Abstract
Purpose
The aim of this study was to investigate the relationship between lower urinary tract symptoms (LUTS) and risk factors for vascular diseases in a population-based cohort study, the Hallym Aging Study (HAS).
Materials and Methods
Among the 1,520 participants in HAS, 280 men aged more than 50 years, who underwent detailed health evaluations, including health-related questionnaires, evaluations of their medical history, and various life style factors, as well as clinical measurements, were included in the study. Vascular risk factors used in the present study including hypertension, diabetes mellitus, hyperlipidemia, and smoking and were assessed by medical history and clinical measurements. LUTS were assessed by validated questionnaires, the International Prostate Symptom Score (IPSS), and the relationship between LUTS and vascular risk factors was investigated.
Results
Of the 280 men, 175 (62.5%) had moderate/severe LUTS (IPSS>7) and 260 (93%) had one or more vascular risk factors. The IPSS was similar in those with no (11.6±9.7) and one or two (11.5±8.5) vascular risk factors, but increased to 15.1±9.3 in those with 3 or more vascular risk factors (p<0.05). The multiple logistic regression analysis, controlling for age and body mass index (BMI) showed that men with 3 or more vascular risk factors were 3 times more likely to have moderate/severe LUTS than men without vascular risk factors (p<0.05).
Conclusions
Men with risk factors for vascular diseases are more likely to have LUTS and these findings suggest that vascular risk factors play a role in the development of LUTS.
Keywords: Diagnosis, Risk factors, Urinary tract, Vascular diseases
INTRODUCTION
Lower urinary tract symptoms (LUTS) are relatively common conditions in aging men, and they have been known to greatly affect the quality of life [1]. Bladder outlet obstruction (BOO) due to benign prostatic hyperplasia (BPH) has been considered to be one of the major causes of LUTS. As a matter of fact, however, it has been reported that there is a lack of significant correlation between prostate size and LUTS [2,3]. Based on these reports, it can be inferred that factors other than BPH are involved in the pathogenesis of LUTS.
Vascular diseases are presumed to be one of the causes which are responsible for the pathogenesis of LUTS, and it has also been reported that aging-related artherosclerosis or endothelial dysfunction in the pelvic vascular system can cause bladder dysfunction [4].
To date, in animal models, artherosclerosis-induced pelvic ischemia has been shown to cause functional and structural alteration of the bladder and some studies have reported that LUTS are significantly correlated with risk factors which are closely related to vascular diseases, such as hypertension, diabetes mellitus (DM), hyperlipidemia, and smoking [5-7]. However, some studies on this question have reported contradictory results and in particular, there are no reports of an association between LUTS and vascular risk factors in a population-based study.
To investigate the potential role of vascular risk factors and the combination of these factors for the development of LUTS, we analyzed the association between LUTS and vascular risk factors (hypertension, DM, hyperlipidemia, smoking) in a population-based study of aging men.
MATERIALS AND METHODS
1. Study population
In the Hallym Aging Study (HAS) on the quality of life in elderly Korean people, conducted by the Institute for Aging Studies at Hallym University, a cohort was composed of 2,519 people aged 45 years or older who were randomly selected in Seoul, the national capital and largest city of South Korea, and Chuncheon, a large city of South Korea. Of these, the Chuncheon-based cohort was considered in the current study. The selection of the study populations can be summarized as follows:
Of the 1,408 study sectors, which were classified based on the 2,000 population census in Chuncheon City, 200 were randomly selected. In accordance with the proportion of the population aged 45 years or older from the individual Eup/Myeon/Dong (Korean neighborhood and sub-neighborhood classifications), the number needed for the study population was determined. Based on a list of study population that were selected for the current study, study participants were systematically sampled. At this time, the number of study population was determined in such a manner that 30% of the study population should be aged between 45 and 64 years and 70% should be aged 65 years or older with consideration of the effects of a long-term follow-up study as well as the calculation of stable epidemiological parameters. Of these, 1,520 people who responded to the primary panel survey, underwent a second in-depth study about health and psychology. Of the cohorts who visited Chuncheon Sacred Heart Hospital, underwent an interview and a clinical test, and completed a health questionnaire, 280 male patients aged 50 years or older were enrolled in the current study.
2. The assessment of LUTS
In all the study participants, LUTS were assessed using a validated questionnaire yielding the International Prostate Symptom Score (IPSS), and the participants were classified into three categories according to their IPSS: mild (IPSS≤7), moderate (IPSS 8-19), and severe (IPSS≥20) [8].
