Abstract
Purpose
We assessed the efficacy and safety of the tyrosine kinase inhibitor sunitinib in Korean patients with metastatic renal cell carcinoma (mRCC).
Materials and Methods
Between September 2007 and December 2009, all twenty-one patients who had mRCC with a clear-cell component were retrospectively reviewed. Sunitinib was administered orally at a dose of 50 mg daily until disease progression or intolerance to treatment occurred. The primary end point of this study was the objective tumor response assessed by Response Evaluation Criteria in Solid Tumors (RECIST), and the secondary end points were progression-free survival (PFS) and overall survival (OS) rates as well as assessment of adverse effects.
Results
After a median of 17.4 months (range, 5.7-33.1 months) of treatment, 11 patients (52.4%) had an objective response with a complete response in 1 patient (4.8%), and a partial response in 10 patients (47.6%) as the best tumor response. The median PFS was 13.4 months (95% confidence interval [CI], range, 12.3-14.5 months), and the median OS was 28.1 months (95% CI, 21.8-34.4 months). All patients experienced adverse events of some sort, but the studied treatment protocol was well tolerated and most patients experienced reversible grade 1 or 2 toxicities.
Conclusions
Sunitinib was efficacious in the treatment of metastatic clear-cell RCC, and was well tolerated in Korean patients. Although sunitinib treatment-related adverse events such as hand-foot syndrome and facial/generalized edema were observed with a higher incidence than in Western trials, they were mainly mild to moderate, and readily managed.
Keywords: Neoplasm metastasis, Renal cell carcinoma, Sunitinib
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignancy of the kidney [1]. Although surgery is curative for localized disease, up to 30% of patients with RCC present with metastatic disease at the time of diagnosis [2,3], and more than 25% of patients with locally advanced RCC develop distant metastasis despite curative resection [4]. Since RCC is highly resistant to chemotherapy, high dose interleukin-2 or interferon-alfa therapy is widely used as a first-line treatment of metastatic disease, but they provide only a modest response rate of less than 20% [5]. Thus, the outcome of metastatic RCC is quietly depressing: The 5-year overall survival rate is less than 10% [3]. Effective systemic therapy for RCC is mandatory.
In conventional RCC, the loss of von Hippel-Lindau protein function leads to overexpression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor that promote tumor angiogenesis [6,7]. Sunitinib is an orally active multi-targeted tyrosine kinase inhibitor that specifically inhibits VEGF receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), FMS-like tyrosine kinase-3 (flt-3), and stem c-Kit protein (c-Kit) [8]. The efficacy on metastatic RCC has been confirmed in both phase II and III trials, where it was shown to improve the median progression-free survival (PFS), yield a higher response rate (RR), and afford a better quality of life over interferon-alfa [9-11]. Based on current data, sunitinib is now the preferred drug for first-line treatment of metastatic RCC [10,11].
However, these studies were performed mainly in Western populations. The difference in behaviors of RCC in different ethnic groups has been demonstrated [12]. Although the nature of these variations and associated molecular basis is not demonstrated, it is reasonable to evaluate the efficacy of sunitinib on metastatic RCC in those of different ethnic backgrounds. Therefore, we perfomed this retrospective study to assess the efficacy and safety of sunitinib in Korean patients with metastatic RCC using an institutional treatment protocol.
MATERIALS AND METHODS
1. Patients
The medical records of patients treated with sunitinib for metastatic RCC were retrospectively reviewed between September 2007 and December 2009. The inclusion criteria were as follows: histologically confirmed clear-cell RCC; metastases measurable on computed tomography (CT) or magnetic resonance imaging (MRI); a performance status of 0-2 based on Eastern Cooperative Oncology Group (ECOG) criteria. Patients were excluded if they had brain metastases. A total of 21 patients were found to fit the study criteria. Patient demographics, prior therapy, best responses, and relevant toxicities were obtained from medical records, and survival data were collected by telephone interviews. Risk factors associated with shorter survival were assessed according to the Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification: a low serum hemoglobin level, an elevated corrected serum calcium level, an elevated serum lactate dehydrogenase level, a poor performance status, and an interval of less than 1 year between diagnosis and treatment [12].
