Abstract
Vaccinations in medicine are commonly administered through the skin. Therefore, the vaccine is immunologically processed by antigen-presenting cells of the skin. There is recent evidence that the clinically less often used intradermal route is effective; in cases even superior to the conventional subcutaneous or intramuscular route. Professional antigen-presenting cells of the skin comprise epidermal Langerhans cells (CD207/langerin+), dermal langerin− and dermal langerin+ dendritic cells (DCs). In human skin, langerin− dermal DCs can be further subdivided on the basis of their reciprocal CD1a and CD14 expression. The relative contributions of these subsets to the generation of immunity or tolerance are still unclear. Langerhans cells in human skin seem to be specialized for induction of cytotoxic T lymphocytes. Likewise, mouse Langerhans cells are capable of cross-presentation and of protecting against experimental tumours. It is desirable to harness these properties for immunotherapy. A promising strategy to dramatically improve the outcome of vaccinations is ‘antigen targeting’. Thereby, the vaccine is delivered directly and selectively to defined types of skin DCs. Targeting is achieved by means of coupling antigen to antibodies that recognize cell surface receptors on DCs. This approach is being widely explored. Little is known, however, about the events that take place in the skin and the DCs subsets involved therein. This topic will be discussed in this article.
Keywords: antigen targeting, dendritic cells, immunogenicity, Langerhans cells, langerin/CD207, vaccine
DEVELOPING RATIONAL VACCINES
Vaccinations in medicine are firmly established and well investigated. The traditional, well-known vaccines induce robust immunity against microbes and they prevent infectious diseases. ‘These vaccines are a major success story and emanate in large part from the discoveries of Louis Pasteur. ….. Pasteur’s research was based on the science of microbiology, i.e., his discovery that distinct microbes are the causes of disease and that an attenuated microbe can induce long-lived protection against infection by the non-attenuated form of that organism. These breakthroughs occurred before there was any clear understanding of vaccine immunity, which began with the discovery of antibodies by von Behring and Kitasato in the 1890s.’ (quotation from Steinman1). The past century brought major advances in our knowledge and understanding of the immune system. This opened a new era of vaccine research that is based on key immune principles.1
A vaccine can be defined as a ‘formulation that induces specific, non-toxic and long-lasting immune responses to prevent or treat disease.’1 Present vaccine research attempts to extend the spectrum of antigens, against which one could vaccinate, from the classical microbial antigens to antigens specific for cancer, autoimmunity or allergy.2 This implies that vaccines will not only serve to enhance immunity in the classical sense, but also to regulate or dampen it or even induce immunological tolerance in patients, as it would be desired in autoimmune diseases. Dendritic cells (DCs), as the prime inducers and regulators of immunity and tolerance are critical in the design of modern vaccines.3–5 Therefore, it is important to study these cells in vivo to progress beyond traditional approaches and devise vaccines that directly take advantage of the specialized properties of DCs to control immunity.1 Thus, the state-of-the-art of vaccine science is characterized by the established and undisputed classical vaccines and by a wide open field of research that attempts to rationally use immunological knowledge to make vaccines helpful in a much wider spectrum of diseases than in the past.
DCs OF THE SKIN AS TYPICAL RECIPIENTS OF VACCINES
Vaccines are commonly administered into the skin by injection. Most vaccines are deposited into the subcutaneous fat or into the muscle beneath the skin. Relatively few vaccines chose the route into the dermis,6 and even less well characterized and applied is the topical route, often called transcutaneous7,8 or epicutaneous (see also companion article by Stoitzner et al.9). Each of these routes of application relies on the presence of DCs in the tissue that take up the vaccine, process it and present it to T lymphocytes in the draining lymphoid organs.
