Abstract
In recent years, there has been substantial progress in transplanting cells into the liver with the ultimate goal of restoring liver mass and function in both inherited and acquired liver diseases. The basis for considering that this might be feasible is that the liver is a highly regenerative organ. After massive liver injury or surgical removal of two-thirds or more of the liver tissue, the organ can restore its mass with completely normal morphologic structure and function. It has also been found under highly selective conditions that transplanted hepatocytes can fully repopulate the liver and cure a metabolic disorder or deficiency state. Fetal liver cells can also substantially repopulate the normal liver, and it is hoped in the future that effective repopulation will be achievable with cultured cells or cell lines, pluripotent stem cells from other somatic tissues, embryonic stem cells, or induced pluripotent stem cells, which can now be generated in vitro by a variety of methods. The purpose of this review is to present the major systems that have been used for liver repopulation, the variables involved in obtaining successful repopulation and what has been achieved in these various systems to date with different cell types.
Keywords: liver repopulation, hepatocytes, stem cells, progenitor cells, selective and non-selective conditions
INTRODUCTION
A method to isolate single hepatocytes that are viable in culture was first developed in the late 1960’s (Berry and Friend, 1969). Subsequently, primary isolated hepatocytes were transplanted into the liver through the portal circulation, leading to transient reduction in serum bilirubin levels in the Gunn rat, a model for the human disorder, Crigler-Najjar Syndrome, Type 1 (Matas et al. 1976). In the 1980’s, primary isolated hepatocytes were transplanted into a variety of ectopic sites, including the dorsal fat pad (Jirtle et al. 1980), spleen (Kusano and Mito, 1982), peritoneal cavity (Demetriou et al., 1986) and under the renal capsule (Ricordi et al, 1989). The cells survived to varying degrees in these sites and demonstrated hepatic morphology and function, including modest proliferation after inducing liver regeneration (Jirtle and Michalopoulos, 1982; Gupta et al, 1987). Studies during the past 15 years have clearly established that the liver can be repopulated by transplanted hepatic cells with restoration of normal structure and function and these studies will be the subject of this review.
Early Studies of Hepatocyte Transplantation into the Liver
Initially, it was shown that primary isolated hepatocytes transplanted into the spleen, traverse to the liver and engraft into the hepatic parenchyma (Gupta et al. 1990; Ponder et al. 1991). However, once the number of transplanted cells exceeded 1–2% of total hepatic mass, there was obstruction of the hepatic sinusoids, portal hypertension and hepatic infarction. Inclusion of a liver proliferative stimulus in conjunction with hepatocyte transplantation (two-thirds partial hepatectomy or acute liver injury through administration of CCl4 or another hepatotoxin), or repeated infusions of hepatocytes, led to a modest increase in liver repopulation, but not to levels sufficient for hepatic cellular therapy (Rajvanshi et al. 1996a; Rajvanshi et al. 1996b). These limitations are reflected in several clinical studies in which hepatocyte transplantation has led to modest but only temporary improvement in hepatic function (Fox et al. 1998; Fisher and Strom, 2006).
Repopulation of the Liver by Transplanted Hepatocytes under Selective Conditions
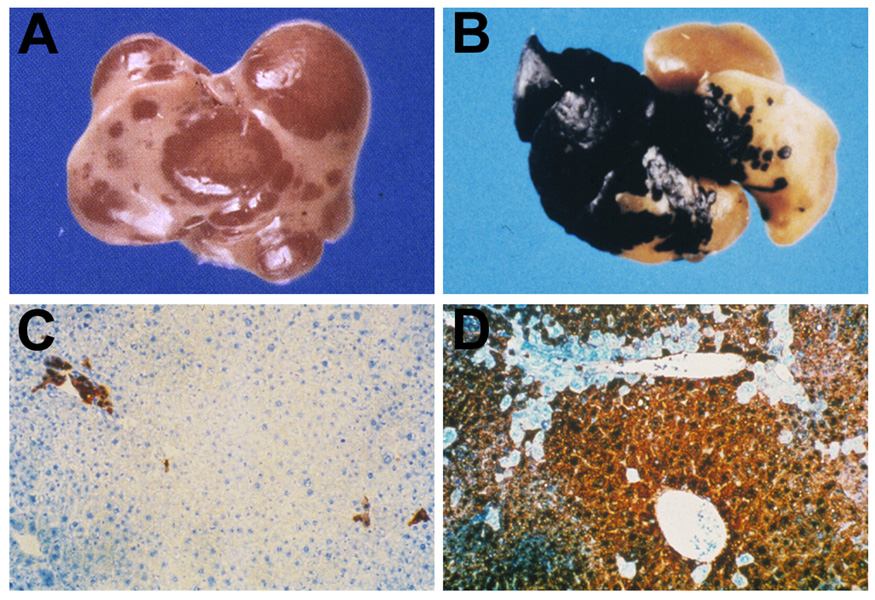
A major breakthrough in hepatic cell transplantation occurred in the early to mid 1990’s through the development of two mouse models for liver repopulation by transplanted hepatocytes. In the first model, Sandgren et al. (1991) developed a transgenic mouse in which a protease, urokinase plasminogen activator (uPA), is expressed exclusively in hepatocytes under control of the albumin promoter and is then secreted into the serum. However, small amounts of uPA remained in the liver tissue causing extensive injury. Most of the mice died between 4–6 weeks of age, but some survived, and in these mice, there were nodules of normal liver tissue of varying sizes scattered throughout the hepatic parenchyma (Fig. 1A). This occurred by deletion of the uPA transgene from individual hepatocytes, and these revertant hepatocytes expanded clonally into large clusters and replaced damaged tissue. This serendipitous finding subsequently led Rhim et al. (1994) to transplant normal hepatocytes (marked with a β-galactosidase transgene) into uPA mice after which they observed extensive liver repopulation (Fig. 1B). Assuming a 10% engraftment efficiency, Rhim et al. estimated that each transplanted hepatocyte that had become incorporated into the uPA host liver underwent ∼12–14 cell divisions (Rhim et al. 1994).
Figure 1.
Alb-uPA transgenic and Fah−/− mouse models for liver repopulation.
A. Spontaneous regeneration of the uPA transgenic mouse liver by endogenous revertant hepatocytes (Sandgren et al. 1991). Hepatocytes that have deleted the uPA transgene replace uPA transgenic hepatocytes because they are not undergoing liver injury and have a selective advantage over uPA expressing hepatocytes that are continually being destroyed.
B. Repopulation of the liver of a uPA transgenic mouse by transplanted wt hepatocytes expressing a marker gene, β-galactosidase (Rhim et al. 1994). Transplanted β-gal expressing, but otherwise normal, mouse hepatocytes have a selective advantage for survival compared to endogenous uPA expressing hepatocytes that are continuously undergoing destruction by expression of the toxic uPA transgene.
C. Fah−/− mouse transplanted with wt FAH+ hepatocytes while on continuous treatment with NTBC (Overturf et al. 1996). Note the presence of small scattered cultures of FAH+ hepatocytes, but no significant liver repopulation by FAH+ cells.
D. Fah−/− mouse transplanted with wt FAH+ hepatocytes followed by withdrawal of NTBC (Overturf et al. 1996). Under these conditions, which are highly selective for survival of FAH expressing hepatocytes, there is massive replacement of the liver by FAH+ hepatocytes at 6 weeks after cell transplantation.
Grompe and colleagues (Overturf et al., 1996) developed a second mouse model to repopulate the liver with mature hepatocytes by targeted disruption of fumarylacetoacetate hydrolase (Fah), the last gene in tyrosine catabolism. Deletion of Fah leads to accumulation of upstream intermediates in tyrosine catabolism, some of which (primarily fumarylacetoacetate) are toxic and cause extensive and continuous liver injury. Administration of 2 (2-nitro-4-trifluoromethylbenzoyl)-cyclohexane-1,3-dione (NTBC), a pharmacologic inhibitor of tyrosine catabolism upstream of homogentisic acid, prevents accumulation of fumarylacetoacetate in the liver of Fah null mice. NTBC was developed originally as an experimental agent to treat patients with hereditary tyrosinemia type 1 (HT1), an inherited metabolic disorder based on mutation of the Fah gene (Lindstedt et al. 1992) and is now being used clinically.
In Fah null mice, Grompe and colleagues demonstrated that liver repopulation by transplanted wt hepatocytes can be totally regulated by NTBC administration. When NTBC is administered, fumarylacetacetate does not accumulate in the liver, liver injury and cell turnover are reduced markedly and there is no advantage for wt hepatocytes to survive over host Fah null hepatocytes. Therefore, when wt hepatocytes are transplanted into Fah null mice maintained on NTBC, only scattered small clusters of transplanted hepatocytes are detected (Fig. 1C), similar to results cited above when wt hepatocytes are transplanted into a normal host liver environment in conjunction with CCl4 administration (Rajvanshi et al. 1996b). However, if NTBC treatment is discontinued at or shortly after cell transplantation, extensive liver injury resumes and transplanted cells proliferate extensively and the transplanted wt hepatocytes selectively repopulate the liver, forming large clusters replacing most of the hepatic mass within 6 weeks (Fig. 1D). Therefore, cyclic administration and withdrawal of NTBC can be used as a tool to control liver failure in Fah null mice and to modulate liver repopulation by transplanted cells. This is an advantage of the Fah null mouse model compared to uPA transgenic mice in which transplanted wt hepatocytes compete with revertant host hepatocytes for liver repopulation. Fah null mice with livers repopulated by wt hepatocytes remain healthy, have normal liver function tests and show a relatively normal liver structure for many months after hepatocyte transplantation (Overturf et al. 1996). Studies in the Fah null mouse were the first to show that liver repopulation can effectively cure a metabolic disease, namely, the mouse equivalent to HT1.
In Fah null mice, not only do transplanted wt hepatocytes replace Fah null hepatocytes, but the transplanted repopulating hepatocytes can also be serially transplanted through seven consecutive Fah null mice, while retaining full ability to proliferate and replace host hepatocytes (Overturf et al. 1997). In these studies, Grompe and colleagues calculated that each serially transplanted hepatocyte underwent an average of at least 69 cell divisions. Previously, it was thought that mature hepatocytes could undergo only two or three cell divisions after which they become terminally differentiated and cannot proliferate further, even under a liver regenerative stimulus. Thus, in both of these mouse models, a paradigm shifting result was obtained; namely, that hepatocytes exhibit extensive capacity to proliferate and restore liver mass and function under highly selective conditions; i.e., when there is massive and continuous injury to host hepatocytes leading to high cell turnover, together with a significant selective advantage for survival of transplanted hepatocytes compared to host hepatocytes.
