Abstract
Background
Resveratrol has been reported to induce angiogenesis in ischemic tissue. We hypothesized that high-dose resveratrol would improve native angiogenesis in a swine model of metabolic syndrome and chronic myocardial ischemia.
Methods
Yorkshire swine were fed a normal diet (Control, n=7), hypercholesterolemic diet (HCD, n=7), or hypercholesterolemic diet with supplemental resveratrol (100 mg/kg/day orally, HCD-R, n=7) beginning one month prior to surgery. Chronic ischemia was created by placing an ameroid constrictor on the left circumflex coronary artery. After 7 weeks, swine underwent functional MRI, coronary angiography, and serum and heart tissue harvest for analysis.
Results
HCD-R animals had lower body mass index (p<0.001), total cholesterol (p<0.001), LDL (p<0.001), blood glucose levels (p<0.001), and systolic blood pressure (p=0.03) than HCD animals. There was no difference in regional myocardial function at 7 weeks (p=0.25). Coronary angiograms revealed no difference in Rentrop collateral scores (p=0.68). Staining for PECAM-1 demonstrated higher capillary density in the Control group (vs. HCD and HCD-R, p=0.02). Immunoblotting demonstrated decreased expression of the pro-angiogenic protein vascular endothelial (VE)-cadherin (p=0.002) and an increase in anti-angiogenic proteins angiostatin (p=0.001) and thrombospondin (p=0.02) in the HCD and HCD-R groups. Matrix metalloprotease 2 (MMP2)(p=0.47) and MMP9 (p=0.12) were not different among groups.
Conclusion
Supplemental resveratrol positively modified cardiovascular risk factors including body mass index, cholesterol, glucose tolerance, and systolic blood pressure. However it did not increase native collateral formation in the ischemic myocardium. This may be a result of increased angiostatin and thrombospondin leading to decreased expression of VE-cadherin and other pro-angiogenic factors.
Keywords: Resveratrol, Metabolic syndrome, Coronary artery disease, Angiogenesis, Angiostatin
INTRODUCTION
Native angiogenesis is the body’s natural response to chronic ischemia. In the heart, chronic hypoxia leads to the activation of a number of molecular pathways, resulting in the formation of collateral vessels to restore blood flow to the ischemic territory. Unfortunately this process is not consistent in patients with advanced coronary artery disease (CAD). In a recent study it was found that only one-third of patients with CAD and one-fifth of patients without CAD developed significant collateral vessels. In addition, patients with well-developed collaterals had a 25% decrease in cardiac mortality at 5 years.1 Therapeutic angiogenesis seeks to induce vessel growth in ischemic myocardium by upregulating pro-angiogenic pathways through gene, protein or cell based treatments. The local delivery of pro-angiogenic growth factors to the ischemic myocardium was a promising method to relieve myocardial ischemia. Unfortunately, the translation of this work from healthy young animals to patients has been disappointing and largely unsuccessful.2
The reason for the lack of a native response to ischemia and therapeutic interventions is multifactorial. Recent data have demonstrated that various factors associated with chronic disease may play a role. Obesity, diabetes mellitus, hypertension, and hypercholesterolemia have been associated with reduced collateral formation.3 These diseases result in endothelial dysfunction, decreased nitric oxide production, increased oxidative stress, and production of specific inhibitors of angiogenesis such as angiostatin and endostatin.
Angiostatin and endostatin are endogenously produced angiostatic proteins that were first described by Folkman.4,5 Angiostatin is a cleavage fragment of plasminogen, and endostatin a product of collagen XVIII cleavage. Both of these anti-angiogenic signaling molecules are increased in diseases commonly associated with CAD such as hypercholesterolemia, hypertension, and diabetes mellitus.6
Resveratrol is a polyphenol found in abundance in red wine. In small animal and cultured cell models the supplement has been shown to enhance angiogenesis.7,8 However, studies have not been performed in large animal models of chronic myocardial ischemia and metabolic syndrome. Additionally, there are few data available on the optimum dosage of resveratrol in large animals. There have been concerns about the bioavailablity of orally delivered resveratrol, and some studies have shown low systemic concentrations.9 Early studies in animal models and humans have demonstrated no adverse effects at daily doses up to 5 grams, the highest dose tested.10 We hypothesize that high-dose supplemental resveratrol will improve collateral formation in the ischemic myocardium.
METHODS
Animal Model
Adult male Yorkshire miniswine (Parsons Research, Amherst, MA) were fed one of three diets daily throughout the 11 weeks of the experiment. The first group was given 500 grams of a hypercholesterolemic diet daily (HCD, n= 7) composed of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow. A second group was fed the same hypercholesterolemic diet supplemented with 100 mg/kg/day resveratrol (HCD-R, n= 7) (Chromadex, Irvine, CA). The third group of swine was fed regular chow (Control, n= 7).
After 4 weeks of dietary modification, animals in the HCD and HCD-R groups underwent cardiovascular magnetic resonance imaging (CMR) and ameroid constrictor placement on the proximal left circumflex coronary artery (LCx). For all surgical procedures, anesthesia was induced with ketamine (10 mg/kg IM) and thiopental 2.5%, and maintained with a gas mixture of oxygen at 1.5–2 L/min and 3.0% isoflurane. The animals were intubated and mechanically ventilated at 12–20 breaths/min. During the first procedure (ameroid placement through a left mini-thoracotomy) gold-labeled microspheres (Biophysics Assay Laboratory, Worcester, MA) were injected into the left atrium during temporary occlusion of the LCx to determine the exact myocardial territory at risk. Next, a titanium ameroid constrictor (1.75 mm internal diameter) was placed around the proximal LCx.
