Abstract
Aims
Young injection drug users (IDU) are at high risk for hepatitis C virus (HCV). We sought to determine whether perceiving one's injecting partner to be HCV positive was associated with decreased odds of engaging in receptive needle/syringe sharing (RNS) or ancillary equipment sharing (AES) with that partner.
Design
We conducted a cross-sectional study from 2003 to 2007 in San Francisco (n=212 participants) to examine whether perceived partner HCV status was associated with RNS and AES within injecting partnerships (n=492 partnerships) of young (under age 30) IDU who are HCV antibody negative.
Findings
RNS and AES (in the absence of RNS) occurred in 23% and 66% of injecting partnerships in the prior month. The odds of engaging in RNS were significantly lower for relationships in which the participant reported that his/her partner was HCV positive (odds ratio [OR] 0.49; 95% confidence interval [CI] 0.25-0.95). This association was attenuated when adjusted for reusing one's own needle/syringe (adjusted OR 0.57; 95% CI 0.28-1.15). The odds of engaging in AES were lower for participants who did not know the HCV status of their partner, only among non-sexual partnerships (OR 0.47; 95% CI 0.29-0.76).
Conclusions
Because perceiving one's partner to be HCV positive was associated with decreased RNS, increased HCV testing and partner disclosure may be warranted. AES was common and was decreased only among non-sexual partnerships in which the HCV status of the partner was not known. This suggests that interventions to reduce AES in young IDU must be widespread.
Introduction
Hepatitis C virus (HCV) is a major cause of morbidity and mortality, especially among injecting drug users (IDU). The majority of IDU in Western countries, 50-95%, are infected with HCV.1 HCV incidence in IDU remains high, and is 26.7 per 100 person years of observation (PYO) (95% CI, 21.5-31.6) in young (under age 30) IDU in San Francisco.2
Injecting with someone else's previously used needle/syringe, termed receptive needle/syringe sharing (RNS), is the most efficient route for acquiring HCV. In addition, sharing ancillary injecting equipment such as cookers to dissolve drugs and cottons used to filter impurities from drug solutions has been associated with HCV infection3, 4 and likely plays a significant role in the current HCV epidemic in injecting drug users (IDU).5 Many studies have characterized the individual level characteristics associated with RNS6-15 and AES.16-18
However, for most IDU, injecting drugs is a highly social activity not conducted in isolation.16, 19 Injecting relationships (which may or may not include sex) may be formed for economic reasons, to share drugs that are more cheaply purchased in larger quantities, or, as was the case in an ethnographic study among young women on the streets of San Francisco, for safety, economic, and romantic reasons.20 Several studies have found that being in a sexual relationship with injecting partners increased the odds of engaging in RNS,11, 14, 15, 21-23 along with other relationship level variables such as injecting frequently with one's injecting partners,14 pooling resources to buy drugs with injecting partners,21 being related to one's injecting partners,11, 21, 23 having a close relationship,14, 21, 22 and having known one's injecting partner for a longer duration.22 Studies that have examined the influence of injecting relationships on AES found that injecting network size,24 pooling money to buy drugs,24 injecting with sex partners or regular injecting partners,25 having injecting partners who engaged in a variety of unsafe injecting behaviors,26 and holding norms that encouraged risk behavior were associated with engaging in AES.25, 27
Very little study has been conducted to examine whether the perceived risk of HCV infection of one's injecting partners is associated with RNS and AES. The impact of knowledge of the HIV infection status of one's sexual partners on sexual behavior has been examined among men who have sex with men,28-31 but the impact of partner HCV serostatus on injecting risk behavior has been examined in only two studies of IDU, with conflicting results.32, 33 We therefore examined the injecting partnerships of young HCV antibody negative IDU to describe the characteristics and risk behaviors within such partnerships and to determine whether perceptions about one's partner's HCV status is associated with RNS or AES with that partner.
Methods
Design, setting and participants
From 2003 to 2007, young (under age 30) IDU were recruited into a cross-sectional study to determine eligibility for a prospective cohort study, one of several studies of young IDU in San Francisco, collectively known as the UFO Study. This study occurred at community-based storefront field sites. Outreach workers visited neighborhoods where young drug users were known to congregate. They approached those young persons who were socializing or panhandling in public spaces; who had visible signs of counter-cultural dress, tattoos, piercings, or hairstyle; who were visibly homeless; and/or who were socializing with those who met the above description. Individuals who were interested in participating in a research study were given a card that included the study contact information. Word of mouth referrals by current and former study participants were also encouraged.
Eligible persons were under age 30, injected drugs in the prior 30 days; and from March 2006 onward, reported not being infected with HCV. Injecting status was verified by field staff through questions about injection practices. Study participants completed an interviewer-administered interview, received client-centered risk reduction counseling, and underwent phlebotomy for HCV testing. Participants returned one week later for viral testing results, counseling, referrals and enrollment in a prospective cohort (if eligible) and were remunerated $10-$20 each visit. All protocols were approved by the Institutional Review Board of the University of California, San Francisco.
Partnership definition
Each participant reported on up to three injecting and/or sexual partners with whom he/she had injected the most or spent the most time with in the prior month. Injecting partners were defined as persons with whom the participant had injected in the same physical space, i.e., both persons were injecting drugs. This definition did not require that drugs or injecting equipment was shared. Sexual partners were those with whom the participant had engaged in oral, vaginal or anal sex in the prior month.
Outcome variables
The main outcome variables for each partnership were: (1) injecting with one's partner's previously used needle/syringe in the prior month (RNS) (yes/no), and; (2) and, among those who had not engaged in RNS with their partner, preparing drugs with one's partner in the same cooker, spoon, or baggie (AES) (yes/no).