A third method to obtain a high level of liver repopulation by transplanted hepatocytes is to impair proliferation of endogenous hepatocytes, i.e., to render them incapable of cell division, and then transplant wt hepatocytes with normal proliferation potential in conjunction with a liver proliferative stimulus to the host liver. These conditions are also highly selective for liver repopulation by transplanted cells compared to endogenous host hepatocytes. This was first achieved in rats using retrorsine, a plant alkaloid that is taken up by hepatocytes, cross links cellular DNA, and blocks proliferation of hepatocytes by disrupting their progression through the cell cycle (Samuel and Jago, 1975; Laconi et al. 1998). When retrorsine or a closely related compound, monocrotaline, is administered to rats or mice (Laconi et al. 1998; Guo et al. 2002; Nierhoff et al. 2005; Witek et al. 2005), there is a long-lived inhibition of hepatocyte proliferation. However, essential metabolic functions are maintained in these DNA damaged hepatocytes and the animals survive. After the effects of acute chemical injury have subsided (2–4 weeks), the animals are subjected to two-thirds PH or CCl4 administration and hepatocytes from normal animals are transplanted into the liver. This leads to a brisk regenerative response by transplanted hepatocytes and there is near total replacement of DNA damaged host hepatocytes within several months (Laconi et al. 1998; Guo et al. 2002; Nierhoff et al. 2005). To follow the fate of transplanted hepatocytes, we used a specific strain of inbred Fischer (F) 344 rats with a spontaneous mutation in the dipeptidyl peptidase (DPP)IV gene, rendering the mutant protein enzymatically inactive. DPPIV is expressed on the apical (bile canalicular) domain of the hepatocyte cell surface membrane in a characteristic pattern that can be readily identified (Gossrau, 1979; Hubbard et al. 1985; Walborg et al. 1985). In 1991, Hixson and colleagues developed a model system for liver repopulation in which they transplanted normal (wt) F344 rat hepatocytes into syngeneic DPPIV− mutant F344 rats and identified transplanted hepatocytes in the host liver by DPPIV enzyme histochemistry or immunohistochemistry (Thompson et al. 1991). Because wt and DPPIV− mutant F344 rats are inbred, there is no rejection of transplanted cells. We have utilized this model extensively, as well as DPPIV−/− mice and DPPIV−/−/Rag2−/− mice, to study repopulation of the liver by transplanted cells (Laconi et al. 1998; Oren et al. 1999; Dabeva et al. 2000; Sandhu et al. 2001; Menthena et al. 2004; Oertel et al. 2006a,b; Oertel et al. 2007; Oertel et al. 2008; Yuan et al. 2003; Gouon-Evans et al. 2006). We also developed a double label enzyme histochemistry assay to simultaneously detect DPPIV and Na+-K+ ATPase, another enzyme that is expressed on the bile canalicular domain of the hepatocyte plasma membrane (Dabeva et al. 1997). Therefore, we could follow the engraftment, proliferation and structural incorporation of transplanted hepatocytes into the host parenchyma (Laconi et al. 1998).
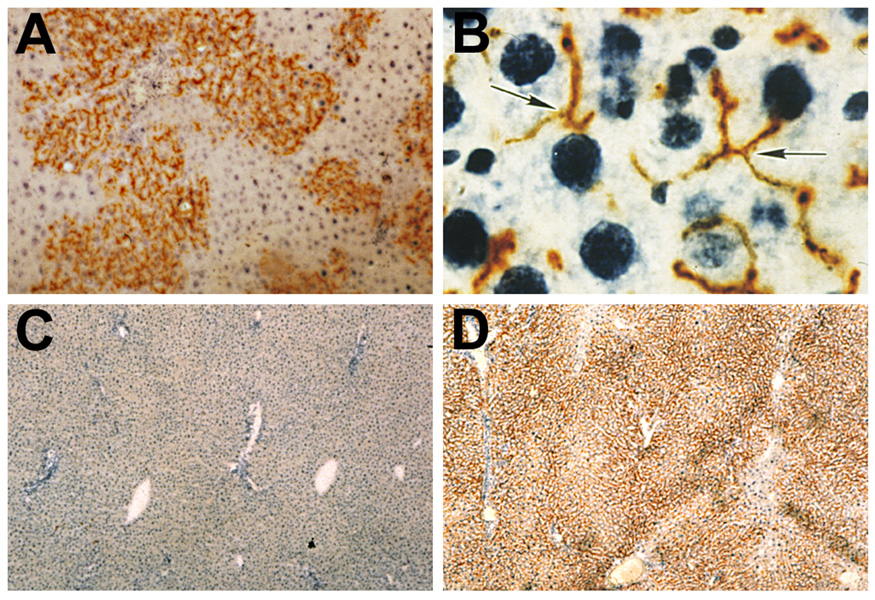
What was most surprising in the retrorsine/PH model is that the transplanted hepatocytes do not develop into hyperplastic nodules (Fig. 2A), the liver remodels and the transplanted hepatocytes become fully integrated into the hepatic plates with transplanted hepatocytes forming hybrid canaliculi with neighboring host hepatocytes (Fig. 2B). The liver structure becomes and remains essentially normal for many months after cell transplantation and 98–99% of host hepatocytes can be replaced in 6 to 9 months (Fig. 2C, D) (Laconi et al. 1998). Using the retrorsine/PH model, we have also transplanted wt allogenic hepatocytes into albumin deficient Sprague Dawley rats, i.e., the Nagase analbuminemic rat, under immunosuppression with cyclosporine. Under these conditions, there was 75–80% replacement of albumin-deficient host hepatocytes by wt albumin expressing hepatocytes (Oren et al. 1999), which led to a 7,000-fold increase in albumin production and restoration of serum albumin levels to the normal range. Other methods to achieve a selective advantage for transplanted cells include x-irradiation of the host liver to induce DNA damage (Guha et al. 1999; Malhi et al. 2002) or use of transgenic or knock-out hepatocytes with augmented proliferative potential or resistance to apoptosis to repopulate the liver (Mignon et al. 1998; Yuan et al. 2003). In these various models of host hepatocyte mitoinhibition, the liver proliferative stimulus can be supplied by two-thirds PH, CCl4 administration, ischemic liver injury or treatment of the host with hormones (tri-iodothyronine) or growth factors (HGF) (Guha et al. 1999; Yuan et al. 2003; Malhi et al. 2002; Oren et al. 1999; Landis et al. 2006).
Figure 2.
Retrorsine/PH model for liver repopulation.
A. Active repopulation of the liver by transplanted wt DPPIV+ hepatocytes without evidence for tissue compression or destruction of the surrounding hepatic DPPIV− parenchyma (Laconi et al. 1998).
B. Transplanted hepatocytes are completely integrated into the host liver parenchymal, forming DPPIV+ hybrid bile canaliculi with adjacent DPPIV− host hepatocytes (Laconi et al. 1998).
C. Liver of DPPIV− mutant host removed by two-thirds PH just prior to hepatocyte transplantation (Laconi et al. 1998).
D. Liver of the same DPPIV− mutant rat at 9 months after transplantation of 1 × 106 wt (DPPIV+) hepatocytes, exhibiting 99% replacement of the host liver by DPPIV+ cells (Laconi et al. 1998).
Repopulation of the Liver by Transplanted Hepatic Cells Under Non-Selective Conditions
A key feature in all the above models is that strong selection conditions existed in the host to enable transplanted hepatocytes to extensively repopulate the liver. Although transplanted hepatocytes can engraft into the liver in a normal host (Gupta et al. 1990; Ponder et al. 1991) and undergo several cell divisions in response to a proliferative stimulus, such as two-thirds PH or CCl4 induced liver injury (Rajvanshi et al. 1996b), very little repopulation occurs under these conditions (Rajvanshi et al. 1996b; Sandhu et al. 2001). We refer to these circumstances as non-selective, because the basic liver function is normal and both host and transplanted hepatocytes can respond equally to a liver proliferative stimulus, i.e., neither cell type exhibits a selective advantage for proliferation, survival or function. Since the number of viable host hepatocytes remaining in the liver after two thirds PH or high dose CCl4 administration is ∼100-fold greater than the number of hepatocytes that can be transplanted and engrafted, the amount of liver repopulation that can be achieved by normal hepatocytes under non-selective conditions is no more than 1–2%. Our goal was to identify cells that were capable of effectively repopulating the liver in a normal, non-selective, host environment.
A logical source for cells that might have high repopulation potential is the fetal liver. During liver development in the mouse, undifferentiated endodermal stem cells begin to proliferate on embryonic day (ED) 8.0 (Fig. 3) (Zhao and Duncan, 2005). Specification of endodermal stem cells to the hepatic lineage occurs at ED8.5 and requires fibroblast growth factor (FGF) and bone morphogenic protein (BMP) signaling (Jung et al. 1999; Rossi et al. 2001; Wandzioch and Zaret 2009). Cells expand from the foregut endoderm and by ED 9.0–9.5, these cells begin to bud and express GATA4 and liver-enriched, nuclear factor HNF4α, as well as liver-specific genes, α-fetoprotein (AFP), followed by albumin (Alb). These hepatic-specified cells (now referred to as hepatoblasts) proliferate massively and invade the septum transversum mesenchyme.
Figure 3.
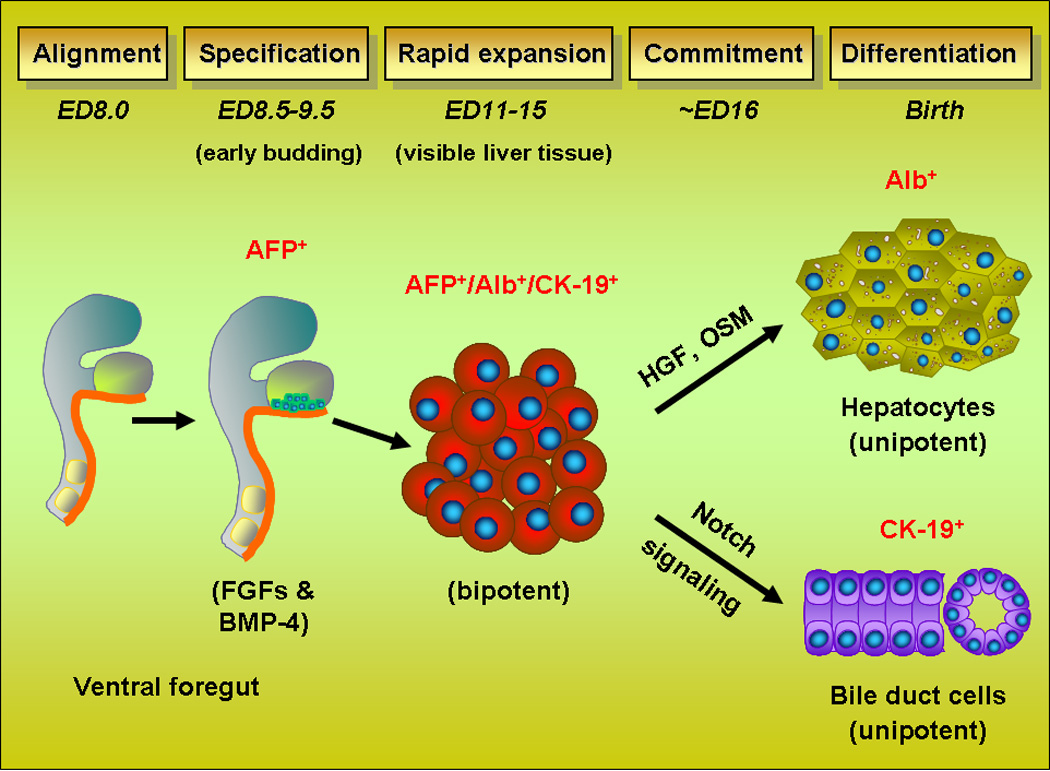
Schematic diagram of fetal liver development in the mouse.
Hepatoblasts continue to proliferate rapidly and begin to express other characteristic genes, including cytokeratins 7, 8, 18 and 19. Just prior to ED16, hepatoblasts diverge along two lineages, hepatocytes (expressing AFP and albumin) and cholangiocytes (expressing CK-19). In the rat, similar events occur one day later (Shiojiri et al. 1991; Marceau et al. 1992). This is associated with a massive and abrupt change in the gene expression profile of rat fetal liver epithelial cells to a more differentiated phenotype, which occurs between ED16 and ED17 (Petkov et al. 2004). After ED16, the percentage of bipotential cells, i.e., those expressing genes in both the hepatocytic and cholangiocytic lineages (i.e., AFP, Alb and CK-19) is markedly reduced (Dabeva et al. 2000; Sandhu et al. 2001). Most of the cells are now unipotential and committed to either the hepatocytic, AFP(Alb)+/CK-19-, or cholangiocytic, AFP(Alb)−/CK-19+, lineage. As organogenesis proceeds, intrahepatic bile ducts are formed in the vicinity of large portal vein branches, and the basic lobular structure is completed, although the parenchymal cords do not become fully mature until several weeks after birth.
