Abstract
Background
Long-lasting increases in dendritic spine density and gene expression in the nucleus accumbens and in the ambulatory response to cocaine occur following chronic cocaine treatment. Despite numerous reports of these findings, the molecular mechanisms leading to these morphological, biochemical and behavioral changes remain unclear.
Methods
We used mice genetically lacking Kalirin7 (Kal7KO), a Rho-GEF which regulates dendritic spine formation and function. Both wildtype (Wt) and Kal7KO mice were given high dose cocaine (20 mg/kg) for four or eight consecutive days. Locomotor sensitization and conditioned place preference elicited by cocaine were evaluated. The nucleus accumbens core was diolistically labeled and spine density and morphology were quantified using confocal microscopy.
Results
Cocaine increased Kalirin7 mRNA and protein expression in the nucleus accumbens of Wt mice. Kal7KO animals showed greater locomotor sensitization to cocaine than Wt mice. In contrast, Kal7KO mice exhibited decreased place preference for cocaine, despite displaying a normal place preference for food. While Wt mice showed a robust increase in dendritic spine density after four and eight days of cocaine treatment, dendritic spine density failed to increase in cocaine-exposed Kal7KO mice. Wt mice treated with cocaine for eight days exhibited larger dendritic spines than cocaine-treated Kal7KO mice.
Conclusions
Kalirin7 is an essential determinant of dendritic spine formation following cocaine treatment. The absence of this single isoform of one of the many Rho-GEFs expressed in the nucleus accumbens results in enhanced locomotor sensitization and diminished place preference in response to cocaine.
Keywords: addiction, cocaine, dendritic spine, sensitization, place-preference, nucleus accumbens
Introduction
Drug addiction is a recalcitrant condition affecting millions of families worldwide. Drugs of abuse are thought to co-opt normal learning and plasticity mechanisms and permanently alter the structure and function of the brain (1). Over the past two decades a number of candidate pathways have emerged that may support these lasting changes. The transcription factor ΔFosB remains elevated at least one month following cocaine treatment in rodents, and levels of ΔFosB dictate behavioral responses to cocaine (2). Similarly, recent work has shown that GluR1-containing AMPA receptors are trafficked to synapses throughout extended withdrawal periods and that this membrane trafficking has effects on the behavioral response to cocaine (3). One of the most consistently reported and longest-lasting changes in animal models of drug addiction is an increase in dendritic spine density in the nucleus accumbens (NAc) (4). In rats these increases in spine number persist up to 3.5 months following drug administration (5). A recent study by Shen and colleagues found that, while cocaine withdrawal itself did not alter spine density, the increase in spine density in response to a cocaine injection was more rapid in cocaine withdrawn rats, compared to saline-treated rats (6). Despite repeated findings of alterations in NAc spine number and morphology, the molecular mechanisms underlying the morphological changes and their physiological significance remain elusive.
Rho-GDP/GTP exchange factors (Rho-GEFs) are known to play critical roles in spine morphogenesis (7,8). Kalirin is one of 31 Rho-GEFs expressed at significant levels in the NAc of the adult mouse and one of 10 Rho-GEFs localized to the PSD (9). Kalirin7 (Kal7), the Kalirin splice variant most prevalent at the PSD, regulates dendritic spine morphogenesis in vitro (10,11) and in vivo (12) (Figure 1A). Examination of the CA1 region of the hippocampus in Kal7 knockout mice (Kal7KO) revealed a decrease in linear spine density (12). These mice exhibited abnormal fear learning as well as decreased anxiety-like behavior, yet maintained normal radial arm maze and object recognition learning (12). The deficit in hippocampal dendritic spine density in these animals resulted in specific losses of function. Given the role that dendritic spine alterations may play in cocaine-mediated behaviors, we explored the possibility that chronic cocaine treatment would affect spine morphology differently in Wt and Kal7KO animals. If so, this would provide a model system to begin to explore both the molecular mechanisms underlying the morphological changes observed in cocaine addiction and their functional consequences.
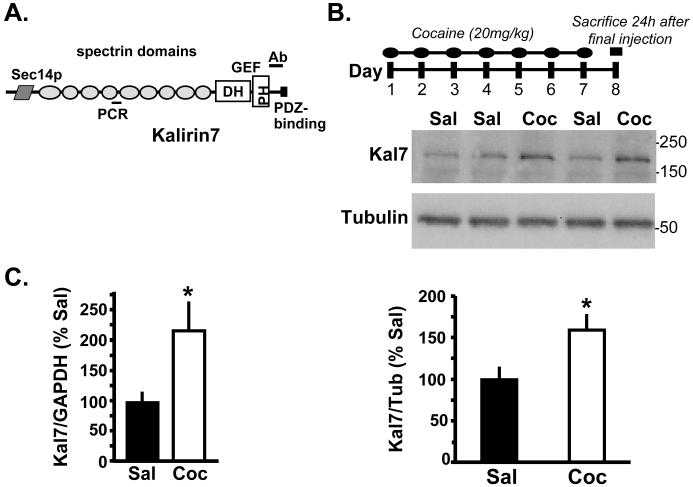
Figure 1.
