Abstract
Background
Modulation of midbrain dopamine neurons by nicotinic acetylcholine receptors (nAChRs) plays an important role in behavior, cognition, motivation and reward. Specifically, nAChRs containing β2 subunits (β2-nAChRs) switch dopamine cells from a resting to an excited state. However, how β2-nAChRs can be modulated and thereby dopamine firing activity be affected is still elusive. Because changes in dopamine cell activity are reflected in the dynamics of micro-circuits generating altered responses to stimuli/inputs, factors regulating their state are fundamental. Among these, endogenous ligands to the nuclear receptor-transcription factor peroxisome proliferator-activated receptors type-alpha (PPARα) have been recently found to suppress nicotine-induced responses of dopamine neurons.
Methods
We used both in vitro and in vivo electrophysiological techniques together with behavioral analysis to investigate on the effects of modulation of PPARα in Sprague Dawley rat and C57BLJ/6 mouse dopamine neurons, and their interactions with β2-nAChRs. To this aim, we took advantage of a selective re-expression of β2-nAChR exclusively in dopamine cells by stereotaxically injecting a lentiviral vector in the mouse ventral tegmental area.
Results
We found that activation of PPARα decreases in vitro both dopamine cell activity and ventral tegmental area net output through negative modulation of β2-nAChRs. Additionally, PPARα activation in vivo reduces both the number of spontaneously active dopamine neurons and nicotine-induced increased locomotion.
Conclusions
Our combined findings suggest PPARα ligands as important negative modulators of β2-nAChRs on dopamine neurons. Thus, PPARα ligands might prove beneficial in treating those disorders where dopamine dysfunction plays a prominent role, such as schizophrenia and nicotine addiction.
Keywords: acetylcholine, dopamine neuron, nicotine, patch-clamp, peroxisome proliferator-activated receptor, ventral tegmental area
Introduction
Dopamine (DA) neurons of the ventral tegmental area (VTA) project to subcortical and cortical structures to form the mesocorticolimbic pathway. VTA DA neurons detect primary rewards, novel and reward-predicting stimuli (1, 2), and respond to these with changes in their firing activity (3), resulting in phasic DA transmission in target regions where the signal is integrated and translated into learned appetitive behaviors (4). VTA DA cell function is shaped in response to both behaviorally relevant events and drugs of abuse, including nicotine. VTA DA cell firing pattern is controlled by extrinsic afferents, among which the excitatory inputs play a major role (5, 6). The excitatory projections to the VTA originate from the prefrontal cortex, the bed nucleus of the stria terminalis, and the cholinergic laterodorsal and pedunculopontine tegmental nuclei (7–9). These latter make synaptic contacts with VTA DA cells (10), which express both muscarinic and nicotinic receptors (11, 12), and whose activation leads to increases in DA neuron activity (13, 14). Importantly, endogenously produced acetylcholine (ACh) controls DA neuron firing pattern and frequency through activation of nicotinic receptors (nAChRs) (15), and an imbalance between DA/ACh function is often associated in diverse brain disorders (7, 16–19).
Nicotine-induced increase in DA neuron firing rate can be suppressed by ligands for the peroxisome-proliferator-activated receptor-α (PPARα) (20), a family of nuclear receptor transcription factors involved in the modulation of various peripheral physiological responses, such as lipolysis, inflammation and energy balance (21). PPARα-induced phosphorylation of nAChRs accounts for the suppression of DA neuron responses to nicotine (20). Because the effects of nicotine on DA neurons were enhanced when PPARα were blocked (20), we hypothesized a constitutive interaction between PPARα and tyrosine kinases regulating the function of nAChRs in VTA DA cells. Thus, tonic control of the ratio of phosphorylated/dephosphorylated nAChRs might allow DA neurons to have access to distinct firing patterns and/or to change their firing frequency.
To explore the possible role of PPARα in the regulation of DA neuron excitability by endogenous acetylcholine, we investigated interactions between nuclear PPARα and surface nAChRs in VTA DA cells both in vitro and in vivo, and the functional consequences of PPARα activation on nicotine-induced increases in spontaneous locomotion, a DA-mediated behavior.
Methods and Materials
All procedures were performed in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2004), EEC Council Directive (219/1990 and 220/1990) and approved by Animalerie centrale and Médecine du travail, Institut Pasteur. We made all efforts to minimize pain and suffering, and to reduce the number of animals used.
Electrophysiological studies in vitro
Whole cell patch clamp recordings from VTA DA cells were as described previously (22) and Supplement 1. Briefly, male Sprague Dawley rats (Harlan Nossan, San Pietro al Natisone, Italy) or β2−/− and β2+/+ mice (see below for detailed information) were anesthetized with halothane and killed. Recordings were made from horizontal slices superfused with artificial cerebrospinal fluid (ACSF, 37° C) saturated with 95% O2 and 5% CO2 containing (in mM): 126 NaCl, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 18 NaHCO3, and 11 glucose. Evoked field potential recordings were as described previously (23) and Supplement 1.
All the drugs were dissolved in DMSO. The final concentration of DMSO was < 0.01 %.
Electrophysiological study in vivo
Extracellular single unit recordings from male Sprague Dawley rat VTA DA neurons were performed as described previously (24) and Supplement 1. Single unit activity of DA neurons located in VTA was recorded extracellularly with glass micropipettes filled with 2% pontamine sky blue dissolved in 0.5 M sodium acetate. Single units were isolated and identified according to the already published criteria (20).
Mice
A line expressing Cre recombinase under the control of the dopamine transporter promoter, line DAT-Cre (Tronche), was backcrossed for several generations with the B2-nAChR knock-out line ACNB2. The resulting mice used in this study were heterozygous for the Cre transgene and homozygous for the B2 knock-out, Cre+−; B2 −/−.
Lentiviral expression vector and stereotaxic procedure
pTripPDGF lentivector (25) was modified by replacing the eGFP with the β2nAChR sequence. Lentivirus was bilaterally injected (26) at a dose of 230ng p24 protein/2µL into the VTA of β2−/−/DAT-Cre transgenic mice, generated by backcrossing β2−/− with DAT-Cre mice (27). Injection of the vector into β2−/−/DAT-Cre mice leads to recombination and subsequent expression of the β2 subunit exclusively in DA neurons (25).
Behavioral study
Mice were pretreated with either WY14643 (40 mg/kg, i.p.) or its vehicle (10 ml/kg, i.p.) 60 min before nicotine administration (0.02 mg/kg, s.c.) and individually tested for motor activity under standardized environmental conditions as previously described (28) and Supplement 1.
Statistical analysis
Numerical data are given as mean ± S.E.M.. Data were compared and analyzed through two-way ANOVA for repeated measures, or one-way ANOVA or Student’s t-test, when appropriate.