3. The assessment of vascular risk factors
Risk factors for vascular diseases used in the present study included hypertension, DM, hyperlipidemia, and smoking. To assess vascular risk factors, a detailed medical history questionnaire was completed by all the study participants. This was followed by blood pressure monitoring and hematologic tests. Blood pressure was measured by an experienced family medicine physician in such a condition that stability was maintained for more than ten minutes and venous blood was sampled following a 12-hour fast. Then, fasting blood sugar, total cholesterol, and triglyceride were measured.
The presence of hypertension, DM, and hyperlipidemia were defined as cases in which there were medical histories or abnormal findings from the blood pressure and hematologic tests based on criteria proposed by the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) [9]. Smokers were defined as participants who annually smoked more than 400 cigarettes.
4. Data analysis
Statistical analysis was performed using SPSS 15.0 for Windows. Among the age groups, comparisons were made for the severity of LUTS and the number of vascular risk factors using a Mantel-Haenszel chi-square test. The differences in IPSS according to the number of vascular risk factors were assessed by using one-way ANOVA, and multiple regression analysis was used to describe the impact of vascular risk factors on LUTS. A value of p<0.05 was considered statistically significant.
RESULTS
1. Study population
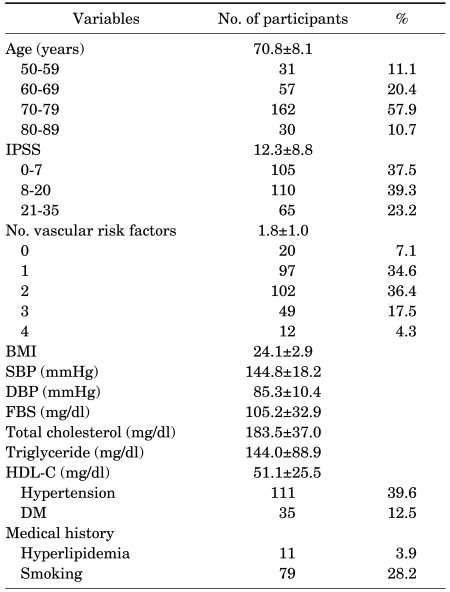
A total of 280 men, aged 50 to 87 years, were included in this study. The mean age was 70.8±8.1 years. Of these 280 men, 31 (11.1%) were 50-59 years, 57 (20.4%) were 60-69 years, 162 (57.9%) were 70-79 years and 30 (10.7%) were 80 years or older. The mean IPSS and the number of vascular risk factors were 12.3 and 1.8, respectively. Baseline characteristics of study participants are summarized in Table 1.
TABLE 1.
Clinical characteristics of participants
Values are presented as Mean±SD. IPSS: International Prostate Symptom Score, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, FBS: fasting blood sugar, HDL-C: high-density lipoprotein cholesterol, DM: diabetes mellitus
2. Prevalence and severity of LUTS and vascular risk factors
Of the 280 men, 175 (62.5%) had moderate/severe LUTS (IPSS>7) and 260 (93%) had one or more vascular risk factors.
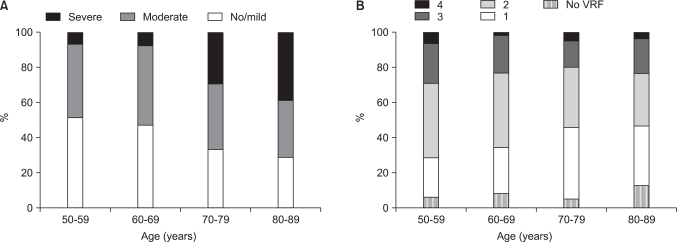
The prevalence of moderate/severe LUTS was 48.4%, 52.6%, 67.3%, and 70%, respectively, among those aged 50 to 59, 60 to 69, 70 to 79, and 80 years or older. The prevalence and severity of LUTS increased statistically significantly with age (Mantel-Haenszel chi-square test, p<0.001) (Fig. 1).
FIG. 1.
(A) Prevalence and severity of lower urinary tract symptoms. (B) Vascular risk factors (VRF) in different age groups of the study population.
In each age group, the prevalence of participants with no vascular risk factors was 6.5%, 8.8%, 5.6%, and 13.3%, that with 1-2 vascular risk factors was 64.5%, 68.4%, 74.7%, and 63.3%, and that with 3-4 vascular risk factors was 29%, 22.8%, 19.7%, and 23.4%, respectively. There was no significant difference in the number of vascular risk factors among the age groups (Mantel-Haenszel chi-square test, p>0.05) (Fig. 1).