Independent variables
Participant characteristics
Demographic characteristics included gender (grouping the single transgender with gender at birth), age, and race/ethnicity. We categorized participants who reported living the majority of the time in the prior three months on the street, in a shelter, drug treatment facility, jail, or prison as marginally housed or homeless. We obtained self-reported HIV and HCV status for participants in 2004 through 2007, and 2006 through 2007 respectively.
Participant drug and alcohol use
Injection drug use variables included duration since first injected, the number of days the participant injected in the prior 30, and in the prior three months, the drugs injected, the number of injecting partners, whether the participant obtained needles/syringes from a needle exchange program, and whether the participant typically reused his/her own needle/syringe. Non-injection drug use variables included the number of days the participant consumed alcohol in the prior 30 days and whether the participant used methamphetamine and crack cocaine in the prior three months.
Partnership characteristics
The participants reported for each partnership: the partner's sex, approximate age, duration of acquaintance, and in the past month, whether the participant and the partner had stayed together for at least one night, and whether the participant and partner had traveled together outside of San Francisco (2003 to 2005 surveys only). The participants also reported whether they had engaged in sexual relations (not explicitly defined) with each partner in the past month.
Injecting behaviors within partnerships
Injecting behaviors (aside from RNS and AES described above) elicited for each partnership in the prior month included the frequency the participant injected with the partner, whether the participant pooled money with the partner to buy drugs (2003 to 2005 surveys only), and whether the participant was injected by the partner (2003 to 2005 surveys only). Participants reported on the perceived HIV and HCV status of each partner.
Laboratory testing
Antibody to HCV (anti-HCV) was detected using a second generation enzyme immunoassay (EIA-2.0; Ortho Diagnostics Systems, Raritan, New Jersey) or using the EIA 3.0 (EIA-3, Ortho Clinical Diagnostics, Raritan NJ). Specimens that were antibody negative were additionally tested for HCV RNA (using the dHCV TMA assay component of the Procleix® HIV-1/HCV assay, Gen-Probe Inc., San Diego, CA).
Analyses
We reported on frequency distributions for categorical variables and medians and interquartile ranges (IQRs) for continuous variables. Several continuous variables (age, years since first injected, number of days injected in the prior month, and number of persons with whom the participant injected in the prior month) were categorized for ease of presentation. We explicitly noted where data were missing due to differing questionnaire versions; other missing data were due to non-response. We examined bivariate associations of the participant characteristics and drug and alcohol behaviors, and partnership variables with the two outcome variables. The participant variables considered were participant age, sex, race/ethnicity, homelessness, travel out of San Francisco, prior or current incarceration, self-reported HCV and HIV status, years injected drugs, safe injecting behaviors such as not reusing one's own needle/syringe and accessing new needles/syringes at needle/syringe exchange programs (NSEPs), and current drug and alcohol use. The partnership level variables included demographic characteristics such as the age of the partner, the age difference between the partners, and the sexual composition of the partnership (male-male, male-female, etc.). These partnership variables were chosen based on the observations of Bourgois et alwho observed that males who were typically older and sexual partners frequently controlled the injecting of their young female IDU partners.15 20 Lastly, because emotional and physical closeness have been previously associated with increased odds of RNS and/or AES, 11, 14, 15, 21-23, 25 we examined whether the injecting partners had engaged in sex in the prior month, how long the partners had been acquainted, whether the partners stayed together overnight, how frequently the partners injected together, and whether the participant reported that their partner injected with other IDU. We used Generalized Estimating Equation (GEE) methods with the logistic link and an exchangeable correlation matrix to calculate odds ratios and confidence intervals (CI) that correct for the correlation between multiple partners reported by the same participant. We created a dummy category to represent the missing data for variables collected only in the 2003 to 2005 data.
We used GEE analyses to examine effect modification of the perceived HCV status of the participants' injecting partners for two variables of interest: sex of the participant and whether the partners had engaged in sex in the prior month. We concluded that there was effect modification if the overall p-value of the interaction was less than 0.10 (set liberally in order to overcome low power to detect interaction)34 or if the p-value for any individual interaction term was less than 0.05.
We examined potential confounding by variables that were associated with the outcomes in the bivariate analyses. We constructed models individually for each potential confounder along with perceived HCV status of the injecting partner for each outcome. In the case of effect modification, we examined confounding within the levels of the effect modifier. We did not examine the potential confounding effect of AES in the model of RNS because almost all (93%) engaging in RNS with their injecting partner also engaged in AES with that partner. We concluded that there was confounding if the p-value for the resulting odds ratios for perceived HCV status of one's injecting partner changed to p>0.10 for any level of that variable that had been statistically significant in the unadjusted model.
Results
Sample description
Two-hundred sixty five of 384 eligible study participants (69%) had negative anti-HCV tests, while 21 (8%) of those were positive on RNA testing. Eighty percent (n=212) of the 265 anti-HCV negative participants reported on at least one injecting partnership in the prior month, for a total of 492 partnerships for this analysis (Figure 1).
Figure 1.
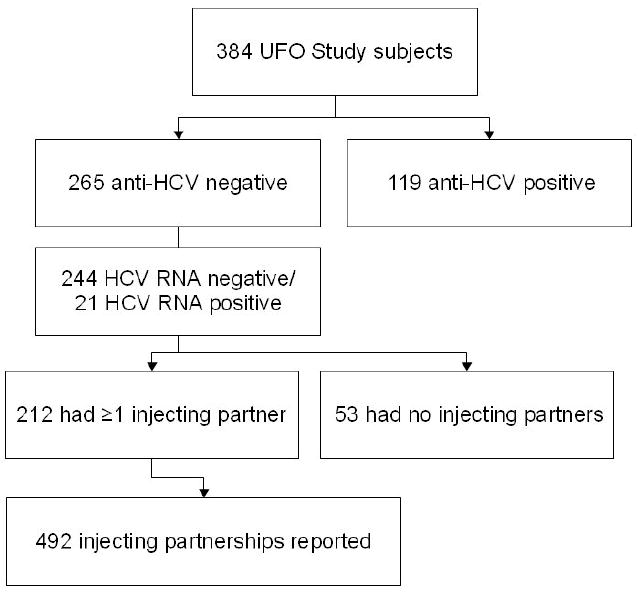
Flow chart of study participants and injecting partnerships.