Whereas subcutaneous fat and muscle tissue10–12 contain relatively few DCs, the dermis and the epidermis are densely populated by different subsets of DCs. The classical skin DCs is the Langerhans cell of the epidermis. In the past few years, other types of DCs have been described and characterized in the skin. Healthy human and murine skin harbours ‘dermal DCs’ that do not express langerin/CD207.13,14 These dermal DCs can be further subdivided into a minority population expressing CD14 and a majority population characterized by strong CD1a expression.15,16 It should be emphasized here that mouse and human dermis generally contain many more macrophages than DCs.17,14 This requires great care in defining these DCs phenotypically as some markers that were earlier thought to be specific for DCs, such as DC-SIGN/CD209, may also be expressed on macrophages, thus confounding analyses. It is, therefore, important to include good macrophage markers such as CD163, a scavenger receptor, in human beings18 or CD301, a galactose-/N-acetylgalactosamine-specific C-type lectin receptor, in mouse dermis.19 In healthy mouse skin, ‘dermal langerin+ DCs’ were recently defined.20–22 The human counterpart for this very rare (at least in situ) population is currently being disclosed (reported in Romani et al.23). Inflamed skin contains additional subsets of DCs such as TNF-α and iNOS-producing DCs (‘Tip-DC’),24 inflammatory dendritic epidermal cells25 and plasmacytoid DCs.26,27 For more in depth reviews about skin DCs, in particular Langerhans cells, the reader is referred to companion articles in this Special Feature and to a few recent reviews.23,14,17,28,29
DISTINCT PROPERTIES OF SKIN DCs
There is an increasing evidence that DCs from the epidermis (that is Langerhans cells) and DCs from other tissues are not identical, importantly also in terms of function. This is mainly based on the investigation of Langerhans cell-like and non-Langerhans cell-like (CD14-expressing, interstitial type, dermal type) DCs grown from human CD34+ haematopoietic stem cells. There, pronounced functional differences were described: for instance, Langerhans cell-like DCs take up less endocytic tracers such as fluorescein isothiocyanate dextran or peroxidase. Another difference is the failure of Langerhans cell-like DCs to induce naive B cells to differentiate into IgM-secreting cells, in response to CD40 triggering and interleukin-2, as opposed to interstitial type DCs.30 This was essentially verified with Langerhans cells and CD14+ dermal DCs isolated from human skin. The former subset was superior in cross-priming CD8+ T cells, and the latter subset was specialized to prime CD4+ helper T cells that in turn induced B cells to become antibody-producing cells.15 It is interesting to note, however, that the CD14+ subset of dermal DCs comprises only about a tenth of all langerin− dermal DCs.5 These in vitro data correspond to in vivo observations in mice, in which skin-derived dermal DCs localized close to the B-cell follicles in the outer paracortex of the lymph node. Langerhans cells, in contrast, settled in the inner paracortex, intermingled with lymph node-resident langerin+ cells.31 It is not known whether these dermal DCs correspond to the human dermal DCs subset that promotes the humoral response. Yet, these findings suggest that a ‘division of labour’ may indeed be operative in vivo. This point is discussed in more detail in the companion article by Ueno et al.32
With reference to the potential harnessing of skin DCs for vaccinations that aim at the generation of cytotoxic T lymphocytes (for example in vaccinations against cancer), some recent observations are of importance. Several laboratories could show that Langerhans cells are especially capable of inducing cytotoxic T lymphocytes, as opposed to dermal DCs. This was first shown with Langerhans cells derived from CD34+ stem cells.33 Recently, these data were confirmed using human Langerhans cells isolated from the epidermis; Langerhans cells were indeed more potent to induce CD8+ T cells that contained increased levels of lytic molecules (perforin, granzymes) and that efficiently killed tumour cell lines. Langerhans cell-derived IL-15 seemed to be a critical factor.15,5 In this regard, it is of note that we found human Langerhans cells to induce substantial levels of INF-γ secretion in naive CD4+ helper T cells in response to CD40 ligation.34 Studies in a mouse tumour model confirmed and emphasized that Langerhans cells (and possibly also dermal langerin+ DCs) are essential for anti-tumour immunity in vivo. Protection from an experimental tumour was lost when Langerhans cells and dermal langerin+ DCs were absent35 because they had been ablated by means of the diphtheria toxin receptor knock-in technology.36 For a detailed description of innovative mouse models for Langerhans cell research, please see companion article by Clausen and Kel.37
Thus, there is good reason to rationally try and address DCs of the skin, especially Langerhans cells, for purposes of vaccination.5 This, however, would imply to shift the emphasis from the subcutis and the muscle to the dermis and epidermis as the preferred site of vaccination.
IMPROVING IMMUNOGENICITY AND TOLERANCE INDUCTION IN VIVO BY ‘ANTIGEN TARGETING’
Principle of antigen targeting
DCs are equipped with varying sets of receptors that help them with the uptake of pathogens, the so-called ‘C-type lectin receptors’. Important examples are langerin (CD207), DC-SIGN (CD209) and DEC-205 (CD205).38 A series of seminal studies from the Rockefeller University laboratories has shown that immune responses can be dramatically enhanced when an antigen is ‘not only’ injected into or under the skin (as in conventional vaccinations), but is delivered (‘targeted’) directly and selectively to DCs. In other words, the antigen or the vaccine is getting an ‘address label’ in the form of a specific antibody against a C-type lectin. Thereby, the antigen or vaccine ‘finds its way’ directly and exclusively to the DCs that expresses the respective C-type lectin on its surface. The coupling of the protein antigen with the monoclonal antibodies can be achieved by chemical conjugation or, preferably, by genetic engineering. In many experiments, the model antigen ovalbumin (OVA) was inserted into a hybrid antibody consisting of the rat immunoglobulin (Ig) variable regions recognizing the C-type lectin and mouse Ig constant regions. Antibodies to different C-type lectins were used.39 Importantly, this strategy is being extended beyond the almost ubiquitous model antigen OVA to other antigens such as hen egg lysozyme,40 the HIV gag protein,41,42 mesothelin, a human tumour antigen,43 or tyrosinase-related protein-1, a mouse melanoma antigen.44 The overcoming of the experimental OVA model is a desirable goal. Data from the OVA model must always be judged with caution, and premature generalizations must be avoided. The widely used OVA peptide-specific TCR transgenic T cells, in particular the CD8+ T cells (‘OT-I cells’), are unphysiologically sensitive to TCR stimulation: they already respond to minute amounts of antigen in the picomolar range.45 Nevertheless, experiments in the OVA model have yielded and are still yielding important insights.