Seven weeks after ameroid placement, swine were anesthetized and CMR and x-ray coronary angiography were completed. The heart was then exposed and microspheres were again injected, followed by euthanasia. The heart was harvested and two 1 cm thick transverse slices were cut at the midventricular level and sectioned into 8 segments each. Samples were divided and rapidly frozen in liquid nitrogen (molecular studies), placed in 4°C Krebs solution (microvessel reactivity studies), 10% formalin (immunohistochemistry studies), or weighed and dried (microsphere perfusion analyses).
All experiments were approved by the hospital Institutional Animal Care and Use Committee. Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication no. 5377-3 1996).
Serum Cholesterol and Intravenous Glucose Tolerance Test
An intravenous glucose tolerance test (IVGTT) was performed on all animals prior to placement of the ameroid and again prior to euthanasia. A fasting baseline blood glucose level was measured and then dextrose, 0.5 grams/kg, was infused. Blood glucose levels were measured at 30 minutes and 60 minutes post infusion. Serum total cholesterol and low-density lipoprotein (LDL) measurements were made by AccelLAB, Boisbriand, Quebec, Canada with a Siemens Advia 1200 chemistry system.
Myocardial Perfusion analysis
Myocardial perfusion was determined during each procedure with isotope-labeled microspheres (ILMs), 15 µm diameter (BioPAL Worcester, MA) using previously reported methods.11 Briefly, 1.5x107 gold-labeled microspheres were injected during temporary LCx occlusion at the time of ameroid placement to identify the area at risk. Labeled ILMs were also injected at the final procedure during rest and ventricular pace (150 beats/min) conditions. Following euthanasia, 10 transmural left ventricular sections were collected for ILM assays. The samples were exposed to neutron beams and microsphere densities were measured using a gamma counter.
Cardiovascular Magnetic Resonance (CMR) Imaging
Animals in the HCD and HCD-R groups underwent a CMR study before placement of the ameroid (pre) and prior to sacrifice (post) 7 weeks later. All animals were scanned using a 1.5T Philips Achieva scanner (Philips Healthcare, Best, Netherlands) with a five-element cardiac phased-array receiver coil. Left ventricular (LV) function were evaluated by acquiring free-breathing cine short axis steady state free processing (SSFP) slices covering the entire LV and a free-breathing phase contrast velocity map in the ascending aorta. MR tagging was performed to evaluate dyssynchrony and local LV myocardial function using three short axis slices with a spiral complementary spatial modulation of magnetization sequence.12 Images were analyzed using a MATLAB (The Mathworks, Natick, MA) program.13 Each LV slice was divided into 6 segments and the circumferential strain of each segment was analyzed. The values obtained represent the amount of regional myocardial contraction in the mid anterolateral region contributing to the overall ejection fraction. Thus, a more negative number represents greater contraction of the myocardial segment. Comparisons of mid anterolateral segment myocardial function were made between the HCD and HCD-R groups prior to induction of ischemia and again after 7 weeks of ischemia.
X-ray coronary angiography
X-ray coronary angiography was carried out in order to ensure occlusion of the LCx and assess collateral formation. Recorded images were interpreted by a blinded interventional cardiologist. Angiographic collateral formation was assessed according to the Rentrop grading system of 0–3, depending on the presence and extension of the collateral filling of coronary epicardial vessels.14
Immunohistochemistry
Formalin fixed tissue samples were processed for immunostaining. Antibodies against platelet endothelial cell adhesion molecule (PECAM-1, CD-31, Santa Cruz Biotechnology, Santa Cruz, CA) were applied to the sections for 2 hours at room temperature. Detection was obtained using a biotinylated goat anti-mouse secondary antibody and the avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA). Color was developed using diaminobenzidine substrate (1mg/ml in PBS and 0.03% H2O2). Sections were then counterstained with hematoxilin, dehydrated and mounted. Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) equipped with a digital camera (Photodoc, Upland, CA) and capillaries were counted in a blinded fashion.
Immunoblotting studies
Whole cell lysates were made from myocardial tissue using radioimmunoprecipitation assay buffer. Sixty micrograms of total protein was fractionated by 4–20% gradient, sodium dodecyl sulfate polyacrylamide gel electrophoresis (Invitrogen, San Diego, CA) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Each membrane was incubated with the following specific antibodies: anti-vascular endothelial (VE) Cadherin, anti-matrix metalloproteinase (MMP) 2, anti-MMP 9, anti-β catenin (Cell Signaling, Beverly, MA), anti-angiopoietin 1, anti-Angiostatin, anti-tissue inhibitor of metalloproteinase (TIMP) 2, anti-thrombospondin (Abcam Inc, Cambridge, MA), and anti-endostatin (Millipore, Billerica, MA). Immune complexes were visualized with an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). Bands were quantified by densitometry of autoradiograph films. Ponceau staining was used to ensure equal protein loading.
Data Analysis
All results are expressed as mean ± standard error of the mean (SEM). Microvessel responses are expressed as percent relaxation of the preconstricted diameter and were analyzed using two way, repeated measures analysis of variance with a post-hoc Bonferroni test. Western blots were analyzed after digitalization (ScanJet 4c; Hewlett-Packard, Palo Alto, CA) with NIH ImageJ 1.33 software (National Institute of Health, Bethesda, MD). Comparisons between the three groups were analyzed by one way, repeated measures analysis of variance with Newman-Keuls Multiple Comparison post-hoc test, with the exception of the circumferential strain data which was analyzed by two tailed t-test, using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA). Probability values less than 0.05 were considered significant.