Chronic cocaine induces increased Kal7 expression in the NAc of Wt mice. A. The structure of Kal7 is diagramed (53) and the locations of the antibody site (JH2959; (35)) and the quantitative polymerase chain reaction (qPCR) primers are indicated. Twenty-four hours following 7 days of cocaine (20mg/kg) or saline injections, Kal7 protein (B; normalized to βIII tubulin – p=0.016) and mRNA (C; normalized to GAPDH – p=0.047) were significantly elevated. Primers used are listed in Supplementary Table 1.
In this study we probed the morphological and behavioral responses of Wt and Kal7KO animals to chronic cocaine treatment. Unlike Wt mice, Kal7KO animals showed no increase in spine density following either a four or eight day course of cocaine administration. Additionally, Kal7KO animals exhibited abnormal spine morphology after the longer cocaine treatment. Behaviorally, the Kal7KO animals were hypersensitive to the locomotor sensitizing effects of cocaine but showed a decrease in conditioned place preference for cocaine, despite their normal place preference for food.
Methods
Animals
For these experiments, all Kal7KO mice had been back-crossed into C57BL/6J (Jackson Labs) for at least four generations (12); further breeding to greater than 10 generations into BL6 has not altered biochemical or behavioral outcomes. As with all back-crossed knockout lines, fragments of 129 genome remain in these largely C57BL/6J mice (13). Since the deleted Kal7 exon is 507 kb and 193 kb distant from the immediately adjacent genes, the possibility that a polymorphism at a locus near the Kal7 exon accounts for our results is slight (13). All animals were housed in the UCHC animal facility on a 12 hour light/dark cycle with food and water ad lib except as noted. All procedures were in accord with the guidelines of the UCHC IACUC.
Behavioral Experiments
Animals were allowed to acclimate to the behavior room for one hour before the start of training or testing. All animals were male littermates between 60-80 days old at the start of testing. Each experimental group included 6-12 animals, with the exception of the Latin-square cocaine and place preference Saline groups, which had five animals per genotype.
Locomotor Sensitization
Procedures for sensitization experiments were adapted from published methods (14). To minimize stress and establish baseline activity, animals were injected with saline for four days with their locomotor activity monitored. On subsequent days, animals received cocaine (National Institute of Drug Abuse, Bethesda, MD) immediately before the 60 min locomotor monitoring. Animals had their locomotor activity monitored for the first five days of cocaine to ensure the development of sensitization and then received two additional injections in their home cages. To test for the persistence of sensitization, animals were returned to the colony room for one week before receiving a challenge dose of cocaine.
Place Preference
Methods for conditioned place preference (CPP) were adapted from published procedures (15). To test cocaine preference, the animals were placed in the central walkway 24 hours after the final cocaine injection with both doors open and allowed to explore freely for twenty minutes. For food preference, the animals were given a 20 minute test similar to the cocaine animals; details are included in Supplemental Methods.
Morphology
Animals
For study #1, mice (N=4/group) were given eight injections of 20mg/kg cocaine or saline and were then anesthetized using ketamine and perfusion fixed 30 minutes after the final dose of cocaine. For study #2, mice (N=3/group) were given 4 daily injections of cocaine (20mg/kg) or saline and perfusion fixed 30 minutes after the final injection. For both morphology studies, animals were brought into the behavioral suite and injected in empty rat cages which had been cleaned with a scented detergent, to allow association of cocaine with a unique context, mimicking the injection conditions used in the behavioral studies.
Tissue Processing
All mice were perfusion fixed with 4% paraformaldehyde followed by 1 hour of post-fixation in 4% paraformaldehyde (12). Slices (100μm) containing the NAc were made using a vibratome and were then diolistically labeled using a Gene Gun [Biorad, Hercules CA] (16,17). A side-by side comparison of several different perfusion and post-fixation conditions (comparing 1.5% and 4% PFA with post-fixation times of 1 to 24 hours) was performed, as an effect of fixation strength on DiI diffusion has been reported (18). In agreement with the majority of the literature, fixation with 4% PFA followed by post-fixation for 1 hour in 4% PFA yielded the clearest images with the most readily visible spines (17,19-21). Neurons fixed with 1.5% and 4% paraformaldehyde are compared in Fig. S1. After ballistic labeling, the dye (DiI; Invitrogen, Carlsbad, CA) was allowed to fill the processes for 2 hours before imaging.
Image Analysis
Collapsed z-stack images were coded; images were then scored by a blinded observer. Quantification of spine density, spine length, and spine area was performed using MetaMorph (10,12). Any spines for which the base and tip were not clearly visible were excluded from length and area measurements, but were included in the spine density measurements. Detailed descriptions of spine length and area measurements as well as image acquisition appear in Supplemental Methods; a graphical depiction of how spine area measurements were made is provided in Fig. S2.
Biochemistry and Statistics
Detailed descriptions are available in Supplemental Methods.
