Abstract
While multiple pathways of dendritic cell (DC) maturation result in transient production of IL-12, fully mature DCs show reduced ability to produce IL-12p70 upon a subsequent interaction with Ag-specific T cells, limiting their in vivo performance as vaccines. Such “DC exhaustion” can be prevented by the presence of IFNγ during the maturation of human DCs (type-1-polarization), resulting in improved induction of tumor-specific Th1 and CTL responses in vitro. Here, we show that type-1 polarization of mouse DCs strongly enhances their ability to induce CTL responses against a model tumor antigen, OVA, in vivo, promoting the induction of protective immunity against OVA-expressing EG7 lymphoma. Interestingly, in contrast to the human system, the induction of mouse DC1s requires the participation of IL-4, a nominal Th2-inducing cytokine. The current data help to explain the previously reported Th1-driving and anti-tumor activities of IL-4, and demonstrate that type-1 polarization increases in vivo activity of DC-based vaccines.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-008-0648-5) contains supplementary material, which is available to authorized users.
Keywords: Dendritic cells, Vaccines, Lymphoma, Mouse, IL-12, IL-4
Introduction
Dendritic cells (DCs) are professional antigen presenting cells that are crucial for inducing immune responses and in directing them to adequately respond to different types of pathogens [2]. In their immature state, DCs are highly efficient in the uptake and processing of antigen (Ag), but express low levels of costimulatory molecules that are required to activate T cells. Upon maturation, DCs upregulate MHC expression, enhancing their ability to engage TCR (“signal 1”), and the expression of CD80/CD86 (B7.1/2) and CD40, to provide the required costimulatory molecules (“signal 2”). In addition, it is becoming increasingly clear that DCs are instructed to preferentially induce type-1 (Th1- and CTL-dominated) or type-2 (Th2 and B cell-dominated) responses (“signal 3”) during maturation [13, 37].
The ability of DCs to generate potent type-1 immune responses has made them highly attractive for use as cancer vaccines where cytolytic immunity is desired for the clearance of tumor cells [1]. Unfortunately, the maturation stimuli commonly used in the preparation of DC-based vaccines induce only transient production of IL-12, the key cytokine promoting type-1 immune responses [36], and limit their physiologically relevant ability to produce IL-12 after subsequent activation at a fully-mature stage during the interaction with T cells [15, 19]. Numerous studies have demonstrated that co-administration of IL-12 [6] or genetic engineering of DCs to produce high levels of IL-12 [21, 22, 42, 43], strongly enhances the antitumor efficacy of cancer vaccines. However, the high toxicity of IL-12 limits the clinical use of therapies relying on systemically-administered or constitutively-produced IL-12 [29, 33]. The use of DCs that have undergone short-term stimulation directly before vaccine administration, allowing the transient IL-12 production after DC injection [10, 20, 23], have been shown to improve anti-tumor responses but do not alleviate concerns of DC exhaustion before they reach the lymph node, particularly in the human system where the LN migration of DCs can take up to 48 h [5].
In order to allow the controlled release of IL-12 and to focus it at the relevant time and site of DC interaction with tumor-specific T cells, we have developed several methods of maturing human DCs while preserving or even enhancing their ability to secrete high levels of IL-12 during subsequent interaction with T cells [14, 17, 24–27, 37, 38]. Such “type-1-polarized DCs” (DC1 s), induced by inflammatory cytokines, memory CD8+ T cells or properly activated NK cells, [17, 24–27, 37, 38] show a strongly-elevated ability to activate the Th1-pathway of differentiation of CD4+ T cells [24–26, 37, 38] and to induce tumor-specific Th1 cells and CTLs during in vitro sensitization [14, 27, 40].
While the above in vitro data provides compelling evidence to argue for the use of this maturation strategy for DC-based cancer vaccines, it remains to be seen if polarized DCs, that rely on the interaction with T cells for optimal IL-12 secretion [25–27, 37], instead of spontaneously producing this factor, are capable of promoting type-1 anti-tumor responses in vivo. To date, there are no mouse models that replicate the DC1 polarized phenotype.
While, similar to the human studies [16, 18], mouse IFNγ and IL-4 have been shown to co-stimulate IL-12 production when applied simultaneously with CD40L [9] or TLR ligands [9, 10], resulting in a transient production of high levels of IL-12, the ability of such factors to prime DCs for high IL-12 production upon a subsequent stimulation has not been determined. Here, we report that IL-4 and IFNγ synergistically act to prime maturing bone marrow-derived DCs for high IL-12 production upon a subsequent CD40 stimulation, providing a mouse model of type-1-polarized DCs. Compared to mouse non-polarized DCs, such type-1-polarized DCs show a superior ability to induce Ag-specific immune responses in vivo and to induce a protective immunity against tumor challenge.