Among the 53 anti-HCV negative participants who reported no injecting partners in the prior month, 40% (n=21) had not injected drugs in the prior month. The remaining who injected drugs but reported no injecting partners (n=32) did not differ from the 212 who had at least one injecting partner on demographic variables, but differed significantly on several behavioral variables, including frequency of injecting (median 7 [IQR 2-20.5] versus median 18 [IQR 6-28] days of the past 30, p=0.01), injecting heroin prior 3 months (59% versus 84%, p<0.01), and drinking alcohol daily or almost daily in the prior 30 days (19% versus 39%, p=0.04).
The 212 anti-HCV negative young IDU who had at least one injecting partner included in this analysis were primarily male (67%) and white (70%), the median age was 22 (IQR 20-25), the participants had injected drugs for a median of 3.9 years (IQR 1.7-6.7) and had injected a median of 18 (IQR 6-28) days of the prior 30 (Table 1). Half (53%) of those who were asked their HCV status (n=87) reported that they were HCV negative, while most of the others (41%) reported that they had never been tested for HCV. Non-injecting drug and alcohol use are also shown on Table 1.
Table 1. Demographic characteristics and injecting behaviors of HCV negative young IDU in San Francisco with ≥1 injecting partner (N=212).
| Demographic characteristic | n (%) |
|---|---|
| Gender | |
| Male | 142 (67) |
| Female | 70 (33) |
| Age | (Median 22.2; IQR 20.0-24.6) |
| 15-19 | 53 (25) |
| 20-24 | 113 (53) |
| 25-29 | 46 (22) |
| Race | |
| White | 148 (70) |
| Mixed/other | 63 (30) |
| Homeless/marginally housed, prior 3 months | |
| No | 61 (29) |
| Yes | 151 (71) |
| Ever incarcerated (jail or prison) | |
| No | 43 (20) |
| Yes, >3 months ago | 112 (53) |
| Yes, in the prior 3 months | 56 (27) |
| Traveled outside of San Francisco, prior 3 months | |
| No | 76 (36) |
| Yes | 136 (64) |
| HCV/HIV status | |
| Self-reported HCV status (n=87) | |
| Negative | 46 (53) |
| Positive (incorrect) | 1 (1) |
| Unknown | 4 (5) |
| Never tested | 36 (41) |
| Self reported HIV status (n=152) | |
| Negative | 109 (72) |
| Positive | 4 (3) |
| Indeterminate | 4 (3) |
| Never tested | 35 (23) |
| Participant drug and alcohol use | |
| Drank alcohol daily or almost daily, prior month | |
| No | 130 (61) |
| Yes | 82 (39) |
| Smoked crack cocaine, prior 3 months | |
| No | 100 (47) |
| Yes | 112 (53) |
| Snorted or smoked methamphetamine, prior 3 months | |
| No | 92 (43) |
| Yes | 120 (57) |
| Participant injecting drug use (prior 3 months unless otherwise specified) | |
| Years since first injected drugs | (Median 3.9; IQR 1.7-6.7) |
| <3 | 86 (41) |
| 3-5 | 63 (30) |
| ≥6 | 62 (29) |
| Number of days injected, past 30 days | (Median 18; IQR 6-28) |
| 0-20 days | 77 (36) |
| 21-30 days | 135 (64) |
| Number of persons with whom injected (prior month) n=196 | (Median 3; IQR 2-5) |
| 1 | 43 (22) |
| 2-4 | 92 (47) |
| ≥5 | 61 (31) |
| Obtained needles/syringes at an NEP | |
| No | 54 (25) |
| Yes | 158 (75) |
| Injected heroin (not mixed with other drugs) | |
| No | 33 (16) |
| Yes | 178 (84) |
| Injected methamphetamine (by itself or mixed with heroin) | |
| No | 68 (32) |
| Yes | 144 (68) |
| Injected powder cocaine | |
| No | 149 (70) |
| Yes | 63 (30) |
| Injected crack cocaine | |
| No | 169 (81) |
| Yes | 40 (19) |
| Injected with the same needle/syringe >1 time | |
| No | 106 (50) |
| Yes | 106 (50) |
Description of partnerships
Partnership characteristics
The characteristics of the 492 injecting partnerships are summarized in Table 2. In twelve percent (12%) of the partnerships, the partner was 5 to 10 years older than the participant, and in 12% the partner was more than 10 years older than the participant. Forty-nine percent (49%) of the partnerships were between male and female IDU, 43% were between two males, and 7% were between two females. The partners had been acquainted for a median of 6 months (IQR 2-25), and 71% had lived or stayed together at least one night in the prior month.
Table 2. Characteristics and behaviors in the prior month of the injecting partnerships of young IDU, N=492 partnerships.
