Summary
Langerhans cells (LCs) are antigen-presenting dendritic cells (DCs) that reside in epithelia. The best studied example is the LC of the epidermis. By electron microscopy, their identifying feature is the unique rod- or tennis racket-shaped Birbeck granule. The phenotypic hallmark is their expression of the C-type lectin receptor langerin/CD207. Langerin, however, is also expressed on a recently discovered population of DC in the dermis and other tissues of the body. These ‘dermal langerin+ dendritic cells’ are unrelated to LCs. The complex field of langerin-negative dermal DCs is not dealt with here. In this article, we briefly review the history, ontogeny, and homeostasis of LCs. More emphasis is laid on the discussion of functional properties in vivo. Novel models using genetically engineered mice are contributing tremendously to our understanding of the role of LCs in eliciting adaptive immune responses against pathogens or tumors and in inducing and maintaining tolerance against self antigens and innocuous substances in vivo. Also, innate effector functions are increasingly being recognized. Current activities in this area are reviewed, and possibilities for future exploitation of LC in medicine, e.g. for the improvement of vaccines, are contemplated.
Keywords: antigen presentation, cell ablation, dendritic cells, immunogenicity, Langerhans cells, langerin/CD207
Introduction
Dendritic cells (DCs) are key regulators in the immune system. They mediate between the innate and the adaptive immune system, and they critically determine whether immunity or tolerance is generated in the body (1-3). DCs were first discovered, unknowingly, in 1868 by Paul Langerhans (4) at Berlin; he described conspicuous dendritically shaped cells in the human epidermis that were eventually named after him (Fig. 1).
Fig. 1.
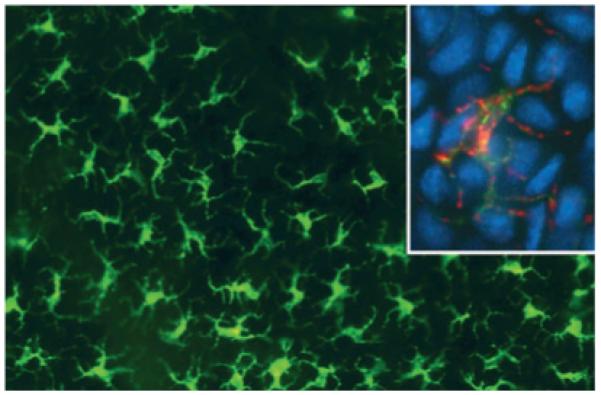
Langerhans cells in an epidermal sheet from mouse skin. They are visualized by immunostaining with anti-major histocompatibility complex (MHC)-II antibodies in the large picture. The inset depicts double-labeling of MHC-II (green fluroescence) and langerin (red fluorescence). Nuclei are counterstained with DAPI (4′,6-diamidino-2-phenylindole) (blue). Conventional epi-fluorescence microscopy.
In 1973, DCs were discovered in the spleens of mice (5). However, it took another 12 years until Langerhans cells (LCs) were definitively recognized as a member of the DC system. This was the seminal paper in 1985 by Gerold Schuler and Ralph Steinman entitled ‘Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro’ (6). This milestone did not appear out of the blue. Rather, it was preceded by a number of important observations and experiments that are summarized in Table 1.
Table 1. Milestones in the history of Langerhans cells 1868–1985.
| Year |
Key findings |
Ref. |
|---|---|---|
| 1868 | Paul Langerhans discovered dendritically shaped cells in the human epidermis by means of gold chloride staining technique and assumed that they were nerve cells |
(4) |
| 1961 | Birbeck et al. described the typical rod- and tennis racket-shaped cytoplasmic organelles in Langerhans cells of the epidermis. Langerhans cells were regarded as ‘effete’ (i.e. worn out, moribund) melanocytes |
(7) |
| 1966 | Basset et al. found Birbeck granules in lesions of ‘Histiocytosis X’, now called‘Langerhans cell histiocytosis’. This was the first link of Langerhans cells with cells of the ‘reticulo-endothelial system’, i.e. with leukocytes and the immune system |
(8) |
| 1967 | Wolff & Winkelmann detected ATPase activity on the surface of Langerhans cells – another piece of evidence for the leukocyte nature of Langerhans cell and their affiliation with the ‘mononuclear phagocyte system’ |
(9) |
| 1977 | The groups of Stingl, Rowden and Klareskog showed the expression of Fc-and complement receptors and MHC-II molecules on Langerhans cells. This further anchored Langerhans cells in the immune system |
(10-12) |
| 1979 | The groups of Katz and Frelinger demonstrated the bone marrow origin of Langerhans cells by chimera studies |
(13, 14) |
| 1985 and 1989 | Schuler & Steinman recognized that Langerhans cells are a special form of DCs, and that they occur in distinct states of maturation (i.e. the ‘Langerhans cell paradigm’) that are characterized by reciprocally expressed antigen processing and T-cell stimulatory capacities |
(6, 15) |
The 1985 breakthrough was not the end of LC research; in fact, it marked the beginning of a new era. Table 2 highlights important findings that facilitated to shape the picture of LC and other langerin+ DC as it is today. This period was characterized by the establishment of a so-called ‘LC paradigm’ (16, 17) and its subsequent challenge. Today, LC research is bustling with exciting activities, and milestones seem to be set at an increasingly rapid pace.
Table 2. Milestones in the history of Langerhans cells from 1985 to today.
| Year |
Key findings |
Ref. |
|---|---|---|
| 1992 | Jacques Banchereau and Christophe Caux were the first ones to generate Langerhans cells with Birbeck granules from human hematopoietic progenitor cells (CD34+ cells). This model was and still is being widely exploited |
(18) |
| 1999 | Sem Saeland and Jenny Valladeau identified the langerin/CD207 molecule as the main ‘molecular brick’ of Birbeck granules. Antibodies against langerin became available and led to a boost in Langerhans cell research |
(19) (20) (21) |
| 2002 | Miriam Merad elegantly showed that in the steady-state Langerhans cells remained in the epidermis for virtually the entire life, and that they slowly renew themselves in situ by proliferation |
(22) |
| 2003 | Allan et al. shook the commonly held belief that Langerhans cells are the ‘one and only’ potent presenters of pathogen-derived antigens in vivo. They demonstrated that herpes virus applied to the epidermis is not presented by Langerhans cells in the lymph nodes |
(23) |
| 2005/2006 | The advent of mouse models where Langerhans cells can be selectively depleted from living mice and the possibility to selectively tag langerin+ cells with GFP laid the basis for much of the ongoing research in the field that still aims at clarifying the role of Langerhans cells in vivo in immunity and tolerance |
(24-26) |
| 2007 | Langerhans cells are not exclusive any longer in their expression of langerin. Dermal langerin+ dendritic cells were discovered in mice and found to be unrelated to epidermal Langerhans cells |
(27-29) |
Review articles on LC have regularly been published over the years (including early and recent reviews in this Journal). Even the old articles are still worthwhile reading, because they allow the reader to follow the historic development of our views on LCs. They sometimes also contain observations that have only recently been substantiated and better understood by more advanced experimental techniques, e.g. the detection of proliferating LCs within the epidermis (Table 3).
Table 3. Selected review articles on Langerhans cells within the past 40 years.
| 1965 | Breathnach AS. The cell of Langerhans. Int Rev Cytol 18:1–28 |
| 1972 | Wolff K. The Langerhans cell. Curr Probl Dermatol 4:79–145 |
| 1980 | Stingl G, Tamaki K, Katz SI. Origin and function of epidermal Langerhans cells. Immunol Rev 53:149–174 |
| 1980 | Silberberg-Sinakin I, Gigli I, Baer RL, Thorbecke GJ. Langerhans cells: role in contact hypersensitivity and relationship to lymphoid dendritic cells and to macrophages. Immunol Rev 53:203–232 |
| 1981 | Rowden G. The Langerhans cell. CRC Crit Rev Immunol 3:95–180 |
| 1983 | Wolff K, Stingl G. The Langerhans Cell. J Invest Dermatol 80:17s–21s |
| 1984 | Streilein JW, Bergstresser PR. Langerhans cells: antigen presenting cells of the epidermis. Immunobiology 168:285–300. |
| 1987 | Stingl G, Romani N, Wolff K. Langerhans cells: A distinctive member of bone marrow-derived dendritic cells. Adv Dermatol 2:269–282 |
| 1991 | Epidermal Langerhans Cells. Schuler G, ed. CRC Press,Inc., Boca Raton, Florida, 1–324 |
| 1995 | The Immune Functions of Epidermal Langerhans Cells. Moll H, ed. R.G.Landes Company/Springer-Verlag, Austin,New York, Berlin, 1–193 |
| 2003 | Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells – dendritic cells of the epidermis. APMIS 111:725–740 |
| 2005 | Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol 17:273–283 |
| 2006 | Romani N, Tripp CH, Ratzinger G, Heufler C, Koch F, Saeland S, Stoitzner P. Epidermal Langerhans Cells. In Handbook of Dendritic Cells. Biology, Diseases and Therapies. Lutz MB, Romani N, Steinkasserer A, editors. Wiley-VCH Verlag, Weinheim, 73–100 |
| 2007 | Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 219:118–142 |
| 2008 | Romani N, Ebner S, Flacher V, Tripp CH, Heufler C, Clausen BE, Stoitzner P. Langerhans cells – dendritic cells of the epidermis and other epi thelia. In: Recent Progress in Skin Immunology, ed. Saeland S, Research Signpost, Trivandrum, Kerala, India, 27–73 |
| 2008 | Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol 8:935–947 |
| 2009 | Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol 10:1237–1244 |
Genetically engineered cell-ablation models
Some 5 years ago, mouse models that significantly stimulated LC research were established (30). As they will recur throughout this article, they will be described in advance. In essence, these models allow the researcher to deliberately ‘remove’ langerin+ cells from a mouse (24, 25) or, alternatively, have a mouse that never possesses LCs (26). The generation of the models is summarized in much detail in an excellent recent review authored by the three protagonists of this approach (31).
Two different principles to generate such mice have been applied. (i) One approach is based on the fact that mouse cells are at least 1000–100 000 times less sensitive to diphtheria toxin as compared with human or simian cells. This is because of a mutation in the rodent diphtheria toxin receptor (DTR) that prevents binding of the toxin (30). The groups of Clausen/Bennett (25) and Malissen/Kissenpfennig (24) knocked the high affinity human DTR into the langerin gene locus. In these ‘Langerin-DTR’ mice, langerin+ cells can be efficiently depleted in response to intraperitoneal administration of the toxin. This inducible ablation strategy is similar to what was previously achieved in CD11c-DTR mice (32). However, in the CD11c-driven model virtually all DCs can be eliminated with the notable exception of LCs that are not depleted from the epidermis (30). Importantly, cell ablation is transient. However, it takes few weeks until the epidermis is repopulated with new LCs. (ii) The group of Kaplan/Shlomchik (26) chose another approach. They engineered mice in which expression of the diphtheria toxin subunit A (DTA) was driven by the regulatory elements of human langerin. These ‘Langerin-DTA’ mice exhibited a constitutive absence of epidermal LCs. For still unknown reasons (presumably because of the use of a human langerin promoter), only epidermal LCs are missing; langerin+ cells in the dermis and in lymphoid organs are still present.
LCs and more
‘Classical’ LCs
The typical LCs occur in the supra-basal layer of the epidermis. This is more apparent in human skin, where the epidermis is thicker and layered in a more pronounced way as compared with mouse epidermis. With regard to the possible encounter of LCs with pathogens, it seems important that they also occur in special types of skin such as rectal skin or foreskin and, most importantly, in various mucosae of the body (33, 34). Some examples for mucosal LCs are presented in Table 4. LCs were mainly recognized by virtue of their human leukocyte antigen (HLA)-DR and CD1a expression which is specific for LCs in healthy human epidermis. Major histocompatibility complex class II (MHC-II) was also the most common antibody marker in murine epidermis until the advent of anti-langerin/CD207 antibodies some years ago (21). Now, the state-of-the-art marker for identifying LCs in mouse and human is Langerin. The ultrastructural presence of Birbeck granules was also an important piece of identification.
Table 4. Langerhans cells in epithelia other than epidermis.
| Location |
Remarks |
Reference |
|---|---|---|
| Nasal mucosa | Mainly studied in the context of allergic disorders | (35, 36) |
| Oral mucosa | Often investigated with regard to oral lichen planus, an inflammatory condition | (35, 37) |
| Esophageal mucosa | (38, 39) | |
| Intestine | Langerhans cells in the muscular layer of the gut (mouse); Langerhans cells in sub-epithelial space (human); langerin+ cells in the lamina propria |
(40) (41, 42) |
| Vaginal mucosa | Well studied in the context of HIV infection; different population kinetics as compared with epidermal Langerhans cells |
(43, 44) (34, 45) |
| Anal mucosa | Langerhans cells defined by CD1a expression | (46) |
| Airways/lung | Co-express CD103 in the murine lung | (47-49) |
| Cornea | Langerin+ cells in the peripheral part of corneal epithelium (human) | (50) |
This list is not complete. It emphasizes more recent work that took advantage of anti-langerin antibodies to characterize the cells. More in-depth descriptions on DCs in general and langerin+ DCs in particular can be found in companion articles by Kweon and MacPherson in this issue.
