Abstract
Previous studies have shown that tolerance to the antinociceptive effect of morphine develops after a prolonged exposure, but its mechanisms remain unclear. In the present study, we examined whether anti-morphine antibody produced by chronic morphine exposure would contribute to the development of morphine antinociceptive tolerance in rats. Our results showed that anti-morphine antibody was present in rats rendered tolerance to antinociception after intrathecal morphine exposure for seven consecutive days. Superfusion of anti-morphine antibody onto spinal cord slice dose-dependently produced an inward excitatory current in spinal cord dorsal horn neurons using whole cell patch-clamp recording, which surpassed morphine-induced outward inhibiting current. Co-administration of morphine with a monoclonal antibody (2.4 G2) against Fc receptors for seven days significantly attenuated the production of anti-morphine antibody as well as the behavioral manifestation of morphine tolerance in same rats. These results indicate that anti-morphine antibody produced by morphine exposure may contribute to the development of morphine tolerance possibly through counteracting the inhibitory morphine effect on spinal cord dorsal horn neurons.
Keywords: morphine, tolerance, antibody, Fc receptor, 2.4G2, membrane current
Introduction
Morphine has long been used to treat severe acute and chronic pain [1,2]. Chronic morphine exposure also has been shown to lead to the development of tolerance resulting in the diminished analgesic efficiency in both clinical and preclinical settings [3,4]. A number of hypotheses [2] have been proposed regarding mechanisms of morphine tolerance. For example, the reduced responsiveness of G protein-coupled opioid receptors may contribute to morphine tolerance through G-protein uncoupling, receptor internalization, down-regulation of intracellular response elements such as protein kinase C. Morphine tolerance may also be mediated through the altered cellular responsiveness involving cAMP, cholecystokinin, neuropeptide FF, nociceptin and glutamate [5].
Production of anti-morphine antibody after chronic morphine exposure has been reported and was thought to either reduce morphine availability in the central nervous system or block the morphine effect through a morphine-hapten-antibody (Ab) complex [6,7]. It is well known that the Fc fragment of an antibody can bind to the Fc receptor expressed in the cell membrane [8], which initiates various responses such as phagocytosis, cytolysis, degranulation, internalization and transport of immune complex [9]. Most mammalian species express Fc receptors in immune cells [10]. Moreover, antibody binding to the Fc receptor has been shown to induce electrophysiological changes including prolonged depolarization in activated T cells [11], increased peak inward current in alveolar macrophages [12], and increased [Ca2+] influx in dorsal root ganglion neurons [13].
These previous reports raise the possibility that anti-morphine antibody might interact with Fc receptors either reversing morphine-induced inhibition of cell membrane excitation or attenuating the production of anti-morphine antibody following chronic morphine exposure. These possibilities were examined in the present study using a rat model of morphine tolerance, detection of serum anti-morphine antibody titer by Enzyme-linked immunosorbent Assay (ELISA), and electrophysiological recording in spinal cord slice with a whole-cell patch-clamp technique. We also used 2.4G2, an antibody known to block the Fc receptor expressed in B cells, to examine whether it would a) attenuate the production of anti-morphine antibody [6; 25], b) alter the electrophysiological response to morphine of spinal dorsal horn neurons, and c) retard the development of morphine tolerance.
Materials and Methods
Animals and Behavioral Test
The study was approved by the Subcommittee on Research Animal Care in the Massachusetts General Hospital. Under pentobarbital sodium (50 mg/kg, intraperitoneally) anesthesia, adult male Sprague-Dawley rats (Charles River Laboratory, MA) weighing 300-350 grams were implanted intrathecally with a PE-10 tubing through the occipito-atlantic membrane downward to the lumbosacral enlargement [14]. Animals were treated with various drug regimens intrathecally twice a day for 7 days. On day 8, tail flick withdrawal latency was measured for the cumulative dose-response effect using the tail-flick test. Animals were injected intrathecally every 30 minutes with cumulative doses of morphine sulfate (1.5 - 96 ug) to determine morphine tolerance [15]. The stimulation intensity was adjusted to result in a baseline latency of 4-6 sec and a cutoff time of 10 sec. The percent of the maximal possible antinociceptive effect (%MPAE) was calculated by comparing the test latency before [baseline (BL)] and after (TL) a drug injection using the equation: %MPAE = [(TL-BL)/(cutoff-BL)]×100.
