right hemisphere network including prefrontal, posterior parietal, medial temporal and median cingulate regions. Patterns of deactivation during response inhibition (go>no-go) were similar in FH− and FH+ control, including regions involved in the DMN. FH+ problem showed fewer deactivated regions.
Figure 1.
Activation (no-go>go) and deactivation (go>no-go) for each group displayed at a statistical threshold of p<.005 and minimum cluster size of 10. Each group activated a primarily right hemisphere network including prefrontal, posterior parietal, medial temporal and median cingulate regions. Patterns of deactivation were similar in the FH− and FH+ control groups, including medial prefrontal regions, precuneus/posterior cingulate and left hippocampus. One notable difference in these results, however, was a significant deactivation in brainstem and ventral striatum in the FH− group, which was not present in the FH+ control group. The FH+ problem group showed fewer regions of significant deactivation during response inhibition – the left precuneus, posterior cingulate and left hippocampus. MedCing, median cingulate; LIPL, left inferior parietal lobe; RIPL, right inferior parietal lobe; RMFG, right medial frontal gyrus; RIFG, right inferior frontal gyrus; LPostCGy, left postcentral gyurs; MTG, medial temporal gyrus; OFG, orbitofrontal gyrus, LHPC, left hippocampus. Color bars represent t-scores.
Table 2.
Activations and Deactivations by Group.
| FH− | FH+ Control | FH+ Problem | |||||
|---|---|---|---|---|---|---|---|
| Activations | x, y, z | t | x, y, z | t | x, y, z | t | |
| Frontal | R Middle Frontal Gy (BA 9/10/46) | 34, 54, 28 | 5.4 | 40, 60, −6 | 4.5* | 40, 54, 14 | 4.8 |
| R Middle Frontal Gy (BA 6/8) | 46, 24, 46 | 4.6 | 32, −2, 60 | 5.7 | |||
| R Inferior Frontal Gy (BA 47)/Insula | 46, 18, −8 | 4.7 | 42, 16, −10 | 4.2* | 38, 14, 6 | 4.6 | |
| R Inferior Frontal Gy (BA 44/45) | 58, 10, 24 | 3.7* | 54, 22, 38 | 6.0 | |||
| L Precentral Gy | −38, −16, 64 | 4.6 | −38, −14, 60 | 3.9* | |||
| R Precentral Gy | 50, 8, 42 | 5.2 | |||||
| Cingulate | Median (BA 23/24/31) | −2, −8, 42 | 5.8 | −2, 2, 44 | 4.2 | 8, −6, 36 | 5.8 |
| Temporal | R Middle Temporal Gy (BA 22) | 64, −28, −12 | 4.8 | 62, −38, −12 | 3.0* | 60, −34, −6 | 4.6 |
| R Middle Temporal Gy (BA 21) | 50, −48, 2 | 4.7 | |||||
| Parietal | R Inferior Parietal Lobe (BA 39/40) | 48, −42, 56 | 6.7 | 52, −48, 52 | 6.0 | 48, −52, 40 | 5.6 |
| L Inferior Parietal Lobe (BA 39/40) | −40, −66, 52 | 5.2 | −44, −48, 56 | 4.2* | |||
| L Postcentral Gy | −40, −22, 54 | 4.6 | −38, −22, 54 | 5.9 | |||
| R Postcentral Gy | 54, −28, 52 | 5.5 | |||||
|
Deactivations | |||||||
| Frontal | Medial Orbital Frontal Ctx (BA 10/11) | −8, 60, −10 | 5.3 | 0, 62, −16 | 4.5* | ||
| L Inferior Frontal Gy (BA 47) | −20, 36, −10 | 4.6 | −36, 34, −16 | 4.5* | |||
| Parietal | L Precuneus | −8, −52, 18 | 5.1 | −8, −52, 10 | 5.2 | −8, −54, 8 | 4.0 |
| R Precuneus | 16, −48, 18 | 4.8 | 8, −50, 10 | 4.9 | |||
| Cingulate | Posterior (BA 29) | −8, −40, 8 | 4.9 | ||||
| Occipital | R Fusiform Gy | 36, −52, −18 | 4.8 | 28, −30, −24 | 4.5 | ||
| Subcortical | Caudate | −12, 24, −4 | 4.5 | ||||
| Nucleus Accumbens | −2, 6, −4 | 5.0 | |||||
| L Thalamus | −12, −20, 20 | 5.3 | −16, −24, 2 | 4.3* | |||
| R Thalamus | 16, −28, 18 | 5.5 | 16, −28, 2 | 4.5* | |||
| L Hippocampus | −30, −26, −8 | 4.9 | −32, −32, −8 | 3.8* | −16, −10, −14 | 4.0* | |
| Brainstem | 2, −22, −14 | 4.9 | |||||
| Cerebellum | Bilateral Lobules 1/6 | 22, −58, −20 | 6.5 | 4, −64, −16 | 7.3 | 24, −60, −20 | 6.0 |
FDR voxel-level corrected p<.01. Note: these clusters did not reach the stated cluster-level corrected threshold of p<.05, but were included to illustrate similarity in pattern of activation across groups.