| Partnership characteristics | n (%) |
|---|---|
| Partner age compared to participant | |
| Partner <5 years older | 351 (76) |
| Partner 5-10 years older | 53 (12) |
| Partner >10 years older | 56 (12) |
| Partner approximate age | |
| <20 | 101 (22) |
| 20-35 | 317 (69) |
| >35 | 42 (9) |
| Partnership sex | |
| Participant male -- partner male | 197 (43) |
| Participant female – partner female | 33 (7) |
| Participant male – partner female | 108 (23) |
| Participant female – partner male | 124 (26) |
| Participant and partner engaged in sexual relations, prior month | |
| No | 343 (70) |
| Yes | 149 (30) |
| Months participant acquainted with partner | (Median 6; IQR 2-25) |
| ≤3 | 183 (37) |
| 3-12 | 135 (27) |
| >=12 | 174 (35) |
| Lived or stayed with partner (at least one night in the prior month) | |
| No | 144 (29) |
| Yes | 348 (71) |
| Traveled with partner outside of San Francisco* | |
| No | 183 (67) |
| Yes | 91 (33) |
| Partnership injecting behaviors, prior month | |
| Frequency of injecting with partner | |
| Less than once a week | 137 (28) |
| 1-2 times/week | 120 (25) |
| 3-5 times/week | 123 (26) |
| >5 times/week | 101 (21) |
| Pooled money to buy drugs with partner* | |
| No | 44 (16) |
| Yes | 230 (84) |
| Injected by partner* | |
| No | 190 (71) |
| Yes | 78 (29) |
| Used partner's needle/syringe (RNS) | |
| No | 101 (67) |
| Yes | 111 (23) |
| Partner injects exclusively with participant | |
| No | 259 (53) |
| Yes | 55 (11) |
| Unknown | 178 (36) |
| Shared drug preparation equipment with partner (AES), among those who did not engage in RNS | |
| No | 130 (36) |
| Yes | 229 (64) |
| Perceptions about partner HCV/HIV status | |
| Perceived HCV status of partner | |
| Negative | 141 (29) |
| Positive | 108 (22) |
| Unknown | 241 (49) |
| Perceived HIV status of partner | |
| Negative | 268 (55) |
| Positive | 16 (3) |
| Unknown | 204 (42) |
Not collected for 2006-2007 surveys
Partnership injecting behaviors
In 47% of the partnerships, the partners injected together at least three times per week in the prior month (Table 2). In 84% of the partnerships, the partners pooled money to buy drugs, and in 29%, the participant was injected by the partner. In 23% of the partnerships, the participant had engaged in RNS with his/her partner, and in the 359 injecting partnerships in which the participant did not engage in RNS, 64% engaged in AES.
Perceptions about partner HCV/HIV status and injecting exclusivity
For half (49%) and 42% of the partnerships, the participant did not know the HCV and HIV status respectively of his/her injecting partner. For 11% of the partnerships, the participant reported that the partner had no other injecting partners, for 53% the participant reported that his/her partner had other injecting partners, and for 36% of the partnerships, the participant did not know if his/her partner had other injecting partners.
Correlates of within partnership RNS
Participant level correlates of RNS within the partnerships in bivariate analysis included female gender, older age (20-24 compared to 15-19), traveling outside of San Francisco, recent incarceration, injecting with only one injecting partner, not obtaining needles/syringes from NEPs, and reusing one's own needle/syringe (Table 3). Partnership level variables associated with RNS within the partnerships included partner age (≤ age 35 compared to >35 years), partnership age difference (partner ≤10 years older than participant), partnership gender (gender discordant compared to male-male partnerships), duration acquainted with one's partner (≥1 year compared to less than one month), living or staying with one's partner, traveling outside of SF with one's partner, injecting frequently with one's partner, being injected by one's partner, and engaging in AES with one's partner. Those who reported that their partner was HCV positive were significantly less likely to engage in RNS with that partner (odds ratio 0.49 (95% CI 0.25-0.95).
Table 3. Correlates of injecting with partner's previously used needle/syringe (RNS) and sharing ancillary injecting equipment (AES) with partner, prior month.
| Overall | Odds ratio for RNS (95% CI) | Odds ratio for AES (95% CI) |
|---|---|---|
| Participant characteristics | ||
| Gender | ||
| Male | 1.00 | 1.00 |
| Female | 1.73 (1.03-2.90)╪ | 1.14 (0.63-2.05) |
| Age | ||
| 15-19 | 1.00 | 1.00 |
| 20-24 | 2.12 (1.06-4.24)╪ | 0.93 (0.45-1.95) |
| 25-29 | 1.11 (0.49-2.50) | 1.12 (0.49-2.58) |
| Race/ethnicity | ||
| White | 1.00 | 1.00 |
| Non-white | 1.59 (0.94-2.70) | 1.20 (0.63-2.29) |
| Homeless/marginally housed, prior 3 months | ||
| No | 1.00 | 1.00 |
| Yes | 1.24 (0.70-2.18) | 0.90 (0.49-1.68) |
| Ever incarcerated (jail or prison) | ||
| No | 1.00 | 1.00 |
| Yes, >3 months ago | 0.77 (0.38-1.53) | 1.02 (0.51-2.01) |
| Yes, in the prior 3 months | 1.94 (1.11-3.40)╪ | 0.56 (0.28-1.11) |
| Traveled outside of San Francisco, prior 3 months | ||
| No | 1.00 | 1.00 |
| Yes | 1.93 (1.10-3.40)╪ | 1.11 (0.62-1.99) |