RESULTS
Experimental Model
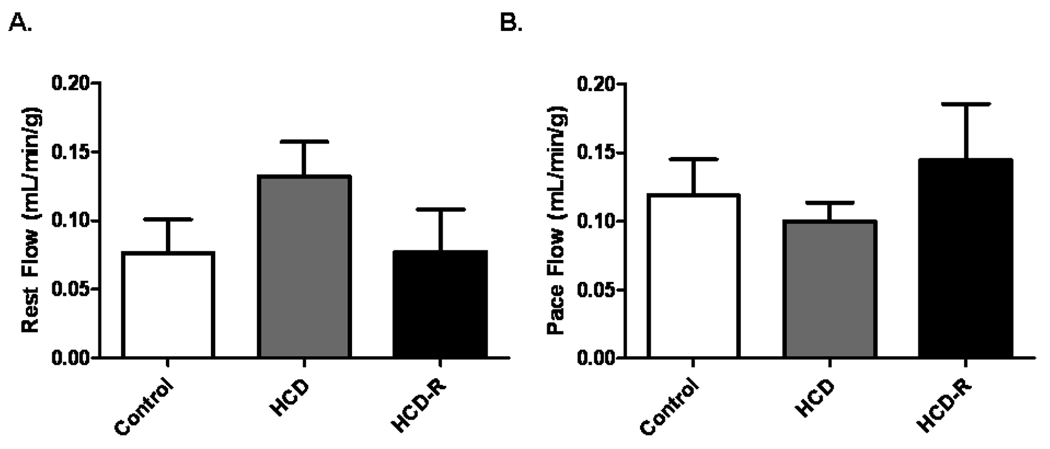
All animals survived all procedures and completed the study. The swine had similar body mass indices (BMI) at the time of the ameroid placement (p= 0.11) (Table 1). After 7 weeks, at the final procedure, animals in the HCD group were significantly larger vs. Control swine (p< 0.001). The HCD group had significantly higher levels of total cholesterol as compared to the Control and HCD-R groups (p< 0.001). There was no statistical difference between the Control and HCD-R groups. The levels of LDL demonstrated a similar pattern, with the HCD group significantly greater than the Control and HCD-R groups (p< 0.001). IVGTT performed during the final procedure demonstrated no differences between the groups at the baseline measurement (p=0.4). Thirty minutes post glucose infusion the HCD group had glucose levels significantly higher than the Control and HCD-R groups (p=0.002). There was no statistical difference between the HCD-R group and the Control group at 30 minutes. Sixty minutes after glucose infusion there was no difference between the groups (p= 0.2). Seven weeks after ameroid placement, myocardial perfusion at rest and during ventricular pacing was similar between groups (p= 0.25 and p= 0.47, respectively) (Figure 1A and B).
Table 1.
Comparison of metabolic changes associated with high cholesterol diet with and without resveratrol treatment after 7 weeks of myocardial ischemia.
| Control | HCD | HCD-R | p-value | |
|---|---|---|---|---|
| Pre BMI (kg/m2) | 27.0 ± 0.5 | 25 ±0.7 | 27 ± 0.7 | 0.11 |
| Post BMI (kg/m2) | 29 ± 0.8 | 36 ± 1.3 | 31 ± 1.4 | 0.001a |
| Total cholesterol (mg/dL) | 79 ± 0.1 | 390 ± 53 | 261 ± 54 | 0.001a,b |
| 30 minute blood glucose (mg/dL) | 108 ± 8 | 171 ± 6.1 | 132 ± 14.1 | 0.001a |
| Systolic blood pressure (mmHg) | 54 ± 2.6 | 83 ± 6.1 | 64 ± 8.5 | 0.03c |
BMI- body mass index. (aHCD significantly increased vs. Control and HCD-R, bHCD-R significantly increased vs. Control, cHCD is increased vs. Control based on Newman-Keuls post hoc test.)
Figure 1. Blood flow to the ischemic myocardium during rest and pace after 7 weeks of ischemia.
A) There was no difference in blood flow to the ischemic myocardium at rest, p= 0.25. B) There was no difference in blood flow to the ischemic territory during ventricular pacing (150 beats/min), p= 0.47.
Cardiac Magnetic Resonance Imaging (CMR)
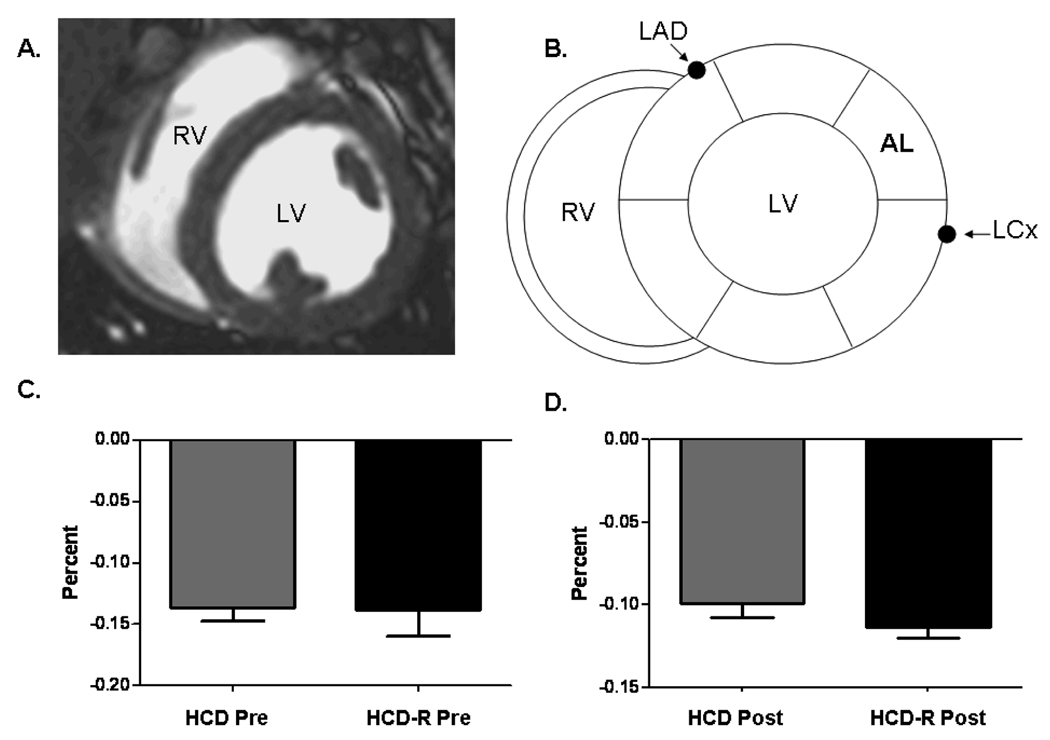
Regional wall motion analysis using CMR circumferential strain demonstrated no difference in anterolateral regional function (region of measurement shown in Figure 2A and B) from baseline (pre) to 7 weeks of chronic ischemia (post) in HCD swine in the basal myocardial slice (−14.1 ± 1% to −12.1 ± 2%, p= 0.40) (Figure 2C). Regional function in HCD-R swine from baseline (pre) to 7 weeks chronic ischemia (post) demonstrated a similar non-significant decrease in regional function (−13.8 ± 7% to −11.3% ± 1%, p= 0.32) (Figure 2D).