ELISA
Whole blood 3 ml was collected from the animal's heart while anesthesia with pentobarbital (50 mg/kg, i.p.) at postoperative day 8 after the behavioral testing. Blood serum was separated by centrifuge at 1200 g for 10 minutes and stored at 4°C. For detection of anti-morphine antibody, the protocol was modified from a previous study [16]. Each 96 well microplate was anchored with 1 ug (10 ug/ml) morphine-BSA (bovine serum albumin) conjugate (Azog, NJ) at 4°C overnight. After washing the well with Well Wash AC® (Thermo Fisher Scientific, Finland), each well was blocked with 3% BSA/PBS (phosphate buffered saline) 200 ul overnight at 4°C, incubated with 100 ul blood serum (1:10) in 3% BSA/PBS overnight 4°C, reacted with 100 ul anti-rat IgG-horse radish peroxidase (1:10,000, Jackson Immuno Research, PA) for an hour at 36°C, and then added with 100 ul 3,3′,5,5′-tetramethylbenzidine (T0440, Sigma, MO) to colorize the reaction. The reaction was stopped 30 minutes later by adding 50 ul 10% H2SO4, and the absorbance was measured at 450 nm wavelength using a microplate reader (Synergy HT, Bio-TEK®, VT).
In order to determine whether the detected anti-morphine antibody specifically binds to morphine, sera from animals (n = 4) treated for 7 days with morphine sulfate (10 ug twice a day) were diluted (1:100) in 3 % BSA/PBS and then added into 4 wells with 100 ul. The wells were kept at room temperature (RT) for 15 minutes. The final concentration of morphine sulfate in these wells was 0, 10-4, 10-7 and 10-10M. The IC50 was calculated to determine the specificity of the morphine binding to anti-morphine antibody by using the Lineweaver-Burk plot,
(V: inhibition reaction, Vmax: maximum reaction of inhibition, IC50: 50% morphine concentration to maximal inhibition, [S]: morphine concentration)
Electrophysiology
Tissue slicing
Lumbar laminectomy was performed in the mouse (B6129SF/J, Jackson Laboratories) under pentobarbital anesthesia (50 mg/kg, ip). Spinal cord lumbar segment was collected and submerged over 10 minutes in an iced artificial cerebrospinal fluid (CSF) solution (mM) composed of NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25 and glucose 11 with pH 7.3 and bubbled with 95% O2 and 5% CO2. Lumbar spinal segment was attached by cyanoacrylate glue to a gel block and fixed at the bottom of the tissue chamber filled with artificial CSF in a vibratome (St. Louis, MO). The horizontal slice of 250 um thickness was kept in 34°C warm artificial CSF for 10 minutes and then cooled down to RT.
Whole cell patch-clamp recording
Spinal cord slice was anchored by a hold-down in a chamber and superfused at 2.5 ml/min with artificial CSF at RT. The spinal cord dorsal horn, a brightly translucent band under light microscope, was visualized using an infrared Differential Interference Contrast (DIC) camera. Neuronal cells were approached with a positive pressure to remove debris by a pipette glass with resistance of 6-8 MΩ and filled with the internal solution (mM) composed of K-gluconate 140, HEPES 10, MgCl2·H2O 2, CaCl2·2H2O 1, EGTA (ethylene glycol tetraacetic acid) 1.1 and ATP (adenosine triphosphate) 2 with pH 7.3 using a micromanipulator (Sutter Instrument, CA). Whole-cell voltage clamp was maintained at -65 mV using MultiClamp 700B with digitizer 1322A (Molecular Devices, CA). Responses were filtered at 2 kHz and digitized at 10 kHz and acquired, amplified and analyzed by Clampex 9.1 and Clampfit 8.0. Series resistance and capacitance as well as I-V curves were measured throughout the process. Data were not included in the final analysis if obtained with more than 20% changes of series resistance and capacitance. Morphine, a purified monoclonal anti-morphine antibody (QED Biosciences Inc., CA), and/or 2.4G2, diluted in artificial CSF, were applied by a syringe pump at 20 ul/min with a total volume of 6 ul. A silicone tube (Polymicro, AZ), connected to the syringe via PE-10 tubing, was placed about 100 um upstream from a target neuron.