Results
The PPARα antagonist MK886 enhances DA neuron spontaneous activity
To characterize the postsynaptic effects on DA neurons, all experiments were performed in the presence of CNQX (10 µM), D-AP5 (100 µM) and picrotoxin (100 µM), in order to block AMPA-,NMDA- and GABAA-mediated postsynaptic responses. Whole-cell current- and voltage- clamp recordings were performed from medial posterior VTA DA neurons in rat horizontal slices containing the midbrain. DA cells displayed electrophysiological characteristics that facilitated their identification (29). Figure 1(A) shows that bath application of the PPARα antagonist MK886 (500 nM, 8 min) significantly increased the spontaneous activity of VTA DA neurons (about 275% above baseline, n=6; F19,100=3.308, P<0.0001, one-way ANOVA). Under voltage-clamp mode (Vholding= −70 mV), MK886 caused an inward current of 93.3 ± 29.3 pA (Figure 1B). Both effects were reversible on wash out and blocked by the synthetic PPARα agonist WY14643 (Figure 1B,C; firing rate: WY14643+MK886 vs MK886 alone: F1,171=7.86, n=6, P=0.02, two-way ANOVA; Iholding: 10.0 ± 2.6 pA, t=2.42, P=0.03, unpaired two-tailed t-test), at a dose (300 nM) that is ineffective (20). MK886 effect was concentration-dependent (Figure 1D; 100.8 ± 6.8 % and 434.9 ± 69.6% of baseline at 0.3 and 1 µM, respectively), and mimicked by the structurally dissimilar PPARα antagonist GW6471 (0.3–1 µM) (Figure 1D; 0.3µM: 161.0 ± 15.97 % of baseline; 0.5 µM: 211.5 ± 25.4% of baseline; 1 µM: 338.2 ± 47 % of baseline).
Figure 1.
PPARα blockade activates VTA dopamine neurons in vitro. (A) MK886 application (0.5 µM) increases dopamine neuron spontaneous activity. Current-clamp recording from a dopamine neuron (left panel) and rate histogram depicting MK886 averaged effects (right panel). (B) In voltage-clamp mode MK886 caused an inward current (Vhold= −70 mV) blocked by the PPARα agonist WY14643 (0.3 µM). (C) WY14643 blocked MK886-induced activation of dopamine neurons. (D) Summary of dose-related effects of PPARα antagonists on dopamine neuronal frequency. Numbers above bars indicate n values. Data expressed as mean ± SEM. *p < 0.05; **p < 0.005.
PPARα blockade induces dephosphorylation of nAChRs and increases efficacy of nAChR agonists
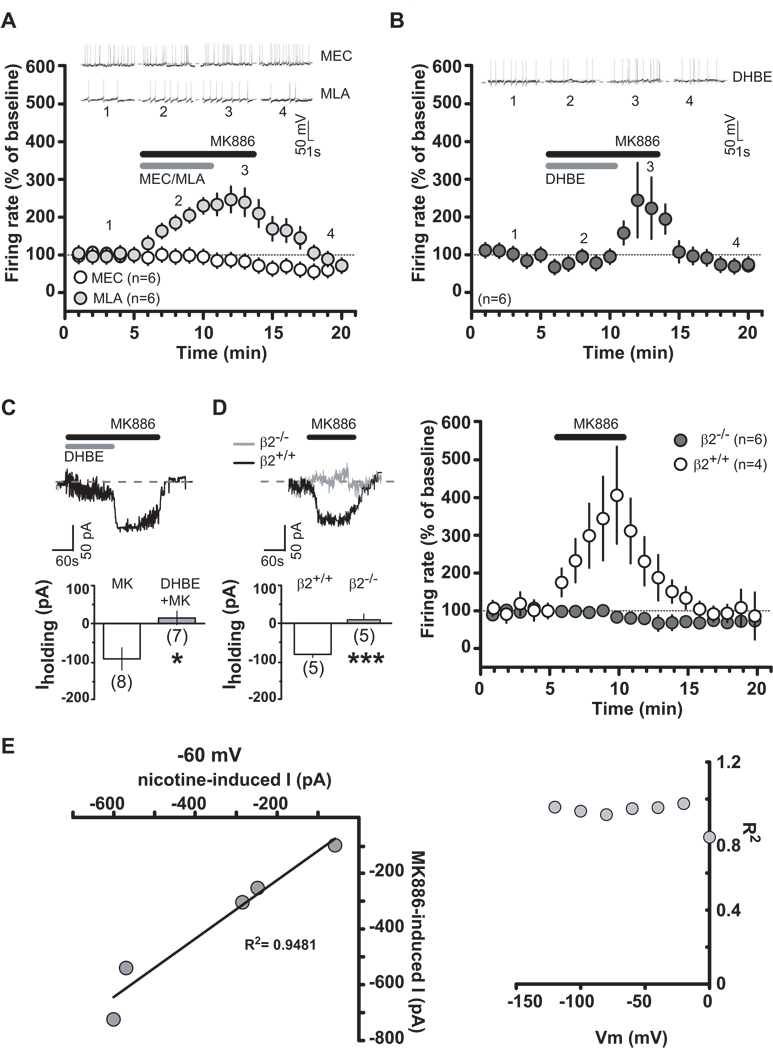
To examine the contribution of nAChRs to the effects of MK886, nAChRs were blocked by bath applying either mecamylamine (MEC, a non-competitive nAChR antagonist), methyllycaconitine (MLA, a specific antagonist for α7 subtype nAChRs) or dihydro-β-erythroidine (DHBE, a competitive antagonist for neuronal α4 containing nAChRs). Figure 2(A,B) shows that MK886 (0.5 µM) actions on DA neuronal firing rate were prevented by MEC (100 µM, 5 min; F2,285=5.7, P=0.01, two-way ANOVA) and DHBE (1µM, a dose that is ineffective on DA cell firing rate (30); 5 min: F1,90=4.97, P=0.05, two-way ANOVA), whereas MLA was ineffective (5 nM, 5 min; F1,190=0.86, P=0.4, two-way ANOVA). Additionally, DHBE blocked MK886-induced inward currents (Figure 2C; MK886+DHBE: −3.857 ± 18.9 pA, P= 0.018), suggesting that most, if not all, of responses in DA neurons to MK886 were due to activation of α4 subunit containing nAChRs. Since the α4 subunit generally assembles with the β2 subunit, we hypothesized that most of the effects would be mediated by postsynaptic α4 β2-nAChRs. Therefore, we examined MK886 effects in β2-nAChR knock out (β2−/−) mice. MK886-induced effects were absent in β2−/− mice (Figure 2D; two-way ANOVA F1,152=8.65, P=0.018; t=5.1, P=0.0005, unpaired t-test), whereas its effects in β2+/+ mice were comparable to those observed in rats. To further characterize the relationship between MK886-induced current and membrane potential, 6-s voltage ramps ranging from −120 to +60 mV were applied to voltage-clamped DA neurons (30) in the absence and presence of MK886 (0.5 µM). The MK886-induced net current was observed by subtracting currents in the presence of MK886 from those in the absence of MK886. When we compared the MK886-induced current with the one produced by nicotine (1 µM) under the same conditions, we found a strong correlation between the currents induced by both MK886 and nicotine at all membrane potentials (Figure 2E), particularly at resting membrane potential (i.e. −60 mV), thus suggesting a common site of action.