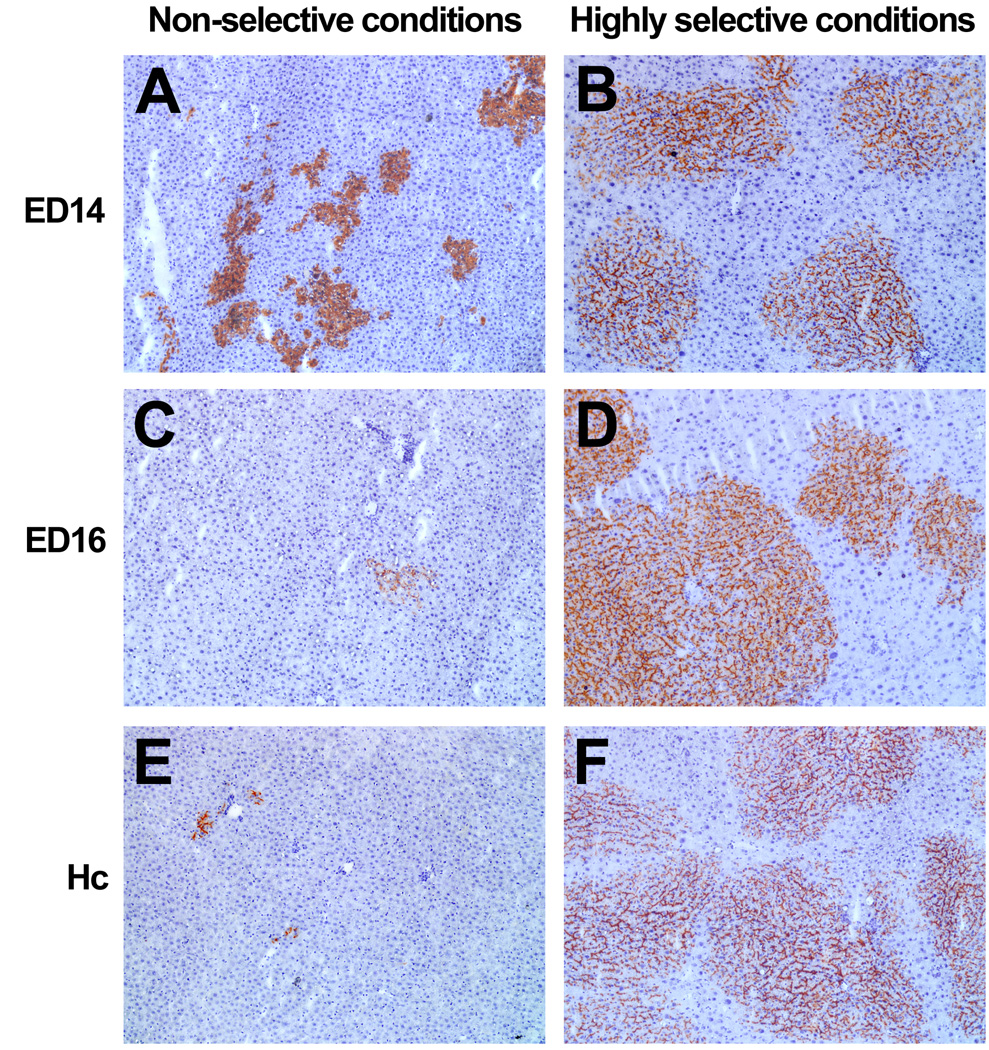
Studies were conducted to determine whether fetal liver cells could effectively repopulate the normal adult liver under non-selective conditions (i.e., in a basically normal liver with no underlying hepatic dysfunction) and to compare the results to those obtained with adult hepatocytes under both non-selective vs. highly selective conditions. For highly selective conditions, rats were pretreated with retrorsine to block proliferation of host hepatocytes. Under both non-selective and selective experimental conditions, two-thirds PH was performed at the time of cell transplantation and in each case comparable numbers of hepatic cells were infused directly into the portal vein. As shown in Fig. 4, under non-selective conditions, there was significant repopulation by ED14 fetal liver cells (Fig. 4A), but much less repopulation with ED16 fetal liver cells (Fig. 4C) and almost no repopulation with adult hepatocytes (Fig. 4E) (data shown at 4 months after cell transplantation because repopulation proceeds at a slower pace under non-selective conditions). Using the same numbers of cells under selective conditions (2 doses of retrorsine 30 mg/kg, two weeks apart, followed by a one month recovery period), there was a 10–100 fold greater liver repopulation with all three cell types, even as soon as 2 months after cell transplantation (Fig. 4B, D & F). These studies demonstrated that after lineage divergence in the fetal liver, there is a dramatic reduction in the repopulation potential of hepatic progenitor cells when transplanted into a normal liver (i.e., under non-selective conditions). However, both ED14 (early) and ED16 (later, more differentiated) liver progenitor cells, as well as fully differentiated, mature hepatocytes exhibit high repopulation potential under highly selective conditions. These studies also demonstrate that there is an enormous difference in the repopulation potential of hepatic cells under selective vs. non-selective conditions. Therefore, one cannot compare the repopulation potential of different cell types when the repopulation studies are performed under different experimental circumstances. Finally, Ott and coworkers have recently reported that for mouse or human cells transplanted into Alb-uPA/Rag2−/−/γc−/− mice (highly selective conditions), repopulation with adult hepatocytes is more efficient than with ED 11.5–13.5 fetal liver cells (Haridass et al. 2009). Our results with adult hepatocytes vs. ED 12.5 mouse fetal liver cells in retrorsine/CCl4 treated mice (Nierhoff et al. 2005) are consistent with the findings of Ott and coworkers. Whether this is due to more efficient engraftment of mature hepatocytes compared to hepatoblasts in the mouse liver or some other factor(s) remains to be determined.
Figure 4.
Photomicrographs showing the level of liver repopulation by rat ED14 and ED16 fetal liver cells and adult rat hepatocytes under non-selective (normal/PH) vs. highly selective (retrorsine/PH) host conditions. In each case, the number of transplanted hepatic epithelial cells was ∼4 × 105 cells. For non-selective conditions (A, C & E), animals underwent two-thirds PH at the time of hepatocyte transplantation and were sacrificed 4 months after cell transplantation. For highly selective conditions (B, D & F), animals were treated with retrorsine/PH prior to hepatocytic transplantation and were sacrificed 2 months after cell transplantation. A & B, ED14 fetal liver cells, C & D, ED16 fetal liver cells and E & F, adult hepatocytes (Sandhu et al. 2001 and previously unpublished). All photomicrographs at 100X original magnification.
What is a stem cell, and how does this cell differ from a progenitor cell?
During development of the mammalian embryo, stem cells originate from the inner cell mass of the blastocyst (Thomson et al. 1998). These cells, termed embryonic stem (ES) cells, are totipotent and give rise to somatic stem cells that differentiate further into multipotent, tissue-specified stem cells. The latter differentiate further into lineage committed progenitor cells and ultimately into adult differentiated cells, comprising the various organs. Tissue specific (or somatic) stem cells have been identified in studies of tissues with high turnover, such as hematopoietic cells of the bone marrow and epithelial cells of the skin and intestinal mucosa. Continuous proliferation and differentiation of stem cells in these organs maintains tissue mass at a steady state as part of normal cellular turnover (Potten, 1997).
Tissue specified stem cells are generally considered to exhibit four basic properties: (1) capacity for self-renewal or self-maintenance; (2) multipotency, producing progeny in at least two lineages; (3) functional, long-term tissue reconstitution; and (4) serial transplantability. Progenitor cells are the progeny of stem cells. These cells may be comprised of distinct subpopulations, some with multilineage potential (multipotent progenitor cells) and others that have differentiated further and give rise to progeny in only a single lineage (unipotent progenitor cells). In contrast to stem cells, progenitor cells do not self-renew; they divide rapidly but are capable of only short-term tissue reconstitution. Progenitor cells cannot be serially transplanted (under normal circumstances), and they have been termed “transit amplifying cells” (Potten, 1997).
Properties of Fetal Liver Epithelial Cells as Hepatic Stem/Progenitor Cells
As implied above, the ultimate test for a putative stem cell is to demonstrate its ability to functionally repopulate a tissue or organ, long-term. Sandhu et al. (2001) reported 5–10% repopulation of DPPIV− mutant F344 rat liver by transplanting wt ED14 fetal liver epithelial cells into the normal adult liver in conjunction with two-thirds PH. The transplanted cells were integrated into the host parenchyma, proliferated extensively, and the majority of repopulating clusters contained both hepatocytes and mature bile duct cells (Sandhu et al. 2001). Liver repopulation by transplanted ED14 fetal liver cells increased progressively and remained stable over six months. These results were obtained in a normal tissue environment, requiring only a two-thirds PH to initiate the repopulation process. These findings are comparable to those obtained in the hematopoietic system, where extensive ablation is required for bone marrow replacement by transplanted hematopoietic stem cells.
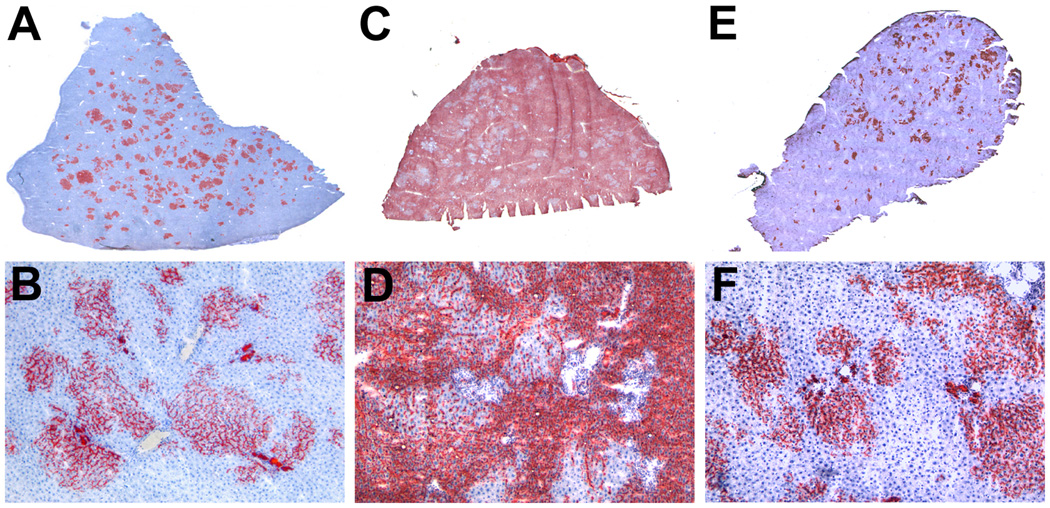
In ED14 rat fetal liver, there are three distinct populations of epithelial cells, those positive for AFP and Alb but negative for CK-19, those positive for AFP, Alb and CK-19 and those positive for CK-19 but negative for AFP and Alb (Dabeva et al. 2000; Sandhu et al. 2001; Oertel et al. 2006a). The number of AFP+/Alb+/CK-19+ cells decreases dramatically at ED16, after which liver repopulation potential of rat fetal liver cells also decreases dramatically (Sandhu, 2001). The level of liver repopulation by rat ED14 fetal liver cells under non-selective conditions can also be increased to 20–25% by increasing the number of ED14 fetal liver cells transplanted (Fig. 5A & B) (Oertel et al. 2006a). A frequently asked question is whether these repopulation results represent a general phenomenon or a unique property of DPPIV− mutant rats which is not apparent, since the liver appears normal morphologically. To address this question, we have reversed the model, so that ED14 fetal liver cells from DPPIV− mutant F344 rats have been transplanted into normal wt (DPPIV+) F344 hosts. In these studies, essentially the same results have been obtained (Fig. 5C & D).