L, left hemisphere; R, right hemisphere; Gy, gyrus, Ctx, cortex; BA, Brodmann’s area;
Whole-brain ANOVA results
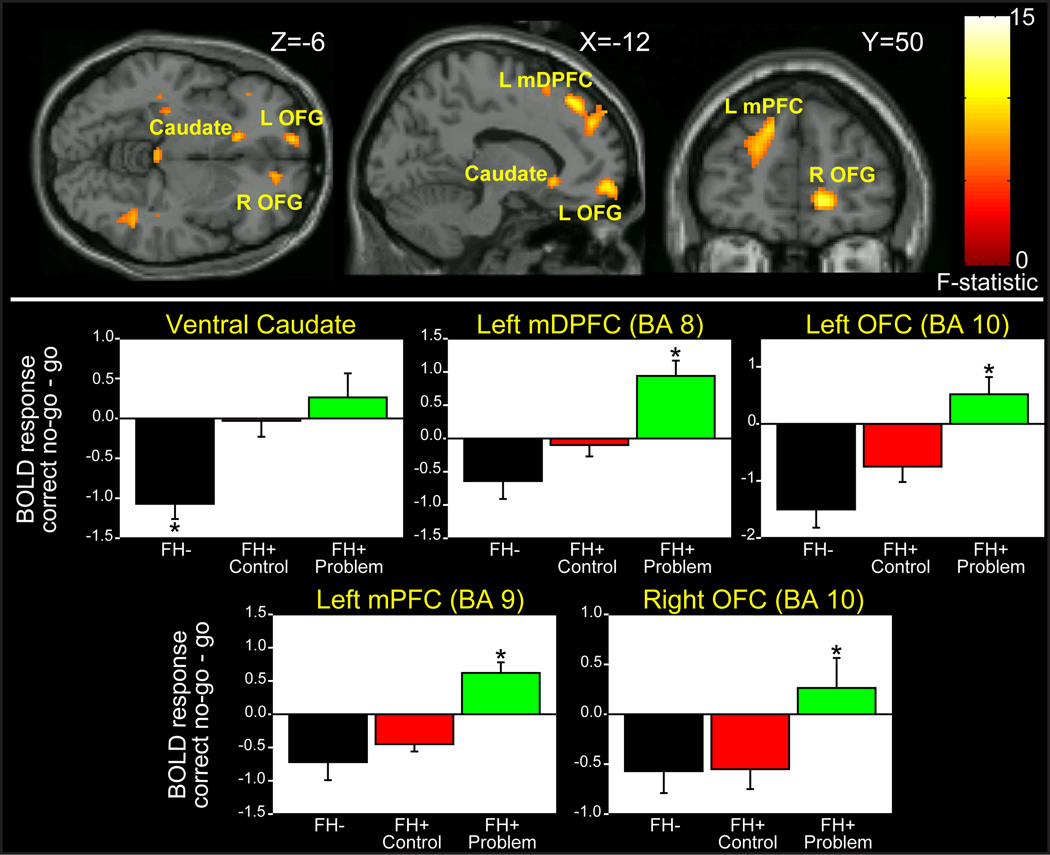
Group effects were obtained in the left and right medial orbitofrontal cortex (OFC), the left dorsomedial prefrontal cortex (mDPFC), left medial prefrontal cortex (mPFC) and the left ventral caudate (Figure 2, Table 3). Tukey’s post-hoc tests showed that ventral caudate activation differed between FH− and both FH+ groups, whereas it did not differ between the two FH+ groups. This difference was in the direction of greater deactivation in FH− but not FH+ groups.