Figure 2. Cardiac Magnetic Resonance (CMR) imaging.
A) Axial mid-papillary myocardial image showing the right (RV) and left ventricle (LV). B) Schematic drawing depicting the segement of interest (mid anterolateral) in relation to the left circumflex coronary artery (LCx). C) Spiral strain analysis of regional myocardial function in the mid anterolateral segment prior to ameroid placement (Pre) and after 7 weeks of ischemia (Post) in the HCD group, p= 0.40. D) Spiral strain analysis of regional myocardial function in the mid anterolateral segment Pre and Post in the HCD-R group, p= 0.32.
X-ray Coronary Angiography
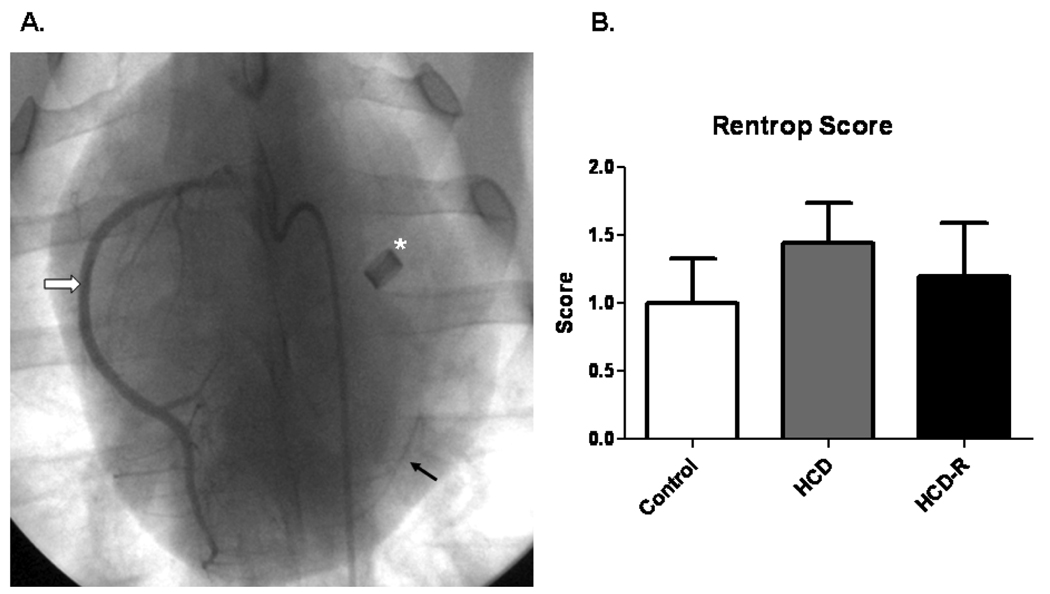
All animals had total occlusion of the proximal LCx by the ameroid constrictors (Figure 3A). Rentrop collateral scores were not significantly different among the groups (Control 0.9 ± 0.3, HCD-R 1.3 ± 0.4, HCD 1.3 ± 0.3, p= 0.68) (Figure 3B).
Figure 3. X-ray coronary angiography.
A) Representative coronary angiogram depicting the ameroid constrictor (asterisk), right coronary artery (white arrow), and retrograde contrast flow into the distal left circumflex artery (black arrow). B) Mean Rentrop scores in each group 7 weeks after ameroid placement, p= 0.68.