Figure 2.
MK886 enhances dopamine neuron activity through α4β2-nAChRs. (A and B) Time course of MK886 effect, alone or together with mecamylamine (MEC) (100 µM), methyllycaconitine (MLA) (5 nM) or dihydro-β-erythroidine (DHBE) (1 µM), on dopamine neuron activity. Grey and black bars represent time of nAChR antagonist or MK886 application, respectively. Insets show representative traces of dopamine neuron frequency. (C) Bar graph illustrating DHBE mean effect on MK886-induced inward current. Inset shows representative DHBE+MK886 effect on dopamine cell. Grey and black bars represent the time of DHBE and MK886 application, respectively. (D) MK886 effects on dopamine neurons in β2−/− and β2+/+ mice under voltage-clamp (left panel) and current-clamp (right panel) modes. Inset shows a representative MK886 effect. (E) Left panel, Magnitude of currents induced by nicotine plotted as function of those induced by MK886 at membrane potential of -60 mV. Data fit by linear regression with r2 = 0.9481 (p < 0.001). Right panel, R2 for five cells in f plotted as a function of voltage membrane. Numbers above bars indicate n values. Data expressed as mean ± SEM. *p < 0.05, ***p < 0.0005.
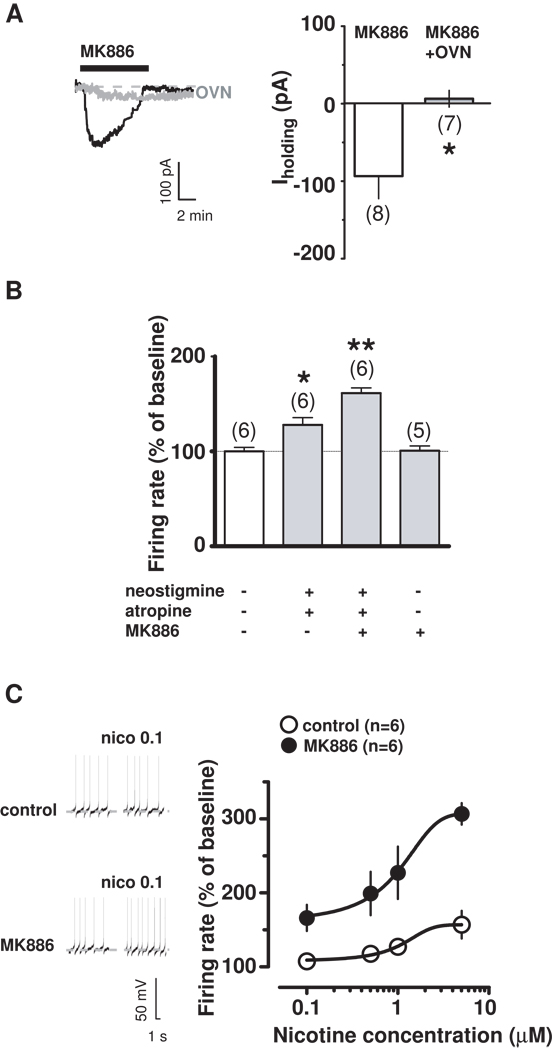
The functional properties of nAChRs depend on the tyrosine phosphorylation status of the receptors, being the result of a balance between tyrosine kinases and phosphatases, which negatively and positively modulate nAChR-mediated currents, respectively (31). To investigate the possibility that MK886 was modifying the status of nAChRs, we incubated, and continuously perfused, rat brain slices with the general tyrosine phosphatase inhibitor sodium orthovanadate (OVN, 1 mM), and then applied MK886 (0.5 µM, 5 min). We only performed voltage-clamp experiments because of OVN actions on hyperpolarization-activated cyclic nucleotide-gated channels, which prevented pacemaker-like spontaneous activity (32). OVN prevented MK886-induced inward currents (Figure 3A; −3.714 ± 10.8 pA; t=2.93, P=0.01, unpaired t-test vs MK886 alone) without affecting nicotine-induced inward currents (OVN+nicotine: 27.29 ± 6.2 pA; nicotine: 40.33 ± 5.6; t=1.53, P=0.15, unpaired t-test vs nicotine alone; data not shown), thus indicating that it blocked phosphatases downstream of PPARα. Thus, our observations suggest that MK886 does not directly act on nAChRs, but increases sensitivity of high-affinity nAChRs to endogenous ACh in midbrain DA neurons. Accordingly, when we enhanced endogenous ACh actions at nAChRs by applying both the ACh esterase inhibitor neostigmine (2 µM) and the muscarinic antagonist atropine (5 µM), we observed an increased DA cell firing rate (Figure 3B; 128.7 ± 7.6 % of basal, t=3.47, P=0.03, paired-t test), which was further enhanced in the presence of an ineffective dose of MK886 (0.3 µM, 5 min) (Figure 3B: 161.3 ± 5.5 % of basal, t=7.53, P=0.008, paired-t test). Consistently, the effects of nicotine (0.1–5 µM) were strongly enhanced in the presence of MK886 (0.3 µM) (Figure 3C: for example, nicotine 0.1 µM: 107.5 ± 10.5 % and 166.2 ± 17.7 % of baseline in the absence or presence of MK886, respectively; n=6 for both groups; Nicotine+MK886 vs Nicotine alone: t=2.85, P=0.008, unpaired t-test; and nicotine 5 µM: 157.7 ± 18.8 % and 306.8 ± 14.5 % of baseline in the absence or presence of MK886, respectively; n=5 for both groups; nicotine+MK886 vs nicotine alone: t=6.27, P=0.0001, unpaired t-test).
Figure 3.
PPARα blockade increases efficacy of nAChR agonists. (A) Under voltage-clamp mode, MK886 effect on dopamine neuron in presence (grey) or absence (black) of sodium-orthovanadate (OVN). (B) Under current-clamp mode, effects of enhanced endogenous ACh levels acting at nAChRs (neostigmine+atropine) in presence or absence of MK886 (0.3 µM). (C) Current-clamp recording of a dopamine neuron (left panel) showing enhanced nicotine response in presence of MK886 (0.3 µM). Dose-response curves depicting averaged effects of nicotine (right panel) on dopamine neurons in presence or absence of MK886. Numbers above bars indicate n values. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.005.