2. Treatment schedule
Sunitinib was administered orally at a dose of 50 mg daily, consisting of 4 weeks of treatment followed by a 2-week rest period in cycles of 6 weeks (schedule 4-2). Dose reduction of sunitinib was allowed to 37.5 mg and then 25 mg daily depending on the type and severity of adverse events. Treatment with sunitinib was continued until the occurrence of disease progression, unacceptable adverse events, or patient withdrawal.
3. Study end points
The primary end point of this study was the objective clinical response (complete response, partial response, stable disease, or progressive disease), assessed by Response Evaluation Criteria in Solid Tumors (RECIST) using regular physical examinations, CT/MRI, and bone scans (if bone metastases were present at baseline) after each cycle for the first 4 cycles thereafter until the end of treatment [13]. The secondary end points were progression free survival (PFS) and overall survival (OS) rates. PFS was defined as the time from the start of treatment to the date of progressive disease or death. OS was defined as the time from start of treatment to death or the date at which patients were last known to be alive.
The adverse events secondary to treatment were evaluated at each visit according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 3.0).
4. Statistical analysis
Estimates of median PFS and OS were calculated using the Kaplan-Meier method, and 95% CIs were considered statistically significant. All statistical analysis was done using SPSS, version 11.0.
RESULTS
1. Patient characteristics
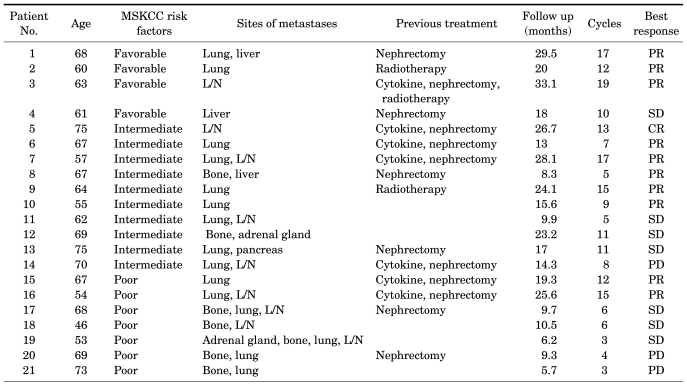
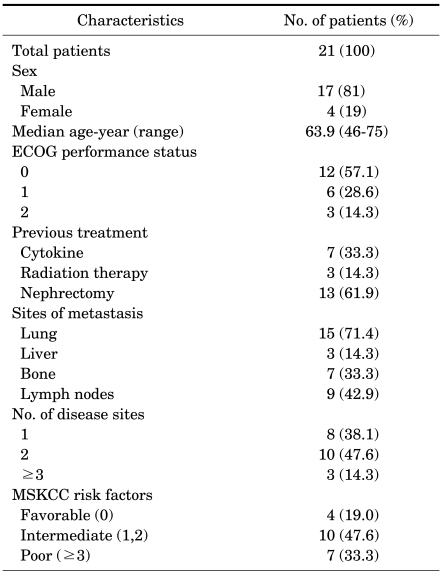
Between July 2007 and March 2010, all twenty-one patients who had metastatic RCC with a clear-cell component were included in the analysis (Table 1, 2). There were 17 males (81%) and 4 females (19%), and the median age was 63.9 (range, 46-75 years). Of those patients, 4 patients (19.0%) had low-risk disease, 10 patients (47.6%) had intermediate-risk disease, and 7 patients (33.3%) had high-risk disease according to the MSKCC criteria. The previous treatments were as follows: previous nephrectomy in 13 patients (61.9%), cytokine therapy in 7 patients (33.3%), and radiation therapy in 3 patients (14.3%). The most prevalent sites of metastases were as follows: the lung in 15 patients (71.4%), lymph nodes in 9 patients (42.9%), bone in 7 patients (33.3%), and liver in 3 patients (14.3%).
TABLE 1.
Patient demographics and disease characteristics
MSKCC: Memorial Sloan-Kettering Cancer Center, PR: partial response, L/N: lymph nodes, SD: stable disease, CR: complete response, PD: progressive disease
TABLE 2.