Figure 2.
Top: SPM ANOVA results. Bottom: Regions from SPM analysis plotted for FH−, FH+ control and FH+ problem groups. Tukey’s post-hoc tests showed that ventral caudate activation differed between FH− and FH+ control (p=.015) and FH+ problem (p=.001), whereas it did not differ between the two FH+ groups (p>.6). Left and right OFG, mPFC and mDPFC differed between FH+ problem and FH+ control (p≤.01) and FH− (p<.01), but not between FH− and FH+ control (all p>.2). L, left; R, right; OFG, orbitofrontal gyrus, mPFC, medial prefrontal cortex; mDPFC medial dorsal prefrontal cortex; * significant difference from both other groups p<.05.
Table 3.
SPM ANOVA Results
| Brain Region | MNI space x, y, z |
Cluster extent |
Voxel-Level | ||
|---|---|---|---|---|---|
| Peak F | Peak Z | p-value | |||
| L OFC (BA 10) | −8, 62, −12 | 83 | 12.1 | 3.9 | <.0001 |
| R OFC (BA 10) | 14, 50, −2 | 55 | 12.0 | 3.9 | <.0001 |
| L medial PFC (BA 9) | −8, 58, 40 | 82 | 11.3 | 3.8 | <.0001 |
| L mDPFC (BA 8) | −10, 38, 50 | 64 | 12.1 | 3.9 | <.0001 |
| Ventral Caudate | −12, 24, −6 | 20 | 8.7 | 3.3 | <.0001 |
L, left hemisphere; R, right hemisphere; OFC, orbitofrontal cortex; PFC, prefrontal cortex; mDPFC, dorsomedial prefrontal cortex; BA, Brodmann’s Area
The four prefrontal regions all differed between FH+ problem and both FH+ control and FH−, but not between FH− and FH+ control. These differences were in the direction of greater deactivation in FH− and FH+ control but not FH+ problem. In the mDPFC, FH+ problem showed an activation to no-go relative to go trials (t=4.0, uncorrected p<.0001), although this did not reach the corrected threshold for significance.
A secondary analysis was conducted in which those in FH+ problem with any diagnosis (AUD, SUD, conduct disorder or ADHD; n=8, see Table 1) were excluded, resulting in n=13. All differences remained significant at p≤.05. This confirms that effects of problem use in frontal regions were not due to comorbid diagnoses in FH+ problem.
Correlations with YSR, DDHx
Only ventral caudate BOLD response correlated with externalizing problems (failure to deactivate this region was related to more externalizing problems). Drinking volume correlated with activity in the left and right OFG and the mDPFC; number of illicit drugs correlated with activation in all four prefrontal regions; and marijuana use correlated with left OFG activity (Table 4).
Table 4.