Immunohistochemistry
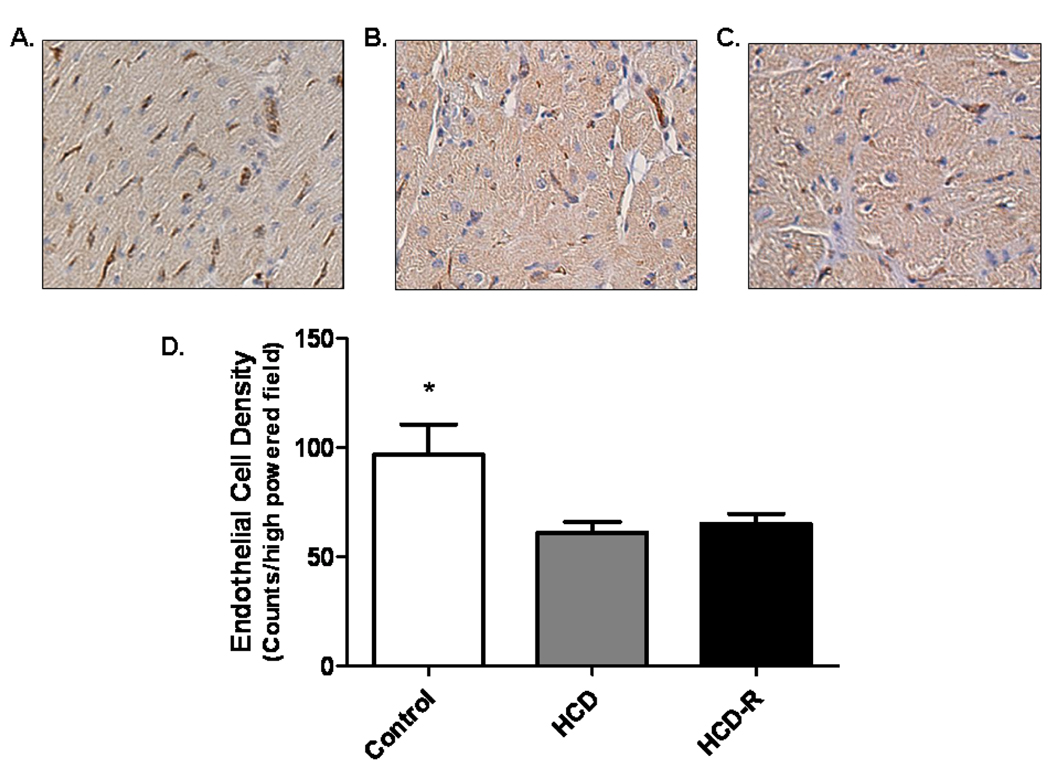
Staining of fixed sections from the ischemic territory for PECAM-1 demonstrated that the HCD-R and HCD groups had significantly fewer capillaries (PECAM-1 positive vessels/200X field) than Control (Control 96 ± 17.1, HCD 61 ± 5.4, HCD-R 65 ± 5.4, p=0.02) (Figure 4 A-D).
Figure 4. Collateral blood vessel formation.
Immunohistochemical staining for PECAM-1 demonstrated significantly fewer vessels in the HCD and HCD-R groups as compared to the Control group, A) Control, B) HCD and C) HCD-R. D) Quantification of PECAM-1 positive vessels per 200x field, *p= 0.02 (Magnification 200x).
Protein Expression
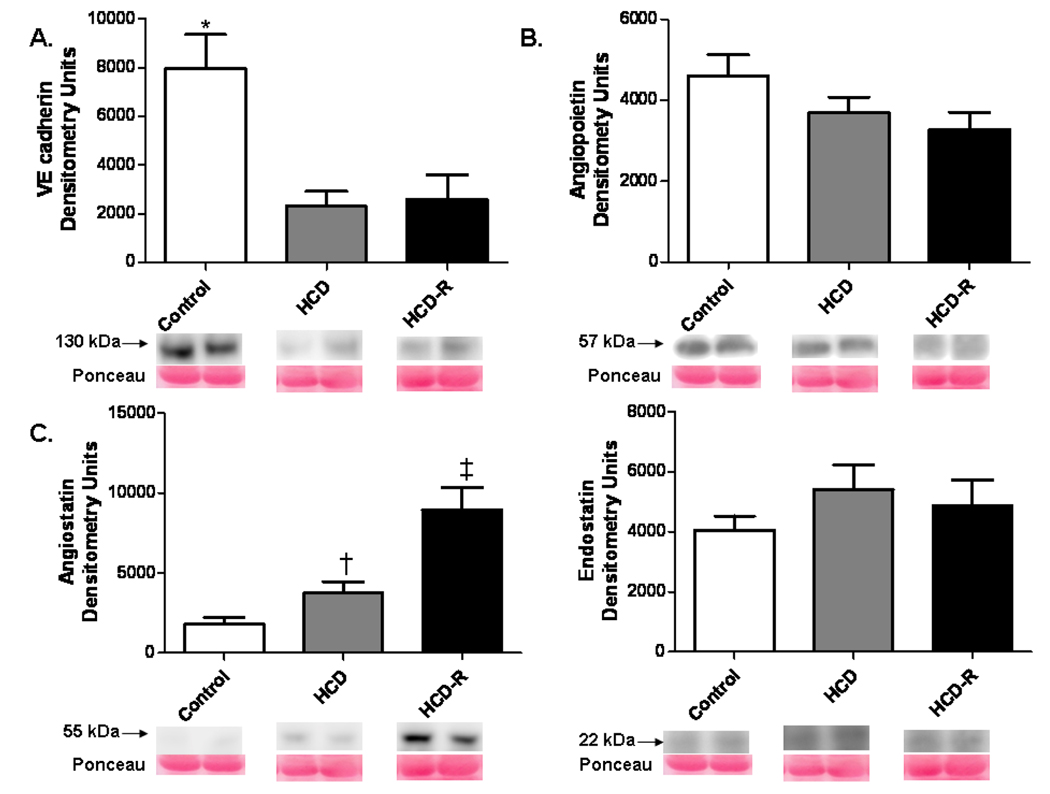
VE cadherin expression was reduced in the HCD and HCD-R groups compared to control (p= 0.02). Angiopoietin 1 demonstrated a non-significant trend for decreased expression in the HC groups, which appeared unaffected by resveratrol treatment. (p= 0.17). Protein levels of angiostatin were increased in HCD-R group v. the Control and HCD groups (p= 0.001). Endostatin expression was not significantly different between groups (p= 0.39) (Figure 5 A-D). There was similar expression of β catenin (p= 0.79) (not shown).