Results
Repeated treatment with cocaine increases Kal7 levels in the NAc
Chronic treatment of laboratory animals with cocaine increases dendritic spine number in the NAc (4,22). Kal7 plays an important role in dendritic spine formation and maintenance in cultured neurons (10) and in the hippocampus of mice (12), but is only one of many Rho-GEFs expressed in the NAc (9). To test the hypothesis that Kal7 could be involved in the formation of new dendritic spines following cocaine administration, we gave Wt mice injections of 20mg/kg cocaine once daily for one week and examined protein and RNA expression 24 h after the final injection. This dosing regimen increased levels of Kal7 protein (t21= −2.60, p=0.016) (Fig. 1B) and mRNA (t6= −2.49, p=0.047) (Fig. 1C) in the NAc. Adult male rats responded to chronic cocaine (20 mg/kg for 8 days) with similar increases in Kal7 mRNA and protein (data not shown).
Kal7 plays an important role in cocaine-induced spine plasticity
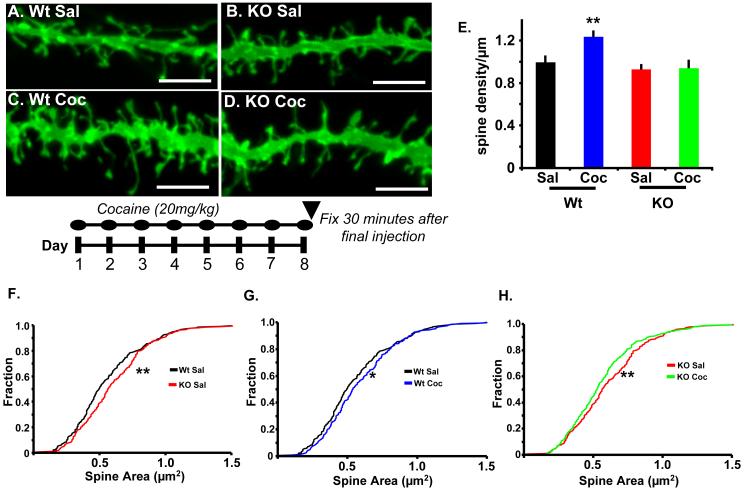
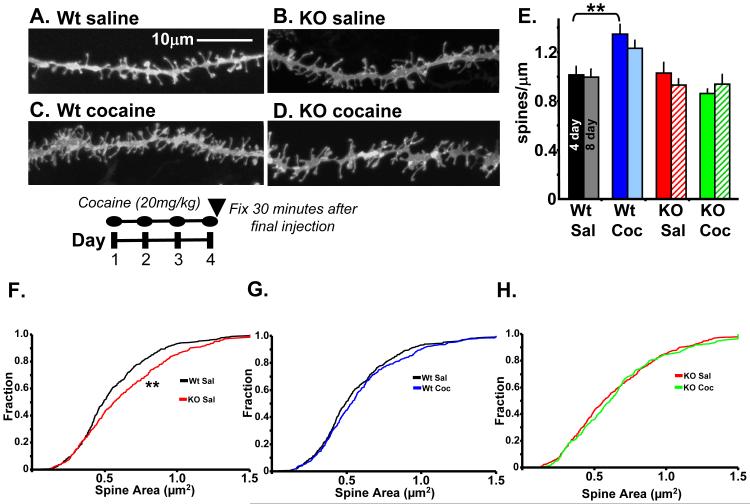
The finding that Kal7 levels increased in the NAc after one week of cocaine injection suggested that Kal7 might be essential for the previously reported increases in dendritic spine density (4,22). To test this hypothesis, we gave Wt and Kal7KO mice injections of saline or 20mg/kg cocaine once daily for eight days and examined them 30 min after the final injection. We selected this treatment time and post-injection time to correlate to induction of behavioral sensitization, an approach that has previously been reported to increase NAc spine density (23). In saline treated animals, linear spine density was indistinguishable in the NAc of Wt and Kal7KO mice (Fig. 2A,B,E). Following eight days of cocaine treatment, Wt animals displayed an increase in dendritic spine density similar in magnitude to the increases observed with longer treatment paradigms (Fig. 2A,C,E) (17,24). In contrast, Kal7KO animals showed no increase in dendritic spine density following eight days of cocaine treatment (Fig. 2B,D,E). Two-way ANOVA analysis showed main effects of genotype (F1,73=13.537; p<0.0001) and treatment (F1,73=14.069; p<0.0001) as well as a significant genotype x treatment interaction (F1,73=8.815; p=0.004). Kal7 is thus one of the few proteins demonstrated to be essential for cocaine-induced increases in dendritic spine density in vivo (25-27).
Figure 2.