The Birbeck granule is a unique organelle of LCs (7). It has the shape of a rod or a tennis racket (Fig. 2). For many years, this organelle remained ‘enigmatic’ until Jenny Valladeau and Sem Saeland finally identified the langerin/CD207 molecule (a C-type lectin receptor) as the main molecular component of these granules and suggested a role in antigen uptake (19, 20, 51), as one would expect from an endocytic C-type lectin receptor. There is evidence that viral particles (HIV) can gain access to and are degraded in Birbeck granules (52). However, thorough cell biologic studies by Daniel Hanau’s group (53, 54) suggest that Birbeck granules are part of the endosomal recycling network and may be not primarily involved in endocytosis. The antibodies against langerin have led to a boost in LCs research because only then (i.e. some 10 years ago) did it become technically possible to unequivocally identify and track LCs by immunohistochemistry/immunofluorescence. Most of the anti-murine langerin antibodies were directed against intracellular portions of the molecule. Only recently has an antibody been generated that recognizes a cell surface-exposed epitope of langerin and may therefore be suitable for cell sorting (55), although one has to be aware of the fast internalization of extracellular langerin (56, 57), which may make sorting tricky. The lack of langerin antibodies was particularly cumbersome in the mouse system. A highly useful antibody against human Birbeck granules (Lag) had already been generated earlier (58, 59). This antibody eventually turned out to recognize an intracellular epitope of the langerin molecule (19).
Fig. 2.
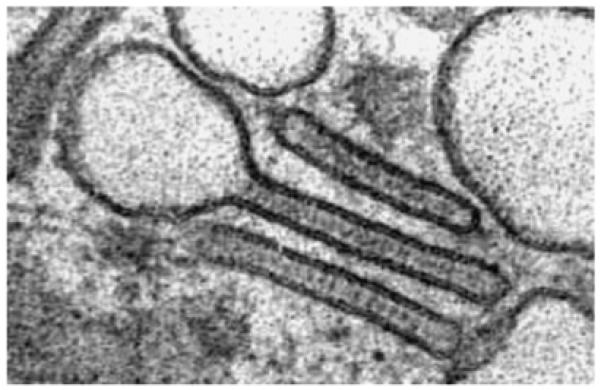
Transmission electronmicroscopy of Birbeck granules in Langerhans cells from human epidermis. Note the conspicuous rod and tennis racket shapes, and the very precisely ‘drawn’ limiting membranes of the structures. The diameter of the rod portion is about 50 nm.
Dermal langerin+ DCs
The picture of the skin DCs has recently become more complex (60). In addition to classical LC and dermal DC [i.e. langerin-negative (61)], a triplet of papers in 2007 (27-29) has identified another population of langerin+ DCs that reside in the dermis. To long-standing members of the LC community this came somewhat as a surprise. Langerin+ cells residing in the steady-state dermis are rare and they have therefore often been overlooked or not been paid much attention to. If ever they were spotted, they were regarded as LCs that were just in transit through the dermis on their way to the lymph nodes (62). This conclusion was plausible indeed, because langerin turned out to be a marker that persisted during LCs maturation and migration and could therefore be used as a tracer (63). Our own unpublished extensive cells counts in human skin sections yielded a rough estimate of one langerin+ dermal cell per each 2 mm of section length. In the overlying epidermis, this frequency is at least 20–30 times higher (64, 65). Obviously, in those days no distinctions could be drawn between epidermal LCs en route through the dermis and the novel dermal langerin+ DCs. The same numerical relations exist in murine skin: ear epidermis of BALB/c mice contains about 1000 LCs per mm2. In contrast, only about 50–80 langerin-expressing cells (both transiting LCs and dermal langerin+ DCs) can be found in 1 mm2 of dermis (66). These numbers emphasize how sporadically langerin-expressing cells appear in the dermis.
Yet, there are several pieces of evidence that firmly establish the novel dermal langerin+ DCs as a distinct subset of cutaneous DCs, not related to LCs (Table 5). (i) Unlike LCs, dermal langerin+ DCs are radiosensitive. This means that in bone marrow radiation chimeras these cells are replaced by donor cells (27, 28), whereas LCs remain of recipient origin and are not exchanged (22). (ii) In the Langerin-DTR mouse model, dermal langerin+ DCs display repopulation kinetics distinct from LCs. After the complete disappearance of all langerin+ cells in response to the administration of diphtheria toxin, dermal langerin+ DCs partly return into the dermis (as well as into the skin-draining lymph nodes) after a few days. At this time point the epidermis is still devoid of LCs, indicating that dermal langerin+ DCs cannot be derived from epidermal LCs (28). (iii) In Langerin-DTA mice, where epidermal LCs are completely missing, dermal langerin+ DCs are present in normal numbers (29). (iv) Likewise, dermal langerin+ DCs develop normally in transforming growth factor (TGF)-β knock-out mice (67) that are otherwise characterized by a complete lack of LCs in the epidermis (68). (v) In sharp contrast to epidermal LCs, the development of dermal langerin+ DCs is dependent on the cytokine Flt3 ligand. This becomes apparent in Flt3L knock-out mice that possess a virtually normal set of epidermal LCs but lack dermal langerin+ DCs (69, 70). (vi) Inversely, LCs are strictly dependent on macrophage colony-stimulating factor (M-CSF)/CSF-1 and interleukin (IL)-34, the second ligand for the M-CSF receptor. Accordingly, M-CSF receptor knock out mice have no LCs (71) in the epidermis, but their dermal langerin+ DCs are present in normal numbers (69, 70). (vii) Also in populations of DCs that migrate out of murine whole skin explants, cultured without additional chemokines, over a period of 3–4 days one can consistently find these cells at values of around 3–10% of all langerin+ cells (i.e. including epidermal LCs) (72, 73). Values for the ratio of dermal langerin+ DCs to epidermal LCs in transit through the dermis fluctuate between 2:1 and 1:2 (27-29). While this may be as a result of many experimental variables, it should be underscored that dermal langerin+ DCs always constitute a substantial population and certainly not a trace subset. As opposed to the scarcity of dermal langerin+ DCs in situ, one can consistently and easily find them in the skin-draining lymph nodes in substantial quantities, i.e. in the order of 30–50% of all CD11c+/langerin+ cells (27, 29, 60; V. Flacher and P. Stoitzner, unpublished data). The apparent discrepancy of ratios observed in skin versus lymph nodes could be as a result of differences in half-life or survival. CD103 is a useful marker for segregating this subset from migratory epidermal LCs, though it may not be ideal (74). A word of caution is warranted when considering skin explant cultures, where percentages of dermal langerin+ DCs vary quite a bit. This may depend critically on the specific culture system that the authors are using, e.g. ear skin with or without CCR7 ligands (67) or body wall skin with CCR7 ligands. Nevertheless, all methodical variants yield sizeable populations of dermal langerin+ DCs.
Table 5. Occurrence in adult skin of Langerhans cells and dermal langerin+ dendritic cells (DCs) in some different mouse models.
|
|
Langerhans cells |
Dermal langerin+ DCs |
Ref. |
|---|---|---|---|
| Langerin-DTA mice | Absent | Present | (29) |
| TGF-β knock-out mice | Absent | Present | (67) |
| TGF-β receptor deletion in CD11c+ DC including langerin+ cells | Largely absent (in adult life) | Present | (70, 75) |
| TGF-β receptor and TGF-β deletion in Langerhans cells (not dermal langerin+ DCs) |
Substantially reduced | Not determined | (76) |
| M-CSF receptor knockout mice | Absent | Present | (71) |
| Flt3 ligand knockout mice | Present | Absent | (69) |
TGF, transforming growth factor; DTA, diphtheria toxin subunit A; M-CSF, macrophage colony-stimulating factor.
Currently, the most useful marker profile to identify and sort dermal langerin+ DCs from murine skin and skin-draining lymph nodes is ‘langerin+/CD103+/CD8negative’. CD8+ DCs need to be excluded, because it has been shown repeatedly that lymph node-resident CD8+ DCs also express langerin (56, 77, 78). High expression levels of CD11c may be used instead of CD8 expression for this distinction (78). The epithelial cell adhesion molecule EpCAM/CD326 is another important marker: epidermal LCs express EpCAM (67, 79); dermal langerin+ DCs do not (67) or only to a minor extent (29). As dermal langerin+ DCs are so rare, it is difficult and cumbersome to isolate large numbers of pure cells for electron microscopy. Likewise, it is extremely difficult to find and identify such infrequent cells in immuno-electron microscopic approaches on skin sections. This is why it is not known, whether dermal langerin+ DCs possess Birbeck granules. Clearly, the expression of langerin in a given cell does not necessarily mean that the cell contains Birbeck granules. For example, murine-cultured LCs have lost virtually all Birbeck granules (6), yet they express substantial levels of langerin (63).
This novel cell type also occurs in other organs and tissues such as lungs (29, 80), liver, or the intestine (47, 81). Interestingly, langerin cannot be regarded as the identifying marker in all tissues. CD103+ DCs in the intestine do not express langerin (69). This topic is reviewed in much more detail by Miriam Merad in an accompanying article in this issue (82, 83).
All published descriptions of dermal langerin+ DCs have so far been in the mouse. Obviously, the question arises whether there is a human counterpart. Given the rarity of these dermal cells and the logistic (and ethical) difficulties in obtaining large amounts of human skin, it is not surprising that knowledge on human skin lags behind. Recently, however a very likely candidate was identified in human dermis, namely a langerin+/EpCAMnegative DCs. CD103 seems to be no reliable marker for these cells in human skin. The dermal langerin+ DC equivalent was even found in human bone marrow transplant patients. Like in the mouse, this cell type turned out to be of donor origin (70; M. Collin, personal communication).
Langerin+ cells in the lymphoid organs
Again, it was the availability of anti-langerin antibodies that caused ‘a great leap forward’ in our understanding of DC subsets in lymphoid organs. These antibodies allowed a direct identification of langerin+ cells outside the skin (19, 21, 63). The traditional view, that all langerin+ cells in lymph nodes would be LC from the epidermis, needed to be modified. Three subsets of langerin-expressing DCs, i.e. CD11c+ cells, can now be distinguished in the skin-draining lymphoid organs of the mouse (Table 6). (i) LCs that have migrated from the epidermis are characterized by high expression levels of MHC-II, CD40, CD205 (84), CD86, CCR7, and the absence of CD8. Moreover, they also express relatively less CD11c (55, 78). (ii) Migrated dermal langerin+ DCs look similar to the phenotype they have while still in the dermis. They express CD103 but largely lack EpCAM, and they are found in the CD11c+/CD8negative fraction of DCs (29). (iii) A resident, presumably blood-derived population of langerin+ DCs has been described in spleens and lymph nodes (56, 77). These cells can be distinguished from migratory langerin+ cells by their expression of CD8, and high levels of CD11c, and by their lack of CCR7. Importantly, the frequency of this subset is markedly mouse strain-dependent. It is substantial in BALB/c mice but small to absent in C57BL/6 mice (55, 78). In lymph nodes that do not drain skin, such as the mesenteric nodes, this CD8+ subset comprises almost all langerin+ cells (56). Likewise, all langerin+ DCs in the spleen of mice belong to the CD8+ subset.
Table 6. Subsets of langerin-expressing dendritic cells (DCs) in the skin-draining lymph nodes of the mouse.
|
|
Langerin |
MHC-II |
CD86 |
CCR7 |
CD11c |
CD8α |
|---|---|---|---|---|---|---|
| Langerhans cells | ++ | ++ | ++ | ++ | + | − |
| Dermal langerin+ DCs |
+ | ++ | ++ | ++ | + | − |
| Resident langerin+ DCs |
+ | + | + | − | ++ | + |
MHC, major histocompatibility complex.
Where are the different subsets of langerin+ DCs localized within the lymph nodes or the spleen? As expected, the bulk of langerin+ cells in the skin-draining lymph nodes can be found in the T-cell areas (21, 55, 63). In the spleen, a detailed recent analysis (85) revealed that in the steady-state most langerin+ DCs localize to the marginal zone around white pulp nodules into which blood vessels flow. This means that they are ideally placed to take up antigens that are flushed into the spleen by the bloodstream (85). Given the extraordinary capacity of this subset to cross-present antigens (86) and to promote Th1 responses (87), this is of great relevance.
Regarding the interesting question whether the occurrence of Birbeck granules may be restricted to the one or the other subset, no answer can be given to date. Occasional Birbeck granules have been found in DCs in the lymph node in response to cutaneous sensitization, but this was long before DCs subsets, let alone langerin was known (88). Back then, these Birbeck granules were taken as a solid argument for the migration of LCs to the lymph nodes (89). Only in the spleen, the highly purified CD8+ subset was investigated by electron microscopy; no Birbeck granules were found (56).
A big discrepancy exists between our knowledge on langerin expression in DCs of murine and human lymphoid organs. All the above-mentioned findings are in the mouse. Occasional spottings of Birbeck granules in human lymph nodes were reported (90, 91). Langerin+ cells were first detected in human lymph nodes in 1986 using antibodies directed against the ‘Lag’ antigen (58), which we now know is a part of the langerin molecule (19). The first study using anti-langerin antibodies was performed by Geissmann et al. (92) looking at lymph nodes in dermatopathic lymphadenitis where they found an accumulation of many langerin+ cells that were phenotypically immature and that localized to the afferent sinus and to the T-cell area. These were pathological nodes, though. Angel et al. (93) recently provided a detailed analysis of normal human lymph nodes. Langerin+ cells, all of them co-expressing CD1a, were found in the paracortex of skin-draining nodes, i.e. in the T-cell area. About half of these langerin+ DC were phenotypically mature as indicated by their expression of DC-LAMP/CD208. As opposed to murine lymph nodes, where epidermal LCs and langerinnegative dermal DCs settle in distinct regions (24), CD1a+/langerin+ and CD1a+/langerinnegative DCs are intermingled in the same area. Subsets of langerin+ DCs in human lymph nodes, in analogy to the mouse, cannot be defined (yet).