Patient characteristics
ECOG: Eastern Cooperative Oncology Group. MSKCC: Memorial Sloan-Kettering Cancer Center
2. Treatment outcomes
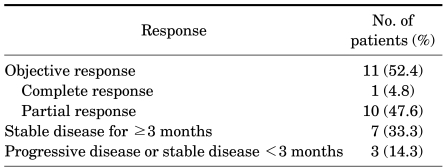
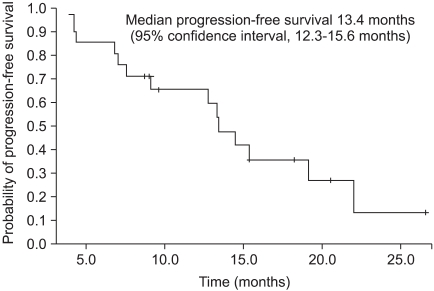
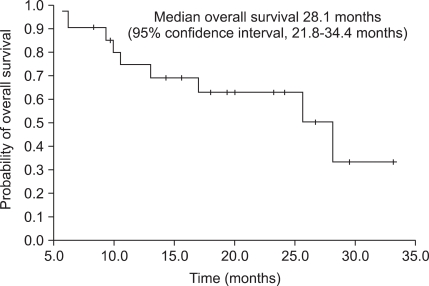
After a median of 17.4 months (range, 5.7-33.1 months) of treatment, 14 patients (66.7%) remained alive with the disease, and treatment was ongoing in 11 patients (52.4%). Reasons for discontinuing treatment were progressive disease in 2 patients and adverse events in 1 patient. According to assessment with the RECIST criteria, 11 patients (52.4%) had an objective response rate, with a complete response in 1 patient (4.8%), and a partial response in 10 patients (47.6%) as the best tumor response rate (Table 3). The median PFS was 13.4 months (95% confidence interval [CI], range, 12.3-14.5 months) (Fig. 1), and the median OS was 28.1 months (95% CI, 21.8-34.4 months) (Fig. 2).
TABLE 3.
Best responsea to sunitinib treatment
a: tumor response was assessed by Response Evaluation Criteria In Solid Tumor
FIG. 1.
Progression-free survival in Korean patients with metastatic clear-cell renal-cell carcinoma.
FIG. 2.
Overall survival in Korean patients with metastatic clear-cell renal-cell carcinoma.
3. Adverse events
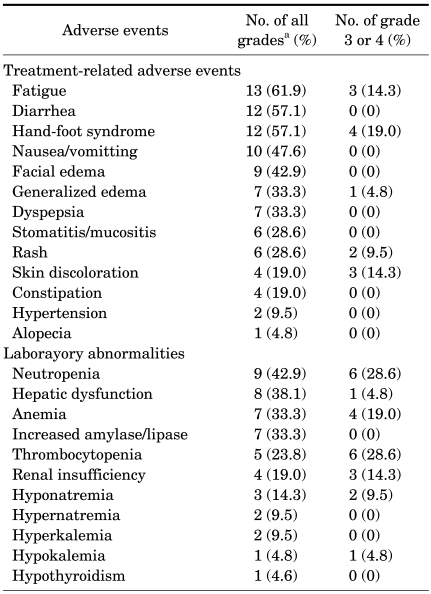
All patients experienced adverse events of some sort: fatigue, diarrhea, and hand-foot syndrome were the most common adverse effects. However, the treatment protocol under study was well tolerated and most patients experienced reversible grade 1 or 2 toxicities (Table 4). The most commonly observed severe adverse events were hand-foot syndrome, fatigue, and generalized edema; hand-foot syndrome was observed in 4 patients (19.0%), fatigue was observed in 3 patients (14.3%), and generalized edema was observed in 3 patients (14.3%) as grade 3 or 4.
TABLE 4.