Kal7 modulates morphological effects of cocaine in NAc. A.-D. Representative DiI labeled dendrites from NAc core after treatment of Wt and Kal7KO mice with saline or cocaine (20mg/kg) for eight days, as shown below the images. Scale bars are 5μm. E. Dendritic spine density in the NAc was increased following cocaine treatment of Wt but not Kal7KO animals. Two-way ANOVA yields main effects of genotype (p<0.0001) and drug (p<0.0001) as well as a drug x genotype interaction (p=0.004). F.-H. Quantification of dendritic spine area in Wt and Kal7KO animals after treatment with cocaine or saline. F. Kolmogorov-Smirnov analysis of cumulative distribution plots of spine areas shows a main effect of genotype at baseline, with Kal7KO animals exhibiting larger spines (p<0.005) Similar analysis shows a main effect of treatment in both genotypes with Wt spines increasing in size (G. p<0.05) and KO spines decreasing in size (H. p<0.005) [N=235-295 spines/group].
In addition to linear spine density, we examined dendritic spine length and area in this group of animals, as these parameters are known to be determinants of synaptic function (28). The effects of cocaine on spine structure in rats are time-dependent (6); data for animals sacrificed 30 minutes after the final dose have not been reported. Analysis of dendritic spine length demonstrated a main effect of genotype (F1,48=23.00; p<0.0001, two-way ANOVA) (Fig. S3) but no effect of treatment (F1,48=2.01; p=0.163) or genotype by treatment interaction (F1,48=0.09; p=0.760). To examine changes in spine area, data were plotted using Kolmogorov-Smirnov cumulative distribution plots, as this is the least biased and most detailed way to examine a large and variable population. In this approach, each spine is weighted equally, diminishing the influence of dendrites with more or fewer measurable spines. Interestingly, Kal7KO animals demonstrated significant increases in spine size at baseline (p<0.005) (Fig. 2F) However, the prolonged cocaine treatment produced opposite effects on spine size between genotypes. While Wt animals showed an increase in spine size after cocaine treatment (p<0.05) (Fig. 2G), the Kal7KO animals showed a marked decrease in spine size (p<0.005) (Fig. 2H). Recent data have suggested that spine number and size increase following learning or LTP induction stimuli, and larger spines have greater surface area for the expression of receptors and other signaling molecules (28-31). While the Kal7KO animals do have larger spines at baseline, the lack of new spine formation and shrinkage of existing spines may be indicative of disrupted synaptic plasticity in these mice.
Kal7 is essential for behavioral plasticity in response to cocaine
Locomotor Behavior
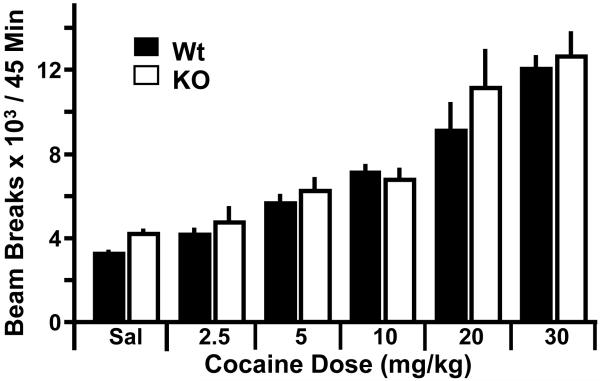
Given the altered morphological plasticity and lack of increase in spine number in the Kal7KO animals, we examined what effect this might have on the behavioral response to cocaine. Initially we examined a range of acute cocaine doses and measured open field locomotor activity to determine the response of Kal7KO mice to acute cocaine. Dose-response studies were performed using a randomized Latin-square cross-over design (32). Briefly, after one day of habituation to the locomotor boxes, the animals were given a single injection of cocaine in a randomized order and locomotor activity was measured for 45 minutes after the injection (Fig. 3). As expected, there was a strong effect of treatment (F5,40=566.29; p<0.0001, two-way RM ANOVA). Interestingly however, there was no effect of genotype (F1,40=1.13; p=0.318) and no genotype x treatment interaction (F5,40=1.42; p=0.267).
Figure 3.
Lack of Kal7 does not affect acute locomotor response to cocaine. Using a randomized cross-over Latin-square design, Wt and Kal7KO animals were given the indicated doses of cocaine and examined in the open field. Two-way RM ANOVA revealed a main effect of treatment (p<0.0001) but no effect of genotype (p=0.318) or genotype x treatment interaction (p=0.267) [N=5/group].