Figure 5. Expression of pro- and anti-angiogenic proteins in the ischemic myocardium.
A) VE cadherin expression in the HCD and HCD-R group was significantly lower than the Control group, * p= 0.002. B) Expression of angiopoietin 1 was unchanged in the HCD and HCD-R groups, p= 0.16. C) Angiostatin was significantly increased in the HCD-R group as compared to the Control and HCD groups, † p< 0.05 vs. Control by Newman-Keuls post-hoc test, ‡ p= 0.001. D) Expression of the anti-angiogenic factor endostatin was not different between groups, p= 0.39.
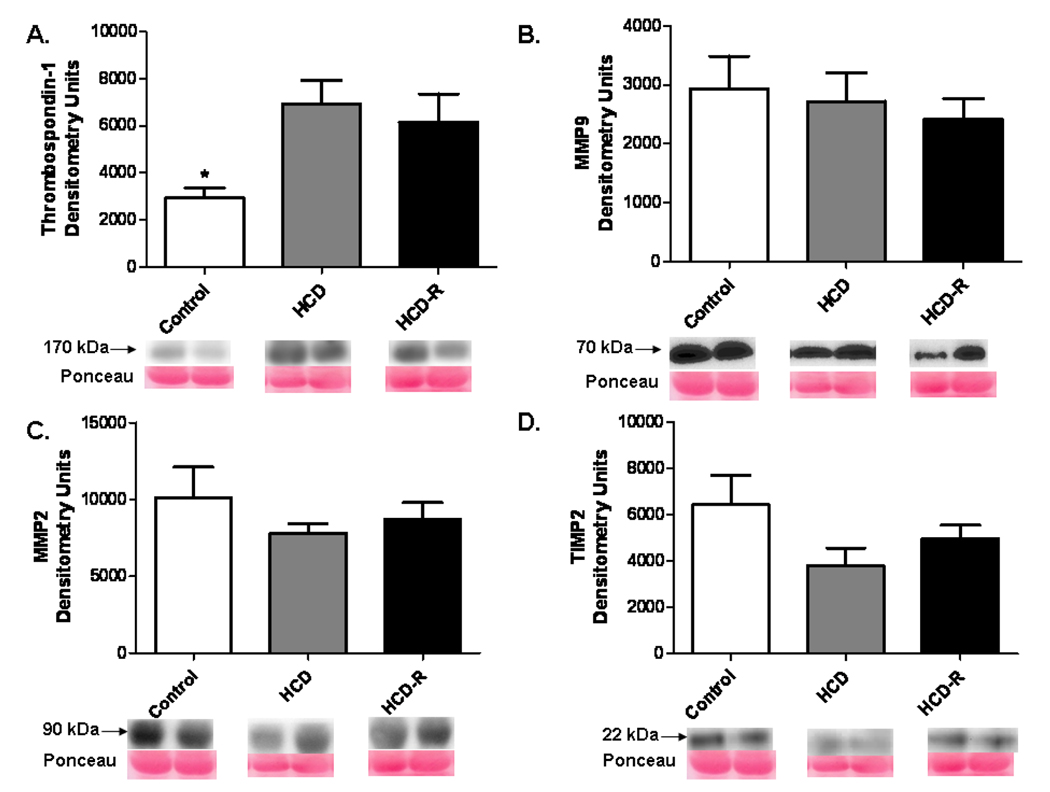
Levels of thrombospondin-1 were increased in the HCD and HCD-R groups as compared to the Control group (p= 0.02). MMP 2 (p= 0.48) and MMP 9 (p= 0.80) expression were not different between groups. There was similar expression of TIMP 2 protein among groups (p= 0.17) (Figure 6 A-D).
Figure 6. Expression of proteins implicated in angiostatin production in the ischemic myocardium.
A) Thrombospondin-1 was increased in the HCD and HCD-R groups as compared to the Control, * p= 0.02. B, C) Expression of MMP 2 and MMP9, were not different between groups, p= 0.48, p= 0.80, respectively. D) TIMP 2 displayed a non-significant trend toward decreased expression in the HCD and HCD-R groups, p= 0.17.
DISCUSSION
In this swine study of chronic ischemia we found that supplemental resveratrol positively modified a number of risk factors for cardiovascular disease including BMI, total cholesterol, glucose tolerance, and systolic blood pressure. However, resveratrol did not increase native collateral development in the ischemic myocardial territory. Both the HCD-R and the HCD groups had significantly lower capillary density in the ischemic myocardium than the Control group. Examination of the molecular signaling pathways was significant for a decrease in the expression of VE cadherin and an increase in angiostatin and thrombospondin-1 in the HCD-R group. There was no difference in the expression of β catenin, endostatin, MMP 2, MMP 9, or TIMP 2.
VE cadherin (also known as cadherin-5 and CD144) is essential for angiogenesis. It functions to form the junctions between endothelial cells which lead to vascular tubule formation.15 VE cadherin mediates endothelial cell adhesion by homophilic calcium dependent interactions with intracellular catenins (α, β, or, γ). Experiments done in vitro with specific antibodies against VE cadherin led to dissociation of the endothelial cell layer.16 Angiopoietin 1 also functions to stabilize endothelial cell contacts by inhibiting dissociation of VE cadherin- β catenin interactions. VE cadherin also has a pro-survival, anti-apoptotic signaling role during angiogenesis. Additionally, increased expression of VE cadherin has been associated with a prolongation of the half-life of VEGFR2, the major receptor responsible for activating the myriad pro-angiogenic pathways of VEGF.17 With decreased expression of VE cadherin these functions that are supportive to angiogenesis may be negated, and could lead to decreased collateral formation in the ischemic myocardium.