Figure 5.
High levels of liver repopulation by rat ED14 fetal liver cells and highly enriched rat ED14 fetal liver stem/progenitor cells under non-selective conditions. A & B, 50 × 106 unfractionated rat ED14 fetal liver cells from a DPPIV+ donor were transplanted into a DPPIV− mutant rat under non-selective conditions (PH without retrorsine pretreatment) (Oertel et al. 2006b). A, whole liver section scanned without magnification and B, photomicrograph at 100X original magnification. C & D, reversal of the DPPIV model system in which 20 × 106 ED rat fetal liver cells from a DPPIV− donor were transplanted into a wt (DPPIV+) host liver under non-selective conditions (PH without prior retrorsine treatment) (Oertel et al., previously unpublished). C, whole liver section scanned without magnification and D, photomicrograph at 100X original magnification. E & F, 1.9 × 106 purified ED14 fetal liver stem/progenitor cells (enriched to 95% purity by immunoselection for Dlk-1 and equivalent to 35 × 106 unfractionated ED14 fetal liver cells) from a DPPIV+ donor transplanted into a DPPIV− mutant rat under non-selective conditions (PH without retrorsine pretreatment) (Oertel et al. 2008). E, whole liver section scanned without magnification and F, photomicrograph at 100X original magnification.
Repopulating fetal liver cells have been enriched to 95% homogeneity by immunoselection for Dlk-1, an early fetal liver epithelial cell surface marker. The enriched cells retain the full repopulation potential of unselected fetal liver cells in the normal adult rat liver (Fig. 5E & F) (Oertel et al. 2008). Repopulation continues to increase for up to one year, reaching an average of ∼30% for the total liver (Oertel et al., unpublished observations). Repopulation remains stable for the life of the animal, which is consistent with the slow turnover of parenchymal cells in this organ. In this normal rat model, there is a several thousand fold amplification of transplanted fetal liver epithelial cells in the repopulated host liver (Oertel et al. 2006b). Both hepatic parenchymal cords and mature bile ducts are formed by transplanted fetal liver cells, as well as whole new liver lobules, and the progeny of the transplanted cells express normal levels of hepatocytic and cholangiocytic genes in the respective cell types (Oertel et al. 2006b; Oertel et al. 2008). Thus, transplanted rat ED14 fetal liver epithelial cells exhibit three major properties of stem cells: 1) extensive proliferation, 2) differentiation into the two mature epithelial cell phenotypes in the liver, i.e., hepatocytes and cholangiocytes, and 3) long-term, in vivo functional reconstitution of the tissue. Since serial transplantation (an indicator of self-renewal) has not yet been demonstrated with fetal liver epithelial cells, they are referred to as fetal liver stem/progenitor cells (Oertel et al. 2006a,b; Oertel et al. 2008).
The mechanism for liver repopulation by rat fetal liver stem/progenitor cells has been shown to be cell competition between the transplanted cells and host hepatocytes (Oertel et al. 2006b), a process originally described in Drosophila during wing development (Moreno and Basler, 2004; de la Cova et al. 2004). Rat fetal liver stem/progenitor cells have been cryopreserved with full ability to repopulate the normal adult liver after thawing (Oertel et al. 2006a).
Induction and Properties of “Oval” (Progenitor) Cells
The term “oval cells” was introduced by Farber in 1956 to describe non-parenchymal cells in the periportal region that were present after treating rats with carcinogenic agents, such as ethionine, α (alpha)-acetaminobenzene [2-AAF] and 3-methyl-4-diethylaminobenzene (Farber, 1956). Other methods to induce proliferation of “oval cells” are treatment of rats with D-galactosamine (Lemire et al. 1991; Dabeva and Shafritz, 1993), a choline deficient/ethionine substituted diet (Sells et al. 1981; Akhurst et al. 2001) or allyl alcohol (Yin et al. 1999) or treatment of mice with dipin (Factor et al. 1994) or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) (Preisegger et al. 1999). Cells that are induced in each of these models have a small oval-shaped, pale-stained blue nucleus and very scant, lightly basophilic cytoplasm. Farber did not believe that “oval cells” are hepatocyte progenitors (Tatematsu et al. 1984), but Thorgeirsson and coworkers (Evarts et al. 1987) demonstrated that “oval cells”, induced to proliferate in the periportal region after treatment of rats with 2-AAF followed by two-thirds PH, subsequently differentiate into distinct clusters of basophilic hepatocytes in the mid-parenchyma (Evarts et al. 1987; Evarts et al. 1989). They subsequently demonstrated that “oval cells”, induced to proliferate by 2-AAF/PH, are derived from undifferentiated cells in the Canals of Hering (Paku et al. 2001). Other evidence that “oval cells” are hepatic progenitors came from reports that “oval cells” express c-kit (Fujio et al. 1994), CD34 (Omori et al. 1997), flt3 receptor (Omori et al. 1997) and LIF (Omori et al. 1996), all known markers for hematopoietic stem cells or their immediate derivatives. Sca-1, another cell surface protein expressed by hematopoietic stem cells in the mouse, is also expressed in fetal liver epithelial cells (Nierhoff et al. 2005) and in “oval cells” of the adult mouse liver (Petersen et al. 2003; Wright et al. 2008). Most recently, using lineage tracing studies in a double transgenic mouse expressing a β (beta)-galactosidase reporter gene under control of the Foxl1 promoter, Greenbaum and coworkers (Sackett et al. 2009) showed that “oval cells”, induced to proliferate by bile duct ligation or feeding mice a DDC containing diet, can differentiate into both hepatocytes and bile duct epithelial cells, and thus represent bipotent progenitor cells.
The term “oval cells” is used to identify a highly heterogenous population of cells, and many authors use the terms, oval cells, oval/progenitor cells and hepatic progenitor cells interchangeably. Attempts to establish specific markers for “oval cells” to distinguish them from mature hepatocytes and bile duct epithelial cells and to determine their lineage origin (mesoderm or endoderm) have led to conflicting findings. All investigators agree that “oval cells” express common liver epithelial progenitor cell markers, such as AFP and albumin for hepatocyte progenitors and CK-19 (and OV6 in the rat) for bile duct progenitor cells. However, it is not clear whether “oval cells” from different animal species or from different hepatic injuries are in fact the same. Although “oval cells” were initially thought to express hematopoietic stem cell markers, c-kit, CD34 and Thy-1 (Fujio et al. 1994; Omori et al. 1997; Crosby et al. 2001; Petersen et al. 1998), several recent studies have shown that both fetal liver progenitor cells and “oval cells” are negative for c-kit, CD34 and Thy-1 (Suzuki et al 2000; Tanimizu et al. 2003; Dezso et al. 2007; Yovchev et al. 2007).
Liver Repopulation by “Oval Cells”
As indicated previously, since hepatocytes do not effectively repopulate the normal adult liver, an obvious alternative would be to transplant progenitor (“oval”) cells that should have a higher proliferative potential than adult hepatocytes. “Oval cells” isolated from the liver of rats treated with D-galactosamine also proliferate and differentiate into hepatocytes after transplantation into rats undergoing two-thirds PH (Dabeva et al. 1997). However, in these studies, which were conducted in a non-selective tissue environment, liver repopulation by transplanted D-galactosamine induced “oval cells” was low. Duct-like epithelial cells, isolated from the atrophic pancreas of rats treated with a copper deficient diet supplemented with a copper chelating agent (Rao et al. 1988; Dabeva et al. 1995), also proliferate modestly after transplantation into the normal rat liver in conjunction with two-thirds PH (Dabeva et al. 1997). Isolated pancreatic cells from normal mice also repopulate the liver of Fah null mice (Wang et al. 2001). “Oval cells” isolated from the liver of DDC-fed mice also repopulate the liver of Fah null mice, although with a reduced efficiency compared with mature hepatocytes (Wang et al. 2003). Similarly, “oval cells” from GFP transgenic mice maintained on a DDC diet also repopulate the liver of wt mice treated with monocrotaline in conjunction with PH (Song et al. 2004). Other recent studies have shown effective repopulation of the liver by purified “oval cells” in retrorsine treated rats (Yovchev et al. 2008).
Most recently, “oval cells” have been isolated from normal mouse and dog liver (Wright et al. 2008; Suzuki et al. 2008; Arends et al. 2009). These cells exhibit properties characteristic of hepatic progenitor cells in culture, but in vivo repopulation data are very limited. Numerous studies have reported the isolation of “oval cell” lines or hepatic progenitor cell lines from rats, mice and humans (Grisham 1980; Yasui et al. 1997; Coleman et al. 1997; Suzuki et al. 2002; Strick-Marchand et al. 2004; Fougere-Deschatrette et al. 2006; Dan et al. 2006; Suzuki et al. 2008). These cells are clonal, bipotent and exhibit other stem and progenitor cell properties in vitro and in vivo. However, liver repopulation by “oval” or hepatic progenitor cell lines has generally been very low, even under highly selective conditions.
Liver Repopulation by Extrahepatic and Embryonic Stem Cells
Various studies have reported that cells released from the BM into the circulation, migrate to the liver and differentiate into hepatocytes. However, the extent to which this occurs and the mechanism(s) involved remain controversial (for reviews, see Goodell, 2003; Wagers and Weissman, 2004; Fausto, 2004; Thorgeirsson and Grisham, 2006). Originally, Petersen and coworkers reported that BM stem cells isolated from DPPIV+ F344 rats and transplanted into sublethally irradiated DPPIV− F344 rats repopulate the BM and then migrate to the liver and “transdifferentiate” into hepatocytes through the liver “oval cell” progenitor pathway (Petersen et al. 1999). These findings were subsequently confirmed by studies in both mice and humans (Theise et al. 2000a,b; Alison et al. 2000). However, studies by Wang et al. (2003) showed that BM cells did not enter the “oval cell” pool in mice treated with DDC. Menthena et al. (2004) also showed that DPPIV+ BM cells transplanted into DPPIV− rats contributed <1% to the “oval cell” pool in three different models of “oval cell” activation: 1) 2-AAF/PH, 2) retrorsine/PH or 3) D-gal induced liver injury. These studies (Wang et al., 2003; Menthena et al., 2004) and others in both mice and rats (Wagers and Weissman, 2004; Fausto, 2004; Thorgeirsson and Grisham, 2006) have demonstrated that “transdifferentiation” of hematopoietic stem cells into “oval cells” is at best a rare event that probably does not have physiologic significance. More recently, Oh et al. (2007) reported that DPPIV+ oval cells derived from bone marrow cells and transplanted into DPPIV− mutant rats treated with 2-AAF/PH, represent 20% of total activated “oval cells”. However, after secondary transplantation of these DPPIV+ “oval cells” into secondary DPPIV deficient hosts under highly selective conditions (monocrotaline/PH), less than 0.5% of hepatocytes in the repopulated liver were DPPIV+. Therefore, bone marrow cells that had “transdifferentiated” into hepatic “oval cells” contributed very little to liver repopulation under highly selective circumstances.