In vitro studies
Most studies, especially the initial ones, were performed with antigens conjugated to antibodies against the DEC-205 (CD205) receptor. In the first description of the cell biology of antigen targeting through C-type lectins,46 the authors investigated the fate of Ig binding to chimeric Fc receptors that possessed the cytosolid domains of C-type lectins. When Ig was given to DCs expressing the cytosolic part of the DEC-205 receptor, its uptake was strongly enhanced. Moreover, in contrast to targeting through the macrophage mannose receptor, antigens were specifically routed to late endosomes or lysosomes that contained abundant MHC II molecules. This resulted in a markedly (up to 100-fold) augmented T-cell response in vitro.46
In vivo studies—targeting in the steady state
Subsequently, mice were immunized with anti-DEC-205/antigen conjugates. Immunization in the absence of DCs maturation stimuli such as CD40 ligation or poly (I:C), that is in the steady state, led to T-cell unresponsiveness in both the CD4+40 and the CD8+47 T-cell compartment. An initial wave of proliferation was not sustained but, rather, T-cell numbers were reduced, and the remaining T cells did not respond any longer to a standard immunization protocol using complete Freund’s adjuvant. This indicated that peripheral tolerance had developed in response to the steady-state administration of the antigen–antibody conjugate.48 These findings have already been validated in a mouse model for type I autoimmune diabetes in which both onset and progression of the disease could be inhibited bytreatment with anti-DEC-205-conjugated antigen.49,50 The underlying mechanism for tolerance induction seems to be not only deletion and anergy, as shown in the original work,40,47 but also induction of regulatory T cells.51
In vivo studies—targeting under inflammatory/immunogenic conditions
The outcome in terms of T-cell responses changed dramatically when DCs maturation stimuli were added at the time of immunization with the anti-DEC-205-antigen conjugate. Importantly, T-cell proliferation in vivo increased several orders of magnitude as compared with immunization with the same amount of unconjugated antigen, be it apeptide40 or the whole-antigenic protein.47 The augmented T-cell proliferation translated into markedly improved anti-tumour immunity in an experimental model in vivo. These authors found that the very infrequent naive antigen-specific T cells that exist in a nonimmune mouse could only be primed if the subcutaneously injected antigen was coupled to an anti-DEC-205 antibody and a strong DCs maturation stimulus (CD40 ligation) was co-administered. Uncoupled antigen plus/minus CD40 ligation as well as immunization with antigen-pulsed cultured DCs did not lead to efficient priming of naive T cells in this setting, thus highlighting the potential of antigen targeting.52 Not unexpectedly, the increased stimulation of helper T cells by the anti-DEC-205-antigen conjugates led to amplified antibody responses in mice.53 These data emphasize that it is feasible to also harness antigen targeting for improving humoral immune responses in vaccinations.
Antigen targeting to different receptors
Further studies showed that C-type lectins other than DEC-205 could be targeted, and they also revealed that the type of targeted antigen uptake receptor can influence the quantity and quality of resulting immune responses. Dudziak et al.54 compared side-by-side immune responses induced by immunization with antigens conjugated to antibodies against DEC-205 versus DCIR2 (DCs inhibitory receptor-2; ‘33D1 antigen’) that are expressed by CD8+ and CD8− DCs in the spleen of mice, respectively. Immunization through DEC-205 favoured CD8+ T-cell responses, whereas DCIR2 targeting preferentially induced CD4+ T-cell responses. In this case, the differential T-cell responses were due to DC-intrinsic properties, CD8+/DEC-205+ DCs expressing more genes associated with processing for the MHC class I pathway (TAP, calreticulin, etc.) and CD8−/DCIR2+ DCs expressing more genes associated with processing for the MHC class II pathway (cathepsins, etc.).54 At another level, it was shown that the selective delivery of antigen to the splenic CD8+/DEC-205+ and CD8−/DCIR2+ DCs subsets by means of the respective antigen–antibody conjugates led to the de novo induction of FoxP3+ Treg and to the expansion of pre-existing FoxP3+ Treg, respectively.51,55
In addition, the outcome may also depend on the molecular nature of the targeted receptor. For example, in the original studies, Mahnke et al.46 found that bone marrow-derived DCs expressed both DEC-205 and macrophage mannose receptor. Yet, antigen targeted to either of these two receptors was taken up differently, routed differently and enhanced T-cell responses were only observed with DEC-205-targeted antigen. A more recent, similar example was provided by Bozzacco et al.56 who compared targeting an HIV antigen (p24 gag) to human DCs with anti-DEC-205 vis-á-vis anti-DC-SIGN/CD209 antibodies. They found that anti-DEC-205-conjugated antigen was more effectively cross-presented than anti-DC-SIGN-conjugated antigen, even though both receptors are expressed on the same DCs at similar levels.