Ontogeny and homeostasis
During their life, LCs need to establish and/or maintain their typical network in the epidermis (Fig. 1) under three different conditions. First, before and around birth the epidermis needs to be populated by LCs or their precursors. Second, the density of LCs in the undisturbed, steady-state adult epidermis needs to be somehow maintained. Third, after an inflammation, in the course of which LCs migrate away from the epidermis towards the draining lymph nodes, the epidermis needs to be replenished with fresh LCs. These three scenarios will be considered separately. The same applies to dermal langerin+ cells as well.
Initial seeding of the epidermis with LCs
This has been best described in the mouse where pregnancy lasts only for about 3 weeks, and where epidermal sheet specimens can be readily prepared starting around day 17 of gestation. The final numbers of LCs are reached some 10 days after birth. At this time point, the densities are maximal with almost 2000 LCs per mm2. Subsequently, they drop to adult levels, i.e. 700–1000 per mm2 (94-96). This is mainly because the given number of LCs remains constant while the area of skin increases as the mouse grows. The initial wave of LCs that enters the epidermis in perinatal life has a high proliferative potential. Many (20–35%) LCs actively divide; Geissmann even speaks of an ‘explosive’ proliferation in the first week after birth with some 60% of LCs in cell cycle as determined by Ki-67 immunolabeling (97). This number falls to 2–5% in adult epidermis (22, 98). Thus, the initial network of LCs is established by immigration of precursors and their subsequent proliferation in situ. LC precursors acquire immunologically relevant molecules in a stepwise fashion, only after arrival in the epidermis (94-96). In murine prenatal epidermis, most LC precursors express only ATPase and CD45. Around birth, MHC-II molecules are added, and a few days later de novo expression of C-type lectin receptors follows, first langerin (96), and then DEC-205/CD205 (99). This sequence of surface molecule expression can also be observed, at least for MHC-II and langerin, when LCs repopulate the epidermis after tape stripping, when bone marrow-derived precursor populations are injected into the skin (100) or when monocytes were experimentally used as LC precursors (71). The development of dermal langerin+ DCs in the perinatal period has not been studied yet.
Less is known about the ontogeny of human LCs. Early work (101, 102) described that dendritically shaped cells in the fetal epidermis first expressed HLA-DR and ATPase and only later acquired CD1a. Recently, Adelheid Elbe-Bürger’s group took on the demanding task to study human prenatal skin and described for the first time in detail the development of the LC network in the epidermis. Similar to the mouse system, there is a high proliferation rate of CD45+ leukocytes in the fetal/embryonic skin that decreases after birth. During the first to second trimester markers on epidermal leukocytes come up in a stepwise order, first HLA-DR, then CD1c, followed by langerin and CD1a. Interestingly, this correlated with TGF-β expression in the epidermis. High expression of IL-10 in embryonic skin seems to keep the LC in a functional blockade (103).
Maintenance of LC homeostasis in the steady-state epidermis
The bone marrow derivation of LCs was proven many years ago in mice (13, 14) and in humans (104). It remained unclear, however, how LCs would maintain their numbers in an undisturbed epidermis. Krueger’s group highlighted the remarkable longevity of LCs for the first time: LCs in human skin transplanted onto nude mice persisted in the grafts for at least 9 weeks (105). Much later, this could be recapitulated in the human system: In a hand transplant patient, LCs of the hand donor survived in the graft for more than 4 years (106). These observations suggested some homeostatic proliferation of LCs within the epidermis. Indeed, over the years, several investigators showed a low degree of LC division in the epidermis. (i) Very rare spottings of mitotic LCs were reported (107-109). (ii) By classical autoradiographic methods or by BrdU labeling, a small percentage of LCs was shown to incorporate labeled nucleotides, indicating cell division (110, 111). (iii) Labeling studies with cell cycle-specific antibodies also detected few dividing LCs within the steady-state epidermis of mice (98) and humans (103). Only recently, Miriam Merad (22) elegantly proved what Krueger et al. (105) anticipated in 1983: ‘....Langerhans cells or Langerhans cell precursors are capable of dividing in the skin or, alternatively, represent an extremely long-lived cell population.’ In bone marrow chimeric mice and in a model of murine parabiosis Merad demonstrated that LCs were radio-resistant and that they, indeed, remained of host origin for almost 2 years, i.e. virtually the entire life of the mouse. No influx of progenitor cells from the blood occurred. In accordance with the scattered previous reports (see above), they found that about one-third of all LCs had divided within the 30 day BrdU-labeling period in the steady-state epidermis (22). This is similar to Kamath et al. (110), who found that about a quarter of all LCs had divided within 2 weeks. This proves indeed that LCs self-renew in the steady-state epidermis. Based on today’s knowledge, one could modify Krueger’ sentence in the following way: ‘…Langerhans cells are capable of dividing in the skin and they represent an extremely long-lived cell population.’ LCs in other epithelia may be different, though. For instance, vaginal mucosal DCs (probably LCs) (45) or DCs in the airway epithelium (112) are not as long-lived and are repopulated by circulating precursor cells. This may reflect the relationship of these DCs to dermal langerin+ DCs rather than to epidermal LCs. Dermal langerin+ DCs are not as long-lived as LCs. This point will be discussed in more detail in the companion paper by Miriam Merad.
Replenishment of inflamed epidermis with LCs
LCs leave the epidermis in response to inflammatory stimuli like contact sensitizers, etc. (113) or gentle tape stripping (100). After some time, the densities of LCs are back to normal. This is the situation where monocytes act as progenitors for LCs (114). Again, it was the seminal work of Merad’s team who showed that, in response to inflammatory stimuli (UV irradiation in their case), progenitor cells from the blood entered the LC-depleted epidermis in a CCR2-dependent way and gave rise to LCs (22). They further defined the progenitor and found it to be a monocyte characterized by high expression of the Gr-1 molecule. This monocyte migrated to inflamed epidermis, proliferated there, and acquired LC features such as langerin expression (71). These data highlight that monocytes can turn into LCs in situ under appropriate conditions, and that this is an important mechanism for ensuring that the epidermis is not left deprived from LCs after an inflammation. These data were foreshadowed by some early observations on the repopulation of human epidermis with LCs after clinical bone marrow transplantation. Returning cells within the epidermis exhibited morphological and phenotypical features of macrophages before they acquired LCs characteristics, in particular Birbeck granules. The authors then concluded that a ‘phenotypic transformation of macrophages to LCs in the skin’ (115) had occurred. in vitro, the transformation of monocytes into LC-like cells, expressing langerin and containing some Birbeck granules was anticipated by Geissmann et al. (116). Whether this is the only and predominant mechanism for the replenishment of LCs remains to be determined. For instance, an additional role for dermal cells possessing features of hematopoietic stem cells may be envisaged (117).
Lineage of LCs
As discussed above (Table 5), the presence of LCs in the epidermis is strictly dependent on the presence of certain cytokines and growth factors. As opposed to all other types of DCs, LCs develop independently of Flt3-ligand.
The development of different hematopoietic cell lineages is regulated by different patterns of transcription factor expression. A few transcription factors were identified, that are critically involved in the development of epidermal LCs. Above all, the differentiation of LCs is dependent on TGF-β1. This becomes most impressively apparent in TGF-β1 knockout mice that totally lack epidermal LCs (68, 118). TGF-β1 appears to be derived from LCs themselves and act in an autocrine way (76). TGF-β1 induces the transcription factor Id2. Therefore, it came not entirely unexpected that Id2 knockout mice did also not have any LCs in their epidermis (119), just like TGF-β1 knockout mice. Runx3 is another transcription factor that is regarded as a key regulator of cell lineage decisions. It is involved in TGF-β1 signaling and again, mice that lack this transcription factor are devoid of LCs (120). The dependence on TGF-β1 and its downstream target Id2 was also found for human LCs derived from CD34+ hematopoietic progenitor cells (121, 122). Other transcription factors, whose absence severely affects DC subsets in the spleen and elsewhere (e.g. Rel-b, Ikaros), do not have an influence on the development of LCs. Some transcription factors that are unrelated to the TGF-β1 signaling pathway also appear to interfere with the development of LCs, though not as dramatically as Id2 or Runx3. There are factors such as Gfi1 whose absence leads to a marked increase in the densities of LCs in the epidermis (123), and other factors such as members of the interferon regulatory factor family whose absence leads to a reduction, though not complete absence of LCs (124). For a much more detailed overview over this intricate topic, the reader is referred to an excellent review by Martin Zenke (125) and to the companion articles by Manz and Liu and Nussenzweig in this volume.
LC function in vitro: the LC paradigm
Schuler and Steinman (6) showed for the first time that murine LCs occur in two distinct states. When freshly isolated from the epidermis by standard trypsinization techniques, they expressed relatively little surface MHC-II, but displayed substantial levels of molecules that are present on macrophages/monocytes at higher levels, namely F4/80, Fc receptors (CD32), and ATPase/ADPase. These LCs were poor stimulators of resting T cells in the allogeneic mixed leukocyte reaction (MLR). In contrast, they were extraordinarily potent in processing protein antigen into immunogenic MHC-II–peptide complexes for presentation to antigen-specific T-cells (T-cell hybridomas in that case) (15). When epidermal cell suspensions, containing LCs or highly purified LC [in the presence of GM-CSF (126, 127)] were cultured for 3 days, this picture was inverted. MHC-II surface expression increased tremendously, F4/80, CD32, and ATPase were strongly downregulated, and so was the ability to process protein antigens. In contrast, T-cell stimulatory capacity in the MLR was dramatically upregulated. Philipp Pierre and Ira Mellman extended this model by one additional important aspect emphasizing the obvious: freshly isolated LCs in suspension are not identical to LCs in situ. These authors [and others (128)] showed that LCs in situ have virtually no MHC-II surface expression; rather, most of these molecules are intracellular. Only upon a maturation stimulus, MHC-II molecules begin to translocate to the cell surface (129).
These in vitro observations allowed for the design a scenario for what could happen in vivo when antigen, in particular pathogen, is recognized by LCs. Upon this encounter, they start to undergo a complex maturation process that involves the upregulation of MHC-II and costimulatory molecules. They set into operation the machinery to process antigens and they begin to express chemokine receptors, in particular CCR7, and downregulate E-cadherin (130) that allow them to leave the skin and migrate to the lymphoid organs, typically the draining lymph nodes (62, 63, 72, 131). During migration they downregulate MHC-II synthesis (132) and processing capacity (133), thereby ensuring that antigen picked up and processed while still in the skin is not displaced and lost on the way to the lymph nodes. Once in the nodes, they efficiently stimulate T lymphocytes and thus start the immune response. This scenario was frequently called the ‘LC paradigm’ (16, 17). It was then thought that LCs are a prototypic example for all other types of DCs.
Starting from the classical experiments by Stingl, Katz, and others, who demonstrated the T-cell stimulatory capacity of epidermal LCs in mouse (134, 135) and humans (136), much evidence for an immunogenic function of LCs in vitro has accumulated over the years. The above-mentioned studies on the differential expression of antigen-presenting functions in immature and mature LCs (6, 15) were widely recapitulated and extended, e.g. by showing the capability of LCs to cross-present (137) or to efficiently induce cytotoxic T lymphocytes (CTLs) in vitro (138). Even though some early studies on LCs may have inadvertently included dermal langerin+ DCs, as sometimes insinuated (139), it should be emphasized here that cross-presenting capacity was indeed demonstrated for defined populations of LCs that did not contain dermal langerin+ DCs (137).
Clearly, much less evidence was acquired in vitro for a possible role for LCs in the development and maintenance of peripheral tolerance. Early observations suggested that LCs cultured in the presence of IL-10 would induce anergy in responding T cells (140). An important hint at a potential role of LCs in tolerance came from experiments by the group of Mellman (141). They found that the disruption of E-cadherin bonds between DC clusters leads to an incomplete maturation of these cells, the main feature being a markedly reduced production of cytokines needed for efficient T-cell stimulation (e.g. IL-12) and, as a consequence, the induction of regulatory T cells. When injected into mice, these DC-induced tolerance (141). Notably, this type of ‘phenotypic maturation’ occurred in the steady-state, i.e. in the absence of micobial stimuli. In the mouse, these seminal data have not yet been verified in LCs or other langerin+ DCs. They would, however, serve as an attractive explanation for a tolerogenic function of LCs in the steady-state. Indeed, LCs are anchored within the epidermis by means of E-cadherin bonds (130, 142) that need to be broken when they leave the epidermis to migrate to the lymph nodes (143). In humans, these observations were also not made in ‘real’ LC but rather in langerin+ DCs derived from CD34+ hematopoietic stem cells (141, 144).