Correlations with BOLD response.
| Drinking and Drug Use | YSR | Performance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drink Volume | Illicit Drugs | Marijuana | Externalizing | RTs | FA Rate | |||||||
| Brain Region | r | p | r | p | r | p | r | p | r | p | r | p |
| L OFG (BA 10) | .42 | .001 | .41 | .001 | .32 | .01 | .14 | .32 | .11 | .39 | −.08 | .52 |
| R OFG (BA 10) | .34 | .008 | .33 | .009 | .22 | .09 | .22 | .11 | .01 | .95 | −.17 | .19 |
| L medPFC (BA 9) | .27 | .04 | .40 | .001 | .27 | .03 | .22 | .10 | .32 | .01 | −.28 | .03 |
| L mDPFC (BA 8) | .33 | .01 | .47 | <.001 | .25 | .05 | .20 | .13 | .35 | .01 | −.23 | .08 |
| Caudate | .23 | .08 | .18 | .17 | .03 | .82 | .34 | .009 | −.03 | .80 | −.02 | .89 |
Note: Drinking volume, marijuana use, illicit drugs used, alcohol problems and externalizing problems were all correlated at p<.0001; r’s ranged from .29 for drinking volume and externalizing to .74 for illicit drug use and alcohol problems. L, left hemisphere; R, right hemisphere; OFC, orbitofrontal cortex; PFC, prefrontal cortex; mDPFC, dorsomedial prefrontal cortex; BA, Brodmann’s Area; bolded values represent significant differences at p<.01.
Multiple regression analyses were conducted to further explore these findings by brain region. Sex and age were entered at the first step for all analyses, but were omitted in subsequent analyses as neither was a significant contributor to the variance. We set out to determine: 1) whether FH contributed to the variance in ventral caudate response above and beyond externalizing problems; and 2) whether alcohol problems contributed to the variance in each of the four prefrontal regions above and beyond drinking volume. Marijuana and illicit drug use were left out of the model because they are strongly correlated with the other variables and we had no clear hypothesis regarding the order of their inclusion.
In the ventral caudate, FH contributed significantly to variance when controlling for externalizing behavior (ΔR2=.18, p<.001; final model: R2=.30, F=11.5, p<.0001; ΒEXT=.21, p=.08; ΒFH=.44, p<.001). In each prefrontal region, alcohol problems did not account for significant variability when controlling for drinking volume (right OFG: ΔR2=.02, p=.32; left OFG: ΔR2=.04, p=.08; mDPFC: ΔR2=.03, p=.14; mPFC: ΔR2=.05, p=.07).
Correlations with performance
Across the entire sample, deactivations in mPFC and mDPFC were correlated with faster RTs (Table 4), indicating that greater activation to go trials compared to correct inhibitions in these regions is associated with faster responding.
Discussion
This study was undertaken to identify differences in frontostriatal functioning during response inhibition in adolescents/young adults with and without a family history of AUD, and to clarify the effects of preexisting vulnerability versus the effects of problem alcohol involvement. Two main findings were obtained. First, the FH− group showed a robust deactivation of ventral caudate during successful inhibition trials, which was not present in the FH+ control and problem groups, suggesting a preexisting vulnerability factor. Second, medial prefrontal regions were deactivated during successful inhibition in the groups with low problem alcohol use regardless of FH, but not in the FH+ problem group. Contrary to our hypotheses, we did not see evidence of weakened prefrontal responding during inhibition as a result of familial risk alone.
Each group showed patterns of activation consistent with those observed in previous studies (e.g., 50, 51, 52) – a primarily right-hemisphere fronto-parietal network. Deactivations were found mainly in the bilateral posterior parietal and inferior frontal regions in FH− and FH+ control groups as previously reported (30, 33). The FH+ problem group showed more widespread activations and fewer regions of deactivation during successful inhibition. Differences between groups emerged in left prefrontal and orbitofrontal regions and the ventral striatum; all areas showing deactivations in the FH− group.
To understand these findings, the functional significance of regional deactivations during response inhibition must first be addressed. Along with task-related activations, Hester et al. (30) reported deactivations prior to successful response inhibition in left medial frontal gyrus, left insula and caudate in a cued go/no-go task. Failure to inhibit the medial frontal and insula regions related to poorer performance. Stevens et al. (31) investigated functional neural networks underlying response inhibition and found an inhibitory influence of three distinct circuits on one another based on task demands. In particular, a frontal-parietal network was found to activate with concurrent nucleus caudate deactivation, consistent with the present findings in FH−. These findings indicate that successful inhibition requires both activation of task-related brain regions and deactivation of irrelevant brain regions. This has been proposed for cognitive tasks more generally, with the DMN reducing its baseline level of activity during effortful, task-relevant processing (19, 53).