Activation of PPARα modulates DA neuronal activity through hydrogen peroxide production
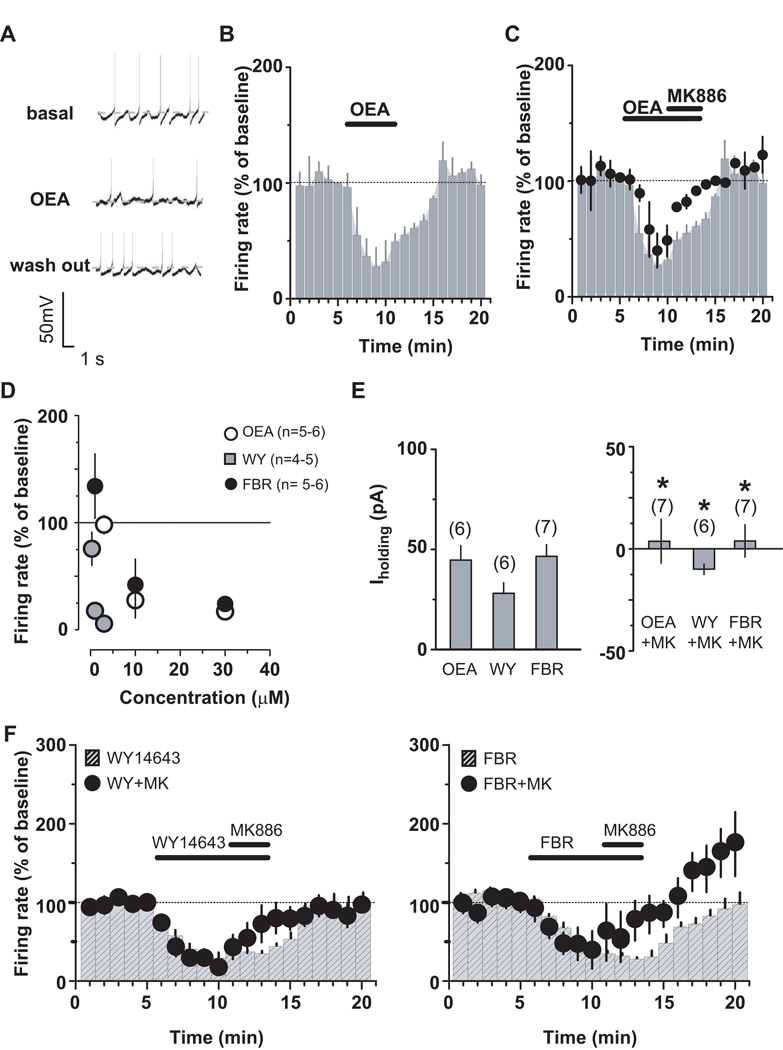
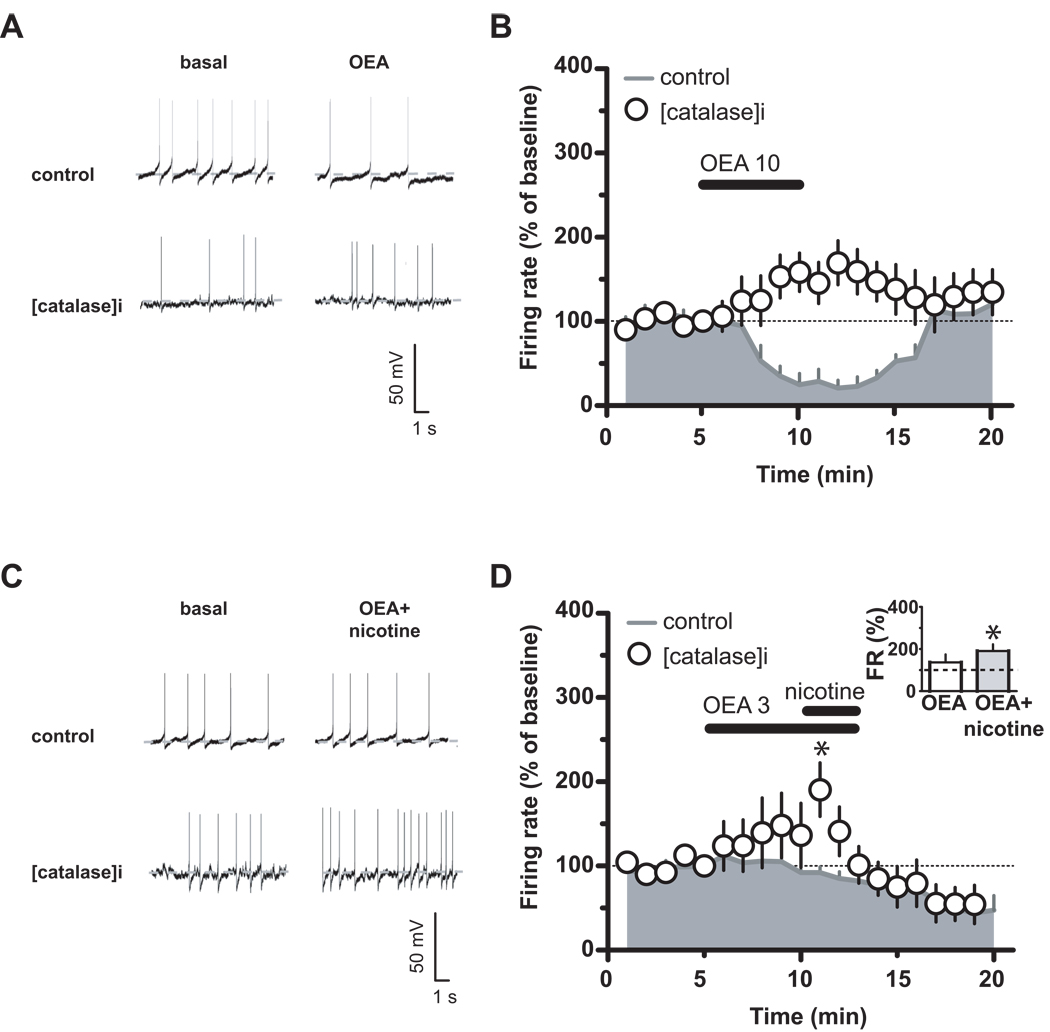
To investigate the role of PPARα in more detail, we then tested the effects of an endogenous ligand for PPARα oleoylethanolamide (OEA) on DA cell firing. OEA (10 µM) decreased DA neuronal discharge (Figure 4A,B: F19,60=17.03, P<0.0001, one-way ANOVA) in a concentration-dependent fashion (Figure 4D: 3 to 30 µM; 3 µM: 98.2±8.6 %, n=4, ; 10 µM: 27.7±16.5 %, n=4; 30 µM: 20.0±5.8 %, n=5), an effect reversed by MK886 (Figure 4C: 500 nM, 3 min; n=5, F1,133=32.40, P=0.0007, two-way ANOVA). The OEA effect was mimicked by two structurally different PPARα agonists, WY14643 (0.3–3 µM) and fenofibrate (1–10 µM) (Figure 4D). Fenofibrate was the least potent (1 µM: 134.2±30 %, n=6; 10 µM: 42.1±23.8 %, n=6; 30 µM: 24.3±4.9 %, n=5), and WY14643 the most potent (0.3 µM: 75.8±15.5 %, n=5 ; 1 µM: 17.9±5 %, n=5; 3 µM: 5.9±0.5 %, n=4). In addition, under voltage-clamp mode, the three PPARα agonists also produced outward currents (Figure 4E; OEA 10 µM: 45.4±5.7 pA; WY14643 1 µM: 27.3±5.1 pA; fenofibrate 3 µM: 43.6±7.05 pA), which were blocked by MK886 (Figure 4E). MK886 also reversed the effects produced by the two agonists on DA cell frequency (Figure 4F; WY14643: F1,171=7.83, P=0.02, two-way ANOVA; fenofibrate: F1,190=5.07, P=0.04, two-way ANOVA). Activation of PPARα has been shown to increase intracellular concentrations of reactive oxygen species (33), such as hydrogen peroxide, which is produced by DA cells in an activity-dependent fashion and depresses firing rate of DA neurons through ATP-sensitive K+ channels (34). Because hydrogen peroxide can also activate a tyrosine kinase (35), we tested the possibility that it might be involved in PPARα ligand actions by filling the DA cell with catalase (500 IU/ml), to rapidly inactivate hydrogen peroxide, and then bath applying OEA. Figure 5 (A,B) shows that, in the presence of catalase, OEA (10 µM, 5 min) failed to produce any inhibitory effect (OEA vs OEA+catalase: n=5, F1,112=16.87, P=0.003, two-way ANOVA), thus indicating hydrogen peroxide as a downstream effector of PPARα. Importantly, because depletion of intracellular hydrogen peroxide by including catalase in the patch pipette increases the spontaneous firing rate of midbrain DA neurons (34), we can assume that OEA-induced increase in firing rate, which is comparable to the previously reported in the presence of catalase into DA cell compartment (34), might be ascribed to a reduced hydrogen peroxide inhibitory physiological role. Nonetheless, if hydrogen peroxide production is also related to PPARα activation, then one would expect that under these conditions the effects of nicotine on