LC function in vivo: challenging the LC paradigm
In the past decade, the LC paradigm has been challenged, or at least extended, in three ways. (i) As opposed to the early days, the situation has become more complex. Besides classical epidermal LCs, we now have to consider the immunologic functions of dermal langerin+ DCs and lymph node-resident langerin+ DCs. (ii) The LC paradigm was mainly about an immunogenic function of these cells. In the meantime we have learned that DCs not only induce immunity but that they can also efficiently mediate and maintain peripheral tolerance (145-147). (iii) The straightforward concept that LCs pick up antigens in the skin and present them to T cells in the draining lymph nodes was put into question by some inconsistent and unexpected observations (23, 24, 26).
It should be strongly emphasized that there is a wide gap between what we know about LC function in vitro and in vivo. Most of our knowledge stems from observations in in vitro models. Only in the past 5 years has there been a marked increase in research activities looking at the in vivo functions of langerin+ cells in the skin. This was mainly because of the availability of the above-mentioned mouse models that allow to selectively deplete langerin+ cells and/or selectively visualize and trace them in the living animal (31).
LC function in vivo: observations in the contact hypersensitivity model
The early years: evidence for an immunogenic role of LCs in vivo
Silberberg was first to postulate a role for LCs in the generation of immunity in delayed type hypersensitivity (DTH) reactions in the skin. In her classical studies, she noted a ‘close apposition of mononuclear cells to LCs in contact allergic reactions’ (148). Almost 40 years later, the role of LCs in vivo in this reaction is still not entirely clear. At first glance this may sound surprising, given the abundance of reports on contact hypersensitivity (CHS), a mouse model that has commonly been regarded as a good, if not the ideal model to study LC biology in vivo. It is important to realize, however, that the outcome of this assay (in general ear swelling) does not only measure the sensitizing capacity of epidermal LCs, but also of langerin+ (27-29) and langerinnegative (61, 149, 150) dermal DCs. This means that experimental manipulations like UV irradiation (151), corticosteroids (152), etc., that previously aimed at depleting LCs, were likely to affect all these types of skin DCs, rather than exclusively LCs. Nevertheless, the potential of LCs to induce CHS in vivo was demonstrated already many years ago by adoptive transfer of haptenized LCs into naive animals (153). Furthermore, detailed early dose-response studies with haptens also hinted strongly at an in vivo function for LCs in CHS when low doses of hapten were applied (154).
The recent years: more ‘cons’ than ‘pros’ for an immunogenic role of LCs in vivo
When the in vivo ablation models of LCs were introduced (see above) (30, 31), the first observations in the classical CHS model came as a big surprise to many LC ‘stalwarts’. In two of these studies, CHS was not impaired at all in the absence of LCs: Kissenpfennig et al. (24) found no difference in the magnitude of ear swelling in the inducible ablation model; Kaplan et al. (26) noted even an increase in the swelling responses in the constitutive ablation model. Only the study of Bennett et al. (25) showed a reduction (but not an absence) of ear swelling when langerin+ cells in the skin were missing. At that time, the population of dermal langerin+ DCs was not yet known and the observed effects were therefore attributed to the presence or absence of LCs. After these initial experiments, the evidence was rather against an essential immunogenic function of LCs in CHS. Even a downregulatory role for LCs was compatible with the observations (26).
The present: recognition of dermal langerin+ DCs and development of a more differentiated view
An active role for cells other than epidermal LCs was inferred from the inducible Langerin-DTR model, where CHS responses could be measured at time points after diphtheria toxin administration when epidermal LCs were still totally absent but dermal langerin+ DCs had already (at least partially) returned to the dermis (29). The initial report of Langerin-DTA mice (26) had already heralded this: good CHS can develop in the total absence of LCs. The explanation was given in subsequent work that showed dermal langerin+ DCs to be perfectly normal in Langerin-DTA mice (29), strongly suggesting sensitizing capacity for this subset, too. The concentrations of DNFB used to sensitize in these experiments were apparently high enough to penetrate through the epidermis and to reach and successfully haptenize dermal DCs. Thus, there is no doubt, that dermal langerin+ DCs can induce CHS in vivo.
Bennett et al. (155) also anticipated the involvement of two types of langerin+ cells in the sensitization for CHS. Four weeks after the depletion of langerin+ cells in their Langerin-DTR mice, i.e. when LCs were still largely absent from the epidermis, ear swelling responses to the hapten were still reduced in spite of the repopulation of lymph nodes by CD8+/langerin+ resident DCs and of the dermis by dermal langerin+ DCs. Importantly, this was especially the case when low concentrations of hapten were applied (155, 156). Very recent observations in a bone marrow chimera model, where LCs are present, but dermal langerin+ DCs are absent, also support an immunogenic role for LCs: Honda et al. (70) find unimpaired CHS responses when there were no dermal langerin+ DCs in the sensitized skin (70). Thus, there is also no more doubt, that epidermal LCs have the capacity to induce CHS in vivo.
These observations inevitably lead to the question which DCs type is the critical one in CHS in vivo. In the subsequent search for explanations, antigen/hapten concentration turned out to be critical – not unexpectedly. As suggested by earlier experiments (154), recent, thorough studies unequivocally revealed a critical role for LCs (156, 157, 158): in the absence of all langerin+ cells (i.e. epidermal and dermal ones) CHS was significantly reduced, in many mice even completely abrogated. So was the transport by langerin+ cells of a topically applied fluorescent hapten (fluorescein isothiocyanate) to the lymph node (155). When low concentrations of hapten were applied, ear swelling responses were also markedly diminished in the absence of LCs (but the presence of dermal langerin+ DCs). At higher hapten doses, LCs turned out not to be necessary any longer (Table 7), because hapten could now diffuse into the dermis and reach dermal langerin+ (and also langerinnegative) DCs that induced CHS (156). We like to emphasize that in the latter experiments ‘French’ (24) and ‘Dutch’ (25) Langerin-DTR-mice were analyzed side-by-side with high and low concentrations of haptens, and they showed no differences in CHS responses. This seemed important because of the many experimental variables that otherwise often lead to seemingly contradictory results. In addition to the crucial role of hapten concentration, some other parameters may influence the in vivo responses in the CHS assay. Mice with a constitutive absence of LCs (26) may respond differently to sensitization and/or may possess different T-cell properties as compared with mice with inducible ablation of LCs (30, 31). The site of sensitization may be of importance: Wang et al. (157) suggest that LCs are more important when immunization (protein antigen, not haptens) is performed through the flank skin of a mouse as opposed to ear skin, most probably caused by the better diffusion of antigen into the dermis of the thinner ear skin in contrast to body wall skin. Finally, one must not forget that in addition to LCs and dermal langerin+ DCs, also langerinnegative dermal DCs contribute to the sensitization of CHS (150), especially when the doses of hapten are high.
Table 7. Contact hypersensitivity responses in the selective presence or absence of different types of skin dendritic cells in mouse models.
| Types of skin dendritic cells (DCs) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Langerhans cells |
Dermal langerin+ DCs |
Dermal DCs (langerinneg) |
Method/mouse model |
Low hapten dose |
High hapten dose |
| Present | Present | Present | Untreated mice | ++ | +++ |
| Absent | Present | Present | Langerin-DTR, sensitization 10–14 d after DT |
⇩ (155, 156, 160) | ⇔(156) |
| Langerin-DTA | ⇧ (26) | ⇧(26) | |||
| Absent | Absent | Present | Langerin-DTR, sensitization right after DT |
⇩ (29, 155-157) | ⇩ (25, 156, 161) ⇔(24) |
| Present | Absent | Present | Langerin-DTR into normal mice; bone marrow chimera |
⇔ (70) | ND |
| Present | Present | Absent | No model available | ND | ND |
| Absent | Absent | Absent | No model available | ND | ND |
DT, administration of diphtheria toxin; ND, not determined; ⇩, ⇧, ⇔, downregulation, upregulation, no change, respectively, in ear swelling responses as compared with wildtype control mice.
Langerin-DTA mice revealed an additional potential property of LCs. Not only could CHS responses (ear swelling) be induced in the total absence of epidermal LCs, but these responses were even markedly exaggerated as compared with wildtype skin (26, 158). From this it was concluded that LCs may have a downregulatory role. When there are no LCs, immune reactions in the skin proceed uninhibited. Similarly, in an H-Y skin transplantation model the donor grafts were efficiently rejected when LCs were not present, also indicating a down-modulatory role in vivo (159). In the highly inflammatory context of a CHS-sensitizing reaction (57), it is difficult to conceive how downregulatory properties could develop in such ‘inflammatory Langerhans cells’. Yet, there is additional evidence for a regulatory LC function in models other than CHS (70, 159) (see below), and LC-derived IL-10 and LC interaction with T cells have been identified as possible mechanisms for suppression (158). Interestingly, in LC-depleted Langerin-DTR mice one cannot observe enhanced CHS. Therefore, more research is needed to fully resolve this important issue.
In terms of CHS reactions there appears to be functional redundancy in the skin. It is primarily the accessibility of the hapten that decides which types of skin DCs are operative in inducing the CHS response. Definitely, all of them are capable of doing so.
LC function in vivo: observations in the ‘OVA model’
The model antigen ovalbumin (OVA) has become very popular. Researchers can use a wide range of readily available and helpful tools such as commercially available peptides, pentamers recognizing OVA peptide-specific CD8+ T cells, transgenic mice expressing OVA peptide-specific T-cell receptors (OT-I and OT-II mice) as a convenient functional read-out, and tumor cells expressing OVA as a tumor antigen (B16-OVA melanoma, E.G7-OVA lymphoma, and others). Finally, there are mice that express OVA as a self antigen in keratinocytes of the epithelia (K14-OVA or K5-OVA mice) or in pancreatic islet cells (RIP-OVA mice). Such antigens can be inducible (162) or constitutive (163-167). Before discussing the important data that have been obtained in this model, we wish to emphasize the fact that this model cannot be perfectly representative for real infection or cancer scenarios in vivo. One must be aware that the OVA peptide-specific T cells are ‘abnormally’ sensitive. This is a concern especially with the MHC class I-restricted CD8+ OT-I cells. They can respond to minute quantities of antigen in the picomolar range rendering them 100 times more sensitive compared with transgenic T cells specific for a pathogen (168). Therefore, all data corroborated in the OVA model need to be viewed with these caveats in mind.
A few recent studies attempted to determine, which type of DC in the draining lymph node would present the OVA antigen when applied onto (epicutaneous) or into (intradermal injection, as a transgene) the skin. This was made possible by the availability of several useful tools.
Langerin-enhanced green fluorescent protein (EGFP) mice (24, 25) elegantly circumvented the lack of anti-langerin antibodies that would be suitable for cell sorting. Only recently has an antibody been described that recognizes a cell surface exposed epitope of the murine langerin molecule and that may be used for cell sorting approaches (55).
Langerin-DTR and Langerin-DTA mice were described above.
Additional insight came from models where OVA is expressed under the promoter for keratin in epidermal keratinocytes. It may be regarded as a self-antigen in these models. Promoters for keratins 14 and 5 are used, i.e. those keratins that are expressed in the basal keratinocytes. This means that LCs are surrounded by keratinocytes that synthesize and secrete the OVA protein (169) or the OVA peptide SIINFEKL (170) or carry the OVA protein in a membrane-bound form (163, 164, 169). It is not entirely clear how dermal langerin+ or langerinnegative DCs get access to the OVA antigen. In the case of OVA-secreting keratinocytes (169), the antigen may diffuse into the dermis. This is certainly possible as suggested by the inverse example: Large proteins (antibodies) that are deposited in the dermis (66) or even injected intraperitoneally (171) can easily diffuse into the epidermis and target epidermal LCs. In addition, keratinocyte material, possibly including the antigen, may ‘drop down’ into the dermis, as it has been observed to happen in human skin (172).
Bone marrow chimeras allow the construction of useful models. As LCs are radioresistant (22), bone marrow transplanted mice possess virtually all leukocytes from the donor, except LCs that remain of recipient origin. Frequently mice are used that have mutated MHC molecules [‘bm’ mice (173)] that cannot present to OT-I or OT-II cells. When the donor has mutated MHC molecules one obtains a mouse where only LCs can present OVA because they are of host origin and therefore possess intact MHC molecules. Inversely, when the recipient has mutated MHC molecules, one gets a mouse where LCs cannot present. Dermal langerin+ and langerinnegative DCs, however, can present.
When one tries to obtain a synopsis of the data in the OVA model, it is important to keep in mind the fact that the early studies were performed at a time when dermal langerin+ DCs were not yet known. Therefore, in these studies the term ‘Langerhans cells’ may often include, unknowingly, also dermal langerin+ DCs, dependent on the timing of the experiments. It is also important to note that those studies that demonstrated antigen uptake by LCs in situ and subsequent (cross)-presentation to antigen-specific T cells in vitro by LCs obtained by migration from epidermal sheets reflect only LC activities (66, 137, 162). Dermal langerin+ DCs do not occur in these preparations.
Irrespective of the final outcome in terms of immunity or tolerance, several of these studies show that LCs as well as dermal langerin+ DCs can indeed present and cross-present OVA to the transgenic T cells in vivo. This was observed in experimental settings where LCs picked up the OVA antigen in the epidermis after intradermal injection (66) or from surrounding, genetically modified keratinocytes expressing OVA or OVA peptide (137, 162) and were then allowed to migrate out of the epidermis into the culture medium. These migrant LCs stimulated TCR transgenic T cells. When looking further down the line into the skin-draining lymph nodes, several studies indicated that langerin+ DCs can present in vivo. Stoitzner et al. (174) showed this for epicutaneously applied OVA protein. Sorted langerin+ (i.e. EGFP+)/CD8negative DCs readily presented to OT-I cells in an in vitro proliferation assay, whereas resident langerin+/CD8+ DCs did not. Waithman et al. (175) essentially confirmed these data with similar methods. Both studies were performed at a time when no distinction could be drawn between epidermal LCs and dermal langerin+ DCs.