Finally, the development of monoclonal antibodies recognizing the extracellular domain of the langerin (CD20757) molecule58 opened the way to study antigen targeting to this molecule expressed on important skin DCs, particularly on Langerhans cells. Given the pronounced properties of Langerhans cells in the induction of cytotoxic responses (see above), targeting this receptor is of high interest. Initial analyses revealed many similarities in targeting properties to DEC-205.39 Targeting the CD8α+ DCs subset in spleen, that is responsible for cross-presentation there,59 will be interesting because this subset specifically co-expresses the langerin receptor.60 Recent data suggest that, indeed, cross-presenting activity is restricted to that CD8α+/langerin+ DCs population in mouse spleen.61
Antigen targeting to other DCs surface receptors including Dectin-1 and -2, CD36, DC-SIGN/CD209, mannose receptor and, importantly, Clec9A62 and Clec12A63 is also being explored (reviewed in Caminschi et al.64). Molecules beyond the field of C-type lectin receptors may also be interesting candidates. For instance, improved immune responses have been reported for antigens that were more broadly targeted to MHC molecules (by means of an MHC-binding superantigen, not with antibodies).65
Conclusions
On the basis of this ample evidence, it is now widely attempted to harness ‘antigen targeting’ for clinical purposes, in particular for the improvement of vaccinations.64,66 With regard to the above-discussed considerations as to the optimal site for vaccinations, it is important to recognize that none of the targeting studies has systematically investigated skin DCs (except a recent paper from our own laboratory67 to be discussed in more detail below.) These antibody–antigen conjugates in the mentioned mouse studies were injected into the skin (typically subcutaneously into the footpad of the mouse), but it was not investigated which specific cell types there would take up, process and transport these conjugates to the lymph nodes. This is particularly true in human beings, in which virtually nothing is known about the relative capacities of skin DCs to be targeted.
SKIN DCs CAN BE SUCCESSFULLY TARGETED WITH ANTIGENS
Expression of ‘targetable’ receptors on skin DCs
Langerhans cells are phenotypically well characterized both in mouse and human skin. Their identifying receptor is Langerin/CD207. In addition, they express Dectin-168 and Dectin-2.69 The DCIR2 molecule is absent from Langerhans cells,70 but importantly, they display surface expression of DEC-205,71,18,27 the prototype targeting receptor. Most phenotypical data in the dermis do not (yet) take into account the newly described subsets of dermal DCs. The ‘classical’, langerin− dermal DCs express DEC-205 both in mouse and man.27 DC-SIGN, that was initially regarded as a marker for dermal DCs,27 occurs more abundantly on macrophages in the dermis as identified by CD1415,72 or CD16318 expression. The expression on skin DCs of additional interesting candidates for targeting such as Clec9,62 Clec12,63 Lox,73 and others (reviewed recently by Caminschi et al.64) has not yet beeninvestigated.
The final judgement as to the feasibility of a DC-expressed molecule to serve as a target can only be made after successful demonstration of antibody binding in situ to this molecule, as described recently by Flacher et al.67 One must be aware that expression of a given molecule on skin DCs can be determined in several different ways, each having its pros and cons. (i) The visualization of receptor expression in situ on sections or in epidermal and dermal sheet specimens has the advantage of showing expression just where it is needed for targeting. On the other hand, true cell surface expression, especially when it is weak, cannot be unequivocally ascertained by this approach. (ii) The search for receptor expression by flow cytometry on freshly isolated DCs from epidermis (typically by trypsinization) or dermis (typically by collagenase treatment) may be more sensitive and can unequivocally detect surface expression. On