Angiostatin was first isolated from mice with Lewis lung carcinoma that demonstrated inhibition of metastatic tumor growth due to its production by the primary lesion.5 It is a cleavage product of plasminogen. MMP 2 and 9 are type IV collagenases that have been implicated in both pro- and anti-angiogenic processes. Initially they are involved with remodeling of the extracellular matrix for vessel extension and growth. Over time, however, their function may change, and in an anti-angiogenic role they have been identified as the terminal step in angiostatin formation.18 MMP 2 and 9 can also activate other matrix derived anti-angiogenic factors such as tumastatin. TIMPs are known to function as inhibitors of active MMPs, and may act to decrease the formation of angiostatin.19 Like MMPs, TIMPs have been found to have a canonical role in angiogenesis. It has been shown that TIMP 2 is required for MMP 2 activation.20 In our current study there was a slight increase in TIMP 2, but its role was not clear. It is possible that the increase in angiostatin was due to decreased metabolism leading to accumulation. We did not measure the breakdown of angiostatin, and the increased levels may be due to decreased degradation.
Angiostatin inhibits angiogenesis by inhibiting endothelial cell migration and tube formation. Previous work has demonstrated that angiostatin interferes with cellular adhesion mediated by VE cadherin.21 By inhibiting cellular adhesion angiostatin can block migration and adhesion of endothelial cells resulting in decreased vessel formation.
PECAM-1 is a transmembrane glycoprotein essential for angiogenesis. It is also involved in endothelial cell adhesion and vascular tube formation. Thrombospondin-1 is a glycoprotein that interacts with endothelial cells and has anti-angiogenic properties. It has been shown to inhibit angiogenesis by downregulating expression of PECAM-1. It also acts to decrease endothelial cell proliferation, block migration and induce apoptosis signaling.22
In previous animal and human studies we and others have demonstrated that hypercholesterolemia and diabetes mellitus are detrimental to the native angiogenic process.23 In these studies, like the current work, common comorbid diseases lead to decreased collateral formation in ischemic myocardium and increased anti-angiogenic factor expression. Resveratrol has been shown to act as a pro-angiogenic supplement in cultured cell and healthy small animal studies 7,8, but this was not the case in the current study. Recently studies have suggested that the angiogenic response to resveratrol is dose dependent. In a study of intracerebral gliomas in rats high-dose resveratrol resulted in decreased expression of VEGF, decreased angiogenesis, and longer survival at a dose of 100 mg/kg/day.24 Dudley et al 25 examined the effects of orally delivered resveratrol on the myocardium in the setting of acute ischemia reperfusion injury. They described dose dependent effects of resveratrol in the heart. Rats fed high-dose resveratrol (>25 mg/kg/day) demonstrated depressed cardiac function and increased myocardial infarct size. Additionally, at high dosages cell death signals predominated in the reperfused myocardium.
There are several limitations to our study. First, it was performed in a porcine model of chronic myocardial ischemia. While in most situations, the porcine coronary circulation closely mimics the physiology and pathophysiology of the human coronary circulation, this may not necessarily be the case in this situation. Secondly, although the observed changes were sufficient to allow determination of statistical significance, the number of animals in each group was relatively small and therefore our findings should be interpreted in this context. Additionally, the fine balance between pro- and anti-angiogenic signaling is complex and not fully understood. There may be unmeasured inhibitors of angiogenesis impacting the overall response. Finally, while we measured levels of protein expression (MMP, TIMP) the activity of these enzymes may be altered, and unaccounted for.
Future planned studies will examine a lower dose of resveratrol on native angiogenesis, as this may result in a pro-angiogenic effect. In addition, we intend to explore the combined effects of supplemental resveratrol and exogenous growth factors in our chronic myocardial ischemia model. The role of this supplement in the setting of chronic comorbid diseases, seen commonly in patients with CAD, has not been established. The pleotropic effects of resveratrol will need to be clarified prior to extensive study in humans.
In conclusion, high dose supplemental resveratrol positively modified a number of components of the metabolic syndrome, but did not increase collateral density in the ischemic myocardium. Production of the anti-angiogenic proteins angiostatin, through pathways independent of MMP 2 and 9, and thrombospondin 1 may explain the inhibition of new vessel formation.
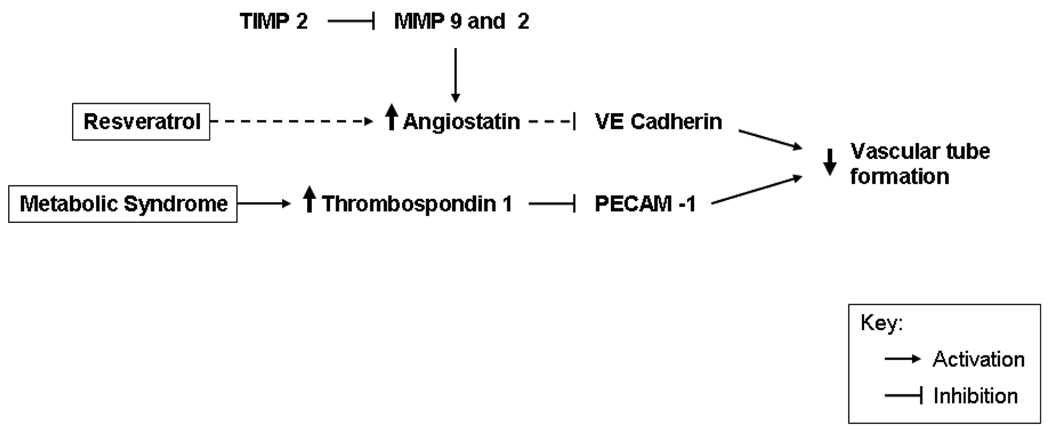
Figure 7. Summary Figure. Schematic representation of angiogenic and anti-angiogenic pathways modified by high cholesterol and or resveratrol.