Amplitudes of recorded peak currents were averaged and compared among groups. Alteration in membrane currents is regarded as excitatory current when the membrane is depolarized (i.e., negatively deflected) or inhibitory current when hyperpolarized (i.e., positively deflected).
Statistical Analysis
SigmaPlot 11 was used to analyze the data from the behavioral test (two-way analysis of variance, ANOVA), ELISA assay of anti-morphine antibody and electrophysiological recording (one-way ANOVA), which was followed by post-hoc Bonferroni test. P<0.05 was regarded as statistically significant.
Results
Effect of 2.4G2 on morphine tolerance
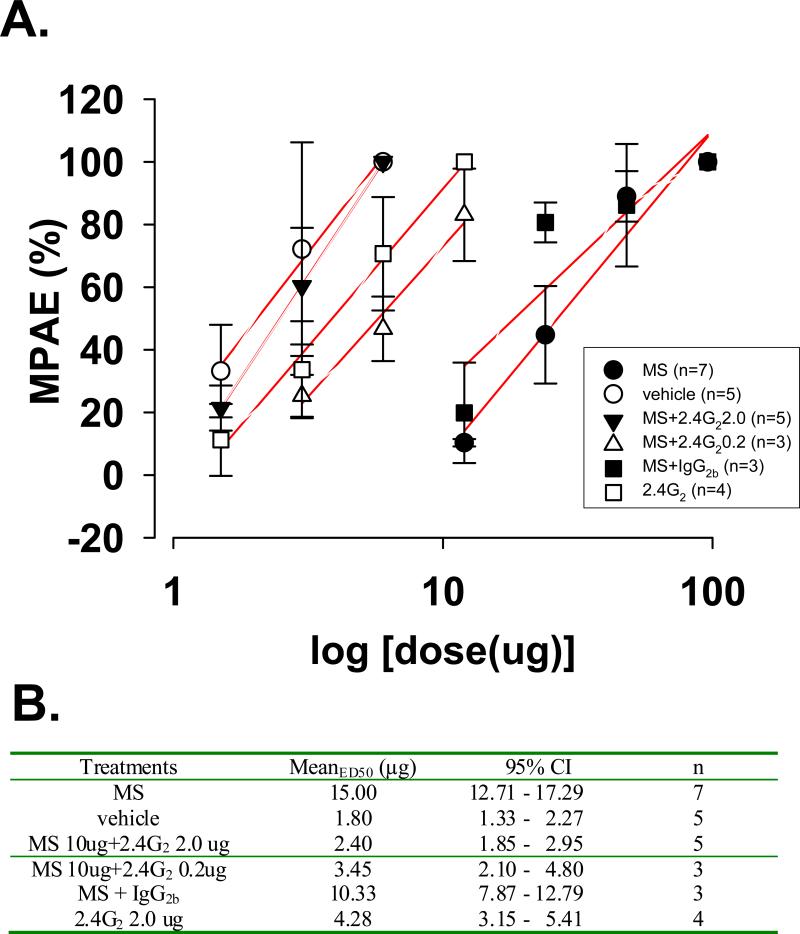
To examine whether the development of tolerance to repeated morphine exposure could be altered by 2.4G2, an antibody interacting with Fc receptors, 2.4G2 was co-administered intrathecally with morphine (10 μg) twice daily for seven consecutive days. The effect of 2.4G2 on morphine tolerance was analyzed on day 8 by generating cumulative dose response curves (Fig. 1A) to compare ED50 values with 95% confidence intervals among groups (Fig. 1B). The rightward shift of the morphine antinociceptive dose-response curve in the morphine alone group was effectively blocked by co-administration of morphine with 2.4G2 in a dose-dependent manner. Administration of 2.4G2 alone did not alter the baseline nociceptive response.
Fig. 1. 2.4G2, an Fc receptor-blocking antibody, inhibited the development of morphine tolerance in rats.