the other hand, enzyme treatment may destroy or at least reduce certain surface molecules. Dispase sensitivity of human DEC-205 or DC-SIGN27 or trypsin sensitivity of murine DEC-20571 are such examples. (iii) Finally, receptor expression can be determined on skin DCs that have migrated from explant cultures of whole-skin, epidermis or dermis over a period of 2–3 days.13,72 This has the advantage that enzyme damage of molecules cannot occur. On the other hand, migratory skin DCs are fully mature and do, therefore, not necessarily correspond to the DCs that reside in the skin at the time of targeting. Moreover, the relative proportions of the different DCs subsets may differ as compared with physiological, in vivo migration towards lymph nodes. We feel that it is important to keep these considerations in mind as several recent papers, that had contributed highly valuable knowledge on skin DCs used isolation techniques that involved incubation periods at 37°C for varying periods of time.15,18,74
Access of the targeted vaccine to skin DCs
The direct approach to access dermal DCs is by injection into the dermis. This procedure is well known to the physician, mainly from the classical tuberculin sensitivity test that requires intradermal injection. The access to epidermal Langerhans cells is less straightforward. Langerhans cells in the epithelium are separated from the well vascularized dermis by the dense collagenous basement membrane. This structure, however, is readily permeable for large molecules such as antibodies. Already a long time ago, Aberer et al.75 showed in mice that intraperitoneally injected antibodies (anti-MHC-II in that case) easily found their way into the epidermis and specifically bound to Langerhans cells there. We could recently show that anti-DEC-205 and anti-langerin antibodies gained access to epidermal Langerhans cells within few hours when either injected intradermally or offered in the culture medium of skin explant cultures (Figures 1 and 2).67 In the latter experimental setting this was also true for human skin. Langerhans cells in the epidermis efficiently captured the antibody proteins (Figure 3). This was presumably by diffusion into the epidermis across the basement membrane, although we could not exclude a possible additional mechanism, namely active sequestration of the antibodies by Langerhans cells reaching ‘down’ through the basement membrane. Such behaviour was shown for DCs in the gut76 and recently even for Langerhans cells that extend their dendrites ‘upwards’ into the stratum corneum for sampling pathogens there.77
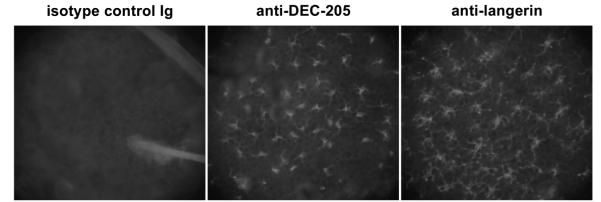
Figure 1.
Langerhans cells in situ are specifically targeted by mAb in murine whole-skin explant cultures. Explants from BALB/c mice were incubated for 4 h with 5 μgml−1 of purified rat anti-mouse DEC-205/CD205 (mAb NLDC145), rat anti-mouse langerin/CD207 (mAb L31) or an isotype-matched control rat Ig. Epidermal sheets were then prepared and stained with fluorescent anti-rat IgG.
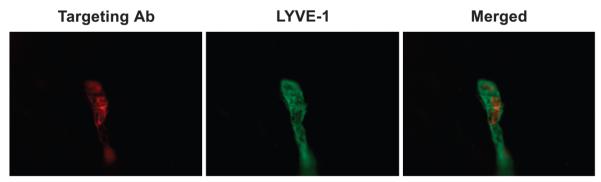
Figure 2.
Langerhans cells targeted in situ by intradermal injection of anti-langerin (L31) antibodies migrate towards lymph nodes in lymphatic vessels of murine ear skin. Analysis of dermal sheets was performed 4 days after intradermal injection. Targeting antibodies were visualized by means of anti-rat Ig (red fluorescence). Dermal lymphatics were identified with antibodies against LYVE-1 (green fluorescence). (Courtesy of Dr Bernhard Haid).
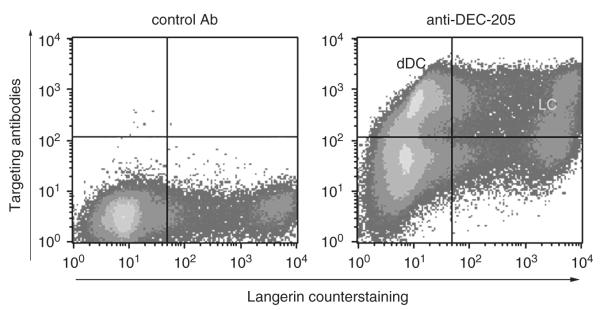
Figure 3.
Human skin DCs bind and transport targeting mAbs. Human whole-skin explants were incubated for 4 h in medium containing antibody MG38 (anti-DEC-205) or IgG isotype control and then cultured for 4 days. Targeting antibodies were detected in permeabilized migratory cells by means of an anti-mouse IgG secondary Ab (y axis). Cells were counterstained with anti-langerin/CD207 (x axis). Note that both Langerhans cells and dermal DCs (not subdivided into subsets here) have captured the targeting antibody. dDC, dermal DCs; LC, Langerhans cells.