Evidence for the capacity of LCs to process and present OVA in vivo came from chimera studies. Under experimental circumstances where only LCs, but not dermal langerin+ DCs can present, proliferation/expansion of adoptively transferred OT-I cells occurred in response to keratinocyte-associated OVA peptide (170) or OVA protein (175). In one study, OT-I cells did not proliferate but started to express acitvation markers CD69 and CD44 indicating antigen presentation (176). Another study by Wang et al. (157), using Langerin-DTR mice, showed a dependence of in vivo cross-presentation on epidermal LCs under defined conditions, which, in that case, meant immunization into the flank skin. In a similar experiment, Kim et al. (169) demonstrated that both LCs and dermal langerin+ DCs are dispensable for OT-I activation after intradermal injection into Langerin-DTR mice expressing membrane-bounds OVA in the epidermis.
Two recent studies specifically attempted to discriminate between LCs and dermal langerin+ DCs. Bedoui et al. (139) found that keratinocyte-derived OVA (from K5-mOVA mice) was presented in vivo in skin-draining lymph nodes by CD103+/CD205+/CD8negative DCs, most likely corresponding to dermal langerin+ DCs. Henri et al. (74) also sorted DC subsets from lymph nodes. They observed that langerin+/CD103+ DCs, i.e. dermal langerin+ DCs presented keratinocyte-associated OVA. Interestingly, in both studies CD103negative LCs, had no activity.
Given the above-mentioned caveats, that are inherent to the OVA system (in particular to OT-I cells), and the fact that many of the here reported data are based on relatively small sample sizes (n often equals no more than 2), the partly contradictory conclusions drawn from these studies must be taken with a pinch of salt. This also emphasizes the importance of experimental attempts that go beyond the OVA system into infection or tumor models, as will be described in the following paragraphs. It is certainly still too early to draw definitive conclusions.
LC function in vivo: observations in viral infection models
It was a viral infection model that cast first doubts on a direct immunogenic role for LCs. In 2003 Allan et al. (23) noted that epicutaneously applied herpes virus is not presented to lymph node T cells by CD8negative, migratory LCs, i.e. those that would have travelled from the site of infection in the skin. In contrast, it was CD8+, lymph node-resident DCs (including langerin+ cells) that presented the herpes antigens. This came unexpected, and it did not fit into the LC paradigm. Although epidermis-derived LCs did not prime the T cells, they were nonetheless required to transport the pathogen or its breakdown products to the nodes (177). Another herpes virus model revealed that LCs of the vaginal epithelium do also not present viral antigens to lymph node T cells. In that case, however, it was a CD8negative population of submucosal DCs that carried and presented viral peptides (178). Regarding the virus infection models, a key issue is whether infected LCs die, as it is the case with the cytopathic herpes viruses (179, 180), or whether they stay alive as, for instance, in a cutaneous lentivirus infection model. In the latter it was shown, that indeed, CD8negative/DEC-205+ skin-derived DCs (containing LCs and langerin+ and langerinnegative dermal DCs) presented the antigen to T cells in the draining lymph nodes (181). The relative presentation capacities of skin DC subsets were not determined in that study. Importantly, when another, apoptosis-inducing viral vector (vaccinia virus) was used, presentation in the draining lymph nodes was taken over by the CD8+ DC fraction, consistent with the hypothesis, that the apoptotic skin DCs (including LCs) are taken up by the resident, presumably blood-derived CD8+ DC population which then cross-presents them to the CD8+ T cells (182). A more refined look at the herpes simplex virus (HSV) model essentially confirmed the above notion, at least for dermal langerin+ DCs. Bedoui et al. (139) investigated the second wave of HSV infection, i.e. the recrudescence phase, when herpes virus infects the keratinocytes of the epidermis from its reservoir, the cutaneous nerves, rather than through a superficial wound (scarification) like in the first wave of infection. In the recrudescence phase, it turned out to be mainly cutaneous CD103+ DCs (containing langerin+ and possibly some langerinnegative DCs) and CD8+ lymph node-resident DCs that presented HSV antigen to CD8+ T cells. LCs played a minor direct role in antigen presentation, possibly because they are infected and then killed by this cytolytic virus as opposed to the dermal langerin+ DCs that remain undamaged during the second wave of viral infection. In the more vigorous primary wave of infection CD8+ DCs are the only ones capable of performing cross-presentation in the node, presumably because both LCs and dermal langerin+ DCs, both CD8negative, become apoptotic.
Based on these observations, Carbone, Heath, and colleagues developed the theory, that antigen-transporting LCs are taken up by resident, CD8+ DCs in the lymph nodes which in turn (cross) present the microbial antigens to CD4 and CD8 cells (182, 183), and that dermal langerin+ DCs play a dominant immunogenic role (139). Clearly, this theory cannot be generalized to all experimental settings, let alone to all in vivo situations. Yet, it is an interesting working hypothesis that sparked valuable follow-up studies. Another point of note is that it is currently not clear whether and how the CD8+ and CD8negative subsets of DCs or langerin+ cells in the murine lymphoid organs relate to the human lymphoid organs. Only few attempts to characterize such subpopulations have been published (184), the C-type lectin Clec9A being a promising marker molecule for the putative human counterpart of CD8+ DCs (185).
LC function in vivo: observations in non-viral infection models
The best studied non-viral pathogen is Leishmania (186). Early observations indicated that LCs transported the protozoans from the skin to the lymph nodes (187). Surprisingly, however, in experimental Leishmania infection of mice, antigen presentation in the lymph nodes was not achieved by skin-derived LCs, but rather by langerinnegative/CD8negative, presumably dermal DCs (186, 188). More recent experiments made use of Langerin-DTR mice. There, it became apparent that indeed, mice recovered clinically from Leishmania infection irrespective of the fact of whether they had langerin+ DCs or not. Proliferation of CD8+ T cells, however, was dependent on the presence of dermal langerin+ DCs but not on LCs (189).
Even though there may be concerns as to the validity of such experimental infection models for ‘real life’, where pathogen doses are likely smaller, LCs appear to be unexpectedly non-protective. The role of dermal langerin+ DCs in these models remains to be studied in more detail.
LC function in vivo: observations in tumor models
Targeting antigens to LCs in situ is an attractive possibility for tumor therapy. The attractivity is based on several observations in vitro indicating that human LCs are very capable of inducing cytotoxic T lymphocytes. This was shown for both CD34-derived LC-like DCs (190, 191) as well as for ‘real’ LCs isolated from human epidermis (138). Moreover, the ability to cross-present protein antigens was shown for murine (137) and human (138) LCs.
To gain more insight into the in vivo function of LCs in the context of a tumor, Stoitzner et al. (174) used the model of inducible LC ablation (Langerin-DTR mice) (24). Immunity against an experimental melanoma tumor was induced by epicutaneous immunization of mice with the tumor antigen. Protection from the tumor was lost when epicutaneous immunization was performed onto epidermis that had been depleted of LCs and dermal langerin+ DCs before, strongly suggesting an immunogenic function of these cells in vivo. Different tumor models may behave differently, e.g. the commonly used transplantable tumor cell lines (e.g. B16-OVA) versus spontaneously arising tumors (192-194). In these latter models, it will be interesting to investigate a possible function of LCs in immunosurveillance. The hypothesis to test would be that LCs are constantly and critically involved in surveying the epidermis to prevent the growth of a tumor.
In the CHS model (see above), LCs serve as the exclusive antigen-presenting cells only when the hapten is applied at a low concentration that precludes access to the hapten for the other, dermal types of skin DCs. Some observations in the tumor model are reminiscent of CHS. When the antigen dose used for immunization is high enough, the dependence on LCs is lost (174), indicating that dermal langerin+ and/or langerinnegative dermal DCs also present the tumor antigen. A contribution of dermal langerin+ DCs was not ruled out in the above-mentioned study; a contribution of dermal langerinnegative DCs was shown to be likely because these cells did present epicutaneously applied antigen in the draining lymph nodes (174). Similarly, when the strength of immunization is increased even more, e.g. by co-administering the immunostimulatory lipid α-galactosyl-ceramide (195), skin DCs appear not to be necessary at all: both the ablation of langerin+ cells in the Langerin-DTR mice and even the removal of the immunization site (the ear, ‘Van Gogh experiment’) (73) did not reduce the magnitude of ensuing T-cell responses. Thus, it seems to be the opportunity for a given type of skin DCs to capture the antigen that decides whether this type of DCs induces a response. There may be differences between the types of skin DCs in the immunogenic potential in tumor models that need to be worked out in future experiments. Also, the surprising observation that in Langerin-DTA mice, that never possess LCs, tumor protection is better will need to be related to other models (196). Importantly, however, epidermal LCs can clearly fulfill an immunogenic role during vaccination against cancer in vivo.
LC function in vivo: induction and maintenance of peripheral tolerance
One of the most straightforward experiments suggesting a tolerogenic function for LCs in vivo was conducted in Steven Katz’s group. Shibaki et al. (164) constructed mice that expressed the OVA antigen under the keratin 14 promoter. When these mice were challenged in a DTH reaction with an intradermal injection of OVA, they turned out to be unresponsive, indicating tolerance. The authors did not go further in defining the mechanism of this tolerance (anergy versus deletion versus regulatory T cells). Nevertheless, these observations strongly suggest that LCs in the steady-state may have presented the OVA antigen to T cells in a tolerogenic context. Migratory skin DCs from whole skin explant cultures containing LCs and dermal langerin+ as well as langerinnegative DCs did indeed efficiently present the antigen to T cells (OT-I cells). This is emphasized by the authors’ observation that transgenic OVA could not be detected in the thymus, making a contribution of central tolerance unlikely.
Waithman et al. (175) examined the consequences of self-antigen presentation (in K5-mOVA mice) by skin DCs on the fate of responding T cells. By using the MHC-I mutated bm1 mice in a bone marrow chimera setting, they followed numbers of injected OT-I cells over a period of 6 weeks. In the presence of OVA-expressing keratinocytes OT-I cells were deleted during that period. Importantly, this deletion also took place when only radioresistant epidermal LCs, but not the other, radiosensitive, skin DCs subsets could present the OVA antigen. A population of radioresistant dermal DCs (langerinnegative) might have contributed; however, this population is minor relative to LCs (197). Therefore, this observation hints at a tolerogenic function for LCs in vivo in the steady-state. Furthermore, in a model where OVA expression in keratinocytes is inducible (162), it was shown that LCs migrating from such epidermal explants induced T cells (OT-I) that produced less interferon-γ, suggestive for tolerance. Recent evidence suggests that radioresistant stromal cells such as lymph node stromal cells or keratinocytes might be important mediators of tolerance induction in some K14-OVA mouse models. The group of Boes (167) demonstrated in K14-mOVA mice that skin-derived DCs as well as lymph node-resident CD8+ DCs were able to present OVA peptide to CD8+ T cells in vitro. In contrast, in vivo only lymph node-resident DCs were important for activation of CD4+ T cells. Cross-presentation in the lymph nodes was possible in the complete absence of DCs and might be performed by stromal cells as supported by few other reports (169, 198). In accordance with these findings, the group of Hogquist reported recently that Langerin+ DCs are dispensable for antigen presentation in their model in which an MHC class I-restricted peptide is expressed under the K14 promoter (176).
LCs were shown to induce regulatory T cells in a mouse model (199). In another study (reviewed in 70) in the murine leishmaniasis model, the depletion of LCs enhanced Th1 responses resulting in smaller lesions after intradermal infection with physiologic doses of parasites. Concomitantly, numbers of regulatory T cells were decreased suggesting a suppressive role of LCs during Leishmania infection (70). Yet another indirect hint comes from experiments by Shklovskaya et al. (200): LCs respond to contact sensitizer by upregulating CD86 and CD40 to a lesser degree as compared with dermal DCs (including langerin+ and langerinnegative DCs).
A word of caution is warranted. When judging (and trying to generalize) the data from currently employed experimental mouse models, it is important to be always aware of potentially confounding factors such as the use of (i) constitutive (26) versus inducible LC ablation (24, 25); (ii) constitutive (163, 164, 167, 170) versus inducible (162) expression of model antigens, typically OVA; (iii) transgenic model antigen in the form of peptide (170) versus soluble (169) or membrane-bound whole protein (163, 164, 167, 169); (iv) CD4-(OT-II, DO11.10 cells) versus CD8-(OT-I) restricted TCR transgenic T cells; (v) low versus high numbers of adoptively transferred transgenic T cells; and (vi) adoptively transferred TCR transgenic T cells (e.g. OT cells)(167, 169, 170, 176) versus endogenous T cells (164).
Clearly, more work is needed to gain a definitive picture about the tolerogenic potential of LCs in vivo. It is more complicated than anticipated, with dermal DCs and resident lymph node DC subsets also playing roles in antigen presentation. Moreover, radioresistant non-LCs, presumably stromal cells in the skin and lymph node may be involved. Nevertheless, the synopsis of the present data does indeed lend some support to a tolerogenic role for LCs in the steady-state, in accordance with the same role proposed for DCs in general (147).