DA cell frequency would be restored. When nicotine (1 µM, 2 min) was applied in the presence of both OEA (the lower dose of 3 µM that is ineffective on firing rate (20), 7 min) and catalase (500 IU/ml), nicotine transiently increased the spontaneous activity of VTA DA neurons (Figure 5C,D: nicotine+OEA vs nicotine+OEA+catalase: n=5, t=3.57, P=0.005, two-tailed t test; nicotine+OEA+catalase vs OEA+catalase at 10min: P=0.0336, t= 2.493, paired t-test) consistently with previous reports (14, 20). As previously reported (20), here we observed a reduction of spontaneous activity following nicotine application in the presence of OEA. Although at this stage we cannot provide a definite explanation for this phenomenon, it is plausible that nicotine would desensitize β2-nAChRs more rapidly once PPARα are activated, with either a possible consequent internalization or run down of these receptors. Nonetheless, the recovery of nicotine effects on DA cell firing supports the hypothesis that activation of PPAR α leads to hydrogen peroxide production and blocks nicotine effects on DA neuronal activity.
Figure 4.
PPARα activation decreases VTA dopamine neuronal activity. (A) Current-clamp traces of a dopamine neuron before, during and after oleoylethanolamide (OEA) application (10 µM). Rate histogram depicting averaged OEA effects on dopamine neuron frequency in absence (B) and presence (C) of MK886 (0.5 µM). (D), Dose-response curves depicting averaged effects of PPARα agonists on dopamine neuron frequency. (E), Under voltage-clamp mode, effects of OEA (10 µM), WY14643 (WY; 1 µM) and fenofibrate (FRB; 10 µM) on dopamine neurons in absence (left panel) and presence (right panel) of MK886 (0.5 µM). (F), Under current-clamp mode, MK886 (0.5 µM) reversed the effects of both WY14643 (left panel) and fenofibrate (right panel) on dopamine neurons. Numbers above bars indicate n values. Data expressed as mean ± SEM. *p < 0.05.
Figure 5.
Hydrogen peroxide is downstream effector of PPARα. (A), Current-clamp recording of a dopamine neuron during bath application of OEA (10 µM) in absence or presence of catalase (500 U/I). (B), Time course of averaged effects of OEA (10 µM) on dopamine neuron frequency in the presence (open circles) and absence (grey area) of catalase. (C) Current-clamp recording of a dopamine neuron during nicotine (1 µM) and OEA (3 µM) application in the absence or presence of catalase. (D) Time course of OEA (3 µM) and OEA+nicotine averaged effects on dopamine neuronal activity in the presence (open circles) and absence (grey area) of catalase. The inset shows that nicotine produced an effect on firing rate (FR) in the presence of OEA (3 µM) and catalase. Catalase was applied through the recording pipette. Data expressed as mean ± SEM. *p < 0.05.