A. Linear expression of dose-dependent curves for the morphine antinociceptive effect after 7-day treatment among groups. Rats were treated with morphine sulfate (MS) 10 ug (•, n=7), MS 10 ug+2.4G2 2.0 (▼, n=5), MS 10 ug+2.4G2 0.2 ug (Δ, n=3), MS 10 ug+IgG2b (■, n=5), 2.4G2 2.0 ug (□, n=3) or vehicle (○, n=5). While the morphine alone group showed a right-ward shift of its antinociceptive morphine dose-response curve, such a right-ward shift was absent in rats co-administered with morphine and 2.4G2 (2.0/0.2 ug). %MPAE (maximum possible antinociceptive effects) = [(TL - BL)/(cut-off - BL)]×100 (BL; a latency to the heat stimuli before a drug injection, TL; a latency after a drug injection). n = animal numbers. B. ED50 with 95% confidence interval of each group in the graph A, which were acquired using the software SigmaPlot 11.
In contrast to the 2.4G2 effect on morphine tolerance, co-administration of morphine with IgG2 (as control for 2.4G2) failed to block the rightward shift of the morphine dose-response curve, indicating that 2.4G2 specifically inhibited the development of morphine tolerance. Collectively, the behavioral data indicated that co-administration of 2.4G2, an antibody blocking the Fc receptor, with morphine attenuated the development of morphine tolerance without changing the baseline nociceptive threshold in rats.
Effect of anti-morphine antibody and 2.4G2 on spinal cord dorsal horn neuronal activity
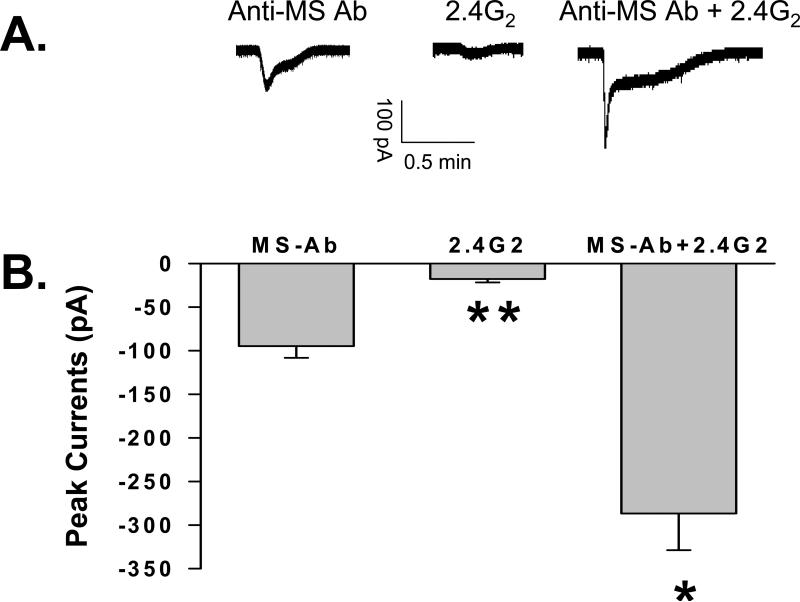
Superfusion of anti-morphine antibody (1.2 μM) onto the spinal cord dorsal horn slice induced cellular excitation as recorded using a whole cell patch clamping preparation (Fig. 2). Since a possible mechanism underlying the effect of 2.4G2 on morphine tolerance could be due to its interaction with Fc receptors at the neuronal level [17], superfusion of 2.4G2 onto spinal cord slice might prevent the excitatory effect of anti-morphine antibody. This possibility was examined by superfusing sub-maximal dose of 2.4G2 (1.2 μM) onto spinal cord slice in the presence of sub-maximal dose of anti-morphine antibody (1.2 μM) (Fig. 2). The result showed that the combined superfusion (-286.75 ± 42.08 pA) of 2.4G2 and anti-morphine antibody further enhanced excitatory current (ANOVA, P < 0.05; Fig. 2B). Also, 2.4G2 itself produced cellular excitation (-17.88 ± 3.91 pA) similar to, but much weaker than, that induced by anti-morphine antibody (-94.72 ± 13.55 pA) (ANOVA, P< 0.001). Collectively, the electrophysiological data indicate that the effect of 2.4G2 on morphine tolerance is unlikely to be mediated by directly blocking the interaction between anti-morphine antibody and Fc receptors within the spinal cord dorsal horn.
Fig. 2. Effect of anti-morphine antibody and 2.4G2 on spinal cord dorsal horn neuronal activity.