Materials and methods
Mice
Female 6–8-week-old C57BL/6 and C57BL/6-IL-12tm1Jm (IL-12p40 knockout) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were maintained in the Hillman Cancer Center Animal Facility and all animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Cell lines
EL4 and EG7 (an OVA-expressing EL4 variant) cell lines, purchased from ATCC (Manassas, VA, USA), were cultured in complete medium (CM; RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, 100 μg/mL streptomycin and 10 mM l-glutamine; all purchased from Invitrogen, Carlsbad, CA, USA) in a humidified incubator at 5% CO2 at 37°C.
Generation of bone marrow-derived dendritic cells (DCs)
Bone marrow was isolated from the femur and tibia of C57BL/6 wild-type (wt) mice (or IL-12p40 KO mice, where indicated). RBCs were lysed with ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2). Cells were washed and resuspended in CM supplemented with 1,000 U/mL GM-CSF (Schering Plough, Kenilworth, NJ, USA), as described [34]. On day 6–7, CD11c+ DCs were isolated using anti-mouse CD11c-coated magnetic beads and MACS™ separation columns (Miltenyi Biotech), according to manufacturer’s protocol. The DCs were matured for 24 h in the presence of 250 ng/mL LPS (Sigma-Aldrich), 1,000 U/mL IL-4, 1,000 U/mL IFNγ, 20 μg/mL poly(I:C) (Sigma-Aldrich), and/or 10 μg/mL CpG (type A, Coley Pharmaceuticals), as indicated. All maturation conditions contained 1,000 U/mL GM-CSF to sustain cell viability.
Antibodies and flow cytometry
The following Abs were used for flow cytometry analysis: anti-CD11c (N418), anti-CD86 (GL1), anti-CD40 (HM40-3), and anti-IAb (AF6-120 1). All antibodies were purchased from BD Biosciences (Mountain View, CA, USA). Directly conjugated Abs (PE or FITC) were added to cells and incubated at 4°C for 20 min, washed, and fixed in 75 μL of 2% paraformaldehyde. Fixed cells were analyzed within 12–72 h on a Beckman Coulter XL. Analyses were performed using WinMDI software, version 2.8.
Induction of IL-12 production
Mature DCs were harvested, washed and stimulated (2 × 104 cells/0.2 mL/well) with 5 × 104 CD40L-transfected J558 cells [16, 27, 30, 39] for 24 h in the absence or presence of maturation cytokines (as indicated). All stimulation conditions contained 1,000 U/mL GM-CSF, to sustain cell viability. IL-12p70 concentrations were determined by ELISA (Endogen), according to the manufacturer’s protocol.
Induction and quantification of OVAp-specific immune responses
The dominant H-2 Kb-restricted OVA epitope (OVAp), OVA257–264 (SIINFEKL) was synthesized by the University of Pittsburgh Peptide Synthesis Facility. Matured DCs were pulsed with OVAp, washed twice with PBS, and injected s.c. (3 × 103 DCs in 0.2 mL PBS). To quantify the OVAp-specific immune responses, spleens were harvested from vaccinated animals (two mice per group, 2 weeks post-vaccination) and splenocyte suspensions were made by dissociation of tissue through a 70 μm filter. RBCs were lysed with ACK lysis buffer and cells were washed and resuspended in CM. Splenocytes were restimulated ex vivo with irradiated OVAp-pulsed EL4 cells. On day 5, cells were harvested and re-plated (1 × 105 cells/well) with OVAp-pulsed or unpulsed EL4 cells (5 × 103 cells/well) for 24 h for IFNγ ELISpot analysis (mAb Tech), according to manufacturer’s protocol.
Tumor protection models
To determine if differentially matured DCs varied in their ability to mediate protective immune responses to a subsequent tumor challenge, C57BL/6 mice were vaccinated with OVAp-pulsed DCs (5–8 mice per group), as described above. Six weeks post-vaccination, mice were inoculated s.c. on the flank with EG7 (3 × 106 cells/0.2 mL). Tumors were measured with vernier calipers every 2–3 days. Data are reported as the mean ± STD of tumor volume: width2 × length × 0.52, where the width is the smaller of two perpendicular diameters.