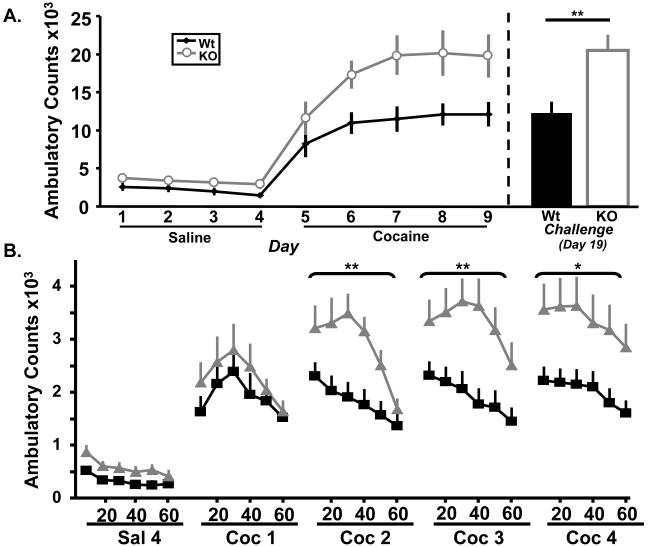
Given that addiction is a chronic condition that develops over time, and that the majority of the literature on dendritic spine changes following cocaine treatment is after a prolonged treatment with cocaine, we examined whether the Kal7KO animals would exhibit abnormal locomotor behavior in response to repeated cocaine injection. For this locomotor sensitization assay, animals were first given four daily injections of saline to allow them to habituate to the locomotor apparatus and injections. Locomotor activity was monitored for one hour after injection on each day of the treatment. While there was a main effect of day (F3,20=5.65; p=0.028, two-way RM ANOVA) there was a stable but insignificant trend towards a genotypic effect during saline injections (F1,20=3.23, p=0.09) (Fig. 4A left). However, when the animals were given a sensitizing dose of cocaine, the Kal7KO animals began to exhibit a robust increase in their locomotor activity. Examination of the locomotor response to cocaine over 5 days yielded main effects of day (F4,20=10.64; p=0.004, two-way RM ANOVA) and genotype (F1,20=7.72; p=0.01) but no day by genotype interaction (F1,20=1.45; p=0.24). To examine the differences between genotypes more thoroughly, we examined the timecourse of the locomotor response during each day of cocaine as well as the final day of saline (Fig. 4B). In this analysis there was a main effect of time on all days except cocaine day 1 (F5,20=47.45, 18.35, 19.30, 9.94; all p≤0.005 – in chronological order) and a main effect of genotype on days 2-4 of cocaine (F1,20=7.77, 7.84, 6.64; all p≤0.018), but not on the final saline day or first day of cocaine. As a control, we examined levels of stereotypies in these animals, and found no significant difference between genotypes (Fig. S4). Taking these and the Latin-square data together, it is clear that the Kal7KO animals display an increased locomotor sensitization response to repeated doses of cocaine, but do not show a difference at any acute dose.
Figure 4.
Kal7 is essential for the normal locomotor sensitization response to cocaine. A left. Wt and Kal7KO animals have similar locomotor responses to repeated treatment with saline (p=0.09), but show a large genotypic difference in their response to repeated cocaine injections (p=0.01). A right. Animals that had been sensitized to cocaine were allowed to withdraw for one week before being given a single challenge injection of cocaine. Kal7KO mice showed elevated responding compared to Wt mice on this day (p=0.007), and showed similar levels of locomotion to the final days of the sensitization regimen. B. A breakdown of the ambulatory response time course on the indicated days shows the increasing separation between Wt and Kal7KO animals over repeated days of cocaine treatment. Main effect of genotype on days 2-4 of cocaine (p≤0.018 for each); (*p<0.02, **p≤0.01) [N=10-12/group].
While the previous experiment tested the development of sensitization, we next wanted to examine the persistence of sensitization in Kal7KO animals. Given their altered spine plasticity, it seemed possible that sensitization would not persist. To test the persistence of sensitization, all animals were returned to their home cages for one week of abstinence before the test injection. The animals were then returned to the behavior room and tested for their locomotor response to a single cocaine injection (20mg/kg) (Fig. 4A right). On this day, Kal7KO animals responded at a higher level than Wt (F1,20=9.09; p=0.007, one-way ANOVA), and displayed levels of locomotor activity similar to those of the final days of the initial sensitization period. Mice lacking this single Rho-GEF develop and maintain cocaine locomotor sensitization at a level higher than their Wt littermates.
Conditioned Place Preference
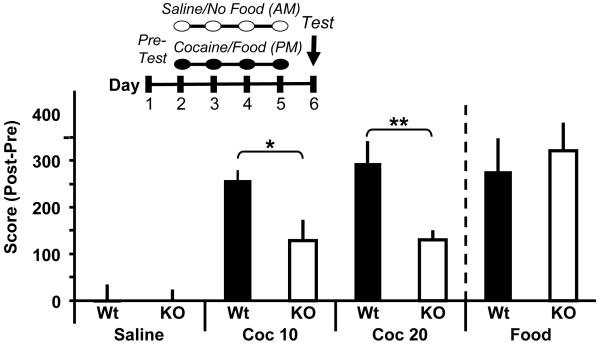
In addition to locomotor sensitization testing, we also tested the ability of the animals to form an association between a context and the reinforcing properties of cocaine, widely considered to be an essential component of addiction (15,27,32). Wt animals showed the expected preference for cocaine (Fig. 5 left). Although the Kal7KO animals showed a preference for cocaine, the magnitude of their preference was markedly reduced for both 10mg/kg (F1,13=6.58; p=0.025) and 20mg/kg (F =5.88; p=0.029) cocaine (Fig. 5 left). The Kal7KO 1,15 animals were not hyperactive on the test day, which could have confounded the finding of decreased preference (Fig. S5). The many other Rho-GEFs expressed in the NAc cannot substitute for Kal7 in the pathways involved in this behavioral response.