LC function in vivo: generating a synthesis
The past few years of research on the in vivo functions of LCs may be viewed from the standpoint of dialectics. The LC paradigm was the ‘thesis’. The various challenges of the paradigm may be considered as the ‘anti-thesis’. We are now in the process of generating a ‘syn-thesis’. This has not yet been achieved. Yet, some main features have become clear. (i) There is no ‘one fits all’ theory for LC function in vivo. LCs are certainly not the ‘ubiquitous T-cell primers’ (201), but the past few years have provided strong evidence for an immunogenic function under certain conditions. (ii) The role of LCs in the skin immune system is manifold. It depends on the circumstances in the skin (steady-state versus inflammation), the type of pathogens, the doses of antigens and on other variables. (iii) LCs are not alone any more. The novel population of dermal langerin+ DCs has clearly been shown to exert immunogenic functions in vivo. It remains to be determined how these two subsets of langerin+ skin DCs and, in addition, langerinnegative dermal DCs share the tasks among each other in different immunologic situations. (iv) The notion (and the evidence) that LCs in vivo can act in a tolerogenic/downregulatory fashion in the steady-state has certainly been reinforced recently.
Importance of LCs for medicine
Four important aspects of LCs that relate directly to clinical medicine are briefly discussed here.
LC histiocytosis
LC histiocytosis (LCH) is characterized by an accumulation of langerin+ DCs in skin, bone or other tissues of the body (202). In the early days, a definitive diagnosis was the ultra-structural proof of Birbeck granules. Now, it can immuno-histologically be recognized by the expression of langerin in the histiocytic cells. Interestingly, it is still not clear whether this disease should be considered a neoplasm or an inflammatory infiltration of the diseased tissue by these langerin+ histiocytes. Research with clinical samples has shown that a ‘cytokine storm’ is involved in the maintenance of this disease (203). Moreover, IL-17A appears to be involved in an as yet unknown way (204), and also regulatory T cells seem to play a role in LCH (205). Mouse models for this disease are being developed (206), but time will tell how useful they will be for the further investigation of the pathophysiology of LCH. This topic is reviewed in much more detail by Maarten Egeler in this issue.
LCs in atopic dermatitis
This common inflammatory skin disorder is often associated with other atopic conditions such as allergic rhinitis and asthma. The clinical manifestation of atopic dermatitis is thought to result from complex interactions of environmental factors, skin barrier defects, and immunologic phenomena. Its pathogenesis is still not well understood. Recent findings highlight the importance of LCs and infiltrating inflammatory DCs in atopic skin lesions. TSLP (thymic stromal lymphopoietin) was identified as a critical cytokine that matures and modifies DCs from human blood such that they promote the development of pro-allergic CD4+ helper T cells, i.e. IL-4/13- and tumor necrosis factor-α (TNFα)-producing cells (207). We could recently show that the same holds true also for human epidermal LCs. TSLP-treated LCs produce the Th2-attracting chemokine TARC (CCL17), and they induce CD4+ T cells that secrete comparably more IL-4, IL-5, IL-13, and TNFα and elaborate markedly less interferon-γ (208). Interestingly, dermal (langerinnegative) DCs did not respond to TSLP in the same way (S. Ebner, unpublished data). Dubrac et al. attempted to validate these observations in a mouse model where the topical administration of vitamin D3 leads to a disease that resembles human atopic dermatitis with eczema, neutrophil, and eosinophil infiltrates and elevated IgE levels in the serum (209). When this treatment was given to Langerin-DTR mice that lacked LCs as well as dermal langerin+ DCs, the atopic disease did not develop. A contribution of dermal langerin+ cells could not formally be ruled out but it was rendered unlikely by the observation that no obvious phenotypic changes could be seen in these cells in response to Vitamin D3, as opposed to epidermal LCs that upregulated maturation markers. Furthermore, the small size of this population in the dermis argues against them being a major contributor (210).
Innate functions of LCs in disease
A key observation was made by Geijtenbeek’s group, who showed that HIV particles could be taken up by human LCs and were routed into the Birbeck granules. But rather than being transmitted to T cells for infection, as expected, the virions were degraded and not passed on to T cells (52). This was contrary to dermal DCs that readily spread the virions to CD4+ T cells. This innate anti-viral activity was dependent on the expression of the langerin molecule. It will be interesting to learn whether such a protective function also applies to viruses other than HIV and whether and how it may eventually be harnessed for therapy or prevention of infection (211, 212). The constitutive opsonization of HIV in vivo, that reduces infectivity, may at the same time also reduce or abrogate the protective function of langerin in vivo (213). These issues need to be studied in the future.
Another unexpected function of human LCs, that as yet stands alone but may also be a starting point for further investigations, was described (214): LCs appear to be involved in the local regulation of dermal blood flow. They make prostanoid synthases required for the formation of vasodilatory prostaglandins E2 and D2. Depletion of epidermal LCs in Langerin-DTR mice abolished nicotinic acid-induced flushing. This model mimics flushing in humans, that occurs as a frequent side effect of niacin treatment for dyslipidemia. These studies may help to develop new ways to suppress the unwanted effects of this widespread therapy.
Langerin-expressing cells as targets for improved vaccinations
Seminal work from Ralph Steinman’s and Michel Nussenzweig’s laboratories over the past few years has revealed and emphasized the potential of targeting antigens directly to DCs. In essence, this is achieved by coupling an antigen to an antibody that recognizes an antigen uptake receptor, typically a C-type lectin, on the surface of DCs. The most widely used target is the DEC-205/CD205 molecule on DCs. It was shown that T and B-cell responses to such conjugated forms of antigen are magnitudes larger as compared with similar concentrations of unconjugated protein antigen (145, 215-218). This concept is currently being exploited for the improvement of vaccines (185, 219). There is evidence that LCs are superior to other skin DCs in inducing cytotoxic T-cell responses in vitro (138, 190), and it was shown that they are involved in protection from tumors (174). LCs and dermal langerin+ DCs express DEC-205 and they can readily be targeted with antibodies against DEC-205, but also against langerin (66). Both targeting pathways lead to increased T-cell responses (220). Combining these different pieces of evidence, it appears attractive and promising to study in more detail how to best target antigens to skin DCs, particularly to LCs, and how to harness most efficiently such boosted immune responses for vaccination purposes against tumors but ultimately also against infectious agents.
Concluding remarks
Since about the turn of the century, LC research has been gaining momentum as impressively illustrated by several fruitful Langerhans Cell Workshops (16, 70, 221), mainly because of the development of useful in vivo research tools. These tools have not brought about an immediate clarification of all the secrets of LC function in vivo. This expectation was probably unrealistic. Rather, the many pieces of data, that are being collected using these tools, are like mosaic stones. It will still take a while until the final picture emerges. We are convinced, however, that the special properties of LCs and dermal langerin+ DCs will soon be better understood and be successfully ‘taken into medicine’ (2) in the foreseeable future.
Acknowledgements
The authors have continuously been supported by the Austrian Science Fund (currently, FWF project P21487 to PS). NR was further supported by the COMET Center ONCOTYROL and funded by the Federal Ministry for Transport Innovation & Technology (BMVIT) and the Federal Ministry of Economics & Labour/the Federal Ministry of Economy, Family & Youth (BMWA/BMWFJ), and the Tiroler Zukunftsstiftung (TZS). We appreciate the participation of the TILAK hospital holding company, who serves as a partner the Oncotyrol research program. Work in the Clausen laboratory has been supported by the Netherlands Organization for Scientific Research (NWO; currently, VIDI 917-76-365) and the Landsteiner Foundation for Blood Transfusion Research (LSBR; 0414F).
References
- 1.Steinman RM. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Langerhans P. Über die Nerven der menschlichen Haut. Virchows Arch [A] 1868;44:325–337. [Google Scholar]
- 5.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birbeck MS, Breathnach AS, Everall JD. An electron microscopic study of basal melanocytes and high level clear cells (Langerhans cell) in vitiligo. J Invest Dermatol. 1961;37:51–64. [Google Scholar]
- 8.Basset F, Nezelof C, Turiaf J. Presence in electron microscopy of odd filamentous structures in pulmonary and osseous lesions of histiocytosis X. Current status of the question. Bull Mem Soc Med Hop Paris. 1966;117:413–426. [PubMed] [Google Scholar]
- 9.Wolff K, Winkelmann RK. Ultrastructural localization of nucleoside triphosphatase in Langerhans cells. J Invest Dermatol. 1967;48:50–54. doi: 10.1038/jid.1967.8. [DOI] [PubMed] [Google Scholar]
- 10.Stingl G, Wolff-Schreiner EC, Pichler WJ, Gschnait F, Knapp W. Epidermal Langerhans cells bear Fc and C3 receptors. Nature. 1977;268:245–246. doi: 10.1038/268245a0. [DOI] [PubMed] [Google Scholar]
- 11.Rowden G, Lewis MG, Sullivan AK. Ia antigen expression on human epidermal Langerhans cells. Nature. 1977;268:247–248. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- 12.Klareskog L, Malmnas-Tjernlund U, Forsum U, Peterson PA. Epidermal Langerhans cells express Ia antigens. Nature. 1977;268:248–250. doi: 10.1038/268248a0. [DOI] [PubMed] [Google Scholar]
- 13.Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979;282:324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- 14.Frelinger JG, Hood L, Hill S, Frelinger JA. Mouse epidermal Ia molecules have a bone marrow origin. Nature. 1979;282:321–323. doi: 10.1038/282321a0. [DOI] [PubMed] [Google Scholar]
- 15.Romani N, et al. Presentation of exogenous protein antigens by dendritic cells to T cell clones: intact protein is presented best by immature epidermal Langerhans cells. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girolomoni G, Caux C, Lebecque S, Dezutter-Dambuyant C, Ricciardi-Castagnoli P. Langerhans cells: still a fundamental paradigm for studying the immunobiology of dendritic cells. Trends Immunol. 2002;23:6–8. doi: 10.1016/s1471-4906(01)02125-1. [DOI] [PubMed] [Google Scholar]
- 17.Wilson NS, Villadangos JA. Lymphoid organ dendritic cells: beyond the Langerhans cells paradigm. Immunol Cell Biol. 2004;82:91–98. doi: 10.1111/j.1440-1711.2004.01216.x. [DOI] [PubMed] [Google Scholar]
- 18.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 19.Valladeau J, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 20.Valladeau J, et al. The monoclonal antibody DCGM4 recognizes Langerin, a protein specific of Langerhans cells, and is rapidly internalized from the cell surface. Eur J Immunol. 1999;29:2695–2704. doi: 10.1002/(SICI)1521-4141(199909)29:09<2695::AID-IMMU2695>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Valladeau J, et al. Identification of mouse Langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- 22.Merad M, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allan RS, et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 24.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginhoux F, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bursch LS, et al. Identification of a novel population of langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett CL, Clausen BE. Dendritic cell ablation in mice: promises, pitfalls, and challenges. Trends Immunol. 2007;28:525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 32.Jung S, et al. in vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 34.Iijima N, Thompson JM, Iwasaki A. Dendritic cells and macrophages in the genitourinary tract. Mucosal Immunol. 2008;1:451–459. doi: 10.1038/mi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allam JP, et al. Comparative analysis of nasal and oral mucosa dendritic cells. Allergy. 2006;61:166–172. doi: 10.1111/j.1398-9995.2005.00965.x. [DOI] [PubMed] [Google Scholar]
- 36.Hellquist HB, Olsen KE, Irander K, Karlsson E, Odkvist LM. Langerhans cells and subsets of lymphocytes in the nasal mucosa. APMIS. 1991;99:449–454. doi: 10.1111/j.1699-0463.1991.tb05174.x. [DOI] [PubMed] [Google Scholar]
- 37.Santoro A, et al. Recruitment of dendritic cells in oral lichen planus. J Pathol. 2005;205:426–434. doi: 10.1002/path.1699. [DOI] [PubMed] [Google Scholar]
- 38.Zavala WD, De Simone DS, Sacerdote FL, Cavicchia JC. Variation in Langerhans cell number and morphology between the upper and lower regions of the human esophageal epithelium. Anat Rec. 2002;268:360–364. doi: 10.1002/ar.10147. [DOI] [PubMed] [Google Scholar]
- 39.Lucendo AJ, et al. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am J Surg Pathol. 2007;31:598–606. doi: 10.1097/01.pas.0000213392.49698.8c. [DOI] [PubMed] [Google Scholar]
- 40.Flores-Langarica A, et al. Network of dendritic cells within the muscular layer of the mouse intestine. Proc Natl Acad Sci USA. 2005;102:19039–19044. doi: 10.1073/pnas.0504253102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaser A, et al. Increased expression of CCL20 in human inflammatory bowel disease. J Clin Immunol. 2004;24:74–85. doi: 10.1023/B:JOCI.0000018066.46279.6b. [DOI] [PubMed] [Google Scholar]