Treatment-related adverse events and laboratory abnormalities
a: adverse effects were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events ver. 3.0
The most common laboratory abnormalities of grade 3 or 4 were neutropenia, thrombocytopenia, and anemia, with neutropenia in 6 patients (28.6%), thrombocytopenia in 6 patients (28.6%), and anemia in 4 patients (19.0%). Neutropenia was observed in 6 patients (28.6%), but there was no occurrence of associated fever or sepsis. Thrombocytopenia was observed in 6 patients (28.6%), but there was no clinical significance such as bleeding tendencies. 1 patient (4.8%) experienced hypothyroidism and needed thyroid hormone replacement. A total of 6 patients (29%) required dose reduction from 50 mg/day to 37.5 mg/day with further reductions to 25 mg/day due to grade 3 or 4 adverse events, and 1 patient (4.8%) discontinued treatment due to severe adverse events. The remaining 14 patients (66.7%) tolerated and continued treatment with a standard dose of sunitinib.
DISCUSSION
RCC is one of the most challenging malignancies with a dismal prognosis because of the modest response to conventional cytotoxic chemotherapeutic agents and limited sensitivity to radiation therapy [14]. Cytokine therapy with interferon-alfa or IL-2 have been the main treatment options for metastatic disease, but these agents have limited efficacy and are associated with considerable toxic effects. Recent advances in understanding the molecular biology of RCC have led to the development of several systemic therapeutic agents targeting the VEGF and mammalian target of rapamycin (mTOR) pathways as first and second line treatments for metastatic disease, with impressive improvements in oncologic outcome [11,15].
Sunitinib is one of several agents, including sorafenib, bevacizumab, and temsirolimus, which target the inhibition of pro-angiogenic growth factor activity and have shown favorable results in clinical trials against metastatic clear-cell RCC [15-17]. Recent studies have confirmed the efficacy of sunitinib as a first line treatment for metastatic clear-cell RCC [10,11], but these series were mainly performed in different ethnicities in Western countries. Thus, the potential ethnic differences in the efficacy and safety of sunitinib have not been established. This study evaluated whether Korean patients with metastatic RCC had comparable oncologic outcomes and safety as patients in previous randomized studies.
In patients with a poor prognosis according to the MSKCC criteria, temsirolimus is recommended as a first-line treatment, with sunitinib as a viable alternative [18], but temsirolimus is not available for Korean patients because of problems related to the insurance system; more patients with poor prognostic factors were included in this study. In terms of he MSKCC criteria, 33.3% of patients were in the poor prognosis group. Considering the selection bias of randomized controlled trials which include relatively more favorable or intermediate risk group, the results of this study seem to reflect more reliable results in Korean clinical practice.
For the treatment outcome, sunitinib treatment showed an objective response rate of 39% (range, 34-44%) and a PFS of 11.0 months (range, 10.7-13.4 months) as well as an OS of 26.4 months (range, 23.0-32.9 months) in previous phase III randomized trials [10,11]. In the current study, the objective response rate was 52.4%, which included a complete response of 4.8%, and a partial response of 47.6% as the best response rates. The median PFS and OS were 13.4 months and 28.1 months, respectively. Even though it is difficult to directly compare this retrospective study with previous randomized trials, it is obvious that metastatic RCC patients benefitted from sunitinib treatment.
All patients experienced treatment-related adverse events, the majority of which were grade 1 or 2 in severity. Fatigue, diarrhea, and hand-foot syndrome were the most frequent adverse events, but grade 3 or 4 adverse events were not frequent and were manageable. Most of patients were able to resume sunitinib treatment following dose reduction, and only 1 patient among all cases discontinued because of serious adverse events. Previous trials have reported that side effects of sunitinib treatment were reversible and tolerable; patients with grade 3 or 4 side effects did not exceed 10% [10,11].
Nevertheless, routine monitoring such as evaluation of blood pressure/ejection fraction and a thyroid function test is recommended because there is possibility of serious adverse events. Emerging safety data indicate that cardiotoxicity may be associated with sunitinib; left ventricular dysfunction is the main cardiac side effect of sunitinib and might be partly a result of cardiomyocyte toxicity exacerbated by hypertension [19]. Therefore, blood pressure and left ventricular ejection fraction should be monitored, especially in patients with cardiac risk factors. It is reported that in patients treated with sunitinib, 85% of patients had abnormal results on thyroid function tests, including elevation of thyroid stimulating hormone levels, decreased T3 levels, and less commonly, decreased T4 or free-thyroxine index levels [20]. Because an abnormal thyroid function test may rapidly progress from mild to profound, regular surveillance is warranted at baseline and every 2-3 months [21].