Figure 5.
Kal7 is essential for formation of cocaine place preference. In a conditioned place preference test carried out on the schedule indicated, mice of both genotypes exhibited a significant preference for the cocaine-paired side, with the response of Kal7KO mice substantially attenuated compared to littermate controls at both 10 and 20mg/kg cocaine (Middle; p=0.025 for 10mg/kg; p=0.029 for 20mg/kg). When food was used as the unconditioned stimulus, both genotypes formed an equal preference for the food paired chamber (Right; p=0.64) indicating that the difference between Wt and Kal7KO mice was specific to drug preference. Animals injected with saline only did not form a preference for either chamber (Left). (*p<0.05; **p<0.01 ANOVA) [N=6-10/group].
Due to the alterations in synaptic architecture displayed in naïve and cocaine-treated Kal7KO mice (Fig. 2), we considered the possibility that the Kal7KO animals might simply be incapable of forming an association between reward and context. To test this possibility, we performed the place preference assay with a different cohort of mice, using food rather than drug as the unconditioned stimulus. In this task, both Wt and Kal7KO mice showed a robust and equal preference for the food paired side (F1,12=0.23; p=0.64) (Fig. 5 right). In several other studies, knockout animals have displayed abnormal drug seeking behavior, yet normal learning for food seeking (33,34). These learning behaviors, which are both dependent on proper striatal function, clearly utilize distinct pathways.
Cocaine alters spine number within four days
Given that Kal7KO mice and Wt mice exhibited a difference in cocaine place preference after only four days of exposure to drug, we also examined dendritic spine density and morphology after four days of cocaine treatment. Examination of spine density in these neurons revealed a very similar pattern to that seen after eight days of cocaine treatment (Fig. 6A-E). Spine density was significantly increased in Wt animals whereas Kal7KO animals showed no increase. Statistical analysis revealed a main effect of genotype (F1,35=9.33; p=0.005) as well as a genotype x treatment interaction (F1,35=10.67; p=0.003) but no main effect of treatment. Comparison of these 4 day treated animals (left bars) to the 8 day treated animals (right bars, repeated from Fig. 2) showed that the spine densities did not differ between the two treatments (Fig. 6E). In addition to spine density we also examined spine length and area. Measurements of length revealed no main effects (Fig. S3). Kolmogorov-Smirnov analyses of spine area showed the same effect of genotype in the saline treated animals (Fig. 6F and 2F), but no effect of the shorter cocaine treatment in either genotype (Fig. 6G,H).
Figure 6.
NAc spine morphology after 4 days of cocaine (20mg/kg) A-D. Representative images are shown; scale bars are 10μm. The treatment paradigm used is indicated below the images. E. Dendritic spine density was quantified as described in Fig. 2E. A two-way ANOVA revealed a main effect of genotype (p=0.005) as well as a genotype x treatment interaction (p=0.003). Spine density data from Fig.2E (8 day) are replotted for comparison. F. Kolmogorov-Smirnov cumulative distribution analysis shows the same effect of genotype at baseline in these animals. G,H. However, the shorter cocaine treatment produced no main effect of treatment on spine area in either genotype [N=321-442 spines/group].
Discussion
Morphology
Kal7 expression in the NAc increased following chronic exposure to cocaine (Fig. 1). Since expression of exogenous Kal7 increased spine density in cultured cortical (35) and hippocampal (10) neurons, we predicted an essential role for Kal7 in the morphological response to cocaine. Pyramidal neurons in the CA1 region of the hippocampus of Kal7KO mice exhibited decreased spine density (12). In the NAc, spine density was indistinguishable in Wt and Kal7KO mice at baseline. While Wt animals showed the expected increase in spine density in the NAc after 4 or 8 daily injections of cocaine, spine density was unaltered in Kal7KO mice (Figs. 2,6). Taken together, our data identify Kal7 as an essential mediator of the cocaine-induced increase in NAc spine density.
In addition to its effects on spine density, Kal7 affects spine morphology (10,12,36). Kal7KO animals displayed larger spines at baseline, a phenomenon that may reflect compensation by other Rho GEFs at the PSD. It is noteworthy spine plasticity can change following a single acute injection of cocaine (6). Since our animals were fixed 30 minutes after the final cocaine injection, some of the observed alterations in spine shape could be due to the acute effects of cocaine. Most interesting to this study, however, is how the spines of the two genotypes responded to cocaine. Neither genotype exhibited an increase in spine area after four days of cocaine. While Wt mice exhibited an increase in spine size after eight days of cocaine treatment (Fig. 2G); spine size was reduced in Kal7KO mice (Fig. 2H). Spines lacking Kal7 displayed an aberrant response to chronic stimulation and were unable to display the same plasticity exhibited by spines with their full complement of Kal7. Given the links that have been made between spine size and synapse strength (28,37,38), this type of disrupted plasticity may play a role in the altered behaviors seen in the Kal7KO animals.