A. Representative membrane current peaks recorded in neurons superfused with anti-morphine (MS) antibody (1.2 uM), anti-MS antibody and 2.4G2 (1.2 uM), or 2.4G2 alone using whole-cell patch clamp at holding potential -65 mV. B. Statistical analysis of membrane currents (mean ± s.e.m.) induced by anti-MS antibody (-94.72 ± 13.55 pA, n = 5), 2.4G2 (-17.88 ± 3.91 pA, n = 4), and anti-MS antibody+2.4G2 (-286.88 ± 42.08 pA, n = 6). * P < 0.05; ** P < 0.001.
Effect of 2.4G2 on production of anti-morphine antibody
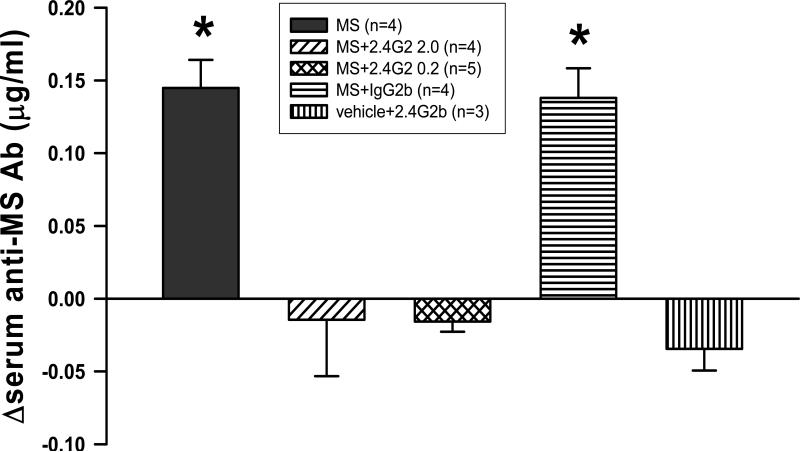
Another possible mechanism underlying the effect of 2.4G2 on morphine tolerance could be due to its ability to reduce the antibody production in response to exogenous morphine exposure [18]. To examine this possibility, the production of anti-morphine antibody was measured following the combined treatment of 2.4G2 and morphine at the same dose used in the behavioral experiment. The results showed that this combined treatment regimen significantly reduced the production of serum anti-morphine antibody compared to that of the morphine alone group (ANOVA; P < 0.001; Fig. 3). All data were expressed as the difference of concentration of anti-morphine antibody in serum (=Ctreatment group - Cvehicle group). The difference of mean serum anti-morphine antibody concentration in the morphine alone group (n = 4, 0.145 ± 0.017 ug/ml) was significantly higher than that of the MS+2.4G2 groups (2.0 ug : n = 4, -0.015 ± 0.033, P < 0.001; 0.2 ug : n = 5, -0.016 ± 0.005, P < 0.001). As a control, IgG2b (n = 4, 0.138 ± 0.010, P = 1.000) failed to reduce the production of anti-morphine antibody. The data indicate that 2.4G2 had a negative effect on the production of anti-morphine antibody following chronic morphine exposure.
Fig. 3. Effect of 2.4G2 on the anti-morphine antibody production.
The production of anti-morphine antibody was assessed in rats treated with a combination of morphine with 2.4G2 or IgG2b. The serum anti-morphine antibody level was significantly higher in the morphine (MS) treatment (intrathecal, twice daily × 7 days) group (n=4, 0.145 ± 0.017 ug/ml as mean ± s.e.m.) as compared with MS 10 ug+2.4G2 2.0 ug (n=4, -0.015 ± 0.033, P < 0.001), MS 10 ug+2.4G2 0.2 ug (n=5, -0.016 ± 0.005, P < 0.001), or 2.4G2 2.0 ug (n=3, -0.035 ± 0.010, P < 0.001). There were no differences in the concentration of morphine antibody between MS alone and MS+IgG2b 2.0 ug (n=5, 0.138 ± 0.018, P = 1.0). The data are expressed as difference values of anti-morphine concentration of a treatment group from that of a vehicle group. * P < 0.05.