- 42.Chang SY, et al. Lack of retinoic acid leads to increased langerin-expressing dendritic cells in gut-associated lymphoid tissues. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.11.006. doi:10.1053/j.gastro.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Wieser F, Hosmann J, Tschugguel W, Czerwenka K, Sedivy R, Huber JC. Progesterone increases the number of Langerhans cells in human vaginal epithelium. Fertil Steril. 2001;75:1234–1235. doi: 10.1016/s0015-0282(01)01796-4. [DOI] [PubMed] [Google Scholar]
- 44.Ildgruben AK, Sjoberg IM, Hammarstrom ML. Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet Gynecol. 2003;102:571–582. doi: 10.1016/s0029-7844(03)00618-5. [DOI] [PubMed] [Google Scholar]
- 45.Iijima N, Linehan MM, Saeland S, Iwasaki A. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc Natl Acad Sci USA. 2007;104:19061–19066. doi: 10.1073/pnas.0707179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobhani I, et al. Anal carcinoma: incidence and effect of cumulative infections. AIDS. 2004;18:1561–1569. doi: 10.1097/01.aids.0000131335.15301.dd. [DOI] [PubMed] [Google Scholar]
- 47.Sung SSJ, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (αE)-β7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 48.Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatability complex antigen (Ia)-bearing dendritic cells (DCs) in the conducting airways. J Exp Med. 1991;173:1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Mayer WJ, et al. Characterization of antigen-presenting cells in fresh and cultured human corneas using novel dendritic cell markers. Invest Ophthalmol Vis Sci. 2007;48:4459–4467. doi: 10.1167/iovs.06-1184. [DOI] [PubMed] [Google Scholar]
- 51.Valladeau J, Dezutter-Dambuyant C, Saeland S. Langerin/CD207 sheds light on formation of Birbeck granules and their possible function in Langerhans cells. Immunol Res. 2003;28:93–107. doi: 10.1385/IR:28:2:93. [DOI] [PubMed] [Google Scholar]
- 52.De Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 53.Uzan-Gafsou S, et al. Rab11A controls the biogenesis of Birbeck granules by regulating Langerin recycling and stability. Mol Biol Cell. 2007;18:3169–3179. doi: 10.1091/mbc.E06-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mc Dermott R, et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where langerin accumulates. Mol Biol Cell. 2002;13:317–335. doi: 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheong C, et al. Production of monoclonal antibodies that recognize the extracellular domain of mouse Langerin/CD207. J Immunol Methods. 2007;324:48–62. doi: 10.1016/j.jim.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Douillard P, et al. Mouse lymphoid tissue contains distinct subsets of Langerin/CD207+ dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 57.Stoitzner P, Tripp CH, Douillard P, Saeland S, Romani N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol. 2005;125:116–125. doi: 10.1111/j.0022-202X.2005.23757.x. [DOI] [PubMed] [Google Scholar]
- 58.Kashihara M, Ueda M, Horiguchi Y, Furukawa F, Hanaoka M, Imamura S. A monoclonal antibody specifically reactive to human Langerhans cells. J Invest Dermatol. 1986;87:602–612. doi: 10.1111/1523-1747.ep12455849. [DOI] [PubMed] [Google Scholar]
- 59.Lukas M, et al. Human cutaneous dendritic cells migrate through dermal lymphatic vessels in a skin organ culture model. J Invest Dermatol. 1996;106:1293–1299. doi: 10.1111/1523-1747.ep12349010. [DOI] [PubMed] [Google Scholar]
- 60.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 61.Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993;92:2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romani N, et al. Migration of dendritic cells into lymphatics – the Langerhans cell example: routes, regulation, and relevance. Int Rev Cytol. 2001;207:237–270. doi: 10.1016/s0074-7696(01)07007-3. [DOI] [PubMed] [Google Scholar]
- 63.Stoitzner P, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 64.Horton JJ, Allen MH, MacDonald DM. An assessment of Langerhans cell quantification in tissue sections. J Am Acad Dermatol. 1984;11:591–593. doi: 10.1016/s0190-9622(84)70211-8. [DOI] [PubMed] [Google Scholar]
- 65.de Jong MCJM, Blanken R, Nanninga J, Van Voorst Vader PC, Poppema S. Defined in situ enumeration of T6 and HLA-DR expressing epidermal Langerhans cells: morphologic and methodologic aspects. J Invest Dermatol. 1986;87:698–702. doi: 10.1111/1523-1747.ep12456649. [DOI] [PubMed] [Google Scholar]
- 66.Flacher V, et al. Epidermal Langerhans cells rapidly capture and present antigens from C-type lectin-targeting antibodies deposited in the dermis. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.343. doi:10.1038/jid.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagao K, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci USA. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor β1 in Langerhans cell biology: the skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romani N, Merad M, Stingl G, Stoitzner P. Langerhans cells at the interface of medicine, science, and industry. J Invest Dermatol. 2010;130:331–335. doi: 10.1038/jid.2009.394. [DOI] [PubMed] [Google Scholar]
- 71.Ginhoux F, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Randolph GJ, Ochando J, Partida-Sánchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 73.Tripp CH, Sparber F, Hermans IF, Romani N, Stoitzner P. Glycolipids injected into the skin are presented to NKT cells in the draining lymph node independently of migratory skin dendritic cells. J Immunol. 2009;182:7644–7654. doi: 10.4049/jimmunol.0900134. [DOI] [PubMed] [Google Scholar]
- 74.Henri S, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kel JM, Noordegraaf M, Girard-Madoux MJH, Reizis B, Clausen BE. TGF-β controls Langerhans cell homeostasis in the epidermis. 2010 submitted. [Google Scholar]
- 76.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLellan AD, et al. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood. 2002;99:2084–2093. doi: 10.1182/blood.v99.6.2084. [DOI] [PubMed] [Google Scholar]
- 78.Flacher V, et al. Expression of Langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borkowski TA, Nelson AJ, Farr AG, Udey MC. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur J Immunol. 1996;26:110–114. doi: 10.1002/eji.1830260117. [DOI] [PubMed] [Google Scholar]
- 80.Geurtsvankessel CH, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD1 1b–but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Helf J, et al. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 83.Ginhoux F, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henri S, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 85.Idoyaga J, Suda N, Suda K, Park CG, Steinman RM. Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2009;106:1524–1529. doi: 10.1073/pnas.0812247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Den Haan JMM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1695. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maldonado-López R, et al. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schuler G, Romani N, Stössel H, Wolff K. Structural organization and biological properties of Langerhans cells. In: Schuler G, editor. Epidermal Langerhans Cells. CRC Press Inc; Boca Raton, FL: 1991. pp. 87–137. [Google Scholar]
- 90.Shamoto M, Shinzato M, Hosokawa S, Kaneko C, Hakuno T, Nomoto K. Langerhans cells in the lymph node: mirror section and immunoelectron microscopic studies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;61:337–341. doi: 10.1007/BF02890436. [DOI] [PubMed] [Google Scholar]
- 91.Vernon ML, Fountain L, Krebs HM, Horta-Barbosa L, Fuccillo DA, Sever JL. Birbeck granules (Langerhans’ cell granules) in human lymph nodes. Am J Clin Pathol. 1973;60:771–779. doi: 10.1093/ajcp/60.6.771. [DOI] [PubMed] [Google Scholar]
- 92.Geissmann F, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–430. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angel CE, et al. Distinctive localization of antigen-presenting cells in human lymph nodes. Blood. 2009;113:1257–1267. doi: 10.1182/blood-2008-06-165266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elbe A, Tschachler E, Steiner G, Binder A, Wolff K, Stingl G. Maturational steps of bone marrow-derived dendritic murine epidermal cells: phenotypic and functional studies on Langerhans cells and Thy-1+ dendritic epidermal cells in the perinatal period. J Immunol. 1989;143:2431–2438. [PubMed] [Google Scholar]
- 95.Romani N, Schuler G, Fritsch PO. The ontogeny of Ia-positive and Thy-1 positive leukocytes of murine epidermis. J Invest Dermatol. 1986;86:129–133. doi: 10.1111/1523-1747.ep12284135. [DOI] [PubMed] [Google Scholar]
- 96.Tripp CH, et al. Ontogeny of Langerin/CD207 expression in the epidermis of mice. J Invest Dermatol. 2004;122:670–672. doi: 10.1111/j.0022-202X.2004.22337.x. [DOI] [PubMed] [Google Scholar]
- 97.Chorro L, et al. Langerhans cell (LCs) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LCs network. J Exp Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang-Rodriguez S, Hoetzenecker W, Schwärzler C, Biedermann T, Saeland S, Elbe-BÜrger A. Fetal and neonatal murine skin harbors Langerhans cell precursors. J Leukoc Biol. 2005;77:352–360. doi: 10.1189/jlb.1004584. [DOI] [PubMed] [Google Scholar]
- 99.Dewar AL, Doherty KV, Woods GM, Lyons AB, Muller HK. Acquisition of immune function during the development of the Langerhans cell network in neonatal mice. Immunology. 2001;103:61–69. doi: 10.1046/j.1365-2567.2001.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holzmann S, et al. A model system using tape stripping for characterization of Langerhans cell-precursors in vivo. J Invest Dermatol. 2004;122:1165–1174. doi: 10.1111/j.0022-202X.2004.22520.x. [DOI] [PubMed] [Google Scholar]
- 101.Foster CA. Ontogeny of Langerhans cells in human embryonic and fetal skin: expression of HLA-DR and OKT-6 determinants. J Invest Dermatol. 1986;86:240–243. doi: 10.1111/1523-1747.ep12285201. [DOI] [PubMed] [Google Scholar]
- 102.Foster CA, Holbrook KA. Ontogeny of Langerhans cells in human embryonic and fetal skin: cell densities and phenotypic expression relative to epidermal growth. Am J Anat. 1989;184:157–164. doi: 10.1002/aja.1001840207. [DOI] [PubMed] [Google Scholar]
- 103.Schuster C, et al. HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J Exp Med. 2009;206:169–181. doi: 10.1084/jem.20081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelletier M, Perreault C, Landry D, David M, Montplaiser S. Ontogeny of human epidermal Langerhans cells. Transplantation. 1984;38:544–546. doi: 10.1097/00007890-198411000-00022. [DOI] [PubMed] [Google Scholar]
- 105.Krueger GG, Daynes RA, Emam M. Biology of Langerhans cells: selective migration of Langerhans cells into allogeneic and xenogeneic grafts on nude mice. Proc Natl Acad Sci USA. 1983;80:1650–1654. doi: 10.1073/pnas.80.6.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanitakis J, Petruzzo P, Dubernard JM. Turnover of epidermal Langerhans’ cells. N Engl J Med. 2004;351:2661–2662. doi: 10.1056/NEJM200412163512523. [DOI] [PubMed] [Google Scholar]
- 107.Miyauchi S, Hashimoto K. Mitotic activities of normal epidermal Langerhans cells. J Invest Dermatol. 1989;92:120–121. doi: 10.1111/1523-1747.ep13071334. [DOI] [PubMed] [Google Scholar]
- 108.Konrad K, Hönigsmann H. Elektronenmikroskopischer Nachweis einer mitotischen Langerhanszelle in normaler menschlicher Epidermis/electron microscopic demonstration of a mitotic Langerhans cell in the normal human epidermis. Arch Dermatol Res. 1973;246:70–76. [PubMed] [Google Scholar]
- 109.Breathnach SM. Origin, cell lineage, ontogeny, tissue distribution and kinetics of Langerhans cells. In: Schuler G, editor. Epidermal Langerhans Cells. CRC Press Inc.; Boca Raton, FL: 1991. pp. 23–47. [Google Scholar]
- 110.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 111.Ghaznawie M, Papadimitriou JM, Heenan PJ. The steady-state turnover of murineepidermal Langerhans cells. Br J Dermatol. 1999;141:57–61. doi: 10.1046/j.1365-2133.1999.02921.x. [DOI] [PubMed] [Google Scholar]
- 112.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 113.Weinlich G, et al. Entry into afferent lymphatics and maturation in situ of migrating cutaneous dendritic cells. J Invest Dermatol. 1998;110:441–448. doi: 10.1046/j.1523-1747.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 114.Palucka AK, Banchereau J. Langerhans cells: daughters of monocytes. Nat Immunol. 2006;7:223–224. doi: 10.1038/ni0306-223. [DOI] [PubMed] [Google Scholar]
- 115.Murphy GF, Messadi D, Fonferko E, Hancock WW. Phenotypic transformation of macrophages to Langerhans cells in the skin. Am J Pathol. 1986;123:401–406. [PMC free article] [PubMed] [Google Scholar]
- 116.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor β1 in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meindl S, Schmidt U, Vaculik C, Elbe-BÜrger A. Characterization, isolation, and differentiation of murine skin cells expressing hematopoietic stem cell markers. J Leukoc Biol. 2006;80:816–826. doi: 10.1189/jlb.0106015. [DOI] [PubMed] [Google Scholar]
- 118.Borkowski TA, et al. A role for TGFβ1 in Langerhans cell biology – further characterization of the epidermal Langerhans cell defect in TGFβ1 null mice. J Clin Invest. 1997;100:575–581. doi: 10.1172/JCI119567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hacker C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 120.Fainaru O, et al. Runx3 regulates mouse TGF-β-mediated dendritic cell function and its absence results in airway inflammation. EMBO J. 2004;23:969–979. doi: 10.1038/sj.emboj.7600085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strobl H, et al. flt3 ligand in cooperation with transforming growth factor-β1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90:1425–1434. [PubMed] [Google Scholar]
- 122.Heinz LX, et al. Differential involvement of PU.1 and Id2 downstream of TGF-beta1 during Langerhans-cell commitment. Blood. 2006;107:1445–1453. doi: 10.1182/blood-2005-04-1721. [DOI] [PubMed] [Google Scholar]
- 123.Rathinam C, et al. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity. 2005;22:717–728. doi: 10.1016/j.immuni.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Schiavoni G, et al. ICSBP is critically involved in the normal development and trafficking of Langerhans cells and dermal dendritic cells. Blood. 2004;103:2221–2228. doi: 10.1182/blood-2003-09-3007. [DOI] [PubMed] [Google Scholar]