In the mouse, anti-DEC-205 antibodies were also captured by langerin+ dermal DCs as well as by langerin− dermal DCs. Interestingly, in mouse skin the majority of DEC-205-targeted cells consisted of Langerhans cells from the epidermis. This was determined by analysing the cells that migrated from whole-skin explants.67 Only relatively few langerin− dermal DCs had bound the antibodies. Yet, such targeted dermal DCs could be visualized on sections of skin that was subcutaneously injected with anti-DEC-205 antibodies.78 When looking at skin-draining lymph nodes a similar picture appeared: most incoming, antibody-targeted DCs (defined as CD11c+/CD8− DCs) were langerin+, including both ‘genuine’ Langerhans cells (CD103−) and dermal langerin+ DCs (CD103+)(Flacher et al., manuscript in preparation). In human skin, the proportions between anti-DEC-205-targeted Langerhans cells and dermal DCs were more variable and not so much in favour of Langerhans cells. This needs to be studied in more detail. Yet, one important experimental detail must be taken into account in this regard. Whereas the thickness of mouse skin, in particular the ears, may be considered by and large constant, human skin can strongly vary in thickness from donor to donor, depending on localization, age, surgical technique (skills) in the preparation of so-called split-thickness skin (0.2–0.3 mm). Even though most dermal DCs reside in the upper, papillary dermis, that is relatively close to the basement membrane, skin thickness may still influence the yields and the subset composition of dermal DCs that can be obtained.
Another way of delivering antigen to epidermal Langerhans cells is the topical application. This modality will be dealt with in more detail in the companion article by Stoitzner et al.9 Unconjugated protein antigen that is topically (epicutaneously) administered readily reached Langerhans cells,79 which processed it and (cross)-presented it to CD8+ and CD4+ T cells in vivo.35 This translated into Langerhans cell-mediated protection in a mouse tumour model.35 Similarly, anti-DEC-205 and anti-langerin antibodies were applied epicutaneously onto tape-stripped mouse skin. Gentle tape stripping has the advantage of simultaneously removing the stratum corneum and inducing inflammation.80 The epidermal barrier is disrupted and the epidermis becomes permeable for the topically deposited antibodies that can then bind to Langerhans cells. Interestingly, under the chosen experimental conditions, anti-DEC-205 antibodies also reached dermal langerin+ DCs, but not dermal langerin− DCs.79
Functional consequences of targeting skin DCs
As mentioned above, virtually all earlier studies on antigen targeting used the classical route of immunization in the mouse, that is injection into the footpad. This corresponds to a subcutaneous injection. It was never thoroughly determined which cells were targeted in this setting. Thus, the relative contributions of different skin DCs to the resulting immunization could not be judged. We addressed this question by injecting the antigen–antibody conjugates (anti-DEC-205/OVA, anti-langerin/OVA, isotype control/OVA) into the dermis of the ear that possesses much less subcutis and where the injection can truly be considered intradermal. Langerhans cells that migrated from epidermal sheets prepared from ear skin 4 h after injection were tested in vitro whether they had taken up and processed the conjugates in situ. OVA peptide-specific CD8+ and CD4+ T cells proliferated in response to Langerhans cells that had migrated from skin injected with very low concentrations of anti-DEC-205/OVA conjugates, indicating both cross-presentation to CD8+ T cells and presentation to CD4+ T cells. (Dermal langerin+ DCs are not present in this experimental setting.) Only 50 (for CD8+ T cells) to 500 ng (for CD4+ T cells) of injected conjugate sufficed to bring about T-cell stimulation.67 Unconjugated OVA at such low concentrations did not lead to T-cell responses; higher concentrations needed to be injected. These data show and emphasize that epidermal Langerhans cells present an antigen targeted to their DEC-205 receptors much better than untargeted antigen. The superior presentation of anti-DEC-205/OVA to CD8+ T cells as compared with CD4+ T cells was originally described for mice that had been immunized subcutaneously.54 We extended these data by defining epidermal Langerhans cells as one potentially responsible skin DCs subset. It is not known whether anti-DEC-205/OVA-targeted dermal langerin+ DCs and dermal langerin− DCs would also stimulate T-cell subsets differentially.
Interestingly, targeting to langerin was less efficient. Neither CD4+ nor CD8+ T cells responded to anti-langerin/OVA-targeted Langerhans cells.67 However, when mice were immunized into the footpads with anti-langerin/OVA, antigen-specific CD4+ and CD8+ T cells vigorously proliferated in the lymph node.39 This discrepancy may be explained by the immunogenic contribution of anti-langerin/OVA-targeted dermal langerin+ DCs that were not present in the epidermal sheet explant cultures.
CONCLUDING REMARKS
Combining vaccination into the dermis or onto the surface of the skin (epicutaneous) with the use of vaccines that are targeted to specific subsets of DCs is promising. Langerhans cells seem to be well capable of initiating cytotoxic T-cell responses and such responses could be potentiated by targeting the antigens to endocytic receptors on the surface of Langerhans cells. Preliminary (unpublished) observations by Stoitzner et al. indicate that targeting tumour antigens to DEC-205 on skin DCs by intradermal injection leads to markedly improved protection from tumour. Thinking beyond vaccination against cancer, one could envisage to target antigen to human dermal DCs, namely the CD14+ subset, to exploit the capacity of that subset to promote antibody responses.5 CD36, the thrombospondin receptor, might be a candidate as this molecule is specifically expressed on dermal DCs13 and it was shown that targeting CD36 can also improve immune responses.81 Most studies are currently performed in mouse models. Others and we are beginning to validate the data in human experimental models. There, the first important step will be to characterize in more detail the subsets of skin DCs that are targeted by the antibodies against DEC-205, langerin and others. Ultimately, the aim will be to translate these findings into clinical medicine and to test targeted vaccines in clinical trials.