Statistical analysis
Comparison of tumor growth on the last day all animals were alive was determined using an exact Jonckheere–Terpstra test for equality of the three groups. If the Jonckheere–Terpstra test was significant at P = 0.05, pairwise contrasts were compared with the exact two-tailed Wilcoxon test. Animal survival was defined as the time in days between tumor cell inoculation and the day of sacrifice, when the tumor diameter reached 2 cm. The comparison of animal survival was performed by an exact log rank test, P ≤ 0.05 was considered as significant.
Results
Combination of IL-4 and IFNγ primes maturing DCs for high IL-12 production
In order to determine whether type-1 polarization of DCs can be used to enhance the induction of type-1 immunity in vivo, we needed to establish the conditions of maturation of mouse bone marrow-derived DCs (DC) leading to the generation of mature DCs with enhanced, rather than exhausted ability to produce IL-12, following their follow-up stimulation. Previous reports have examined the IL-12-enhancing impact of IL-4 when present during the CD40L stimulation [2, 29, 31]. However, the ability of this cytokine to prime DCs for subsequent IL-12 production (DC polarization) has not been determined.
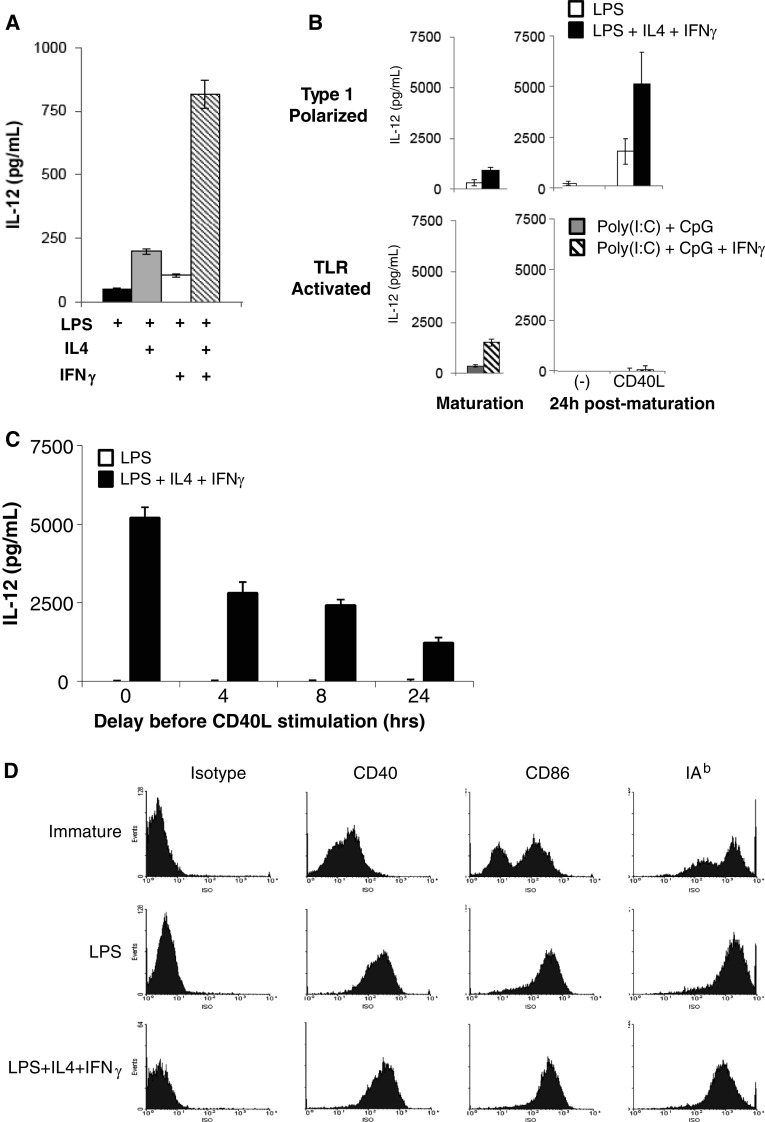
As shown in Fig. 1a, the presence of IL-4 during the LPS-induced DC maturation resulted in a strong enhancement of the ability of the resulting mature DCs to produce IL-12 after the removal of this factor and subsequent stimulation of DCs with CD40L. Interestingly, in contrast to the human system where IFNγ, rather than IL-4, is the key factor responsible for costimulation of IL-12 production [16] and is required for priming of DCs for IL-12 production [37], the presence of IFNγ alone at the maturation stage had only marginal impact on the ability of mature DCs to produce IL-12 (Fig. 1a). However, IFNγ strongly synergized with IL-4 in priming DCs for high IL-12 production. This key requirement for IL-4 at the stage of DC maturation was similarly evident in the DCs that were obtained from bone marrow precursors in each of the alternative protocols of immature DC generation, either in the absence [11, 12, 34] or in the presence [34, 35] of IL-4 at the early stage of DC cultures (Electronic Supplementary Material (ESM) Fig. 1). The priming effects of IL-4 and IFNγ were also seen when the DCs were stimulated with CD40L in the additional presence of high concentrations of these cytokines (ESM Fig. 2), arguing against the role of possible carry-over of minor amounts of these factors from the maturation cultures in determining the differences in IL-12-producing capacity of the differentially matured DCs.