We observed a difference between FH− and FH+ control groups in left ventral caudate, suggestive of a family history effect, which was present after controlling for externalizing behavior. The ventral striatum is part of a system involved in evaluating and responding to motivational stimuli (54) and is dysregulated in substance abuse (e.g., 55, also see review in 56) and other disorders of impulse control, such as pathologic gambling (57) and ADHD (58, 59). In addition, a relationship between impulsivity and ventral striatal activation to monetary reward in non-pathological children (16) and adults (60, 61) has been observed. Our finding that externalizing problems correlated with activation in the ventral caudate - but did not completely account for the effects of FH − suggests that the attenuated deactivation of the left ventral striatum in FH+ groups is associated with pre-existing familial risk for AUD via an externalizing pathway.
We also observed a difference between FH+ control and FH+ problem in orbitofrontal and medial prefrontal regions; again following the direction of lesser deactivation in the riskier group. This finding suggests a dysregulation in the normal response of these regions during response inhibition in the problem group, independent of family history. Furthermore, BOLD response in these regions was related to alcohol consumption and other drug use, but not externalizing problems. Number of alcohol problems did not account for additional variability beyond alcohol consumption, suggesting that the effects of alcohol (and perhaps other drug involvement) on the brain in the problem group is responsible for the differences. Indeed, a number of studies indicate that the frontal lobes are more vulnerable to alcohol-related impairment than other cortical regions (62–64, also see review in 65). Furthermore, there were no differences between groups in the right hemisphere, which is traditionally considered central for inhibitory control (e.g., 66). Therefore, the problem group may be recruiting additional left hemisphere resources - particularly the left dorsomedial PFC, which showed inhibition-related activation - to successfully inhibit responses, an effect that is not present in the FH+ control group. Increased bilateral activation during response inhibition has been observed in the elderly (67) and is typically interpreted as a compensatory mechanism secondary to age-associated neurodegeneration. Similar developmental effects are found from childhood to adulthood, a period during which inhibitory responding shifts from more diffuse, bilateral regions to focal, right-hemipshere circuits; occurring concurrent with improvement in response inhibition (47, 68). Thus, one interpretation of the present finding is that alcohol exposure in the problem group is associated with a disruption of a normally right-lateralized prefrontal cortical system involved in response inhibition, necessitating recruitment of additional resources for successful performance. The overall pattern of activation was more diffuse and bilateral in this group, which supports this hypothesis.
It is possible that the present findings additionally represent a variation in the modulation of the DMN during effortful tasks. Activation in midline structures have been found to decrease as a function of greater task difficulty (69). Given that the main differences in the present study were in the direction of impaired deactivation in midline regions involved in the DMN to the more effortful cognitive task of inhibiting a response versus continuous responding to go trials, this is a likely explanation. This was shown recently in responding to vigilance relative to spatial working memory as a function of family history and may represent an impaired ability to modulate the DMN in response to increasing task demands (18).
There are some study limitations that should be mentioned. First, the study was not specifically design to investigate the functioning of the DMN. Therefore, interpretations regarding differences in the functioning of the DMN between groups are inferential. Confirmation of this hypothesis will need to be borne out with studies which include rest conditions and differing levels of cognitive load. Further, the contribution of factors typically linked with a family environment of alcoholism (e.g., stress) would also need to be addressed in subsequent studies, as they are beyond the scope of the present report. Similarly, internalizing, emotional dysregulation effects have been reported to be associated with alcoholism and drug use risk (70). No difference in internalizing problems was evident in this sample. However, the exclusion of volunteers with diagnoses of mood and anxiety disorders may have preferentially eliminated those at greatest risk via an internalizing pathway, reducing the variability that could be attributed to this factor. Therefore, future studies would be necessary to investigate familial risk as it relates to internalizing symptomatology. Finally, as noted in Supplement 1, the YSR data were collected approximately 3 years before scanning. Therefore, these data are not an exact measure of externalizing problems at the time the functional imaging data were collected. However, externalizing behavior problems have been found to be stable through adolescence and young adulthood (71–74), suggesting that data collected between ages 15 and 17 is a useful indicator of externalizing behavior throughout this developmental period.