Increased cycling of the actin cytoskeleton occurs following cocaine administration (39). As a GEF for the small GTPase Rac1, Kal7 is an important modulator of the actin cytoskeleton and its absence would be expected to alter cytoskeletal dynamics. Cdk5, which forms a complex with activated Rac and Pak, a downstream target of Rac, plays an essential role in cocaine-induced morphological plasticity (25,32,40,41). Cdk5 phosphorylates Kal7, increasing its GEF activity (36). Kal7 mutated to block phosphorylation at its only Cdk5 target site still increases spine formation, but the new spines formed are smaller than normal spines. PSDs prepared from the cortex of Kal7KO mice contained decreased levels of Cdk5 (12), suggesting that the impaired spine morphogenesis observed in the NAc of Kal7KO mice may reflect both the absence of Kal7 and diminished Cdk5 function.
Behavior
These studies shed new light on the potential behavioral roles of altered dendritic spine density and morphology following cocaine. It is interesting that animals such as the Kal7KO mice, which show decreased synaptic plasticity, also show increased locomotor sensitization to cocaine. In MEF2 over-expressing mice, increased behavioral sensitization to cocaine was also accompanied by decreased spine density (26). Additionally, intra-NAc infusions of a Cdk5 inhibitor, roscovitine, reduced cocaine-induced dendritic spine formation (25) and potentiated the locomotor response to cocaine (41). These studies all suggest that increased spine density serves as a homeostatic adaptation tempering the psychomotor activation that follows repeated cocaine exposure; by removing factors that contribute to homeostatic adaptation, the animal may become hypersensitive to repeated doses of cocaine. One theory is that increased glutamatergic signaling from the PFC following chronic cocaine treatment imbalances the NAc circuitry (42). In response, the NAc may undergo a type of synaptic redistribution (43) as a form of homeostatic plasticity to prevent the entire neural network from being destabilized. While we are certainly not the first to suggest this theory (26,44), our findings lend strong credence to this idea.
In animal models of drug addiction, increases in locomotor sensitization and conditioned place preference have long been used to evaluate the effect of factors that contribute to addiction. One of the most informative results of this study is the observation that eliminating Kal7 expression has distinctly different effects on these two behavioral responses to cocaine. While both of these behaviors are heavily reliant on synaptic transmission in the NAc (45,46), both behaviors are also driven by systems-wide processes. As a direct comparison, we examined the behavior of the Kal7KO animals in a radial arm maze task and contextual fear conditioning (12); both behaviors rely heavily on normal hippocampal functioning. The Kal7KO animals showed perfectly normal learning curves in the radial arm maze but showed an inability to consolidate fully a context-fear memory. Our results with cocaine provide another example of how behaviors thought to rely on similar structures must be regulated by distinct pathways within those structures. Future studies of Kal7 interactions and functions at the PSD should clarify some of these mechanisms.
Interestingly, the Kal7KO animals showed a markedly decreased place preference for cocaine yet normal place preference for food. Thus Kal7-dependent plasticity is necessary for cocaine, but not food, to have its full reinforcing value. Intriguingly, animals that learn to self-administer cocaine demonstrate an increase in spine density in the NAc, while animals performing the same task for food reward show no change in spine density (47). Since alterations in spine density are widely considered to underlie normal learning, increased spine density may be essential for the “learned” drive to seek drugs of abuse (48). While we cannot directly demonstrate that lack of Kal7 leads to a decrease in drug craving, we have shown that its absence does affect the place preference for cocaine, a behavior thought to be correlated to drug seeking.
While the alterations in dendritic spines are a striking feature of the Kal7KO animals, it is important to bear in mind that other adaptations in these mice may contribute to these behavioral findings. In particular, Kal7KO animals exhibit decreased PSD levels of Cdk5 and NR2B (12). Cdk5 has been shown to have a wide ranging role in cocaine-mediated behaviors (40,49), and decreases in Cdk5 levels or activity lead to enhanced sensitization and altered place preference behaviors (41,50). Additionally, inhibition of NR2B signaling leads to a sensitized response to amphetamine (51), and disrupts morphine conditioned place preference (52). Kal7 is normally intercalated into the PSD, which is a finely tuned molecular machine. It is likely that Kal7 interacts with numerous other components of the PSD, and that deletion of Kal7 leads to decreases or mislocalization of other components that are important for drug-induced behaviors.
Conclusion
Numerous studies have suggested that changes in spine number and size underlie new learning (28) and that drug addiction is simply a corruption of normal learning mechanisms (1). This study identifies Kal7 as an essential modulator of this pathological plasticity and provides a new model system, the Kal7KO mouse, with which to explore the diverse roles of the dendritic spines that form anew and change shape following cocaine treatment.