In terms of adverse events, neutropenia, thrombocytopenia, and anemia were the most frequent grade 3 or 4 laboratory abnormalities reported previously [10,11], and most of them were improved by dose reduction. Neutropenia was observed in 6 patients (28.6%), but there was no event of associated fever or sepsis; thrombocytopenia was observed in 6 patients (28.6%), but there was no clinical significance such as bleeding tendencies; anemia increased amylase/lipase was observed in 7 patients (33.3%), but there were no associated clinical manifestations of pancreatitis.
Compared to Western trials, sunitinib treatment in Korean patients achieved favorable oncological outcomes in terms of objective response rate, PFS, and OS. It is interesting to note that the favorable outcomes of the current study are consistent with previous Korean studies [22,23]. Although the underlying reason for such a discrepancy remains unclear at this time, one possible explanation is the inherent differences caused by ethnic backgrounds. Further large prospective studies are required to clarify whether the ethnic background and its associated molecular mechanisms contribute to the difference in the efficacy of sunitinib across ethnic groups. In terms of adverse effects, a higher incidence of hand-foot syndrome and facial/generalized edema were observed relative to Western trials, but they were mainly mild to moderate, and managed readily.
There are some limitations to this analysis. This was a retrospective analysis of a small number of patients treated at a single institution, which allows for potential selection bias. Furthermore, survival benefit may not be weighted properly because this was not a randomized controlled trial. However, this study provides evidence of the clinical benefits of sunitinib treatment. Sunitinib was efficacious and was well tolerated in Korean patients; the objective response rate, PFS, and OS in Korean patients were compatible with the oncologic outcomes in previous randomized trials. Further large prospective investigations are required to clarify such favorable efficacy profiles in Korean patients.
CONCLUSIONS
Sunitinib was efficacious in the treatment of metastatic clear-cell RCC, and was well tolerated in Korean patients. The objective response rate, PFS, and OS in Korean patients were compatible with the benefits shown in previous randomized trials. Although sunitinib treatment-related adverse events such as hand-foot syndrome and facial/generalized edema were observed in higher incidence than in Western trials, they were mainly mild to moderate, and readily managed. This study encourages the target therapy of sunitinib in Koreans for metastatic RCC.
Footnotes
The authors have nothing to disclose.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23:202–212. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Russo P, Nanus DM, Berg WJ. Renal cell carcinoma. Curr Probl Cancer. 1997;21:185–232. doi: 10.1016/s0147-0272(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 4.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 5.Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O'Connell M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer. 1995;76:824–832. doi: 10.1002/1097-0142(19950901)76:5<824::aid-cncr2820760517>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Linehan WM, Vasselli J, Srinivasan R, Walther MM, Merino M, Choyke P, et al. Genetic basis of cancer of the kidney: disease-specific approaches to therapy. Clin Cancer Res. 2004;10:6282S–6289S. doi: 10.1158/1078-0432.CCR-050013. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 8.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 9.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 15.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 16.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 18.Bellmunt J, Flodgren P, Roigas J, Oudard S. Optimal management of metastatic renal cell carcinoma: an algorithm for treatment. BJU Int. 2009;104:10–18. doi: 10.1111/j.1464-410X.2009.08563.x. [DOI] [PubMed] [Google Scholar]
- 19.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 21.Kollmannsberger C, Soulieres D, Wong R, Scalera A, Gaspo R, Bjarnason G. Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J. 2007;1(2 Suppl):41S–54S. doi: 10.5489/cuaj.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong MH, Kim HS, Kim C, Ahn JR, Chon HJ, Shin SJ, et al. Treatment outcomes of sunitinib treatment in advanced renal cell carcinoma patients: a single cancer center experience in Korea. Cancer Res Treat. 2009;41:67–72. doi: 10.4143/crt.2009.41.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo C, Kim JE, Lee JL, Ahn JH, Lee DH, Lee JS, et al. The efficacy and safety of sunitinib in Korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn J Clin Oncol. 2010 doi: 10.1093/jjco/hyq073. Epub ahead of print. [DOI] [PubMed] [Google Scholar]