In conclusion, we found dissociation between circuitry associated with vulnerability for AUD and brain regions related to problem alcohol use. A lesser magnitude of ventral striatal deactivation during inhibition was associated with preexisting vulnerability in COA. In contrast, abnormal responding in the orbital and medial prefrontal regions was observed only in COA showing problem use, and was associated with the magnitude of alcohol involvement. Findings indicate a preexisting abnormality in ventral striatal function in youth at risk for AUD, which may lead to inappropriate motivational responding; and suggest that with alcohol use, the prefrontal “control” mechanism loses efficiency, further dysregulating the frontostriatal motivational circuitry.
Supplementary Material
Acknowledgements
This work was supported by K01 DA020088 to MMH, R01 AA12217 and R37 AA07065 to RAZ, and a NARSAD Independent Investigator award to JKZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures.
The authors report no biomedical financial interests or potential conflicts of interest relevant to the content of this manuscript.
References
- 1.National Institute on Alcohol Abuse and Alcoholism. NIAAA, Tenth Special Report to the US Congress on Alcohol and Health: Highlights from current research. Bethesda, MD: U.S. Department of Health and Human Services; 2000. Alcohol involvement over the life course; pp. 28–53. [Google Scholar]
- 2.Russell M. Prevalence of alcoholism among children of alcoholics. In: Windle M, Searles J, editors. Children of alcoholics: Critical perspectives. New York: Guilford Press; 1990. pp. 9–38. [Google Scholar]
- 3.Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- 4.Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcohol Clin Exp Res. 1988;12:494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 5.Chassin L, Ritter J. Vulerability to Substance Use Disorders in Childhood and Adolescence. In: Ingram R, Price J, editors. Vulnerability to Psychopathology: Risk across the lifespan. New York: The Guilford Press; 2001. pp. 107–134. [Google Scholar]
- 6.Zucker R. National Institute on Alcohol Abuse and Alcoholism, Tenth Special Report to the US Congress on Alcohol and Health: Highlights from current research. Bethesda, MD: US Department of Health and Human Services; 2000. Alcohol involvement over the life course; pp. 28–53. [Google Scholar]
- 7.Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Ab Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- 8.Zucker RA. Alcohol use and alcohol use disorders: A developmental-biopsychosocial systems formulation covering the life course. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. 2nd ed. Hoboken, NJ: Wiley & Sons; 2006. pp. 620–656. [Google Scholar]
- 9.Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- 10.Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, et al. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, et al. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- 15.McNamee RL, Dunfee KL, Luna B, Clark DB, Eddy WF, Tarter RE. Brain activation, response inhibition, and increased risk for substance use disorder. Alcohol Clin Exp Res. 2008;32:405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- 16.Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- 17.Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin Exp Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National survey results on drug use, 1975–2008: Volume11, College students and adults ages 19–50. Washington DC: US Government Printing Office; 2009. [Google Scholar]
- 22.Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- 23.Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin Exp Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 25.Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- 26.Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- 27.Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- 28.Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 29.Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. [Google Scholar]
- 30.Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- 31.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Fitzgerald HE, Lester BM, Zucker RA, editors. Children of addiction: Research, Health and Policy Issues. New York: Routledge Falmer; 2000. pp. 109–141. [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Krueger RF. Personality traits in late adolescence predict mental disorders in early adulthood: a prospective-epidemiological study. J Pers. 1999;67:39–65. doi: 10.1111/1467-6494.00047. [DOI] [PubMed] [Google Scholar]