Discussion
The present results demonstrate that chronic morphine exposure resulted in the production of anti-morphine antibody in rats with behavioral manifestation of morphine tolerance. At the cellular level, anti-morphine antibody induced excitatory inward current in spinal cord dorsal horn neurons. Moreover, the combined treatment of morphine with 2.4G2, an antibody that binds to Fc receptors [18], attenuated the development of morphine tolerance as well as the production of anti-morphine antibody following a seven-day treatment regimen. Thus, production of anti-morphine antibody following chronic morphine exposure may contribute to the development of morphine tolerance.
It has been reported that chronic morphine treatment combined with complete Freund adjuvant (CFA) produces anti-morphine antibody [6]. In the present study, anti-morphine antibody was detected following chronic morphine but not vehicle exposure, suggesting that morphine exposure alone was sufficient to produce anti-morphine antibody as reported in previous studies [7,19]. It should be noted that a small amount (0.0162 μg/ml) of anti-morphine antibody was also detected in the vehicle group, which could be due to naturally occurring antibodies [7] or a low degree of cross-reactivity of the ELISA protocol. However, IC50 (4.99×10-10 ± 9.4×10-11M; mean ± s.e.m.), as calculated to measure the specificity of anti-morphine antibody to morphine using sera collected from the morphine alone group, suggests that the antibody is highly specific to morphine. Of significance, anti-morphine antibody has also been detected in subjects with opioid addiction, suggesting a possible clinical implication [20]. Morphine was administered intrathecally rather than systemically to induce tolerance because intrathecal approach was already established in this lab and allows us to focus on the spinal mechanisms of morphine tolerance. Although morphine was administered intrathecally, the detection of serum anti-morphine antibody may be due to the fact that IgG can rapidly exit from brain to blood through the blood-brain barrier [21] or enter the central nervous system (CNS) from the periphery [22].
In an earlier study (1972), Berkowitz and Spector proposed that a complex of anti-morphine antibody and morphine may be formed and that the presence of an antigen-hapten-antibody complex could inactivate the morphine effect through an unknown mechanism. In the present study, we demonstrated that the production of anti-morphine antibody was associated with the development of morphine tolerance. Moreover, the combined treatment of morphine with 2.4G2 significantly attenuated the development of morphine tolerance with a concurrent reduction of serum anti-morphine antibody, suggesting that there may exist a functional relationship between the production of anti-morphine antibody and the development of morphine tolerance in rats.
One possible mechanisms underlying the relationship between anti-morphine antibody and morphine tolerance is that anti-morphine antibody could bind to Fc receptors expressed in the spinal cord dorsal horn neurons, which would in turn produce neuronal excitation and reverse neuronal inhibition induced by morphine alone. Indeed, an inward current was identified using the patch-clamp technique by applying heat-aggregated human IgG to alveolar macrophages and the macrophage IgG Fc receptor itself was an IgG-dependent non-selective ion channel in cell-attached patches [12]. Moreover, it was reported that the Fcγ receptor could form ligand-dependent cation-selective ion channels, suggesting a receptor-ion channel model for the biological responses to a target antibody at the cellular level [23].
Indeed, Fc receptors are present in the cell membrane, allowing the constant Fc portion of an immunoglobulin like IgG to bind to Fcγ receptors [8]. There are several subtypes of Fc receptors such as Fcα, Fcε , Fcγ, Fcμ to bind its antibody ligand IgA, IgE, IgG and IgM, respectively. Especially, the Fcγ receptor is divided into several members depending on their antibody affinities due to their different molecular structures. Functionally, Fcγ receptors are classified into two categories, activating or inhibitory receptors, in order to balance immune responses [10]. Engagement of activating Fc receptors can trigger phagocytosis, cytolysis, degranulation, and transcriptional activation of cytokines in inflammatory reactions [9]. Fc receptors also uptake and transport immune complexes [9]. On the other hand, engagement of inhibitory Fcγ receptor IIb down-regulates immune complex-triggered activation by regulating B cell responses, as most Fc receptors are present in immunologic granulocytes [9]. Neuronal cells, oligodendrocytes, astrocytes and microglia also have been reported to express Fc receptors [24-28], so are neuronal cells in the dorsal root ganglion and cerebellum in mice [13,28] and the spinal cord dorsal horn in rats in our present experiment (data not shown).