ACKNOWLEDGEMENTS
We (NR and VF) have continuously been supported by the Austrian Science Fund (currently, FWF project P21487 to P Stoitzner). NR and MT are further supported by the COMET Center ONCOTYROL and funded by the Federal Ministry for Transport Innovation and Technology (BMVIT) and the Federal Ministry of Economics and Labour/the Federal Ministry of Economy, Family and Youth (BMWA/BMWFJ), and the Tiroler Zukunftsstiftung (TZS). We appreciate the participation of the TILAK hospital holding company, who serves as a partner to the Oncotyrol research programme. We apologize to all colleagues whose important contributions to Langerhans cell biology could not be cited due to space constraints. RMS and JI are supported by the Center for AIDS Vaccine Discovery, National Institutes of Health (Grants AI 13013, AI 40874 and AI 057158 to RMS) and the Canadian Histiocytosis Association.
References
- 1.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. Dendritic cells and vaccines. Proc (Bayl Univ Med Cent) 2008;21:3–8. doi: 10.1080/08998280.2008.11928346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno H, Schmitt N, Klechevsky E, Pedroza-Gonzalez A, Matsui T, Zurawski G, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7:1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 7.Warger T, Schild H, Rechtsteiner G. Initiation of adaptive immune responses by transcutaneous immunization. Immunol Lett. 2007;109:13–20. doi: 10.1016/j.imlet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers’ diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371:2019–2025. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 9.Stoitzner P, Sparber F, Tripp CH. Langerhans cells as targets for immunotherapy against skin cancer. Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.31. (e-pub ahead of print 30 March 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, Van Nest G, Ott G, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186:18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 11.Casares S, Inaba K, Brumeanu TD, Steinman RM, Bona CA. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J Exp Med. 1997;186:1481–1486. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart DNJ, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981;154:347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993;92:2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupasquier M, Prens EP, Leenen PJM. Dermal mononuclear phagocytes. In: Saeland S, editor. Recent Advances in Skin Immunology. Research Signpost; Trivandrum, Kerala, India: 2008. pp. 75–104. [Google Scholar]
- 15.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angel CE, Lala A, Chen CJ, Edgar SG, Ostrovsky LL, Dunbar PR. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int Immunol. 2007;19:1271–1279. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- 17.Zaba LC, Krueger JG, Lowes MA. Resident and inflammatory dendritic cells in human skin. J Invest Dermatol. 2009;129:302–308. doi: 10.1038/jid.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupasquier M, Stoitzner P, Van Oudenaren A, Romani N, Leenen PJM. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol. 2004;123:876–879. doi: 10.1111/j.0022-202X.2004.23427.x. [DOI] [PubMed] [Google Scholar]
- 20.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux F, Collin M, Bogunovic M, Abel M, Leboef M, Helft J, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romani N, Clausen BE, Stoitzner P. Langerhans cells & more: Langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowes MA, Chamian F, Abello MV, J Fuentes-Duculan, Lin SL, Nussbaum R, et al. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) Proc Natl Acad Sci USA. 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wollenberg A, Kraft S, Hanau D, Bieber T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol. 1996;106:446–453. doi: 10.1111/1523-1747.ep12343596. [DOI] [PubMed] [Google Scholar]
- 26.Palamara F, Meindl S, Holcmann M, Lührs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–3061. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 27.Ebner S, Ehammer Z, Holzmann S, Schwingshackl P, Forstner M, Stoitzner P, et al. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int Immunol. 2004;16:877–887. doi: 10.1093/intimm/dxh088. [DOI] [PubMed] [Google Scholar]
- 28.Romani N, Ebner S, Flacher V, Tripp CH, Heufler C, Clausen BE, et al. Langerhans cells—dendritic cells of the epidermis and other epithelia. In: Saeland S, editor. Recent Advances in Skin Immunology. Research Signpost; Trivandrum, Kerala, India: 2008. pp. 27–73. [Google Scholar]
- 29.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 30.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor α. 2. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 31.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Ueno H, Schmitt N, Palucka AK, Banchereau J. Dendritic cells and humoral immunity in humans. Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.28. (e-pub ahead of print 23 March 2010; doi:10.1038/icb.2010.28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, et al. Mature human Langerhans cells derived from CD34+ hemopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 34.Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, Liu Y-J, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen presenting cells that induce pro-allergic T cells. J Allergy Clin Immunol. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, et al. Tumor immunotherapy by epicutaneous immunization requires Langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 37.Clausen BE, Kel JM. Langerhans cells: critical regulators of skin immunity? Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.40. (e-pub ahead of print 30 March 2010) [DOI] [PubMed] [Google Scholar]