In Fah−/− mice and other model systems, cell fusion has been shown to be the predominant mechanism by which hematopoietic cells acquire an hepatocytic phenotype. Initial studies in cell culture showed that BM and neuronal cells can fuse with ES cells (Terada et al. 2002; Ying et al. 2002). Wang et al. (2003) and Vassilopoulos et al. (2003) subsequently showed that hematopoietic stem cells fuse with hepatocytes in Fah null mice to produce cells that express the deficient enzyme, which then expand massively to restore liver tissue. Fusion also occurs between hematopoietic cells and neurons or muscle cells (Alvarez-Dolado et al. 2003; Weinmann et al. 2003) and it has been shown that myelomonocytic cells can fuse with hepatocytes (Camargo et al. 2004; Willenbring et al. 2004) or muscle cells (Camargo et al. 2003) to produce somatic hybrids expressing genes from both parental cell types.
Other studies have reported that fusion is not required for BM-derived cells to differentiate into hepatocytes (Newsome et al. 2003; Harris et al. 2004; Jang et al. 2004; Oh et al. 2007). Unfractionated or CD34+ enriched cells from human cord blood (Danet et al. 2002; Wang et al., 2003; Kakinuma et al. 2003; Kollet et al. 2003), multipotent adult progenitor cells (MAPC) (Schwartz et al. 2002; Jiang et al. 2002) or mesenchymal stem cells from human cord blood or bone marrow (Lee et al. 2004; Aurich et al. 2007; Brulport et al. 2007; Kuo et al. 2008) have been transplanted into the liver of immunodeficient mice. These transplanted cells express a differentiated hepatocytic phenotype (Danet et al. 2002; Wang et al. 2003; Kakinuma et al. 2003; Kollet et al. 2003; Schwartz et al. 2002; Jiang et al. 2002; Lee et al. 2004; Kogler et al. 2004; Lee et al. 2004; Anjos-Afonso et al. 2004; Sato et al. 2005; Aurich et al. 2007), but liver repopulation was once again very low. Several studies have reported that mesenchymal stem cells isolated from adipose tissue can also engraft in the liver parenchyma and contribute to liver regeneration (Banas et al. 2007; Sgodda et al. 2007). One of these studies (Sgodda et al. 2007) reported large repopulation clusters with hepatocyte-differentiated mesenchymal stem cells, but this required retrorsine pretreatment of the recipients and the overall level of liver repopulation was not reported.
Many studies have demonstrated that ES cells in culture can be induced along the endodermal and hepatocytic lineages by addition of specific cytokines and growth factors (Hamazaki et al. 2001; Jones et al. 2002; Yamada et al. 2002; Yamamoto et al. 2003; Rambhatla et al. 2003; Kubo et al. 2004; Guon-Evans et al. 2006; Heo et al. 2006; Basma et al. 2009). This typically involves the generation of embryoid bodies, followed by the induction of definitive endoderm using activin A. The endodermal population is further specified towards the hepatic lineage using BMP-4 and basic FGF. Cells produced in this fashion express typical hepatocytic markers, such as AFP and albumin. They have been transplanted into the liver under highly selective conditions and differentiate into both mature hepatocytes (Gouon-Evans et al. 2006; Heo et al. 2006; Basma et al. 2009) and bile duct epithelial cells (Heo et al. 2006). However, the level of liver repopulation obtained with hepatocyte-differentiated ES cells is very low, although somewhat higher when the cells are transplanted into MUP-uPA/SCID mice (Heo et al. 2006). To date, all ES differentiation protocols generate “hepatocyte-like” cells, but not fully mature hepatocytes. More mature “hepatocyte-like” cells have been selected using surface markers, such as the asialoglycoprotein receptor, and selected cells show higher levels of differentiated function after their transplantation (Basma et al. 2009).
Induced Pluripotent Cells (iPS)
Because of their extensive proliferative capacity, pluripotent stem cells are an attractive potential source of transplantable hepatocytes. Not only can these cells divide extensively, but they also retain the ability to differentiate into many different mature cell types (Slack, 2008). Pluripotent cells can now be derived by direct genetic reprogramming of somatic cell types, such as dermal fibroblasts (Takahashi et al. 2007; Takahashi and Yamanaka, 2006; Yu et al. 2007). Initially, this required the introduction and ectopic expression of four genes, Oct4, Klf4, Sox2 and c-myc (Takahashi et al. 2006; Takahashi et al. 2007), but more recently the same effect has been produced using recombinant proteins (Zhou et al. 2009) and some of these genes have been induced in somatic cells by treatment with small molecules (Shi et al. 2008; Ding 2009). A substantial literature is now evolving concerning the potential use of iPS cells to treat monogenic metabolic disorders. In two most recent studies (Sullivan et al. 2010; Si-Tayeb et al. 2010), iPS cells, derived from human skin fibroblasts, can be differentiated into hepatocyte-like cells in vitro at very high efficiency (80–90%), using differentiation protocols similar to those used for ES cells. In one of these studies (Si-Tayeb et al. 2010), hepatocyte differentiated hiPS cells were injected directly into the hepatic parenchyma of newborn mice and exhibited human albumin expression 7 days later. These studies are just a beginning but have major implications concerning the potential feasibility of using hepatocyte-differentiated iPS cells for therapeutic liver cell repopulation.
Hepatic Stem Cells from Human Liver
Several investigators have isolated, cultured and/or passaged human fetal liver epithelial cells with bipotential properties, and several of these studies have demonstrated their differentiation into hepatocytes after transplantation into SCID or nude mice (Malhi et al. 2002; Dan et al. 2006; Mahieu-Caputo et al. 2004). Schmelzer et al. (Schmelzer et al. 2006) have identified two populations of hepatic progenitor cells from human fetal, neonatal and pediatric liver that exhibit stem cell properties. One population is thought to represent an hepatic stem cell (AFP−/Alb+) and the other a slightly more differentiated hepatoblast (AFP+/Alb+). More recently, Schmelzer et al. (2007) reported data suggesting that these cells may reside in the Canals of Hering. One unexpected difference from their rodent counterpart is that human hepatic-specified stem cells are Alb positive but AFP negative. This is opposite to what one might expect based on expression of AFP before Alb during liver development (Shiojiri et al. 1991). Nonetheless, these studies suggest that a human liver somatic stem cell might exist and hopefully future studies will demonstrate in vivo self-renewal and long-term repopulation of the liver by these cells, proving that they are indeed stem cells.
Xenorepopulation Models
Two models demonstrating repopulation of the mouse liver with human hepatocytes have been reported. In 2001, Dandri et al. (2001) showed that immune-deficient uPA transgenic mice could be engrafted with human hepatocytes and they used this system as a model for hepatitis B virus infection; Mercer et al. (2001) used a similar model for hepatitis C virus infection. Subsequently, this model has been developed further to permit extensive repopulation, reaching levels as high as 90% human cells (Tateno et al. 2004; Meuleman et al. 2005). Highly immunodeficient Fah null mice can also be repopulated extensively with human hepatocytes (Azuma et al. 2007). This model has an advantage over uPA transgenic mice in that the level of hepatic injury can be titrated by NTBC. An additional difficulty in using the uPA transgenic mouse model has been poor breeding efficiency. However, this has recently been overcome by producing an immunodeficient, alb promoter driven-rt TA/SCID mouse that can be transduced at very high efficiency with an adenovirus TRE-uPA vector to produce an hepatocyte specific, doxycycline regulatable-uPA mouse that can be used for liver repopulation studies with xenographic cells (Song et al., 2009).
Summary of Repopulation Results from Studies Transplanting Isolated Primary Cells and Cell Lines into the Liver
Table 1 summarizes the results of selected cell transplantation/liver repopulation studies reviewed above and indicates the model systems and specific physiologic or pathophysiologic circumstances, i.e., selective or non-selective conditions, under which liver repopulation has been achieved. Under non-selective conditions, transplanted hepatocytes engraft into the liver and undergo a few cell divisions, but they do not effectively repopulate the liver. However, under highly selective conditions, hepatocytes transplanted into mice or rats can massively repopulate the liver. In most instances, the liver needs to be undergoing a high level of injury/cell turnover, but similar results can be achieved in other selective models in which the proliferation of host hepatocytes is disrupted, while transplanted exhibit normal proliferative activity. In the latter host mitoinhibitory models, a proliferative stimulus substantially accelerates liver repopulation by transplanted cells (Laconi et al 1998) but is not required (Laconi et al. 2001). “Oval cells” transplanted into the liver under non-selective conditions engraft but also undergo only limited expansion. In contrast, under strong selective conditions, “oval cells” highly repopulate the liver. Fetal liver epithelial (stem/progenitor) cells before divergence into the hepatocytic and biliary lineages exhibit substantial repopulation potential in the adult rat liver under non-selective conditions. This results from cell competition between transplanted fetal liver cells and host hepatocytes in which the fetal liver cells induce apoptosis of host hepatocytes, while they are resistant to apoptosis and continue to proliferatate (Oertel et al. 2006b). After lineage divergence, liver repopulation by fetal liver cells is markedly reduced (Sandhu et al. 2001).
TABLE 1.