- 125.Zenke M, Hieronymus T. Towards an understanding of the transcription factor network of dendritic cell development. Trends Immunol. 2006;27:140–145. doi: 10.1016/j.it.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 126.Heufler C, Koch F, Schuler G. Granulocyte-macrophage colony-stimulating factor and interleukin-1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167:700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Witmer-Pack MD, Olivier W, Valinsky J, Schuler G, Steinman RM. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987;166:1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mommaas AM, et al. Distribution of HLA class II molecules in epidermal Langerhans cells in situ. Eur J Immunol. 1995;25:520–525. doi: 10.1002/eji.1830250232. [DOI] [PubMed] [Google Scholar]
- 129.Pierre P, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 130.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 131.Stoitzner P, Pfaller K, Stössel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol. 2002;118:117–125. doi: 10.1046/j.0022-202x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- 132.Kämpgen E, et al. Class II major histocompatibility complex molecules of murine dendritic cells: synthesis, sialylation of invariant chain, and antigen processing capacity are down-regulated upon culture. Proc Natl Acad Sci USA. 1991;88:3014–3018. doi: 10.1073/pnas.88.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stössel H, et al. Disappearance of certain acidic organelles (endosomes and Langerhans cell granules) accompanies loss of antigen processing capacity upon culture of epidermal Langerhans cells. J Exp Med. 1990;172:1471–1482. doi: 10.1084/jem.172.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stingl G, Katz SI, Clements L, Green I, Shevach EM. Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol. 1978;121:2005–2013. [PubMed] [Google Scholar]
- 135.Pehamberger H, Stingl LA, Pogantsch S, Steiner G, Wolff K, Stingl G. Epidermal cell-induced generation of cytotoxic T-lymphocyte responses against alloantigens or TNP-modified syngeneic cells: requirement for Ia-positive Langerhans cells. J Invest Dermatol. 1983;81:208–211. doi: 10.1111/1523-1747.ep12517984. [DOI] [PubMed] [Google Scholar]
- 136.Braathen LR, Thorsby E. Studies on human epidermal Langerhans cells. I. Allo-activating and antigen-presenting capacity. Scand J Immunol. 1980;11:401–408. doi: 10.1111/j.1365-3083.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 137.Stoitzner P, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci USA. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 140.Enk AH, Angeloni VL, Udey MC, Katz SI. Inhibition of Langerhans cell antigen-presenting function by IL-10: a role for IL-10 in induction of tolerance. J Immunol. 1993;151:2390–2398. [PubMed] [Google Scholar]
- 141.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blauvelt A, Katz SI, Udey MC. Human Langerhans cells express E-cadherin. J Invest Dermatol. 1995;104:293–296. doi: 10.1111/1523-1747.ep12612830. [DOI] [PubMed] [Google Scholar]
- 143.Schwarzenberger K, Udey MC. Contact allergens and epidermal proinflammatory cytokines modulate Langerhans cell E-cadherin expression in situ. J Invest Dermatol. 1996;106:553–558. doi: 10.1111/1523-1747.ep12344019. [DOI] [PubMed] [Google Scholar]
- 144.Riedl E, Stöckl J, Majdic O, Scheinecker C, Knapp W, Strobl H. Ligation of E-cadherin on in vitro-generated immature Langerhans-type dendritic cells inhibits their maturation. Blood. 2000;96:4276–4284. [PubMed] [Google Scholar]
- 145.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 148.Silberberg I, An ultrastructural study Apposition of mononuclear cells to Langerhans cells in contact allergic reactions. Acta Derm Venereol. 1973;53:1–12. [PubMed] [Google Scholar]
- 149.Nestle FO, Zheng X-G, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–6545. [PubMed] [Google Scholar]
- 150.Tse Y, Cooper KD. Cutaneous dermal Ia+ cells are capable of initiating delayed type hypersensitivity responses. J Invest Dermatol. 1990;94:267–272. doi: 10.1111/1523-1747.ep12874114. [DOI] [PubMed] [Google Scholar]
- 151.Schwarz A, Maeda A, Kernebeck K, Van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grabbe S, Steinbrink K, Steinert M, Luger TA, Schwarz T. Removal of the majority of epidermal Langerhans cells by topical or systemic steroid application enhances the effector phase of murine contact hypersensitivity. J Immunol. 1995;155:4207–4217. [PubMed] [Google Scholar]
- 153.Sullivan S, Bergstresser PR, Tigelaar R, Streilein JW. Induction and regulation of contact hypersensitivity by resident, bone marrow-derived, dendritic epidermal cells: Langerhans cells and Thy-1+ epidermal cells. J Immunol. 1986;137:2460–2467. [PubMed] [Google Scholar]
- 154.Bacci S, Alard P, Dai RP, Nakamura T, Streilein JW. High and low doses of haptens dictate whether dermal or epidermal antigen-presenting cells promote contact hypersensitivity. Eur J Immunol. 1997;27:442–448. doi: 10.1002/eji.1830270214. [DOI] [PubMed] [Google Scholar]
- 155.Bennett CL, Noordegraaf M, Martina CAE, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 156.Noordegraaf M, Flacher V, Stoitzner P, Clausen BE. Functional redundancy of Langerhans cells and Langerin+ dermal dendritic cells in contact hypersensitivity. 2010 doi: 10.1038/jid.2010.223. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 158.Igyarto BZ, et al. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183:5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Obhrai JS, et al. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang L, Jameson SC, Hogquist KA. Epidermal Langerhans cells are not required for UV-induced immunosuppression. J Immunol. 2009;183:5548–5553. doi: 10.4049/jimmunol.0900235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schwarz A, Noordegraaf M, Maeda A, Torii K, Clausen BE, Schwarz T. Langerhans cells are required for UVR-induced immunosuppression. J Invest Dermatol. 2010 doi: 10.1038/jid.2009.429. doi:10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 162.Holcmann M, et al. Skin inflammation is not sufficient to break tolerance induced against a novel antigen. J Immunol. 2009;183:1133–1143. doi: 10.4049/jimmunol.0713351. [DOI] [PubMed] [Google Scholar]
- 163.Azukizawa H, et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33:1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 164.Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred T cell receptor transgenic CD8+ T cells into keratin-14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123:109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 165.McGargill MA, et al. A spontaneous CD8 T cell-dependent autoimmune disease to an antigen expressed under the human keratin 14 promoter. J Immunol. 2003;169:2141–2147. doi: 10.4049/jimmunol.169.4.2141. [DOI] [PubMed] [Google Scholar]
- 166.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bianchi T, et al. Maintenance of peripheral tolerance through controlled tissue homing of antigen-specific T cells in K14-mOVA mice. J Immunol. 2009;182:4665–4674. doi: 10.4049/jimmunol.0803628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Choi JH, et al. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim BS, Miyagawa F, Cho YH, Bennett CL, Clausen BE, Katz SI. Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis. J Invest Dermatol. 2009;129:2805–2817. doi: 10.1038/jid.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Langerhans cells activate naïve self-antigen specific CD8 T cells in the steady state. Immunity. 2004;21:391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 171.Aberer W, Kruisbeek AM, Shimada S, Katz SI. in vivo treatment with anti-I-A antibodies: differential effects on Ia antigens and antigen-presenting cell function of spleen cells and epidermal Langerhans cells. J Immunol. 1986;136:830–836. [PubMed] [Google Scholar]
- 172.Grubauer G, Romani N, Kofler H, Stanzl U, Fritsch P, Hintner H. Apoptotic keratin bodies as autoantigen causing the production of IgM-anti-keratin intermediate filament autoantibodies. J Invest Dermatol. 1986;87:466–471. doi: 10.1111/1523-1747.ep12455510. [DOI] [PubMed] [Google Scholar]
- 173.Nikolic-Zugic J, Carbone FR. The effect of mutations in the MHC class I peptide binding groove on the cytotoxic T lymphocyte recognition of the Kb-restricted ovalbumin determinant. Eur J Immunol. 1990;20:2431–2437. doi: 10.1002/eji.1830201111. [DOI] [PubMed] [Google Scholar]
- 174.Stoitzner P, et al. Tumor immunotherapy by epicutaneous immunization requires Langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 175.Waithman J, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J Immunol. 2007;179:4535–4541. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 176.Bursch LS, Rich BE, Hogquist KA. Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens. J Immunol. 2009;182:4657–4664. doi: 10.4049/jimmunol.0803656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 178.Zhao XY, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jones CA, et al. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J Virol. 2003;77:11139–11149. doi: 10.1128/JVI.77.20.11139-11149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 181.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells Induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 183.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 184.Galibert L, et al. Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule. J Biol Chem. 2005;280:21955–21964. doi: 10.1074/jbc.M502095200. [DOI] [PubMed] [Google Scholar]
- 185.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DCs. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 186.Ritter U, Osterloh A. A new view on cutaneous dendritic cell subsets in experimental leishmaniasis. Med Microbiol Immunol (Berl) 2007;196:51–59. doi: 10.1007/s00430-006-0023-0. [DOI] [PubMed] [Google Scholar]
- 187.Moll H, Fuchs H, Blank C, Röllinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 188.Ritter U, Meissner A, Scheidig C, Körner H. CD8α- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 189.Brewig N, et al. Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell sub-types. J Immunol. 2009;182:774–783. doi: 10.4049/jimmunol.182.2.774. [DOI] [PubMed] [Google Scholar]
- 190.Ratzinger G, et al. Mature human Langerhans cells derived from CD34+ hemopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 191.Cao T, Ueno H, Glaser C, Fay JW, Palucka AK, Banchereau J. Both Langerhans cells and interstitial DCs cross-present melanoma antigens and efficiently activate antigen-specific CTL. Eur J Immunol. 2007;37:2657–2667. doi: 10.1002/eji.200636499. [DOI] [PubMed] [Google Scholar]
- 192.Huijbers IJ, et al. An inducible mouse model of melanoma expressing a defined tumor antigen. Cancer Res. 2006;66:3278–3286. doi: 10.1158/0008-5472.CAN-05-3216. [DOI] [PubMed] [Google Scholar]
- 193.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 194.Pollock PM, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 195.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 197.Bogunovic M, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 199.Loser K, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2007;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 200.Shklovskaya E, Roediger B, Fazekas de St GB. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181:418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- 201.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 202.Weitzman S, Egeler RM. Langerhans cell histiocytosis: update for the pediatrician. Curr Opin Pediatr. 2008;20:23–29. doi: 10.1097/MOP.0b013e3282f45ba4. [DOI] [PubMed] [Google Scholar]
- 203.Rolland A, et al. Increased blood myeloid dendritic cells and dendritic cell-poietins in Langerhans cell histiocytosis. J Immunol. 2005;174:3067–3071. doi: 10.4049/jimmunol.174.5.3067. [DOI] [PubMed] [Google Scholar]
- 204.Coury F, et al. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med. 2008;14:81–87. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 205.Senechal B, et al. Expansion of regulatory T cells in patients with Langerhans cell histiocytosis. PLoS Med. 2007;4(e253):1374–1384. doi: 10.1371/journal.pmed.0040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Steiner QG, et al. in vivo transformation of mouse conventional CD8alpha+ dendritic cells leads to progressive multisystem histiocytosis. Blood. 2008;111:2073–2083. doi: 10.1182/blood-2007-06-097576. [DOI] [PubMed] [Google Scholar]
- 207.Soumelis V, Liu YJ. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol. 2004;25:325–333. doi: 10.1007/s00281-003-0152-0. [DOI] [PubMed] [Google Scholar]
- 208.Ebner S, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen presenting cells that induce pro-allergic T cells. J Allergy Clin Immunol. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 209.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Elentner A, Finke D, Schmuth M, et al. Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00797.x. doi:10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.de Jong MA, Geijtenbeek TB. Human immunodeficiency virus-1 acquisition in genital mucosa: Langerhans cells as key-players. J Intern Med. 2009;265:18–28. doi: 10.1111/j.1365-2796.2008.02046.x. [DOI] [PubMed] [Google Scholar]
- 213.Wilflingseder D, et al. IgG opsonization of HIV impedes provirus formation in and infection of dendritic cells and subsequent long-term transfer to T cells. J Immunol. 2007;178:7840–7848. doi: 10.4049/jimmunol.178.12.7840. [DOI] [PubMed] [Google Scholar]
- 214.Benyo Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal Langerhans cells. Mol Pharmacol. 2006;70:1844–1849. doi: 10.1124/mol.106.030833. [DOI] [PubMed] [Google Scholar]
- 215.Bonifaz LC, et al. in vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boscardin SB, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bozzacco L, et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc Natl Acad Sci USA. 2007;104:1289–1294. doi: 10.1073/pnas.0610383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 219.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 220.Idoyaga J, et al. The Langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and MHC II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 221.Merad M, Romani N, Randolph GJ. Langerhans cells at the interface of medicine, science, and industry. J Invest Dermatol. 2008;128:251–255. doi: 10.1038/sj.jid.5701229. [DOI] [PubMed] [Google Scholar]