Using the patch-clamp technique, we examined whether monoclonal IgG anti-morphine antibody would modulate neuronal responses to morphine. Our data indicate that anti-morphine antibody dose-dependently excited spinal cord dorsal horn neurons, which is consistent with a previous report showing that IgG microperfusion activated inward current at the range of 200 to 800 pA as recorded from human alveolar macrophage using a whole-cell patch-clamp setup [12]. In addition, an IgG-antigen complex also increased the Ca2+ concentration in DRG neurons [13] and binding of immune complex to the Fc receptor changed membrane potential in activated T cells [11]. If there were interactions between anti-morphine antibody and Fc receptors, 2.4G2, an antibody blocking antibody-Fc receptor interaction, would be expected to prevent the effect of anti-morphine antibody on neuronal responses. This possibility was not supported by our results showing that the combination of 2.4G2 and anti-morphine antibody led to an enhanced excitatory current peak since 2.4G2 alone at the current dose induced neuronal excitation in spinal cord dorsal slice. Therefore, the effect of 2.4G2 on morphine tolerance is unlikely due to its direct effect on the prevention of anti-morphine antibody-induced cellular excitation.
However, our results showed that co-administration of morphine with 2.4G2 substantially reduced the production of anti-morphine antibody, a finding that is consistent with the regulatory role of Fc receptors (e.g., Fcγ receptor IIb, a primary site of 2.4G2 action) in inhibiting B-lymphocytes and subsequent antibody secretion [18,29]. It is of significance to point out that the effect of 2.4G2 on anti-morphine antibody is a specific effect because a) the combination of morphine and IgG2b (a control immunoglobulin to 2.4G2) failed to alter the production of anti-morphine antibody and b) 2.4G2 is a specific IgG which binds to the Fc receptor in B cells with a high affinity, especially FcγRIIb [30], whereas IgG2b has a much lower binding affinity to the Fc receptor. The exact mechanism underlying the effect of 2.4G2 on anti-morphine antibody production remains to be elucidated in future studies.
In summery, the present study indicates that chronic exposure to morphine resulted in the production of anti-morphine antibody, which produces cellular excitation in spinal cord dorsal horn neurons, thereby contributing to the development of morphine tolerance. While the exact mechanism of this effect remains unclear, our data suggest that immunologic responses following chronic morphine exposure could potentially influence spinal cord neuronal activity and antinociceptive morphine tolerance.
Acknowledgement
This work was supported in part by US PHS RO1 grants NS45681, DE 18214, and DE18538
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.McQuay HJ. Opioids in chronic pain. Br. J. Anaesth. 1989;63:213–226. doi: 10.1093/bja/63.2.213. [DOI] [PubMed] [Google Scholar]
- 2.Collett BJ. Opioid tolerance: the clinical perspective. Br. J. Anaesth. 1998;81:58–68. doi: 10.1093/bja/81.1.58. [DOI] [PubMed] [Google Scholar]
- 3.Yaksh TL, Kohl RL, Rudy TA. Induction of tolerance and withdrawal in rats receiving morphine in the spinal subarachnoid space. Eur. J. Pharmacol. 1977;42:275–284. doi: 10.1016/0014-2999(77)90294-1. [DOI] [PubMed] [Google Scholar]
- 4.Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth. Analg. 1998;86:1307–1311. doi: 10.1097/00000539-199806000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Ueda H, Inoue M, Mizuno K. New approaches to study the development of morphine tolerance and dependence. Life Sci. 2003;74:313–320. doi: 10.1016/j.lfs.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972;178:1290–1292. doi: 10.1126/science.178.4067.1290. [DOI] [PubMed] [Google Scholar]
- 7.Gamaleya NB, Parshin AN, Tronnikov SI, Yusupov DV. Induction of antibodies to morphine during chronic morphine treatment in rodents and opiate addicts. Drug Alcohol Depend. 1993;32:59–64. doi: 10.1016/0376-8716(93)90022-i. [DOI] [PubMed] [Google Scholar]