Liver repopulation by transplanted cells of hepatic and non-hepatic origin
| REPORTING GROUP | SPECIES OF TRANSPLANTED CELLS |
HOST MODEL SYSTEM | % REPOPULATION | COMMENTS |
|---|---|---|---|---|
| ADULT HEPATOCYTES | ||||
| Rhim et al.1994 | mouse | Alb-uPA mouse (highly selective) |
massive (30–80%) |
first study reporting large clusters of repopulating hepatocytes throughout liver parenchyma |
| Overturf et al.1996 | mouse | Fah−/− mouse (highly selective) |
massive (> 80%) |
normal regenerated liver structure and function |
| Rajvanshi et al. 1996b | rat | DPPIV− rat/CCl4 (non-selective) |
low | scattered single cells and small clusters of DPPIV+ hepatocytes, repopulation modestly increased after 3 doses of CCl4 at weekly intervals |
| Laconi et al.1998 | rat | PH alone (non-selective) |
no significant repopulation |
isolated single cells or very small clusters of heatocytes |
| DPPIV− rat retrorsine/PH (highly selective) |
massive (up to 99%) |
normal regenerated liver structure | ||
| Mignon et al.1998 | Bcl2 transgenic mouse |
normal adult mouse/ anti-Fas Ab (8–12 weekly treatments) (moderately selective) |
moderate 3–16% |
% repopulation increased progressively with the number of anti-Fas Ad treatments |
| Guha et al.1999 | rat | DPPIV− rat x-irradiation/PH (highly selective) |
massive | similar to results obtained with retrorsine/PH |
| Dandri et al.1999 2001 |
Woodchuck human |
Alb-uPA/Rag2−/− mouse Alb-uPA/Rag2−/− mouse (highly selective) |
massive (up to 90%) 1–15% |
humanized mouse liver can be infected with hepatitis B virus |
| Malhi et al. 2002 | rat | DPPIV− rat x-irradiation/ischemic injury (highly selective) |
massive | similar to results obtained with either retrorsine/PH or x-irradiation/PH |
| Yuan et al. 2003 | P27 Kip1 null | normal adult mouse/ CCl4 x 4–8 weekly treatments (weakly selective) |
modest 2–6% |
number and size of repopulation cell clusters increased with number of CCl4 treatments |
| Tateno et al. 2004 | human | Alb-uPA/SCID mouse (highly selective) |
massive (up to 90%) | improved results based on use of Futhon, an anti-human complement factor antibody, to prevent cell rejection and kidney failure |
| Azuma et al. 2007 | human | Fah−/−/Rag2−/−/Il2rg−/− mouse (highly selective) |
7/43 recipients highly repopulated (30–90%) |
level of liver repopulation can be modulated by NTBC administration |
| Haridass et al. 2009 | mouse human |
Alb-uPA/Rag2−/−/γc−/−mouse (highly selective) |
46% 10% |
human cells less effective than mouse cells in this model system |
| Song et al. 2009 | mouse | rt TA/SCID mouse transduced with Ad. TRE-uPA (highly selective) |
highly variable | new TET-regulatable model system with improved breeding compared to Alb-uPA mouse |
| OVAL/PROGENITOR CELLS | ||||
| Dabeva et al.1997 | D-gal activated rat liver |
DPPIV− rat/PH (non-selective) |
very low (< 1%) |
scattered clusters of hepatocytes and ductular cells |
| Wang et al. 2003 | DCC treated mouse liver |
Fah−/− mouse (highly selective) |
high | somewhat lower than adult hepatocytes |
| Song et al. 2004 | DDC treated mouse liver |
mouse/monocrotaline/CCl4 (highly selective) |
high (∼40% β-gal+, 10% α1-AT+) |
β-gal transgenes mouse oval cells transduced ex vivo with human α1- antitrypsin gene |
| Oh et al. 2007 | rat BM-derived, 2-AAF/PH activated oval cells |
DPPIV− mutant rat, monocrotaline/PH (highly selective) |
<0.5% | BM-derived oval cells exhibit much lower repopulation potential than liver-derived oval cells |
| Yovchev et al. 2008 | 2-AAF or D-gal activated rat liver oval cells |
DPPIV− rats retrorsine/PH (highly selective) |
high (60–80%) |
very large clusters of repopulating hepatocytes throughout liver parenchyma |
| FETAL HEPATOBLASTS (STEM/PROGENITOR CELLS) | ||||
| Sandhu et al. 2001 | ED14 rat | DPPIV− rat PH alone (non-selective) |
5–10% | hepatocytes and bile ducts, whole new liver lobules; |
| Malhi et al. 2002 | cultured human | SCID mice/CCl4 (non-selective) |
not determined | scattered cells and small cell clusters |
| Mahieu-Caputo et al. 2004 | cultured human | nude mouse or Rag2−/−/γc−/−/50% PH (non-selective) |
up to 10%, mean ∼1% |
small clusters of transplanted human cells expressing Hep Par1, albumin and other liver specific genes |
| Nierhoff et al. 2005 | ED12.5 fetal mouse |
DPPIV−/− mouse CCl4 (non-selective) |
<1% | scattered hepatocytic and endothelial cell clusters |
| retrorsine/CCl4 (highly selective) |
up to 80% av. 10–30% |
large clusters of transplanted hepatocytes and endothelial cells |
||
| Oertel et al. 2006 | ED14 rat | DPPIV− rat PH alone (non-selective) |
20–25% | repopulation increases with number of cells transplanted; cell competition between transplanted fetal liver cells and normal host hepatocytes is mechanism responsible for liver repopulation in normal host environment |
| Haridass et al. 2009 | ED13.5 fetal mouse |
Alb-uPA/Rag2−/−/γc−/− mouse (highly selective) |
range 3–16% (mean 12.1%) |
detailed study comparing results with hepatocytes, fetal liver cells and ES cells of mouse and human origin |
| human 13–17 weeks gestation |
Alb-uPA/Rag2−/−/γc−/− (highly selective) |
2.7 ± 1.1% | cells or small clusters of cells with hepatoblast morphology throughout the liver |
|
| “STEM” CELLS (FETAL, NEONATAL AND ADULT LIVER) | ||||
| Schmelzer et al. 2007 | human | NOD/SCID mice/± CCl4 (non-selective) |
not determined | short-term repopulation (2–8 days) similar results with or without CCl4 |
| CULTURED HEPATIC EPITHELIAL CELLS AND CELL LINES | ||||
| Yasui et al. 1997 | Long-Evans Cinnamon (LEC) rat |
Nagase Analbuminemic Rat (non-selective) |
not determined | cells injected into the liver parenchyma, scattered cells and small cell clusters expressing β- galactosidase or albumin |
| Coleman et al. 1997 | WB-344 rat epithelial cell line |
DPPIV- rats (non-selective) |
not determined | cells injected into liver parenchyma |
| Suzuki et al. 2002 | mouse liver cell line |
mouse/CCl4 (non-selective) |
not determined | scattered clusters of transplanted hepatocytes and bile duct clusters |
| Strick-Marchand et al. 2004 | mouse fetal liver cell line |
Alb-uPA/SCID mouse (highly selective) |
0.8–2.1% | cells with either an hepatocytic or bile ductular phenotype reported |
| Fougère-Deschatrette et al. 2006 |
adult mouse liver cell line |
Alb-uPA/SCID mouse (highly selective) |
0.3–0.4% | hepatocytes and bile ducts |
| Dan et al. 2006 | cloned human fetal liver cell line |
Rag2−/−/γc−/−/retrorsine/CCl4 (highly selective) |
0.8–1.7% | in vitro, cloned cells differentiate into hepatocytes or bile duct epithelial cells; in vivo, clusters of cells with hepatic morphology and human albumin expression observed, as well as human albumin in mouse serum |
| Suzuki et al. 2008 | cloned CD133+ cell lines from DDC treated mice |
Fah−/− mice (highly selective) |
0.03% | moderately sized hepatocyte clusters |
| EMBRYONIC STEM (ES) CELLS | ||||
| Gouon-Evans et al. 2006 |
mouse hepatocyte- differentiated cells |
DPPIV−/−/Rag2−/− (highly selective) Fah−/− mouse (highly selective) |
not determined | scattered DPPIV+ hepatocytic and endothelial cell clusters |
| Heo et al. 2006 | mouse hepatocyte- differentiated cells |
MUP-uPA/SCID (highly selective) |
1.94% | small hepatocytic and bile duct cell clusters; increased cluster size with time |
| Duan et al. 2007 | human hepatocyte- differentiated cells |
NOD/SCID mouse (non-selective) |
not determined | cells injected under liver capsule |
| Basma et al. 2009 | human hepatocyte- differentiated cells |
Alb-uPA/SCID (highly selective) |
not determined | clusters of human Alb+ cells throughout parenchyma |
| Nagase Analbuminemic Rat/retrorsine/PH (highly selective) |
not determined | clusters of human Alb+ cells throughout parenchyma |
||
| Haridass et al. 2009 | mouse and human hepatocyte- differentiated cells |
Alb-uPA/Rag2−/−/γc−/− (highly selective) |
no significant repopulation |
mouse, a few scattered cells with hepatocytic phenotype; human, no cells observed with hepatocytic phenotype |
| INDUCED PLURIPOTENT STEM (iPS) CELLS | ||||
| Si-Tayeb et al. 2010 | human | newborn mouse | Not studied | hepatocyte-differentiated hiPS cells injected directly into the hepatic parenchyma expressed human albumin |
| NON-HEPATIC CELLS AND CELL LINES – BM OR CORD (HEMATOPOIETIC) STEM CELLS | ||||
| Petersen et al.1999 | rat | 2-AAF/CCl4 (highly selective) |
∼0.1–0.2% | scattered cells expressing transplanted cell makers |
| Theise et al. 2000 | mouse | bone marrow ablation (non-selective) |
0.37–2.2% | data corrected for 50% efficiency of Y chromosome detected by FISH in mouse tissue |
| Theise et al. 2000 | human | females transplanted with male BM cells |
5–40% | 5/6 cases studied from liver needle biopsy specimens |
| or males transplanted with a female liver |
data corrected for 20% efficiency of Y chromosome detection by FISH in human tissue |
|||
| Alison et al. 2000 | human | BM transplant recipients | 0.5–2% | scattered cells and small clusters of Y chromosome+ hepatocytes |
| Lagasse et al. 2000 | mouse | Fah−/− mouse (highly selective) |
∼30–50% | selection of FAH expressing cells produced by fusion of hematopoietic cells to hepatocytes; (40–70 very large clusters scattered throughout liver parenchyma) |
| Danet et al. 2002 | human | NOD/SCID/CCl4 (non-selective) |
<0.1% | % repopulation increased by CCl4 administration |
| Kakinuma et al. 2003 | human | SCID mice/2-AAF/1/3 PH (highly selective) |
0.1–1.0% | scattered cells and small cell clusters |
| Wang et al. 2003 | human | NOD/SCID/+ B2M+/CCl4 (non-selective) |
0.05–0.5% | scattered cells and small cell clusters |
| Newsome et al. 2003 | human | NOD/SCID mouse (non selective) |
0.01–0.02% | ∼50 human Hepar1 Ab+ cells, cell fusion not observed |
| Kollet et al. 2003 | human | NOD/SCID mice (non-selective) |
≤0.01% | rare Alb+ cells or cell clusters |
| MESENCHYMAL STEM CELLS | ||||
| Jiang et al. 2002 | cultured mouse | NOD/SCID mice (non-selective) |
5–10% in selected periportal regions in 25% of sections examined |
human/mouse cell fusion not observed |
| Aurich et al. 2007 | cultured/ differentiated human |
Pfp/Rag2−/− mice/CCl4 (non-selective) |
very low | isolated Alb+ cells and small clusters |
| Brulport et al. 2007 | human adherent, proliferating cord blood cells |
NOD/SCID or uPA/ Rag2−/− (non-selective or selective) |
not studied | human Alb+ cells were not hepatocytic |
| Kuo et al. 2008 | cultured/ differentiated human |
NOD/SCID mice/CCl4 (non-selective) |
not measured directly | rare small clusters of human Alb+ cells |
| ADIPOSE STEM CELLS | ||||
| Banas et al. 2007 | cultured human CD105+ cells |
Balb/c nude mice/CCl4 (non-selective) |
not studied | rare human Alb+ cells seen one day after cell transplantation |
| Sgodda et al. 2007 | cultured/ differentiated rat |
DPPIV− rat/retrorsine (highly selective) |
not studied | large clusters of Alb+ or α1-AT+ human cells |
Studies with cultured hepatic epithelial cells and cell lines have led to only very limited liver repopulation under either non-selective or selective conditions. The same is true for hematopoietic stem cells (with one notable exception), embryonic stem cells or mesencymal stem cells. Under most highly selective conditions, Lagasse et al. (2000) showed extensive repopulation of Fah−/− mouse liver by hematopoietic cells, which was later shown to result from fusion of hematopoietic progenitor cells with host hepatocytes (Wang et al. 2003; Vassilopoulos et al. 2003).
Figure 6 is a schematic diagram that highlights the inverse relationship between the proliferative/repopulation potential of fetal liver epithelial cells and their differentiation. After endodermal cells become specified to the hepatic lineage, there is a progressive decrease in the repopulation potential as the cells differentiate. Initially, the cells (early progenitor cells or stem/progenitor cells) are capable of repopulating a normal liver tissue environment, but as the cells differentiate further and diverge along the hepatocytic or cholangiocytic lineage, their repopulation potential declines substantially and they do not effectively repopulate the normal liver under non-selective conditions. Both unipotential hepatic progenitor cells and fully differentiated hepatocytes repopulate the liver only under highly selective conditions. Results obtained with rat fetal liver “progenitor cells” after ED16 are comparable to those obtained with “oval cells” isolated from the adult liver (see Table I). In both instances, some of the cells are bipotential (expressing hepatocytic and cholangiocytic genes) and can be clonally propagated. However, they have lesser repopulation potential than fetal liver stem/progenitor cells (ED14) isolated before lineage divergence. This is a potential area for future investigation. Genetic signals in the transplanted cells and paracrine signals emanating from the host liver environment in which the cells have engrafted undoubtedly contribute to the differences in repopulation potential under non-selective vs. selective conditions, and this is also an important area for future investigation.
Figure 6.