Fig. 1.
Polarized DCs produce increased amounts of IL-12 as compared to equally mature, non-polarized DCs. CD11c+ DCs were positively isolated from day eight bulk cultures differentiated in presence of GM-CSF. a After 1 day of maturation with LPS or polarization with LPS, IL-4 and/or IFNγ, cells were harvested and co-incubated with a CD40L-transfected cell line (J558-CD40L) without co-stimulating cytokines for 24 h. IL-12 in the supernatant was measured by ELISA. b Spontaneous and induced IL-12 production measured for cytokine-matured (upper panels, LPS or LPS, IL-4, IFNγ) and TLR ligand-matured (lower panels, poly-(I:C)/CpG or poly-(I:C)/CpG/IFNγ) DCs. Supernatants were harvested after 24 h maturation (spontaneous) and after matured DCs were stimulated with J558-CD40L cells for an additional 24 h. c After 1 day of maturation with LPS or LPS, IL4 and IFNγ, cells were harvested and replated in medium containing only GM-CSF. J558-CD40L cells were added at the indicated times and supernatants were harvested 24 h later to measure IL-12 production after delayed CD40L stimulation. d Expression of maturation markers on immature DCs, non-polarized (LPS-matured) and polarized (LPS, IL-4 and IFNγ-matured) DCs. Representative histograms are shown. Data represent one of three independent experiments
Similar to human type-1 polarized DCs that rely on the secondary stimulation to produce high levels of IL-12 [37], mouse DC1 s generated in the presence of LPS, IL-4 and IFNγ produced only low levels of IL-12 spontaneously but produce very high levels of IL-12 when stimulated with CD40L, even when such stimulation is delayed up to 24 h after maturation (Fig. 1b, c). In this regard, these cells were clearly a superior model of human polarized DC1s, compared to the recently proposed mouse model involving the DCs matured in combination of distinct of TLR ligands [10]. In contrast to such type-1-polarized DCs, DCs matured in the presence of a combination of TLR ligands showed a transient production of IL-12 during maturation, but did not continue to produce IL-12 following their removal from the maturation cultures, neither spontaneously nor upon their subsequent stimulation with CD40L (Fig. 1b).
In accordance with their fully-mature phenotype, non-polarized and type-1-polarized DCs generated in the presence of LPS, IL-4 and IFNγ showed uniformly high levels of CD86 and I-Ab (Fig. 1d), similar to their LPS-matured non-polarized counterparts, further indicating their similarity to human type-1 polarized DCs [27, 37].
Superior immunostimulatory activity of polarized DC1s in vivo: key role of IL-12/IL-23
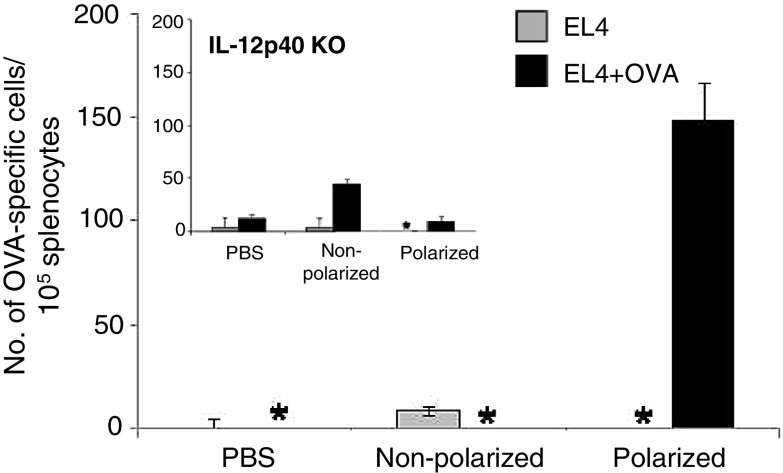
In order to determine if the DCs matured by LPS in the presence of IL-4 and IFNγ have an enhanced capacity to induce antigen-specific type-1 immune responses in vivo, C57BL/6 mice were vaccinated with the differentially matured DCs (3 × 103 DCs) pulsed with the H-2 Kb-restricted dominant CD8+ T cell epitope of chicken ovalbumin (OVA257–264; SIINFEKL). The frequency of OVAp-specific T cells was determined two weeks post-vaccination, using IFNγ ELISPOT of the ex vivo restimulated cultures (Fig. 2). Even with the low number of DCs used for the vaccination, the polarized DCs were capable of inducing a robust immune response to OVA257–264 whereas the non-polarized DCs were not. There was very low induction of non-specific T cell activation by either DC vaccine (Fig. 2).