- 8.Daeron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 9.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J. Clin. Immunol. 2005;25:1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Huckel C, Sandor M, Jenssen HL, Rychly J, Brock J, Gergely J. The binding of IgG1 containing immune complexes to the FcR of allogenically activated T cells induces changes in the membrane potential and the cell surface charge. Mol. Immunol. 1988;25:517–525. doi: 10.1016/0161-5890(88)90073-9. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DJ, Jacobs ER, Tang JM, Zeller JM, Bone RC. Immunoglobulin G-induced single ionic channels in human alveolar macrophage membranes. J. Clin. Invest. 1985;76:500–507. doi: 10.1172/JCI111999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 2004;18:182–184. doi: 10.1096/fj.02-1169fje. [DOI] [PubMed] [Google Scholar]
- 14.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 15.Mao J, Price DD, Mayer DJ. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995;61:353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 16.Gamaleya N, Dmitrieva I, Borg S, Ericcson N. Induction of antibodies to methadone during methadone maintenance treatment of heroin addicts and its possible clinical implications. Eur. J. Pharmacol. 1999;369:357–364. doi: 10.1016/s0014-2999(99)00066-7. [DOI] [PubMed] [Google Scholar]
- 17.Kurlander RJ, Ellison DM, Hall J. The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J. Immunol. 1984;133:855–862. [PubMed] [Google Scholar]
- 18.Dickler HB, Kubicek MT. Effects of various forms of monoclonal anti-Fc gamma R II (2.4G2) on B lymphocyte responses. Mol. Immunol. 1988;25:1169–1174. doi: 10.1016/0161-5890(88)90152-6. [DOI] [PubMed] [Google Scholar]
- 19.Ringle DA, Herndon BL. In vitro morphine binding by sera from morphine-treated rabbits. J. Immunol. 1972;109:174–175. [PubMed] [Google Scholar]
- 20.Gamaleya N, Tronnikov S, Ulyanova L, Klimova S, Dmitrieva I. Antibodies to morphine as indicators of chronic morphine intoxication and impaired immune reactivity. Addict. Biol. 1996;1:437–445. doi: 10.1080/1355621961000125046. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J. Neuroimmunol. 2001;114:168–172. doi: 10.1016/s0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 22.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 23.Young JD, Unkeless JC, Young TM, Mauro A, Cohn ZA. Role for mouse macrophage IgG Fc receptor as ligand-dependent ion channel. Nature. 1983;306:186–189. doi: 10.1038/306186a0. [DOI] [PubMed] [Google Scholar]
- 24.Perl A, Fekete B, Gergely P, Kovach AG. Demonstration of Fc and C3b receptors on rat perikarya. Acta Physiol Acad. Sci. Hung. 1982;60:53–56. [PubMed] [Google Scholar]
- 25.Nitta T, Yagita H, Sato K, Okumura K. Expression of Fc gamma receptors on astroglial cell lines and their role in the central nervous system. Neurosurgery. 1992;31:83–87. doi: 10.1227/00006123-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Vedeler C, Ulvestad E, Grundt I, Conti G, Nyland H, Matre R, Pleasure D. Fc receptor for IgG (FcR) on rat microglia. J. Neuroimmunol. 1994;49:19–24. doi: 10.1016/0165-5728(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 27.Nakahara J, Seiwa C, Shibuya A, Aiso S, Asou H. Expression of Fc receptor for immunoglobulin M in oligodendrocytes and myelin of mouse central nervous system. Neurosci. Lett. 2003;337:73–76. doi: 10.1016/s0304-3940(02)01312-5. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Hirai H, Torashima T, Miyazaki T, Tsurui H, Xiu Y, Ohtsuji M, Lin QS, Tsukamoto K, Nishimura H, Ono M, Watanabe M, Hirose S. CD3 and immunoglobulin G Fc receptor regulate cerebellar functions. Mol. Cell Biol. 2007;27:5128–5134. doi: 10.1128/MCB.01072-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uher F, Lamers MC, Dickler HB. Antigen-antibody complexes bound to B-lymphocyte Fc gamma receptors regulate B-lymphocyte differentiation. Cell Immunol. 1985;95:368–379. doi: 10.1016/0008-8749(85)90324-7. [DOI] [PubMed] [Google Scholar]
- 30.Kurlander RJ, Hall J. Comparison of intravenous gamma globulin and a monoclonal anti-Fc receptor antibody as inhibitors of immune clearance in vivo in mice. J. Clin. Invest. 1986;77:2010–2018. doi: 10.1172/JCI112530. [DOI] [PMC free article] [PubMed] [Google Scholar]