| Participant HCV/HIV status | ||
| Self-reported HCV status, last test (n=201) | ||
| Negative | 1.00 | 1.00 |
| Indeterminate/Incorrect/Unknown | 1.60 (0.74-3.43) | 0.67 (0.28-1.60) |
| Self-reported HIV status, last test | ||
| Negative | 1.00 | 1.00 |
| Positive | 1.59 (0.79-3.17) | 1.19 (0.12-11.92) |
| Indeterminate/Unknown | 1.05 (0.63-1.77) | 0.71 (0.40-1.27) |
| Participant drug and alcohol use | ||
| Drank alcohol daily/almost daily, prior month | ||
| No | 1.00 | 1.00 |
| Yes | 0.85 (0.51-1.41) | 1.09 (0.60-1.97) |
| Smoked crack cocaine, prior 3 months | ||
| No | 1.00 | 1.00 |
| Yes | 1.05 (0.64-1.74) | 1.15 (0.65-2.03) |
| Snorted or smoked methamphetamine, prior 3 months | ||
| No | 1.00 | 1.00 |
| Yes | 0.92 (0.56-1.52) | 1.93 (1.09-3.41)╪ |
| Participant injecting drug use (prior 3 months unless otherwise specified) | ||
| Years since first injected drugs | ||
| <3 | 0.80 (0.45-1.43) | 0.60 (0.30-1.19) |
| 3-5 | 1.04 (0.55-1.95) | 0.40 (0.19-0.85)╪ |
| ≥6 | 1.00 | 1.00 |
| Injected daily or almost daily, prior 30 days | ||
| No | 1.00 | 1.00 |
| Yes | 1.02 (0.62-1.69) | 1.85 (1.04-3.28)╪ |
| Injected heroin (not mixed with other drugs) | ||
| No | 1.00 | 1.00 |
| Yes | 2.09 (0.89-4.89) | 1.06 (0.50-2.26) |
| Injected methamphetamine (by itself or mixed with heroin) | ||
| No | 1.00 | 1.00 |
| Yes | 0.63 (0.38-1.06) | 1.85 (1.01-3.42)╪ |
| Injected powder cocaine | ||
| No | 1.00 | 1.00 |
| Yes | 1.46 (0.87-2.46) | 0.86 (0.46-1.59) |
| Injected crack cocaine | ||
| No | 1.00 | 1.00 |
| Yes | 1.47 (0.84-2.59) | 1.13 (0.54-2.35) |
| Number of persons injected with prior month | ||
| 1 | 1.00 | 1.00 |
| 2-4 | 0.58 (0.27-1.20) | 1.16 (0.49-2.72) |
| ≥5 | 0.60 (0.28-1.30) | 1.21 (0.49-2.97) |
| Obtained needles/syringes from a needle exchange | ||
| No | 1.00 | 1.00 |
| Yes | 0.53 (0.30-0.91)╪ | 1.55 (0.80-3.33) |
| Used needle/syringe >1 time | ||
| No | 1.00 | 1.00 |
| Yes | 3.45 (2.03-5.86)╪ | 1.24 (0.70-2.21) |
| Partnership characteristics | ||
| Partner approximate age | ||
| <20 | 4.40 (1.24-15.65)╪ | 0.85 (0.42-1.73) |
| 20-35 | 5.59 (1.63-19.19)╪ | 1.14 (0.62-2.11) |
| >35 | 1.00 | 1.00 |
| Partner age compared to participant | ||
| Partner <5 years older | 5.53 (1.59-19.24)╪ | 1.25 (0.82-1.91) |
| Partner 5-10 years older | 5.15 (1.65-16.06)╪ | 1.19 (0.64-2.23) |
| Partner >10 years older | 1.00 | 1.00 |
| Partnership sex | ||
| Participant male -- partner male | 1.00 | 1.00 |
| Participant female – partner female | 1.95 (0.82-4.64) | 1.26 (0.50-3.18) |
| Participant male – partner female | 2.79 (1.56-5.00)╪ | 1.30 (0.66-2.57) |
| Participant female -- partner male | 3.01 (1.64-5.53)╪ | 1.22 (0.73-2.03) |
| Participant and partner engaged in sexual relations, prior month | ||
| No | 1.00 | 1.00 |
| Yes | 3.68 (2.40-5.64)╪ | 1.50 (0.93-2.42) |
| Months acquainted with partner | ||
| ≤3 | 1.00 | 0.81 (0.51-1.31) |
| 3-12 | 1.27 (0.72-2.27) | 0.73 (0.45-1.19) |
| >=12 | 1.77 (1.01-3.11) | 1.00 |
| Lived or stayed with partner | ||
| No | 1.00 | 1.00 |
| Yes | 3.29 (1.68-6.41)╪ | 1.81 (1.09-3.02)╪ |
| Traveled with partner outside of San Francisco* | ||
| No | 1.00 | 1.00 |
| Yes | 2.90 (1.48-5.68)╪ | 1.06 (0.62-1.84) |
| Partnership injecting behaviors (prior month) | ||
| Frequency of injecting with partner | ||
| Less than once a week | 1.00 | 1.00 |
| 1-2 times/week | 1.10 (0.46-2.62) | 1.90 (1.00-3.64)╪ |
| 3-5 times/week | 2.38 (1.15-4.95)╪ | 0.91 (0.51-1.62) |
| >5 times/week | 4.80 (2.27-10.13)╪ | 3.03 (1.44-6.38)╪ |
| Pooled money to buy drugs with partner* | ||
| No | 1.00 | 1.00 |
| Yes | 1.18 (0.51-2.71) | 1.22 (0.80-1.87) |
| Injected by partner* | ||
| No | 1.00 | 1.00 |
| Yes | 2.03 (1.08-3.79)╪ | 1.84 (0.97-3.49)╪ |
| Partner injects exclusively with participant | ||
| No | 0.49 (0.29-0.83)╪ | 1.08 (0.53-2.20) |
| Yes | 1.00 | 1.00 |
| Unknown | 0.22 (0.11-0.45)╪ | 0.70 (0.31-1.58) |
| Shared drug preparation equipment with partner | ||
| No | 1.00 | |
| Yes | 7.53 (3.37-16.87)╪ | NA |
| Perceptions about partner HCV/HIV status | ||
| Perceived HCV status of partner | ||
| Negative | 1.00 | 1.00 |
| Positive | 0.49 (0.25-0.95)╪ | 0.91 (0.57-1.44) |
| Unknown | 0.68 (0.40-1.16) | 0.53 (0.33-0.84)╪ |
| Perceived HIV status of partner | ||
| Negative | 1.00 | 1.00 |
| Positive | 0.58 (0.18-1.87) | 0.67 (0.42-1.07) |
| Unknown | 0.82 (0.49-1.39) | 0.60 (0.37-0.97)╪ |
Not collected for 2006-2007 surveys; dummy variable created to represent missing data in the regression analysis
p<0.05
We found no significant effect modification of sex or engaging in sex with one's partner with the perceived HCV status of one's partner (Table 4). When we conducted adjusted analyses, we found only re-using one's own needle/syringe had a significant effect on the association between perceiving one's partner to be HCV positive and engaging in RNS with that partner. Adjusting for this variable, the odds ratio for perceiving one's partner to be HCV positive on engaging in RNS with that partner increased to 0.57 (95% CI 0.28-1.15).