Fig. 2.
Polarized DCs induce superior antigen-specific responses in vivo. Polarized or LPS matured DCs were prepared as in Fig. 1, pulsed with the OVA257–264 peptide (OVAp) and injected s.c. (3 × 103 cells per mouse) into two animals per group. Two weeks later, splenocytes were harvested and restimulated for 5 days with irradiated EL4 cells pulsed with OVAp (EL4 + OVAp). The number of OVAp-specific T cells was determined by IFNγ ELISPOT. Data represent one of three independent experiments. Inset DCs generated from IL-12/23p40 KO mice were used for vaccination, as described above. *Below detectable level
Since IL-12 is a key cytokine involved in the generation of type-1 immunity [36], we tested the relative role of the elevated production of IL-12 family members by polarized DC1s in their superior ability to induce antigen specific immune responses in vivo. It has recently been shown that human type-1 polarized DCs produce high levels of IL-12 and IL-23 [40]. To this aim, C57BL/6 mice were vaccinated using OVAp-loaded differentially matured DCs generated from the bone marrow of IL-12p40-deficient animals, which lack the common subunit for IL-12p70 and IL-23. As expected, IL-12/23p40 deficiency completely abolished the advantage of type-1 polarized DCs in inducing enhanced levels of Ag-specific immune responses (Fig. 2, inset).
Polarized DCs have superior ability to induce protective anti-tumor immunity
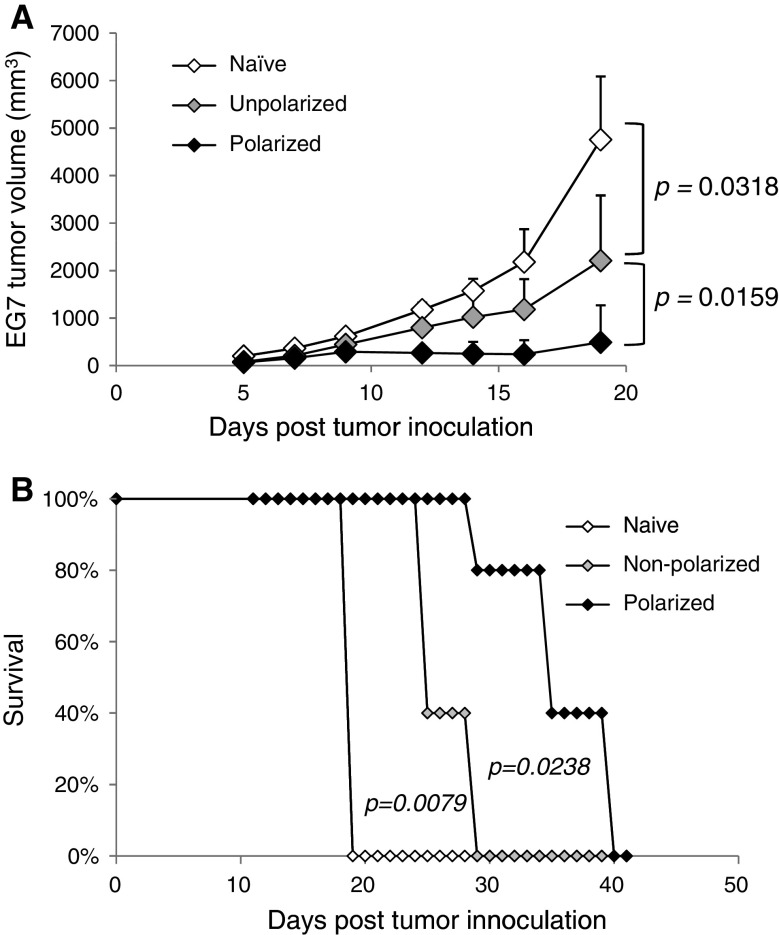
In order to determine if the enhanced immune response generated by the polarized DCs translated into improved immunity to a subsequent tumor challenge, C57BL/6 mice were pre-vaccinated with OVAp-loaded DCs and, 6 weeks post-vaccination, they were inoculated with EG7, an OVA-expressing lymphoma. As shown in Fig. 3a, vaccination with polarized DCs had a significant reduction in tumor size compared to both the untreated control animals and to the animals receiving the analogous non-polarized vaccine. The difference in tumor growth was reflected by the doubling of the survival benefit of vaccination. Compared to the median survival of the untreated animals of 19 days (Fig. 3b), the survival time resulting from DC polarization increased from 25 days (6 day survival benefit observed in mice vaccinated with non-polarized DCs) to 35 days (16 days survival benefit observed in mice vaccinated with polarized DCs). Taken together, these data demonstrate the superior ability of polarized DCs to induce antigen-specific immune responses and protection from tumor challenge.