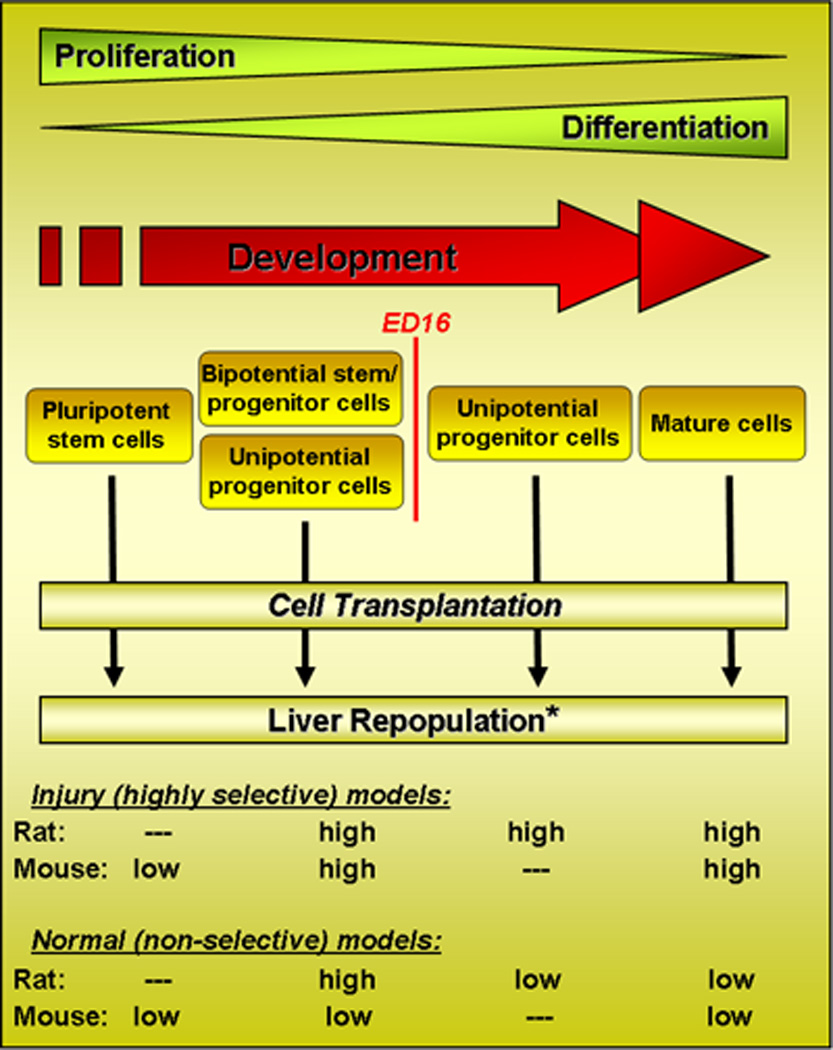
Schematic diagram illustrating the relationship of fetal liver hepatic lineage progression to the repopulation potential of isolated hepatic cells under non-selective vs. highly selective repopulation conditions.
Future Perspective
From many recent studies, it is clear that mammalian cells exhibit a high degree of phenotypic plasticity and that previous distinctions between stem cells, progenitor cells and mature differentiated cells are somewhat arbitrary. Based on the experimental conditions under which cells are tested for proliferative and differentiated functions, either in vitro or in vivo, mammalian cells exhibit different patterns of gene expression, resulting in different cellular behavior. Since the functional properties of the cells depend upon the context in which the cells are maintained and the extracellular signals that they receive from their surrounding environment, the ability of such cells to repopulate a tissue or organ will depend not only on their innate properties, but also on the tissue environment in which the cells are located. Therefore, for effective tissue repopulation to be achieved, it will be important to identify the signals and mechanisms that regulate the specific properties of the cells and to determine how the behavior of the cells can be modulated by external signals.
The emphasis of this review was to indicate the importance of the tissue environment in determining how a specific cell type might behave in terms of specific tissue repopulation. The presentation focused on whether the host tissue environment was either selective or non-selective for repopulation by a particular transplanted cell, at what stage in specific lineage progression was the transplanted cell at the time it was transplanted and how does this impact on the level of tissue repopulation achieved. The next steps will be to determine how these processes are regulated and to identify methods to modulate these processes and enhance the repopulation potential of a given cell type. Effective modulation of these processes in vivo may seem like science fiction today, but at some time in the future, this will become routine medical practice.
Acknowledgements
Research reported from the author’s laboratories was supported in part by NIH grants R01 DK17609 and P30 DK041296 to DAS and an AFAR Research Grant from the American Federation for Aging Research (AFAR) and a Pilot and Feasibility Study from P30DK041296 to MO. The authors thank Anna Caponigro for editorial assistance in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akhurst B, Croager EJ, Farley-Roche CA, Ong JK, Dumble ML, Knight B, et al. A modified choline deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology. 2001;34:519–522. doi: 10.1053/jhep.2001.26751. [DOI] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell types under minimal damage conditions. J Cell Sci. 2004;117:5655–5664. doi: 10.1242/jcs.01488. [DOI] [PubMed] [Google Scholar]
- Arends B, Vankelecom H, Vander Borght S, Roskams T, Penning LC, Rothuizen J, et al. The dog liver contains a “side population” of cells with hepatic progenitor-like characteristics. Stem Cells Dev. 2009;18:343–350. doi: 10.1089/scd.2008.0022. [DOI] [PubMed] [Google Scholar]
- Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger M, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56:405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- Berry MN, Friend DS. High yield preparation of rat liver parenchyma cells: A biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basma H, Soto-Gutiérrez A, Yannam GR, Liu L, Ito R, Yamamoto T, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brulport M, Schormann W, Bauer A, Hermes M, Elsner C, Hammersen FJ, et al. Fate of extrahepatic human stem and precursor cells after transplantation into mouse livers. Hepatology. 2007;46:861–870. doi: 10.1002/hep.21745. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J Clin Invest. 2004;113:1266–1270. doi: 10.1172/JCI21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- Coleman WB, McCullough KD, Esoh GL, Faris RA, Hixson DC, Smith GJ, et al. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- Crosby HA, Kelly DA, Strain AJ. Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology. 2001;120:534–544. doi: 10.1053/gast.2001.21175. [DOI] [PubMed] [Google Scholar]
- Dabeva MD, Hwang S-G, Vasa SRG, Hurston E, Novikoff PM, Hixson DC, et al. Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci USA. 1997;94:7356–7361. doi: 10.1073/pnas.94.14.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, et al. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol. 2000;156:2017–2031. doi: 10.1016/S0002-9440(10)65074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeva MD, Shafritz DA. Liver reconstitution by hepatic stem and progenitor cells, Chapter 10. In: Garcia-Olmo D, Garcia-Verdugo JM, Alemany J, Gutierrez-Fuentes JA, editors. Cell therapy. McGraw-Hill: 2008. pp. 107–122. [Google Scholar]
- Dabeva MD, Shafritz DA. Activation, proliferation and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol. 1993;143:1606–1620. [PMC free article] [PubMed] [Google Scholar]
- Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, et al. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci USA. 2006;103:9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- Danet GH, Luongo JL, Butler G, Lu MM, Tenner AJ, Simon MC, et al. ClqRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc Natl Acad Sci USA. 2002;99:10441–10445. doi: 10.1073/pnas.162104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Demetriou AA, Levenson SM, Novikoff PM, Novikoff AB, Chowdhury NR, Whiting J, et al. Survival, organization and function of microcarrier-attached hepatocytes transplanted in rats. Proc Natl Acad Sci USA. 1986;83:7475–7479. doi: 10.1073/pnas.83.19.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezso K, Jelnes P, László V, Baghy K, Bödör C, Paku S, et al. Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver regeneration. Am J Pathol. 2007;171:1529–1537. doi: 10.2353/ajpath.2007.070273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S. A chemical approach to pluripotency and reprogramming. Fourth Annual Translational Stem Cell Research Conference; October 13–14, 2009; The New York Stem Cell Foundation: [Google Scholar]
- Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, et al. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- Factor VM, Radaeva SA, Thorgeirsson SS. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol. 1994;145:409–422. [PMC free article] [PubMed] [Google Scholar]
- Farber E. Similarities of the sequence of the early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3’-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler-Najjar Syndrome Type 1 with hepatocyte transplantation. New Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- Fougere-Deschatrette C, Imaizumi-Scherrer T, Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, et al. Plasticity of hepatic cell differentiation: bipotential adult mouse liver clonal cell lines competent to differentiate in vitro and in vivo. Stem Cells. 2006;24:2098–2109. doi: 10.1634/stemcells.2006-0009. [DOI] [PubMed] [Google Scholar]
- Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511–516. [PubMed] [Google Scholar]
- Goodell MA. Stem-cell “plasticity”: befuddled by the muddle. Curr Opin Hematol. 2003;10:208–213. doi: 10.1097/00062752-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Gossrau R. Peptidases II. Localization of dipeptidylpeptidase IV (DPP IV). Histochemical and biochemical study. Histochemistry. 1979;60:231–248. doi: 10.1007/BF00495756. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Grisham JW. Cell types in long-term propagable cultures of rat liver. Ann NY Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59:5871–5874. [PubMed] [Google Scholar]
- Guo D, Fu T, Nelson JA, Superina RA, Soriano HE. Liver repopulation after cell transplantation in mice treated with retrorsine and carbon tetrachloride. Transplantation. 2002;73:1818–1824. doi: 10.1097/00007890-200206150-00020. [DOI] [PubMed] [Google Scholar]
- Gupta S, Chowdhury NR, Jagtiani R, Gustin K, Aragona E, Shafritz DA, et al. A novel system for transplantation of isolated hepatocytes utilizing HBsAg producing transgenic donor cells. Transplantation. 1990;50:472–475. [PubMed] [Google Scholar]
- Gupta S, Johnstone R, Darby H, Selden C, Price Y, Hodgson HJ. Transplanted isolated hepatocytes: Effect of partial hepatectomy on proliferation of long-term syngeneic implants in rat spleen. Pathology. 1987;19:28–30. doi: 10.3109/00313028709065131. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacheam AM, Zon LI, et al. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497:15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- Haridass D, Yuan Q, Becker PD, Cantz T, Iken M, Rothe M, et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am J Pathol. 2009;175:1483–1492. doi: 10.2353/ajpath.2009.090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- Heo J, Factor VM, Uren T, Takahama Y, Lee JS, Major M, et al. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology. 2006;44:1478–1486. doi: 10.1002/hep.21441. [DOI] [PubMed] [Google Scholar]
- Hubbard AL, Bartles JR, Braiterman LT. Identification of rat hepatocyte plasma membrane proteins using monoclonal antibodies. J Cell Biol. 1985;100:1115–1125. doi: 10.1083/jcb.100.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- Jiang J, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Biles C, Michalopoulos G. Morphologic and histochemical analysis of hepatocytes transplanted into syngeneic hosts. Am J Pathol. 1980;101:115–126. [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Michalopoulos G. Effects of partial hepatectomy on transplanted hepatocytes. Cancer Res. 1982;42:3000–3004. [PubMed] [Google Scholar]
- Jones EA, Tosh D, Wilson DI, Lindsay S, Forrester LM. Hepatic differentiation of murine embryonic stem cells. Exp Cell Res. 2002;272:15–22. doi: 10.1006/excr.2001.5396. [DOI] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblasts growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, et al. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217–227. doi: 10.1634/stemcells.21-2-217. [DOI] [PubMed] [Google Scholar]
- Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano M, Mito M. Observations on the fine structure of long-survived isolated hepatocytes inoculated into rat spleen. Gastroenterology. 1982;82:616–628. [PubMed] [Google Scholar]
- Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol. 2001;158:771–777. doi: 10.1016/s0002-9440(10)64019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis CS, Yamanouchi K, Zhou H, Mohan S, Roy-Chowdhury N, Shafritz DA, et al. Noninvasive evaluation of liver repopulation by transplanted hepatocytes using 31P MRS imaging in mice. Hepatology. 2006;44:1250–1258. doi: 10.1002/hep.21382. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Shiojiri N, Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- Mahieu-Caputo D, Allain JE, Branger J, Coulomb A, Delgado JP, Andreoletti M, et al. Repopulation of athymic mouse liver by cryopreserved early human fetal hepatoblasts. Hum Gene Ther. 2004;15:1219–1228. doi: 10.1089/hum.2004.15.1219. [DOI] [PubMed] [Google Scholar]
- Malhi H, Gorla GR, Irani AN, Annamaneni P, Gupta S. Cell transplantation after oxidative hepatic preconditioning with radiation and ischemia-reperfusion leads to extensive liver repopulation. Proc Natl Acad Sci USA. 2002;99:13114–13119. doi: 10.1073/pnas.192365499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679–2688. doi: 10.1242/jcs.115.13.2679. [DOI] [PubMed] [Google Scholar]