Table 4. Effect modification of the association of perceived HCV status of one's injecting partner on engaging in RNS and AES with that partner, by sex of the partner and by whether the partner was a sexual partner.
| Odds ratio for RNS (95% CI) | Odds ratio for AES (95% CI) | |
|---|---|---|
| Overall | ||
| Perceived HCV status of partner | ||
| Negative | 1.00 | 1.00 |
| Positive | 0.49 (0.25-0.95)╪ | 0.91 (0.57-1.44) |
| Unknown | 0.68 (0.40-1.16) | 0.53 (0.33-0.84)╪ |
| By sex of participant | p-value for interaction=0.59 | p-value for interaction=0.91 |
| Female | ||
| Perceived HCV status of partner | ||
| Negative | 1.00 | 1.00 |
| Positive | 0.53 (0.23-1.25) | 0.74 (0.35-1.55) |
| Unknown | 0.95 (0.40-2.26) | 0.45 (0.20-1.04) |
| Male | ||
| Perceived HCV status of partner | ||
| Negative | 1.00 | 1.00 |
| Positive | 0.50 (0.19-1.28) | 0.97 (0.55-1.71) |
| Unknown | 0.58 (0.29-1.15) | 0.55 (0.31-0.97) ╪ |
| By whether partner was a sexual partner of participant | p-value for interaction=0.68 | p-value for interaction=0.10 |
| Yes | ||
| Perceived HCV status of partner | ||
| Negative | 1.00 | 1.00 |
| Positive | 0.57 (0.24-1.36) | 1.00 (0.99-1.00) |
| Unknown | 0.77 (0.37-1.60) | 1.00 (0.99-1.00) |
| No | ||
| Perceived HCV status of partner | ||
| Negative | 1.00 | 1.00 |
| Positive | 0.68 (0.24-1.90) | 0.94 (0.58-1.53) |
| Unknown | 1.18 (0.51-2.71) | 0.47 (0.29-0.76) ╪ |
Correlates of AES within the partnerships with no RNS
Participant level variables that were associated with engaging in AES within partnerships in bivariate analysis (Table 3) included snorting or smoking methamphetamine, injecting methamphetamine, years since first injected (any drug), and injecting daily or almost daily. Partnership level variables that were associated with AES included having had sex with one's partner, living or staying with one's partner, injecting frequently with one's partner, and being injected by one's partner. Participants who did not know the HCV or HIV status of their partner were significantly less likely than those who reported that their partner was HCV negative to report engaging in AES with that partner (odds ratio 0.60; 95% CI 0.37-0.97).
We found no effect modification by sex, but we did find significant effect modification on the association between reporting unknown partner HCV and engaging in AES with that partner by whether the participant had engaged in sex with his/her injecting partner (Table 4). We did not find any evidence of confounding within the levels of the effect modifier.
Discussion
This study found that injecting partnerships in which the participant thought that their injecting partner was HCV positive had lower odds of engaging in RNS with that partner, compared to those partnerships in which the participants reported that their partner was HCV negative. While some studies have examined the effects of one's own HCV status on injecting risk behavior,35, 36 only two other studies that we are aware of have examined the effect of perceived partner serostatus on injecting risk behavior32, 33 The study by De et al found no difference in needle/syringe sharing (both borrowing and lending studied together) by the HCV status of the participant and their partners32 The study by Burt et al found that IDU were more likely to share (borrow or lend) injection equipment (needles/syringes, cookers, cottons and water) with those of the same HCV status.33 Our finding is consistent with that of Burt et al33 and is an indication that young IDU may engage in some amount of risk calculation in order to avoid infection with HCV. However, it is not encouraging that young IDU who did not know their partner's HCV status had equal odds of engaging in RNS as those who reported that their partner was HCV negative. We found that using one's own needle/syringe only one time attenuated the association between perceiving one's partner to be HCV positive and engaging in RNS with that partner and therefore re-using one's needle/syringe may be a mediator. Those who believe that their partner is HCV positive are more likely to practice safer injecting habits overall, however the directionality of this association cannot be concluded from these cross-sectional data. None of the other variables that were associated with engaging in RNS with one's partner affected the association with believing one's partner was HCV positive.
Our second finding was those who reported that they did not know the HCV status of their injecting partner had significantly lower odds of engaging in AES with that partner as compared to those who reported that their partner was HCV negative, among those partnerships in which the partners had not engaged in sex. This association was not attenuated after adjusting for variables that were associated with engaging in AES in bivariate analyses. There was no association between the respondent's perceived HCV status of their injecting partners and engaging in AES with those partners, among the partnerships where the partners had engaged in sex in the prior month. This finding is consistent with previous studies showing increased injecting risk behavior among sexual partners.11, 14, 15, 21-23
The majority of the injecting partnerships of young IDU were between persons who were close in age, who had known each other for a median of 6 months, and who were male and female IDU (rather than the same sex), and 30% of the injecting partners had also engaged in sex. Just over half (53%) of the participants had previously been tested for HCV, and the participants did not know the HCV status of almost half (49%) of their injecting partners. This highlights that while there is a fair degree of closeness in several aspects of the injecting relationships in young IDU, HCV testing and HCV status disclosure is not the norm.