- 38.Figdor CG, Van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 39.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, et al. The Langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and MHC II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 40.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B, Kuroiwa JM, He LZ, Charalambous A, Keler T, Steinman RM. The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann N Y Acad Sci. 2009;1174:6–17. doi: 10.1111/j.1749-6632.2009.04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahnke K, Qian YJ, Fondel S, Brueck J, Becker C, Enk AH. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res. 2005;65:7007–7012. doi: 10.1158/0008-5472.CAN-05-0938. [DOI] [PubMed] [Google Scholar]
- 45.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–683. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 49.Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian YJ, et al. On the edge of autoimmunity—T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–3401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- 50.Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, et al. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci USA. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii SI, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boscardin SB, Hafalla JCR, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 55.Yamazaki S, Steinman RM. Dendritic cells as controllers of antigen-specific Foxp3+ regulatory T cells. J Dermatol Sci. 2009;54:69–75. doi: 10.1016/j.jdermsci.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 58.Cheong C, Idoyaga J, Do Y, Pack M, Park SH, Lee H, et al. Production of monoclonal antibodies that recognize the extracellular domain of mouse Langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 60.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8α+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farrand KJ, Dickgreber N, Stoitzner P, Ronchese F, Petersen TR, Hermans IF. Langerin+CD8α+ dendritic cells are critical for cross-priming and IL-12 production in response to systemic antigens. J Immunol. 2009;183:7732–7742. doi: 10.4049/jimmunol.0902707. [DOI] [PubMed] [Google Scholar]
- 62.Caminschi I, Proietto AI, Ahmet F, Kitsoulis S, Teh JS, Lo JCY, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L, et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. 2009;182:7587–7594. doi: 10.4049/jimmunol.0900464. [DOI] [PubMed] [Google Scholar]
- 64.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to dendritic cells. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 65.Dickgreber N, Stoitzner P, Bai Y, Price KM, Farrand KJ, Manning K, et al. Targeting antigen to MHC class II molecules promotes efficient cross-presentation and enhances immunotherapy. J Immunol. 2009;182:1260–1269. doi: 10.4049/jimmunol.182.3.1260. [DOI] [PubMed] [Google Scholar]
- 66.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 67.Flacher V, Tripp CH, Stoitzner P, Haid B, Ebner S, Del Frari B, et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol. 2010;130:755–762. doi: 10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, III, Kumamoto T, et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 69.Ariizumi K, Shen GL, Shikano S, Ritter R, III, Zukas P, Edelbaum D, et al. Cloning of a second dendritic cell-associated C-type lectin (Dectin-2) and its alternatively spliced isoforms. J Biol Chem. 2000;275:11957–11963. doi: 10.1074/jbc.275.16.11957. [DOI] [PubMed] [Google Scholar]
- 70.Witmer-Pack MD, Valinsky J, Olivier W, Steinman RM. Quantitation of surface antigens on cultured murine epidermal Langerhans cells: rapid and selective increase in the level of surface MHC products. J Invest Dermatol. 1987;90:387–394. doi: 10.1111/1523-1747.ep12456460. [DOI] [PubMed] [Google Scholar]
- 71.Inaba K, Swiggard WJ, Inaba M, Meltzer J, Mirza A, Sasagawa T, et al. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol. 1995;163:148–156. doi: 10.1006/cimm.1995.1109. [DOI] [PubMed] [Google Scholar]
- 72.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, et al. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 73.Delneste Y, Magistrelli G, Gauchat JF, Haeuw JF, Aubry JP, Nakamura K, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 74.Angel CE, George E, Brooks AE, Ostrovsky LL, Brown TL, Dunbar PR. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol. 2006;176:5730–5734. doi: 10.4049/jimmunol.176.10.5730. [DOI] [PubMed] [Google Scholar]
- 75.Aberer W, Kruisbeek AM, Shimada S, Katz SI. In vivo treatment with anti-I-A antibodies: differential effects on Ia antigens and antigen-presenting cell function of spleen cells and epidermal Langerhans cells. J Immunol. 1986;136:830–836. [PubMed] [Google Scholar]
- 76.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 77.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206:2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 79.Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins—attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holzmann S, Tripp CH, Schmuth M, Janke K, Koch F, Saeland S, et al. A model system using tape stripping for characterization of Langerhans cell-precursors in vivo. J Invest Dermatol. 2004;122:1165–1174. doi: 10.1111/j.0022-202X.2004.22520.x. [DOI] [PubMed] [Google Scholar]
- 81.Tagliani E, Guermonprez P, Sepulveda J, Lopez-Bravo M, Ardavin C, Amigorena S, et al. Selection of an antibody library identifies a pathway to induce immunity by targeting CD36 on steady-state CD8α+ dendritic cells. J Immunol. 2008;180:3201–3209. doi: 10.4049/jimmunol.180.5.3201. [DOI] [PubMed] [Google Scholar]