Fig. 3.
Polarized DCs elicit protective immunity in EG7 cancer model. Polarized or LPS matured DCs were prepared as in Fig. 1, pulsed with OVAp and injected s.c. (3 × 103 cells per mouse) into 5–8 animals per group. Six weeks later, mice were challenged with 3 × 106 EG7 cells. a Tumor growth kinetics were monitored every 2–3 days. An exact two-tailed Wilcoxon test was used to analyze the statistical significance of tumor growth between the treatment groups. b Survival was monitored daily and mice were sacrificed when tumors reached 2 cm in diameter, in accordance with the University of Pittsburgh IACUC protocol. An exact log rank test was used to analyze the survival differences between the polarized and non-polarized treatment groups (P = 0.0238). Data represent one of three independent experiments
Discussion
It was previously shown that mouse antigen-carrying DCs can be found in draining LN within hours [8, 28, 41], while human DC-based cancer vaccines can take as long as 48 h to migrate from the site of a subcutaneous (or intradermal) injection to the LN and initiate an immune response [5]. Thus, the ability of the DCs to retain the capacity for high IL-12 secretion upon delayed interaction with T cells, in the absence of the original maturation stimulus, is likely to be crucial for their ability to induce type-1 immune responses in vivo.
In attempt to develop cancer vaccines with selectively-enhanced ability to promote Th1, CTL, and NK cell-dominated type-1 immunity, desirable in cancer, we have recently described several ways of inducing fully mature DCs capable of producing high levels of bioactive IL-12p70 after subsequent interaction with T cells or the stimulation with T cell-representing stimuli, such as CD40L [2, 19, 20, 26]. In contrast to the previously-applied strategies (such as DC pre-stimulation with CD40L or with a combination of TLR ligands or transduction of DCs with both IL-12 genes), polarized DC1s do not produce IL-12p70 spontaneously but are primed for secretion of high levels of IL-12 after migration [27], upon contact with T cells or subsequent stimulation, resulting in their ability to convert pre-existing Th2-type responses to Th1 responses, and superior activity in inducing tumor-specific CTLs [2, 19, 20, 26].
While such DC1s showed superior immunostimulatory activity in vitro, the evaluation of their immunogenic effectiveness in vivo, and the design of the most effective DC1-based strategies of cancer vaccination has been hampered by the lack of suitable mouse models of human polarized DCs. Our current data demonstrate that the combination of IL-4 and IFNγ is highly effective in driving type-1 polarization of LPS-matured DCs, in analogy to the human system, manifested by the combination of mature phenotype and elevated ability to produce high levels of IL-12 following subsequent CD40L-stimulation, even if such stimulation is delayed for up to 24 h. It has previously been shown that mouse DCs injected s.c. can take 12-24 h to reach the LN [8, 41], so the ability of polarized DCs to produce high IL-12 after delayed CD40L stimulation is crucial in directing type-1 responses in vivo, with important implications for the use of polarized DCs as cancer vaccines. Moreover, our data show that in addition to their superior ability to drive CTL and Th1 responses demonstrated in the human system in vitro [2, 19, 20], DC1s are superior inducers of CTL responses in vivo (Fig. 2), which translates into enhanced induction of protective anti-tumor immunity (Fig. 3).