Proteins modified by high cholesterol are indicated with solid black arrows. Modification of specific pathways by resveratrol are indicated by small dashed arrows. Resveratrol appears to act by increasing angiostatin, and does not change the increase in thrombospondin associated with metabolic syndrome. The overall effect of these integrated anti-angiogenic signals potentially acts to decrease vascular tube formation resulting in decreased vessel formation in the ischemic myocardium.
Acknowledgments
Financial support: Funding for this project was provided to F.W.S by NHLBI (RO1HL46716, RO1HL69024, and RO1HL85647), and and T32-HL0074 (M.P.R) and the Irving Bard Memorial Fellowship (M.P.R, L.M.C).
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- CMR
cardiovascular magnetic resonance imaging
- HCD
high cholesterol diet
- HCD-R
high cholesterol diet and supplemental resveratrol
- ILM
isotope labeled microsphere
- LCx
left circumflex coronary artery
- LV
left ventricle
- MMP
matrix metalloproteinase
- RV
right ventricle
- TIMP
tissue inhibitor of metalloproteinases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Frank W. Sellke has research support from Ikaria (Clinton, NJ) and Orthologic (Tempe, AZ), and is a consultant for Novo Nordisk (Princeton, NJ), Cubist Pharmaceuticals (Lexington, MA), and Pfizer (Princeton, NJ).
REFERENCES
- 1.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116(9):975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 2.Boodhwani M, Sodha NR, Laham RJ, Sellke FW. The future of therapeutic myocardial angiogenesis. Shock. 2006;26(4):332–341. doi: 10.1097/01.shk.0000225318.08681.a7. [DOI] [PubMed] [Google Scholar]
- 3.Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, et al. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296(2):H428–H434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 6.Matsunaga T, Chilian WM, March K. Angiostatin is negatively associated with coronary collateral growth in patients with coronary artery disease. Am J Physiol Heart Circ Physiol. 2005;288(5):H2042–H2046. doi: 10.1152/ajpheart.00669.2004. [DOI] [PubMed] [Google Scholar]
- 7.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21(20):2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penumathsa SV, Koneru S, Samuel SM, Maulik G, Bagchi D, Yet SF, et al. Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic Biol Med. 2008;45(7):1027–1034. doi: 10.1016/j.freeradbiomed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr., Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 10.Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9(4):371–378. [PubMed] [Google Scholar]
- 11.Boodhwani M, Voisine P, Ruel M, Sodha NR, Feng J, Xu SH, et al. Comparison of vascular endothelial growth factor and fibroblast growth factor-2 in a swine model of endothelial dysfunction. Eur J Cardiothorac Surg. 2008;33(4):645–650. doi: 10.1016/j.ejcts.2007.12.016. discussion 251-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryf S, Kissinger KV, Spiegel MA, Bornert P, Manning WJ, Boesiger P, et al. Spiral MR myocardial tagging. Magn Reson Med. 2004;51(2):237–242. doi: 10.1002/mrm.10688. [DOI] [PubMed] [Google Scholar]
- 13.Haber I, Metaxas DN, Axel L. Three-dimensional motion reconstruction and analysis of the right ventricle using tagged MRI. Med Image Anal. 2000;4(4):335–355. doi: 10.1016/s1361-8415(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 14.Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986;74(3):469–476. doi: 10.1161/01.cir.74.3.469. [DOI] [PubMed] [Google Scholar]
- 15.Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends in Cell Biology. 2009;19(1):8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, et al. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. 1995;15(8):1229–1239. doi: 10.1161/01.atv.15.8.1229. [DOI] [PubMed] [Google Scholar]
- 17.Rahimi N, Kazlauskas A. A role for cadherin-5 in regulation of vascular endothelial growth factor receptor 2 activity in endothelial cells. Mol Biol Cell. 1999;10(10):3401–3407. doi: 10.1091/mbc.10.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahl ML, Kenan DJ, Gonzalez-Gronow M, Pizzo SV. Angiostatin's molecular mechanism: aspects of specificity and regulation elucidated. J Cell Biochem. 2005;96(2):242–261. doi: 10.1002/jcb.20480. [DOI] [PubMed] [Google Scholar]
- 19.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1–2):267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 20.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 21.Eliceiri BP, Cheresh DA. Adhesion events in angiogenesis. Curr Opin Cell Biol. 2001;13(5):563–568. doi: 10.1016/s0955-0674(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22(1):63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 23.Sodha NR, Boodhwani M, Clements RT, Xu SH, Khabbaz KR, Sellke FW. Increased antiangiogenic protein expression in the skeletal muscle of diabetic swine and patients. Arch Surg. 2008;143(5):463–470. doi: 10.1001/archsurg.143.5.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng S-H, Lin S-M, Chen J-C, Su Y-H, Huang H-Y, Chen C-K, et al. Resveratrol Suppresses the Angiogenesis and Tumor Growth of Gliomas in Rats. Clinical Cancer Research. 2004;10(6):2190–2202. doi: 10.1158/1078-0432.ccr-03-0105. [DOI] [PubMed] [Google Scholar]
- 25.Dudley J, Das S, Mukherjee S, Das DK. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J Nutr Biochem. 2009;20(6):443–452. doi: 10.1016/j.jnutbio.2008.05.003. [DOI] [PubMed] [Google Scholar]