- 37.Costello A, Edelbrook C, Dulcan M, Kalas R, Klanc S. Development and testing of the NIMH Diagnostic Interview Schedule for children in a clinic population. Rockville, MD: Center for Epidemiological Studies, NIMH; 1984. [Google Scholar]
- 38.Robins L, Cottler LB, Bucholz KK, Compton WM, North CS, M. RK. Diagnostic Interview Schedule for the DSM-IV (DSM-IV) St. Louis, MO: Washington University School of Medicine; 2000. [Google Scholar]
- 39.Zucker R, Fitzgerald H, Noll R. Drinking and Drug History. (Revised edition, Version 4) Ann Arbor: University of Michigan Department of Psychiatry, Addiction Research Center; 1990. [Google Scholar]
- 40.Zucker RA, Fitzgerald HE. Drinking and Drug History Form for Children. Ann Arbor: University of Michigan Department of Psychiatry, Addiction Research Center; 1994. [Google Scholar]
- 41.Achenbach TM. Manual for the Youth Self-report Form and 1991 Profile. Burlington, VT: University Associates in Psychiatry; 1991. [Google Scholar]
- 42.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 43.Sutton BP, Noll DC, Fessler JA. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Trans Med Imaging. 2003;22:178–188. doi: 10.1109/tmi.2002.808360. [DOI] [PubMed] [Google Scholar]
- 44.Noll DC, Fessler JA, Sutton BP. Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Trans Med Imaging. 2005;24:325–336. doi: 10.1109/tmi.2004.842452. [DOI] [PubMed] [Google Scholar]
- 45.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 46.Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, et al. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. Am J Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- 47.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 48.Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ. Comparing functional (PET) images: The assessment of significant change. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 49.Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox; 8th International Conference on Functional Mapping of the Human Brain; Japan: Sendai; 2002. [Google Scholar]
- 50.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 57.Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 58.Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 59.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 60.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hahn T, Dresler T, Ehlis AC, Plichta MM, Heinzel S, Polak T, et al. Neural response to reward anticipation is modulated by Gray's impulsivity. Neuroimage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 62.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 63.Gilman S, Koeppe RA, Adams K, Johnson-Greene D, Junck L, Kluin KJ, et al. Positron emission tomographic studies of cerebral benzodiazepine-receptor binding in chronic alcoholics. Ann Neurol. 1996;40:163–171. doi: 10.1002/ana.410400207. [DOI] [PubMed] [Google Scholar]
- 64.Gansler DA, Harris GJ, Oscar-Berman M, Streeter C, Lewis RF, Ahmed I, et al. Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: a pilot SPECT study. J Stud Alcohol. 2000;61:32–37. doi: 10.15288/jsa.2000.61.32. [DOI] [PubMed] [Google Scholar]
- 65.Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res. 2006;1105:130–142. doi: 10.1016/j.brainres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 67.Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- 68.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 70.Mezzich A, Tarter R, Kirisci L, Clark D, Buckstein O, Martin C. Subtypes of early age onset alcoholism. Alcohol Clin Exp Res. 1993;17:767–770. doi: 10.1111/j.1530-0277.1993.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 71.Ferdinand RF, Verhulst FC, Wiznitzer M. Continuity and change of self-reported problem behaviors from adolescence into young adulthood. J Am Acad Child Adolesc Psychiatry. 1995;34:680–690. doi: 10.1097/00004583-199505000-00020. [DOI] [PubMed] [Google Scholar]
- 72.Osgood D, Johnston L, O'Malley PM, Bachman JG. The generality of deviance in late adolescence and early adulthood. Am Sociol Rev. 1988;53:81–93. [Google Scholar]
- 73.Verhulst FC, van Wattum PJ. Two-year stability of self-reported problems in an epidemiological sample of adolescents. Acta Psychiatr Scand. 1993;87:322–328. doi: 10.1111/j.1600-0447.1993.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 74.Reitz E, Dekovic M, Meijer AM. The structure and stability of externalizing and internalizing problem behavior during early adolescence. Journal of Youth and Adolescence. 2005;34:577–588. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.