The effects of PPARα on population spike amplitude in the VTA require the expression of β2-containing nAChRs in DA neurons
To ensure that PPARα modulation of DA neuronal firing was not restricted to a certain DA subpopulation within the VTA, the effects of both MK886 and WY14643 were also investigated using extracellular evoked field potential recordings in horizontal brain slices (23). Figure 6 (A) shows that MK886 (500 nM, 5 min) induced a significant change in both negative components of the field potential (i.e. N1 and N2) (N1 amplitude at time = 5 min was enhanced by about 25% of baseline, n= 6, t= 2.098, P=0.04, paired t-test; N2 amplitude at time = 5 min was enhanced by about 50% of baseline, n= 6, t= 2.24, P=0.03, paired t-test). Conversely, WY14643 (1 µM, 5 min) significantly reduced both components of the field potential (Figure 6B: N1 amplitude at time = 5 min was reduced by about 10% of basal, n=6, t=3.82, P=0.006, paired t-test; N2 amplitude at time = 5 min was reduced by about 15% of basal, n=6, t=3.81, P=0.01, paired t-test). Although both components are similarly affected by PPARα modulation, in the next set of experiments we measured only the second negative potential (i.e. N2) because it has been shown to largely represent the postsynaptic responses of DA neurons (36). Nonetheless, it should be noted that N2 component is the summation of stimulation-evoked postsynaptic responses of DA cells and of antidromic action potentials (36), and, therefore, represents both synaptic and population spike components. To further examine the role played by nAChRs in the effect of PPARα activation, we bath applied WY14643 (1 µM, 5 min) in β2−/− mice. Figure 6 (C) shows that WY14643 failed to produce any effect on N2 amplitude when compared with β2+/+ mice (F1,133=6.61, P=0.03, two-way ANOVA). Next, we took advantage of the β2−/− mice in which the Cre-inducible switching on for β2-nAChR gene was allowed only in DA neurons, thus selectively re-expressing the corresponding nAChR subunit exclusively in VTA DA cells by stereotaxically injecting a lentiviral vector (β2-DA-VEC mice)(25). Importantly, in β2-DA-VEC mice WY14643 effect was restored (Figure 6C: N2 amplitude at time = 5 min was reduced by about 25 % of basal, F4,76=18.30, P<0.0001, one-way ANOVA), and similar to its effect in β2+/+ mice (F1,133=0.01, P=0.998, two-way ANOVA). Remarkably, this gene-target strategy allows not only to dissect the functional role of β2-nAChRs on DA cells of the VTA (25), but also to discriminate the contribution of DA neuronal activity to net VTA output (36), and, ultimately, of PPARα.
Figure 6.
PPARα activation reduces VTA output and nicotine induced stimulation of locomotion. (A and B). Typical evoked field potential recordings showing effects of MK886 (0.5 µM, A) and WY14643 (1 µM, B) on field potential amplitude. Bin= 10 s. Traces from typical experiments (top), time-courses of the effects of MK886 and WY14643 on N1 (middle) and N2 (bottom) components, and the mean averaged responses (bar graph in insets) are shown. (C) Averaged N2 amplitude from the VTA of β2−/−, β2+/+ and β2-DA-VEC mice in response to WY14643 (1 µM). Bin= 1 min. (D) WY14643 (40 mg/kg i.p.) decreases the number of VTA DA cells encountered during neuronal sampling in anesthetized rats (left panel), but not averaged firing frequency (right panel). (E) Time-course curve of nicotine (0.02 mg/kg, s.c.) effects on locomotor activity in WY14643- and vehicle- treated mice. (F) Time-course curve of nicotine (0.02 mg/kg, s.c.) effects on locomotor activity in WY14643- and vehicle- treated β2-DA-VEC mice compared with β2−/− mice. Arrows indicate time of nicotine administration. Data expressed as mean ± SEM. *p < 0.05.
PPARα activation reduces the number of spontaneously active DA neurons in vivo
Because β2-nAChRs are key in controlling the firing rate/pattern of VTA DA cells (15), we examined whether PPARα activation affected the number of spontaneously active VTA DA neurons in vivo. We performed neuronal sampling of the VTA in anesthetized rats following administration of either WY14643 (40 mg/kg i.p., 15 min before the experiment) or vehicle. Acute administration of WY14643 reduced the number of spontaneously active DA neurons, since the mean (± S.E.M.) number of DA neurons encountered in the VTA was 1.51±0.2 and 0.97±0.2 cell/track in vehicle- and WY14643-treated animals, respectively (Figure 6D: t=2.21, P=0.02, unpaired t-test). The average spontaneous firing rate of DA cells was not different in vehicle- and WY14643-treated rats (Figure 6D; t=0.29, P=0.38, unpaired t-test), in agreement with the ineffectiveness of the dose tested on firing frequency (20).
The PPARα agonist blocks nicotine-induced locomotor activity
Since midbrain DA neuron activity is thought to be a substrate for nicotine-induced increases in spontaneous activity, we investigated whether activation of PPARα was involved in control of locomotion. We tested nicotine (0.02 mg/kg s.c.) for its effects on locomotor activity of vehicle- and WY14643-treated mice. Figure 6 (E) shows that in WY14643-treated mice (40 mg/kg i.p., 60 min before nicotine) nicotine-induced locomotor stimulation was markedly reduced (F1,112=11.60, P=0.0043, two-way ANOVA). Because β2-nAChRs on VTA DA neurons are critical for nicotine-induced locomotor activation (37, 38), we tested nicotine effects on locomotion in β2−/− and β2-DA-VEC mice. Figure 6 (F) shows that re-expression of β2-nAChRs limited to DA cells of the VTA is sufficient to restore nicotine-dependent locomotor activation, which was absent in β2−/− mice (F1,96=9.16, P=0.01, two-way ANOVA). Remarkably, when we investigated whether PPARα activation was involved in control of locomotion by β2-nAChRs, we observed that in WY14643-treated β2-DA-VEC mice nicotine-induced locomotor stimulation was markedly reduced (F1,96=5.02, P=0.04, two-way ANOVA, Fig.6F) and comparable with β2−/− mice (F1,96=0.49, P=0.5, two-way ANOVA, Fig. 6F). Notably, when WY14643 (40 mg/kg i.p.) was tested for its effects on spontaneous locomotor activity, we observed no effect on this behaviour (40 mg/kg i.p. vs vehicle: F1,70=2.41, P=0.1427, two-way ANOVA; data not shown).
Discussion
Here we show that PPARα significantly contribute to the effects of endogenous cholinergic transmission mediated by β2-nAChRs on DA neuron excitability, and to the effects of nicotine on locomotor activity, a DA associated behavior. The changes in number of spontaneously active DA neurons in vivo following PPARα activation are consistent with both the decreased spontaneous activity of individual VTA DA neurons and the diminished VTA net output observed in vitro. A functional consequence of modulation of DA neuron excitability by PPARα was the reversal of nicotine-induced increased locomotor activity by administration of a synthetic PPARα ligand in β2-DA-VEC mice. Results obtained with selective antagonists for α4 versus α7 nAChRs and from mice lacking the β2 subunit further show that modulation of cholinergic transmission by PPARα, and the resulting modulation of DA neuron excitability, is mediated by α4β2-nAChRs.