- Marceau N, Blouin M-J, Noel M, Torok N, Loranger A. The role of bipotential progenitor cells in liver oncogenesis and neoplasia. In: Sirica AE, editor. The role of cell types in hepatocarinogenesis. Boca Raton: CRC Press; 1992. pp. 121–149. [Google Scholar]
- Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasma bilirubin in Gunn rats. Science. 1976;192:892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- Menthena A, Deb N, Oertel M, Grozdanov PN, Sandhu J, Shah S, et al. Bone marrow progenitors are not the source of expanding oval cells in injured liver. Stem Cells. 2004;22:1049–1061. doi: 10.1634/stemcells.22-6-1049. [DOI] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41:847–856. doi: 10.1002/hep.20657. [DOI] [PubMed] [Google Scholar]
- Mignon A, Guidotti JE, Mitchell C, Fabre M, Wernet A, De La Coste A, et al. Selective repopulation of normal mouse liver by Fas/CD-95 resistant hepatocytes. Nat Med. 1998;4:1185–1188. doi: 10.1038/2681. [DOI] [PubMed] [Google Scholar]
- Moreno E, Basler K. dMyc transforms cells into super-competitors. Cell. 2004;117:117–129. doi: 10.1016/s0092-8674(04)00262-4. [DOI] [PubMed] [Google Scholar]
- Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891–1900. doi: 10.1016/s0016-5085(03)00401-3. [DOI] [PubMed] [Google Scholar]
- Nierhoff D, Ogawa A, Oertel M, Chen YQ, Shafritz DA. Purification and characterization of mouse fetal liver epithelial cells with high in vivo repopulation capacity. Hepatology. 2005;42:130–139. doi: 10.1002/hep.20735. [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Chen Y-Q, Shafritz DA. Properties of cryopreserved fetal liver stem/progenitor cells that exhibit long-term repopulation of the normal rat liver. Stem Cells. 2006;24:2244–2251. doi: 10.1634/stemcells.2006-0141. [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Chen Y-Q, Shafritz DA. Comparison of hepatic properties and transplantation of Thy1+ and Thy-1 cells isolated from ED14 rat fetal liver. Hepatology. 2007;46:1236–1245. doi: 10.1002/hep.21775. [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Chen Y-Q, Teisner B, Harken-Jensen C, Shafritz DA. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology. 2008;134:823–832. doi: 10.1053/j.gastro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Oertel M, Menthena A, Dabeva MD, Shafritz DA. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology. 2006;130:507–520. doi: 10.1053/j.gastro.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, et al. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroentoerology. 2007;132:1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Omori M, Omori N, Evarts RP, Teramoto T, Thorgeirsson SS. Co-expression of flt-3 ligand/flt-3 and SCF/c-kit signal transduction system in bile-duct-ligated SI and W mice. Am J Pathol. 1997;150:1179–1187. [PMC free article] [PubMed] [Google Scholar]
- Omori N, Evarts RP, Omori M, Hu Z, Marsden ER, Thorgeirsson SS. Expression of leukemia inhibitory factor and its receptor during liver regeneration in the adult rat. Lab Invest. 1996;75:15–24. [PubMed] [Google Scholar]
- Omori N, Omori M, Evarts RP, Teramoto T, Miller MJ, Hoang TN, et al. Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver cell regeneration in the adult rat. Hepatology. 1997;26:720–727. doi: 10.1002/hep.510260325. [DOI] [PubMed] [Google Scholar]
- Oren R, Dabeva M, Petkov P, Hurston E, Laconi E, Shafritz DA. Restoration of normal serum albumin levels in Nagase analbuminemic rats using a newly described strategy for hepatocyte transplantation. Hepatology. 1999;29:75–81. doi: 10.1002/hep.510290147. [DOI] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;51:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol. 2001;158:1313–1323. doi: 10.1016/S0002-9440(10)64082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- Petkov PM, Zavadil J, Goetz D, Chu T, Carver R, Rogler CE, et al. Gene expression pattern in hepatic stem/progenitor cells during rat fetal development using complementary DNA microarrays. Hepatology. 2004;39:617–627. doi: 10.1002/hep.20088. [DOI] [PubMed] [Google Scholar]
- Ponder KP, Gupta S, Leland F, Darlington G, Finegold M, DeMayo J, et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci USA. 1991;88:1217–1221. doi: 10.1073/pnas.88.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Stem cells. 1st ed. London: Academic Press, Ltd; 1997. [Google Scholar]
- Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest. 1999;79:103–109. [PubMed] [Google Scholar]
- Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. Efficacy and safety of repeated hepatocyte transplantation for significant liver repopulation in rodents. Gastroenterology. 1996;111:1092–1102. doi: 10.1016/s0016-5085(96)70078-1. [DOI] [PubMed] [Google Scholar]
- Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. Studies of liver repopulation using the dipeptidyl peptidase IV-deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology. 1996;23:482–496. doi: 10.1002/hep.510230313. [DOI] [PubMed] [Google Scholar]
- Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- Rao MS, Dwivedi RS, Subbarao V, Usman MI, Scarpelli DG, Nemali MR, et al. Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation. Biochem Biophys Res Commun. 1988;156:131–136. doi: 10.1016/s0006-291x(88)80814-3. [DOI] [PubMed] [Google Scholar]
- Rhim J, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Lacy PE, Callery MP, Park PW, Flye MW. Trophic factors from pancreatic islets in combined hepatocyte-islet allografts enhance hepatocellular survival. Surgery. 1989;105:218–223. [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BML, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–929. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel A, Jago MW. Localization in the cell cycle of the antimitotic action of the pyrrolizidine alkaloid, lasiocarpine and of its metabolite, dehydroheliotridine. Chem Biol Interact. 1975;10:185–197. doi: 10.1016/0009-2797(75)90112-x. [DOI] [PubMed] [Google Scholar]
- Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell. 1991;66:245–256. doi: 10.1016/0092-8674(91)90615-6. [DOI] [PubMed] [Google Scholar]
- Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am J Pathol. 2001;159:1323–1334. doi: 10.1016/S0002-9440(10)62519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Katyal SL, Shinozuka H, Estes LW, Sell S, Lombardi B. Isolation of oval cells and transitional cells from the livers of rats fed the carcinogen DL-ethionine. J Natl Cancer Inst. 1981;66:355–362. [PubMed] [Google Scholar]
- Sgodda M, Aurich H, Kleist S, Aurich I, König S, Dollinger MM, et al. Hepatocyte differentiation of mesencymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313:2875–2886. doi: 10.1016/j.yexcr.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Shafritz DA, Oertel M, Menthena A, Nierhoff D, Dabeva MD. Liver stem cells and prospects for liver reconstitution by transplanted cells. Hepatology. 2006;43:S89–S98. doi: 10.1002/hep.21047. [DOI] [PubMed] [Google Scholar]
- Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Shiojiri N, Lemire JM, Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991;51:2611–2620. [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Hepatology. 2010. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells (p NA) in press, PMID: 19998274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM. Origin of stem cells in organogenesis. Organ Development. 2008;322:1498–1501. doi: 10.1126/science.1162782. [DOI] [PubMed] [Google Scholar]
- Song S, Witek RP, Lu Y, Choi YK, Zheng D, Jorgensen M, et al. Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice. Hepatology. 2004;40:918–924. doi: 10.1002/hep.20404. [DOI] [PubMed] [Google Scholar]
- Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, Weiss MC. Bipotential mouse embryonic liver stem cell lines contribute to liver regeneration and differentiate as bile ducts and hepatocytes. Proc Natl Acad Sci USA. 2004;101:8360–8365. doi: 10.1073/pnas.0401092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K, Wang G, Daley GQ, Lee JH, Church GM, Forbes SJ, Iredale JP, Wilmut I. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010 doi: 10.1002/hep.23335. in press, PMID: 19877180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H, et al. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology. 2008;48:1964–1978. doi: 10.1002/hep.22558. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–1239. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- Tatematsu M, Ho RH, Kaku T, Ekem JK, Farber E. Studies on the proliferation and fate of oval cells in the liver of rats treated with 2-acetylaminofluorine and partial hepatectomy. J Pathol. 1984;114:418–430. [PMC free article] [PubMed] [Google Scholar]
- Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 2004;165:901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, et al. Deriviation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocyst. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thompson NL, Hixson DC, Callanan H, Panzica M, Flanagan D, Faris RA, et al. A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein. Use as a liver-cell transplantation model. Biochem. J. 1991;273:497–502. doi: 10.1042/bj2730497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson SS, Grisham JW. Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology. 2006;43:2–8. doi: 10.1002/hep.21015. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;42:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Walborg EF, Jr, Tsuchida S, Weeden DS, Thomas MW, Barrick A, McEntire KD, et al. Identification of dipeptidyl peptidase IV as a protein shared by the plasma membrane of hepatocytes and liver biomatrix. Exp Cell Res. 1985;158:509–518. doi: 10.1016/0014-4827(85)90474-4. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Liver repopulation and correction of metabolic liver disease by transplanted adult mouse pancreatic cells. Am J Pathol. 2001;158:571–579. doi: 10.1016/S0002-9440(10)63999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100:11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959–966. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- Witek RP, Fisher SH, Petersen BE. Monocrotaline, an alternative to retrorsine-based hepatocyte transplantation in rodents. Cell Transpl. 2005;14:41–47. doi: 10.3727/000000005783983278. [DOI] [PubMed] [Google Scholar]
- Wright N, Samuelson L, Walkup MH, Chandrasekaran P, Gerber DA. Enrichment of a bipotent hepatic progenitor cell from naïve adult liver tissue. Biochem Biophys Res Comm. 2008;366:367–372. doi: 10.1016/j.bbrc.2007.11.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, et al. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T. Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology. 1997;25:329–334. doi: 10.1053/jhep.1997.v25.pm0009021943. [DOI] [PubMed] [Google Scholar]
- Yin L, Lynch D, Sell S. Participation of different cell types in the restitutive response of the rat liver to periportal injury induced by allyl alcohol. J Hepatol. 1999;31:497–507. doi: 10.1016/s0168-8278(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Ying Q-L, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2007;45:139–149. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47:636–647. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yuan RH, Ogawa A, Ogawa E, Neufeld D, Zhu L, Shafritz DA. p27Kip1 inactivation provides a proliferative advantage to transplanted hepatocytes in DPPIV/Rag2 double knockout mice after repeated host liver injury. Cell Transplant. 2003;12:907–919. doi: 10.3727/000000003771000147. [DOI] [PubMed] [Google Scholar]
- Zhao R, Duncan SA. Embryonic development of the liver. Hepatology. 2005;41:956–967. doi: 10.1002/hep.20691. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, et al. Cell Stem Cell. Brief Report. Cell Press: Elsevier, Inc; 2009. Generation of induced pluripotent stem cells using recombinant proteins; pp. 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]