Recently, Hokey et al. [10] proposed the use of mouse DCs matured under the influence of two TLR ligands, poly(I:C) and CpG, as a model of human DC1s. However, our current data demonstrate that while such TLR ligand-matured DCs do have enhanced production of IL-12 during the period of the TLR exposure, they become “exhausted” within 24 h and lose the ability to produce IL-12, and thus, do not develop a stable “polarized” character (Fig. 1b). The stable ability to produce IL-12 is dependent on IL-4, which can not be substituted for by type-1 interferons or TLR ligands which induce type-1 interferons, in contrast to the human system [27]. While these DCs exhibit enhanced anti-tumor activity when used as a therapeutic vaccine, the above study did not differentiate between the role of IL-12 production and elevated maturation stage of the DCs activated by the combination of two TLR ligands. Our current data showing that DCs matured by LPS in the presence of IFNγ and IL-4 show similar (to LPS-alone matured DCs) expression of the maturation-associated costimulatory molecules, and demonstrates the dependence of their elevated stimulatory function on IL-12 production, help to overcome these concerns, further indicating that the LPS, IFNγ and IL-4-matured DCs represent a mouse model of human type-1-polarized DCs.
While IFNγ is the prototypic Th1 cytokine and has been known to enhance IL-12 [7], resulting in a positive feedback loop where IL12 enhances the secretion of IFNγ by T cells [4], IL-4 is nominally considered as a factor with Th2-driving properties [32]. However, the data from IL-4 knockout mice revealed that IL-4 has also a paradoxical role in the development of Th1 responses [3, 31]. Our current data helps to explain this paradox: while the presence of IL-4 at the time of DC:T cell interaction may enhance production of IL-12 [9, 16], its dominant effect is the shutting down of the IL-12 responsiveness, resulting in the promotion of Th2 responses. In contrast, the pre-exposure of DCs to IL-4 prior to the interaction with Th cells polarizes the DCs for high IL-12 production, allowing such polarized DC1s to promote Th1 responses in another compartment (such as LN) in the absence of IL-4 itself and its antagonistic direct effects on Th1 cells.
In summary, the current data provide a model for the generation of mouse type-1-polarized DCs and demonstrate that ex vivo polarization of DCs allows for boosting their subsequent activity in vivo as cancer vaccines. As such, the current results facilitate an in depth analysis of the application of DC1-based vaccines against cancer and chronic infections in preclinical mouse models.
Electronic Supplementary Material
Acknowledgments
The authors thank Dr. Pia Bjork for stimulating discussions and Erik Berk for critically reading the manuscript. This work was supported by the NIH grants CA095128 and CA101944.
Footnotes
Adam S. Giermasz and Julie A. Urban contributed equally to this work.
References
- 1.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Biedermann T, Zimmermann S, Himmelrich H, Gumy A, Egeter O, Sakrauski AK, Seegmuller I, Voigt H, Launois P, Levine AD, Wagner H, Heeg K, Louis JA, Rocken M. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol. 2001;2:1054–1060. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 4.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vries IJ, Krooshoop DJ, Scharenborg NM, Lesterhuis WJ, Diepstra JH, Van Muijen GN, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, Adema GJ, Punt CJ, Figdor CG. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 6.Fallarino F, Uyttenhove C, Boon T, Gajewski TF. Improved efficacy of dendritic cell vaccines and successful immunization with tumor antigen peptide-pulsed peripheral blood mononuclear cells by coadministration of recombinant murine interleukin-12. Int J Cancer. 1999;80:324–333. doi: 10.1002/(SICI)1097-0215(19990118)80:2<324::AID-IJC25>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 8.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 9.Hochrein H, O’Keeffe M, Luft T, Vandenabeele S, Grumont RJ, Maraskovsky E, Shortman K. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–833. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hokey DA, Larregina AT, Erdos G, Watkins SC, Falo LD., Jr Tumor cell loaded type-1 polarized dendritic cells induce Th1-mediated tumor immunity. Cancer Res. 2005;65:10059–10067. doi: 10.1158/0008-5472.CAN-05-1692. [DOI] [PubMed] [Google Scholar]
- 11.Inaba K, Inaba M, Naito M, Steinman RM. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inaba K, Steinman RM, Pack MW, Aya H, Inaba M, Sudo T, Wolpe S, Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992;175:1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/S0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 14.Kalinski P, Nakamura Y, Watchmaker P, Giermasz A, Muthuswamy R, Mailliard RB. Helper roles of NK and CD8+ T cells in the induction of tumor immunity polarized dendritic cells as cancer vaccines. Immunol Res. 2006;36:137–146. doi: 10.1385/IR:36:1:137. [DOI] [PubMed] [Google Scholar]