Supplementary Material
Acknowledgments
Supported by DA-15464, DA-18274, DA-23082 and NS-41224. We thank Darlene D’Amato and Yanping Wang for incomparable technical support and the Neuropeptide Lab for helpful discussions. Eun-Ji Kim and Jianping Huang provided additional experimental assistance. Cocaine was provided by the National Institutes of Drug Abuse (NIH).
Footnotes
Financial disclosures:
The authors have no biomedical financial interests or potential conflicts of interest.
Reference List
- 1.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 2.Kelz MB, Chen J, Carlezon WJ, Whisler K, Gilden L, Beckmann AM, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 3.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tashiro A, Yuste R. Role of Rho GTPases in the morphogenesis and motility of dendritic spines. Mtds Enzymol. 2008;439:285–302. doi: 10.1016/S0076-6879(07)00421-1. [DOI] [PubMed] [Google Scholar]
- 8.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 9.Kiraly DD, Eipper-Mains JE, Mains RE, Eipper BA. Synaptic Plasticity: A Symphony in GEF. ACS Chemical Neuroscience. 2010 doi: 10.1021/cn100012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma XM, Ferraro F, Mains RE, Eipper BA. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J Neurosci. 2008;28:711–724. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Z, Photowala H, Cahill ME, Srivastava DP, Woolfrey KM, Shum CY, et al. Coordination of synaptic adhesion with dendritic spine remodeling by AF-6 and kalirin-7. J Neurosci. 2008;28:6079–6091. doi: 10.1523/JNEUROSCI.1170-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, et al. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008;28:12368–12382. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crusio WE. Flanking gene and genetic background problems in genetically manipulated mice. Biol Psychiatry. 2004;56:381–385. doi: 10.1016/j.biopsych.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Hiroi N, Fienberg AA, Haile CN, Alburges M, Hanson GR, Greengard P, et al. Neuronal and behavioural abnormalities in striatal function in DARPP-32-mutant mice. Eur J Neurosci. 1999;11:1114–1118. doi: 10.1046/j.1460-9568.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 15.Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien JA, Lummis SC. Diolistic labeling of neuronal cultures and intact tissue using a hand-held gene gun. Nat Protoc. 2006;1:1517–1521. doi: 10.1038/nprot.2006.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci U S A. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Labeling of dendritic spines with the carbocyanine dye DiI for confocal microscopic imaging in lightly fixed cortical slices. J Neurosci Methods. 2007;162:237–243. doi: 10.1016/j.jneumeth.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci U S A. 2009;106:2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryu J, Futai K, Feliu M, Weinberg R, Sheng M. Constitutively active Rap2 transgenic mice display fewer dendritic spines, reduced extracellular signal-regulated kinase signaling, enhanced long-term depression, and impaired spatial learning and fear extinction. J Neurosci. 2008;28:8178–8188. doi: 10.1523/JNEUROSCI.1944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietz DM, Dietz KC, Nestler EJ, Russo SJ. Molecular mechanisms of psychostimulant-induced structural plasticity. Pharmacopsychiatry. 2009;42(Suppl 1):S69–S78. doi: 10.1055/s-0029-1202847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 24.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 25.Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, et al. Nuclear factor kappaB signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 31.Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, et al. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mead AN, Brown G, Le MJ, Stephens DN. Effects of deletion of gria1 or gria2 genes encoding glutamatergic AMPA-receptor subunits on place preference conditioning in mice. Psychopharmacology (Berl) 2005;179:164–171. doi: 10.1007/s00213-004-2071-8. [DOI] [PubMed] [Google Scholar]
- 34.Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, et al. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, et al. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 36.Xin X, Wang Y, Ma XM, Rompolas P, Keutmann HT, Mains RE, et al. Regulation of Kalirin by Cdk5. J Cell Sci. 2008;121:2601–2611. doi: 10.1242/jcs.016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 38.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci U S A. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalivas PW, Volkow N, Seamens J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 44.Chandler LJ, Kalivas PW. Neuroscience: Brain’s defence against cocaine. Nature. 2008;455:743–744. doi: 10.1038/455743a. [DOI] [PubMed] [Google Scholar]
- 45.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 46.Kaddis FG, Uretsky NJ, Wallace LJ. DNQX in the nucleus accumbens inhibits cocaine-induced conditioned place preference. Brain Res. 1995;697:76–82. doi: 10.1016/0006-8993(95)00786-p. [DOI] [PubMed] [Google Scholar]
- 47.Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 49.Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–344. doi: 10.1196/annals.1316.041. [DOI] [PubMed] [Google Scholar]
- 50.Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, et al. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma YY, Guo CY, Yu P, Lee DY, Han JS, Cui CL. The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Exp Neurol. 2006;200:343–355. doi: 10.1016/j.expneurol.2006.02.117. [DOI] [PubMed] [Google Scholar]
- 53.Johnson RC, Penzes P, Eipper BA, Mains RE. Isoforms of kalirin, a neuronal Dbl family member, generated through use of different 5′- and 3′-ends along with an internal translational initiation site. J Biol Chem. 2000;275:19324–19333. doi: 10.1074/jbc.M000676200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.