Our observations support a constitutive interaction between PPARα and β2-nAChRs in VTA DA cells. Particularly, our behavioral experiments show that β2 is a critical subunit for PPARα effects. Indeed, re-expression of β2-nAChRs exclusively in VTA DA cells is sufficient to rescue nicotine effects on locomotion and, thereby, PPARα actions. Accordingly, our electrophysiological experiments show a decreased VTA net output following PPARα activation in rats, but not in β2−/− mice. Remarkably, restoration of β2-nAChR only in the DA cells within the VTA re-established effects of PPARα activation, thus indicating β2-nAChRs as targets of PPARα ligands in VTA DA neurons. Also, our results confirm the versatility and reliability of the genetarget system to achieve lentiviral gene targeting exclusively in DA cells of the VTA (25). Additionally, our whole cell patch clamp experiments show that MK886 produces both an increased firing rate and an inward current in DA cells, which are blocked by DHBE, and are absent in β2−/− mice. Conversely, a global decreased VTA DA cell excitability were also reported in β2−/− mice, thus, suggesting a hierarchical control by β2-nAChR of DA neuron activity (15). Lastly, in agreement with the hypothesis that the number of functional cell surface nAChRs can be indirectly controlled through processes involving tyrosine phosphorylation (39), we found that when the PPARα were blocked, both endogenous ACh and nicotine responses were enhanced (Figure 3C,D).
The conclusion that PPARα activation might help in controlling state and/or number of β2-nAChRs is compatible with the distribution of PPARα in the midbrain (40–42), and provides a functional correlate for their cytoplasmic expression (42). Indeed, although belonging to the family of nuclear receptor transcription factors (21), non-genomic actions of PPARα activation have also been described (20, 43, 44), and likely mediate the rapid-onset effects described in the present study. Based on our findings, we propose both endogenous and synthetic (e.g. fibrates) PPARα ligands as important negative modulators of β2-nAChRs in the VTA. Endogenous ligands for PPARα are endocannabinoids and their cognate compounds, specifically belonging to N-acyl-ethanolamine (NAE) group, such as OEA and palmitoylethanolamide (45), which are signaling molecules involved in the regulation of diverse physiological functions in both CNS and peripheral tissues. Like endocannabinoids, NAEs are synthesized on demand (46, 47) and rapidly degraded (48). Both brain and peripheral tissue levels of NAEs change with high fat diets (49, 50). Particularly, a 30% fat diet enriched in olive oil directly raises rat brain OEA (49). Thus, one can predict that changes to fatty acid profile following ketogenic diet (KD: high fat/adequate protein/low carbohydrate) may also directly modify NAE levels, which in turn would behave like brain PPARα-activating molecules (51). Accordingly, KD diminished neuronal excitability of dentate gyrus and proved to be both anticonvulsant and antiepileptogenic (52). Further, both fenofibrate and KD exerted anticonvulsive properties, thus suggesting a common action on a PPARα-driven pathway (53).
The physiological relevance of the present findings lies in the dynamic interplay between the dopamine and cholinergic systems in major psychiatric disorders (7, 54, 55). Our current hypothesis is that small bioactive lipid molecules acting at PPARα, with consequent hydrogen peroxide production, are ultimately responsible for negative modulation of β2-nAChRs. Hence, the cellular effects resulting from PPAR αactivation, such as decreased DA cell spontaneous activity and blockade of nicotine-induced excitation of DA neurons, are fully prevented when hydrogen peroxide half-life is reduced by raising the intracellular levels of catalase.
β2-nAChRs play a crucial role in mediating the switch from “basal” to “excited” states of VTA DA neurons (15), and serve as a gate enabling these cells to respond to excitatory afferents and, thereby, to switch between phasic and tonic activity (7). Thus, PPARα activation, by making DA neurons less sensitive to external information, might translate into prevention of an erroneous attribution of saliency to otherwise irrelevant stimuli/events. Negative modulation of β2-nAChRs located on VTA DA neurons might, therefore, prove beneficial in those pathophysiological conditions, such as stress or psychiatric disorders such as schizophrenia, attention-deficit hyperactivity disorder and binge eating, where a dysfunctional DA system plays a prominent role. Additionally, targeting PPARα might represent a promising therapeutic approach to prevent relapse to nicotine, which is often related to stress and/or conditioned stimuli associated with previous drug use.
Supplementary Material
Acknowledgements
In loving memory of Dr. Giuseppe Melis. This study was supported in part by the Division of Geriatric Medicine and Gerontology of Johns Hopkins University School of Medicine, and the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. U.M. was supported by the European Commission FP7 Neurocypres project, ANR Blanc, ANR MNP 2009, Institut Pasteur, CNRS URA 2182, and INCa BIO-SILC. S.T acknowledges support from FRM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Rebec GV, Grabner CP, Johnson M, Pierce RC, Bardo MT. Transient increases in catecholaminergic activity in medial prefrontal cortex and nucleus accumbens shell during novelty. Neuroscience. 1997;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- 2.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 4.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 5.Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- 7.Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153 Suppl 1:S438–S445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mash DC, Potter LT. Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience. 1986;19:551–564. doi: 10.1016/0306-4522(86)90280-0. [DOI] [PubMed] [Google Scholar]
- 13.Mereu G, Yoon KW, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–399. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- 14.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 15.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 16.McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avena NM. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp Clin Psychopharmacol. 2007;15:481–491. doi: 10.1037/1064-1297.15.5.481. [DOI] [PubMed] [Google Scholar]
- 18.Sarter M, Bruno JP, Parikh V, Martinez V, Kozak R, Richards JB. Forebrain dopaminergic-cholinergic interactions, attentional effort, psychostimulant addiction and schizophrenia. Exs. 2006;98:65–86. doi: 10.1007/978-3-7643-7772-4_4. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Rill E, Biedermann JA, Chambers T, Skinner RD, Mrak RE, Husain M, et al. Mesopontine neurons in schizophrenia. Neuroscience. 1995;66:321–335. doi: 10.1016/0306-4522(94)00564-l. [DOI] [PubMed] [Google Scholar]
- 20.Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, et al. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 2008;28:13985–13994. doi: 10.1523/JNEUROSCI.3221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Kan L, Qi C, Kanwar YS, Yeldandi AV, Rao MS, et al. Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a coactivator for PPAR. J Biol Chem. 2000;275:13510–13516. doi: 10.1074/jbc.275.18.13510. [DOI] [PubMed] [Google Scholar]