There are several limitations to note in interpreting these results. First, the data are collected by self-report, and social desirability bias may affect the results. Young IDU who have been exposed to risk reduction counseling may under-report engaging in risk behaviors, especially with known HCV positive partners, which would bias our results away from the null. However, the level of risk behavior reported is quite consistent with that observed in other studies of young IDU.25 Participants were asked to report on partnership characteristics for those partners with whom they injected most often, with a limit of three partnerships. Therefore for those with more than three partnerships, lower frequency partnerships were excluded and we therefore cannot generalize our results to all injecting partnerships. The results are also limited by the cross-sectional nature of the study.
The results from this study suggest that knowledge that one's partner is HCV positive may foster avoidance of RNS. This study highlights the potential need for broader programs of HCV testing and emphasis on partner disclosure, given that such high proportions of young IDU knew neither their own nor their partners' HCV status. However, no studies have been conducted to examine the impact of partner HCV status disclosure, while studies of the impact of knowing one's own HCV status have been mixed. A recent ethnographic study which assessed IDU's views of HCV infection in young IDU illustrated that for some young IDU, knowledge that they are infected with HCV leads them to attempt to prevent spreading the infection to others.37 On the other hand, in our population, we found that young IDU did not reduce their level of lending injecting equipment to others or engaging in AES after they were told they were infected with HCV.38
It would be important to conduct modeling studies to determine the plausible effect of a serosorting approach on reducing HCV incidence in IDU. We caution that HIV serosorting of sexual partners has been shown to have clear limitations.31, 39, 40 The results also suggest that young IDU engage in AES with sexual partners regardless of the perceived HCV status of that partner. As AES appears to be a significant route of transmission for HCV,5 this route of infection deserves more emphasis in designing programs to reduce HCV transmission.
Acknowledgments
Funding: This research was funded by National Institutes of Health grants R01DA016017 and K01DA023365.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
References
- 1.Hagan H, Thiede H, Des Jarlais DC. HIV/hepatitis C virus co-infection in drug users: risk behavior and prevention. Aids. 2005 Oct;19(3):S199–207. doi: 10.1097/01.aids.0000192090.61753.d4. [DOI] [PubMed] [Google Scholar]
- 2.Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incidence and spontaneous resolution of infection. 2009 doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De P, Roy E, Boivin JF, Cox J, Morissette C. Risk of hepatitis C virus transmission through drug preparation equipment: a systematic and methodological review. J Viral Hepat. 2008 Jan 20; doi: 10.1111/j.1365-2893.2007.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagan H, Pouget ER, Williams IT, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. Feb 1;201(3):378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 5.Hahn JA, Wylie D, Dill J, et al. Potential impact of vaccination on the hepatitis C virus epidemic in injecting drug users. Epidemics. 2008 doi: 10.1016/j.epidem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kral AH, Lorvick J, Edlin BR. Sex- and drug-related risk among populations of younger and older injection drug users in adjacent neighborhoods in San Francisco. Journal of Acquired Immune Deficiency Syndromes. 2000;24(2):162–167. doi: 10.1097/00126334-200006010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Fennema JS, Van Ameijden EJ, Van Den Hoek A, Coutinho RA. Young and recent-onset injecting drug users are at higher risk for HIV. Addiction. 1997;92(11):1457–1465. [PubMed] [Google Scholar]
- 8.Lum PJ, Sears C, Guydish J. Injection risk behavior among women syringe exchangers in San Francisco. Subst Use Misuse. 2005;40(11):1681–1696. doi: 10.1080/10826080500222834. [DOI] [PubMed] [Google Scholar]
- 9.Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003 Mar;80(1):137–146. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery SB, Hyde J, De Rosa CJ, et al. Gender differences in HIV risk behaviors among young injectors and their social network members. Am J Drug Alcohol Abuse. 2002;28(3):453–475. doi: 10.1081/ada-120006736. [DOI] [PubMed] [Google Scholar]
- 11.Sherman SG, Latkin CA, Gielen AC. Social factors related to syringe sharing among injecting partners: a focus on gender. Subst Use Misuse. 2001 Dec;36(14):2113–2136. doi: 10.1081/ja-100108439. [DOI] [PubMed] [Google Scholar]
- 12.Milloy MJ, Buxton J, Wood E, Li K, Montaner JS, Kerr T. Elevated HIV risk behaviour among recently incarcerated injection drug users in a Canadian setting: a longitudinal analysis. BMC Public Health. 2009;9:156. doi: 10.1186/1471-2458-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milloy MJ, Wood E, Small W, et al. Incarceration experiences in a cohort of active injection drug users. Drug Alcohol Rev. 2008 Mar 31;:1–7. doi: 10.1080/09595230801956157. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RA, Gerstein DR, Pach A, 3rd, Cerbone FG, Brown J. HIV risk behaviors in African-American drug injector networks: implications of injection-partner mixing and partnership characteristics. Addiction. 2002 Aug;97(8):1011–1024. doi: 10.1046/j.1360-0443.2002.00165.x. [DOI] [PubMed] [Google Scholar]
- 15.Bailey SL, Ouellet LJ, Mackesy-Amiti ME, et al. Perceived risk, peer influences, and injection partner type predict receptive syringe sharing among young adult injection drug users in five U.S. cities. Drug Alcohol Depend. 2007 Apr 12; doi: 10.1016/j.drugalcdep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Koester S, Glanz J, Baron A. Drug sharing among heroin networks: implications for HIV and hepatitis B and C prevention. AIDS Behav. 2005 Mar;9(1):27–39. doi: 10.1007/s10461-005-1679-y. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe LE, Bailey SL, Huo D, Monterroso ER, Ouellet LJ. Injection-related risk behaviors in young urban and suburban injection drug users in Chicago (1997-1999) J Acquir Immune Defic Syndr. 2001 May 1;27(1):71–78. doi: 10.1097/00126334-200105010-00012. [DOI] [PubMed] [Google Scholar]