- 15.Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- 16.Kalinski P, Smits HH, Schuitemaker JH, Vieira PL, van Eijk M, de Jong EC, Wierenga EA, Kapsenberg ML. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J Immunol. 2000;165:1877–1881. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 17.Kalinski P, Vieira P, Schuitemaker JH, Cai Q, Kapsenberg M. Generation of human type 1- and type 2-polarized dendritic cells from peripheral blood. Methods Mol Biol. 2003;215:427–436. doi: 10.1385/1-59259-345-3:427. [DOI] [PubMed] [Google Scholar]
- 18.Kiertscher SM, Gitlitz BJ, Figlin RA, Roth MD. Granulocyte/macrophage-colony stimulating factor and interleukin-4 expand and activate type-1 dendritic cells (DC1) when administered in vivo to cancer patients. Int J Cancer. 2003;107:256–261. doi: 10.1002/ijc.11379. [DOI] [PubMed] [Google Scholar]
- 19.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 20.Lapteva N, Seethammagari MR, Hanks BA, Jiang J, Levitt JM, Slawin KM, Spencer DM. Enhanced activation of human dendritic cells by inducible CD40 and Toll-like receptor-4 ligation. Cancer Res. 2007;67:10528–10537. doi: 10.1158/0008-5472.CAN-07-0833. [DOI] [PubMed] [Google Scholar]
- 21.Lotze MT, Hellerstedt B, Stolinski L, Tueting T, Wilson C, Kinzler D, Vu H, Rubin JT, Storkus W, Tahara H, Elder E, Whiteside T. The role of interleukin-2, interleukin-12, and dendritic cells in cancer therapy. Cancer J Sci Am. 1997;3(Suppl 1):S109–S114. [PubMed] [Google Scholar]
- 22.Lotze MT, Zitvogel L, Campbell R, Robbins PD, Elder E, Haluszczak C, Martin D, Whiteside TL, Storkus WJ, Tahara H. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann N Y Acad Sci. 1996;795:440–454. doi: 10.1111/j.1749-6632.1996.tb52715.x. [DOI] [PubMed] [Google Scholar]
- 23.Mackey MF, Gunn JR, Maliszewsky C, Kikutani H, Noelle RJ, Barth RJ., Jr Dendritic cells require maturation via CD40 to generate protective antitumor immunity. J Immunol. 1998;161:2094–2098. [PubMed] [Google Scholar]
- 24.Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83+ CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, Kalinski P. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366–2373. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 27.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 28.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzolini G, Prieto J, Melero I. Gene therapy of cancer with interleukin-12. Curr Pharm Des. 2003;9:1981–1991. doi: 10.2174/1381612033454261. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Watchmaker P, Urban J, Sheridan B, Giermasz A, Nishimura F, Sasaki K, Cumberland R, Muthuswamy R, Mailliard RB, Larregina AT, Falo LD, Gooding W, Storkus WJ, Okada H, Hendricks RL, Kalinski P. Helper function of memory CD8+ T cells: heterologous CD8+ T cells support the induction of therapeutic cancer immunity. Cancer Res. 2007;67:10012–10018. doi: 10.1158/0008-5472.CAN-07-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 32.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 33.Sangro B, Melero I, Qian C, Prieto J. Gene therapy of cancer based on interleukin 12. Curr Gene Ther. 2005;5:573–581. doi: 10.2174/156652305774964712. [DOI] [PubMed] [Google Scholar]
- 34.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr, Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–157. doi: 10.1016/S0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 35.Takahara K, Omatsu Y, Yashima Y, Maeda Y, Tanaka S, Iyoda T, Clausen BE, Matsubara K, Letterio J, Steinman RM, Matsuda Y, Inaba K. Identification and expression of mouse Langerin (CD207) in dendritic cells. Int Immunol. 2002;14:433–444. doi: 10.1093/intimm/14.5.433. [DOI] [PubMed] [Google Scholar]
- 36.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 37.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 38.Watchmaker P, Urban J, Berk E, Nakamura Y, Mailliard RB, Watkins SC, Van Ham SM, Kalinski P. Memory CD8+ T cells protect dendritic cells from CTL killing. J Immunol. 2008;180:3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watchmaker PB, Urban JA, Berk E, Nakamura Y, Mailliard RB, Watkins SC, van Ham SM, Kalinski P. Memory CD8+ T cells protect dendritic cells from CTL killing. J Immunol. 2008;180:3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J Immunother. 2007;30:75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Huck SP, McHugh RS, Hermans IF, Ronchese F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc Natl Acad Sci USA. 2006;103:147–152. doi: 10.1073/pnas.0509054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zitvogel L, Couderc B, Mayordomo JI, Robbins PD, Lotze MT, Storkus WJ. IL-12-engineered dendritic cells serve as effective tumor vaccine adjuvants in vivo. Ann N Y Acad Sci. 1996;795:284–293. doi: 10.1111/j.1749-6632.1996.tb52678.x. [DOI] [PubMed] [Google Scholar]
- 43.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.