- 22.Melis M, Pillolla G, Bisogno T, Minassi A, Petrosino S, Perra S, et al. Protective activation of the endocannabinoid system during ischemia in dopamine neurons. Neurobiol Dis. 2006;24:15–27. doi: 10.1016/j.nbd.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Nugent FS, Hwong AR, Udaka Y, Kauer JA. High-frequency afferent stimulation induces long-term potentiation of field potentials in the ventral tegmental area. Neuropsychopharmacology. 2008;33:1704–1712. doi: 10.1038/sj.npp.1301561. [DOI] [PubMed] [Google Scholar]
- 24.Melis M, Pillolla G, Perra S, Colombo G, Muntoni AL, Pistis M. Electrophysiological properties of dopamine neurons in the ventral tegmental area of Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2009;201:471–481. doi: 10.1007/s00213-008-1309-2. [DOI] [PubMed] [Google Scholar]
- 25.Tolu S, Avale ME, Nakatani H, Pons S, Parnaudeau S, Tronche F, et al. A versatile system for the neuronal subtype specific expression of lentiviral vectors. Faseb J. 2009 doi: 10.1096/fj.09-139790. [DOI] [PubMed] [Google Scholar]
- 26.Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- 27.Turiault M, Parnaudeau S, Milet A, Parlato R, Rouzeau JD, Lazar M, et al. Analysis of dopamine transporter gene expression pattern -- generation of DAT-iCre transgenic mice. Febs J. 2007;274:3568–3577. doi: 10.1111/j.1742-4658.2007.05886.x. [DOI] [PubMed] [Google Scholar]
- 28.Fattore L, Melis M, Diana M, Fratta W, Gessa G. The cyclo-oxygenase inhibitor nimesulide induces conditioned place preference in rats. Eur J Pharmacol. 2000;406:75–77. doi: 10.1016/s0014-2999(00)00665-8. [DOI] [PubMed] [Google Scholar]
- 29.Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsubayashi H, Amano T, Seki T, Sasa M, Sakai N. Electrophysiological characterization of nicotine-induced excitation of dopaminergic neurons in the rat substantia nigra. J Pharmacol Sci. 2003;93:143–148. doi: 10.1254/jphs.93.143. [DOI] [PubMed] [Google Scholar]
- 31.Charpantier E, Wiesner A, Huh KH, Ogier R, Hoda JC, Allaman G, et al. Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J Neurosci. 2005;25:9836–9849. doi: 10.1523/JNEUROSCI.3497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J, Huang A, Zhang Q, Lin YC, Yu HG. Novel mechanism for suppression of hyperpolarization-activated cyclic nucleotide-gated pacemaker channels by receptor-like tyrosine phosphatase-alpha. J Biol Chem. 2008;283:29912–29919. doi: 10.1074/jbc.M804205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teissier E, Nohara A, Chinetti G, Paumelle R, Cariou B, Fruchart JC, et al. Peroxisome proliferator-activated receptor alpha induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-alpha activation properties. Circ Res. 2004;95:1174–1182. doi: 10.1161/01.RES.0000150594.95988.45. [DOI] [PubMed] [Google Scholar]
- 34.Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu YN, Martella G, Johnson SW. Rotenone enhances N-methyl-D-aspartate currents by activating a tyrosine kinase in rat dopamine neurons. Neuroreport. 2007;18:1813–1816. doi: 10.1097/WNR.0b013e3282f0d28f. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y, Sudou K, Nawa H, Namba H. Field potential recording in the ventral tegmental area: pharmacological and toxicological evaluations of postsynaptic dopaminergic neuron activity. Neurosci Res. 2006;55:426–433. doi: 10.1016/j.neures.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Mineur YS, Brunzell DH, Grady SR, Lindstrom JM, McIntosh JM, Marks MJ, et al. Localized low-level re-expression of high-affinity mesolimbic nicotinic acetylcholine receptors restores nicotine-induced locomotion but not place conditioning. Genes Brain Behav. 2009;8:257–266. doi: 10.1111/j.1601-183X.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King SL, Caldarone BJ, Picciotto MR. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47 Suppl 1:132–139. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Wiesner A, Fuhrer C. Regulation of nicotinic acetylcholine receptors by tyrosine kinases in the peripheral and central nervous system: same players, different roles. Cell Mol Life Sci. 2006;63:2818–2828. doi: 10.1007/s00018-006-6081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem. 1998;70:1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- 41.Kainu T, Wikstrom AC, Gustafsson JA, Pelto-Huikko M. Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport. 1994;5:2481–2485. doi: 10.1097/00001756-199412000-00019. [DOI] [PubMed] [Google Scholar]
- 42.Galan-Rodriguez B, Suarez J, Gonzalez-Aparicio R, Bermudez-Silva FJ, Maldonado R, Robledo P, et al. Oleoylethanolamide exerts partial and dose-dependent neuroprotection of substantia nigra dopamine neurons. Neuropharmacology. 2009;56:653–664. doi: 10.1016/j.neuropharm.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Ropero AB, Juan-Pico P, Rafacho A, Fuentes E, Bermudez-Silva FJ, Roche E, et al. Rapid non-genomic regulation of Ca2+ signals and insulin secretion by PPAR alpha ligands in mouse pancreatic islets of Langerhans. J Endocrinol. 2009;200:127–138. doi: 10.1677/JOE-08-0397. [DOI] [PubMed] [Google Scholar]
- 44.Gardner OS, Dewar BJ, Graves LM. Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: an example of nongenomic signaling. Mol Pharmacol. 2005;68:933–941. doi: 10.1124/mol.105.012260. [DOI] [PubMed] [Google Scholar]
- 45.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 46.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 47.Hansen HS, Lauritzen L, Strand AM, Vinggaard AM, Frandsen A, Schousboe A. Characterization of glutamate-induced formation of N-acylphosphatidylethanolamine and N-acylethanolamine in cultured neocortical neurons. J Neurochem. 1997;69:753–761. doi: 10.1046/j.1471-4159.1997.69020753.x. [DOI] [PubMed] [Google Scholar]
- 48.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 49.Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Hansen SL, Nielsen AH, Knudsen KE, Artmann A, Petersen G, Kristiansen U, et al. Ketogenic diet is antiepileptogenic in pentylenetetrazole kindled mice and decrease levels of N-acylethanolamines in hippocampus. Neurochem Int. 2009;54:199–204. doi: 10.1016/j.neuint.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Cullingford T. Peroxisome proliferator-activated receptor alpha and the ketogenic diet. Epilepsia. 2008;49 Suppl 8:70–72. doi: 10.1111/j.1528-1167.2008.01840.x. [DOI] [PubMed] [Google Scholar]
- 52.Bough KJ, Schwartzkroin PA, Rho JM. Calorie restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia. 2003;44:752–760. doi: 10.1046/j.1528-1157.2003.55502.x. [DOI] [PubMed] [Google Scholar]
- 53.Porta N, Vallee L, Lecointe C, Bouchaert E, Staels B, Bordet R, et al. Fenofibrate, a peroxisome proliferator-activated receptor-alpha agonist, exerts anticonvulsive properties. Epilepsia. 2009;50:943–948. doi: 10.1111/j.1528-1167.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 54.Terry AV., Jr Role of the central cholinergic system in the therapeutics of schizophrenia. Curr Neuropharmacol. 2008;6:286–292. doi: 10.2174/157015908785777247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman S, Lopez-Hernandez GY, Corrigall WA, Papke RL. Neuronal nicotinic receptors as brain targets for pharmacotherapy of drug addiction. CNS Neurol Disord Drug Targets. 2008;7:422–441. doi: 10.2174/187152708786927831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.