- 18.Koester S, Booth RE, Zhang Y. The prevalence of additional injection-related HIV risk behaviors among injection drug users. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1996;12(2):202–207. doi: 10.1097/00042560-199606010-00015. [DOI] [PubMed] [Google Scholar]
- 19.McKeganey N, Friedman SR, Mesquita F. The social context of injectors' risk behavior. In: Stimson GV, Des Jarlais DC, Ball AL, editors. Drug Injecting and HIV Infection. London: UCL Press Limited; 1998. pp. 22–41. [Google Scholar]
- 20.Bourgois P, Prince B, Moss A. The everyday violence of hepatitis C among young women in who inject drugs in San Francisco. Human Organization. 2004 Fall 2004;63(3):253–264. doi: 10.17730/humo.63.3.h1phxbhrb7m4mlv0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw SY, Shah L, Jolly AM, Wylie JL. Determinants of injection drug user (IDU) syringe sharing: the relationship between availability of syringes and risk network member characteristics in Winnipeg, Canada. Addiction. 2007 Oct;102(10):1626–1635. doi: 10.1111/j.1360-0443.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- 22.De P, Cox J, Boivin JF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007 Nov;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 23.Unger JB, Kipke MD, De Rosa CJ, Hyde J, Ritt-Olson A, Montgomery S. Needle-sharing among young IV drug users and their social network members: The influence of the injection partner's characteristics on HIV risk behavior. Addict Behav. 2006 Sep;31(9):1607–1618. doi: 10.1016/j.addbeh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Needle RH, Coyle S, Cesari H, et al. HIV risk behaviors associated with the injection process: multiperson use of drug injection equipment and paraphernalia in injection drug user networks. Substance Use and Misuse. 1998;33(12):2403–2423. doi: 10.3109/10826089809059332. [DOI] [PubMed] [Google Scholar]
- 25.Thiede H, Hagan H, Campbell JV, et al. Prevalence and correlates of indirect sharing practices among young adult injection drug users in five U.S. cities. Drug Alcohol Depend. 2007 Apr 25; doi: 10.1016/j.drugalcdep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Davey-Rothwell MA, Latkin CA, Tobin KE. Longitudinal Analysis of the Relationship Between Perceived Norms and Sharing Injection Paraphernalia. AIDS Behav. 2009 Jan 16; doi: 10.1007/s10461-008-9520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andia JF, Deren S, Robles RR, Kang SY, Colon HM. Peer norms and sharing of injection paraphernalia among Puerto Rican injection drug users in New York and Puerto Rico. AIDS Educ Prev. 2008 Jun;20(3):249–257. doi: 10.1521/aeap.2008.20.3.249. [DOI] [PubMed] [Google Scholar]
- 28.Jin F, Crawford J, Prestage GP, et al. Unprotected anal intercourse, risk reduction behaviours, and subsequent HIV infection in a cohort of homosexual men. Aids. 2009 Jan 14;23(2):243–252. doi: 10.1097/QAD.0b013e32831fb51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morin SF, Shade SB, Steward WT, et al. A Behavioral Intervention Reduces HIV Transmission Risk by Promoting Sustained Serosorting Practices Among HIV-Infected Men Who Have Sex With Men. J Acquir Immune Defic Syndr. 2008 Nov 4; doi: 10.1097/QAI.0b013e31818d5def. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton LA, West TV, Kenny DA, Kalichman SC. HIV Transmission Risk among HIV Seroconcordant and Serodiscordant Couples: Dyadic Processes of Partner Selection. AIDS Behav. 2008 Oct 25; doi: 10.1007/s10461-008-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eaton LA, Kalichman SC, Cain DN, et al. Serosorting sexual partners and risk for HIV among men who have sex with men. Am J Prev Med. 2007 Dec;33(6):479–485. doi: 10.1016/j.amepre.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De P, Cox J, Boivin JF, Platt RW, Jolly AM, Alexander PE. HIV and HCV discordant injecting partners and their association to drug equipment sharing. Scand J Infect Dis. 2009 Jan 26;:1–9. doi: 10.1080/00365540902721376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burt RD, Thiede H, Hagan H. Serosorting for hepatitis C status in the sharing of injection equipment among Seattle area injection drug users. Drug Alcohol Depend. 2009 Dec 1;105(3):215–220. doi: 10.1016/j.drugalcdep.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Selvin S. Statistical Analysis of Epidemiologic Data. New York: Oxford University Press; 1996. [Google Scholar]
- 35.Cox J, Morissette C, De P, et al. Access to sterile injecting equipment is more important than awareness of HCV status for injection risk behaviors among drug users. Subst Use Misuse. 2009;44(4):548–568. doi: 10.1080/10826080802544349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carruthers SJ. Preventing hepatitis C: what do positive injectors do? Drug Alcohol Rev. 2005 Mar;24(2):193–198. doi: 10.1080/09595230500102673. [DOI] [PubMed] [Google Scholar]
- 37.Roy E, Nonn E, Haley N, Cox J. Hepatitis C meanings and preventive strategies among street-involved young injection drug users in Montreal. Int J Drug Policy. 2007 Oct;18(5):397–405. doi: 10.1016/j.drugpo.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Tsui JI, Vittinghoff E, Hahn JA, Evans JL, Davidson PJ, Page K. Risk behaviors after hepatitis C virus seroconversion in young injection drug users in San Francisco. Drug Alcohol Depend. 2009 Nov 1;105(1-2):160–163. doi: 10.1016/j.drugalcdep.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zablotska IB, Imrie J, Prestage G, et al. Gay men's current practice of HIV seroconcordant unprotected anal intercourse: serosorting or seroguessing? AIDS Care. 2009 Mar 6;6:1–10. doi: 10.1080/09540120802270292. [DOI] [PubMed] [Google Scholar]
- 40.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008 Oct 1;49(2):212–218. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]