3. IPSS according to the number of vascular risk factors
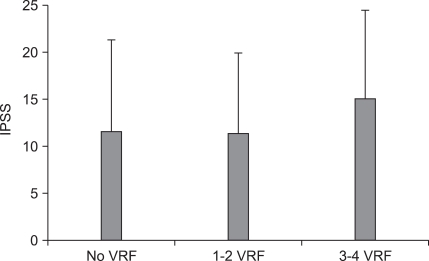
The mean IPSS in men with no vascular risk factors, 1-2 vascular risk factors, and 3-4 vascular risk factors was 11.6±9.7, 11.5±8.5, and 15.1±9.3, respectively and the mean IPSS in men with 3-4 vascular risk factors was significantly higher than that with no or 1-2 vascular risk factors (ANOVA, p<0.05) (Fig. 2).
FIG. 2.
The International Prostate Symptom Score (IPSS) according to the number of vascular risk factors (VRF).
4. Correlation between IPSS and vascular risk factors
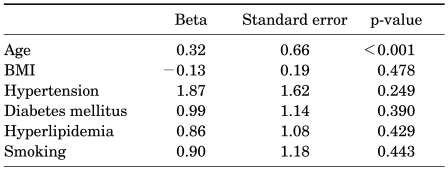
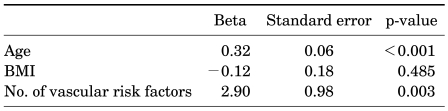
When adjusting for age and body mass index (BMI) in multiple linear regression analysis, none of the individual vascular risk factors, that is, hypertension, DM, hyperlipidemia, or smoking, was shown to be independently correlated with IPSS (Table 2), but the number of vascular risk factors was significantly correlated with IPSS (Beta=2.90, p=0.003) (Table 3).
TABLE 2.
Multiple linear regression analysis of individual vascular risk factors affecting IPSS
IPSS: International Prostate Symptom Score, BMI: body mass index
TABLE 3.
Multiple linear regression analysis of the number of vascular risk factors affecting IPSS
IPSS: International Prostate Symptom Score, BMI: body mass index
5. Association between LUTS and vascular risk factors
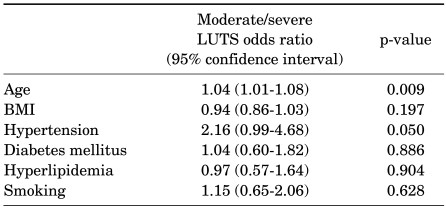
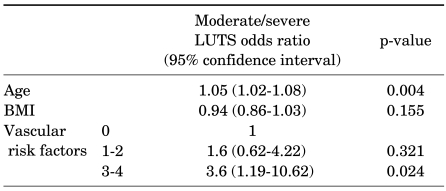
When adjusting for age and BMI in multiple logistic regression analysis, none of the individual vascular risk factors was shown to be associated with the presence of moderate/severe LUTS (Table 4). However, men with 3 or more vascular risk factors were 3 times more likely to have moderate/severe LUTS than men without vascular risk factors (OR=3.6, 95% CI 1.19-10.62, p=0.024) (Table 5).
TABLE 4.
Multiple logistic regression results for relative risk of moderate/severe LUTS by age, BMI, and individual vascular risk factors
LUTS: lower urinary tract symptoms, BMI: body mass index
TABLE 5.
Multiple logistic regression results for relative risk of moderate/severe LUTS by age, BMI, and number of vascular risk factors
LUTS: lower urinary tract symptoms, BMI: body mass index
DISCUSSION
LUTS are relatively common conditions in the field of urology, and it is well established that the prevalence of LUTS increases with age. Rosen et al conducted a large-scale study in 12,815 men aged 50-80 years from six countries [10]. According to this study, the prevalence of moderate/severe LUTS was 22% in men aged 50-59 years, but it was significantly higher (45.3%) in men aged 70-80 years. Also in Korea, according to a study that was conducted in 1,356 men aged between 40 and 79 from Seoul and its suburban areas, the prevalence of moderate/severe LUTS increased with age (10%, 16%, 29%, and 45% in each age group) [11]. Also in our series, as shown in previous reports, the prevalence of moderate/severe LUTS increased significantly with age.
In aging men with LUTS, BOO due to BPH has been considered one of the major causes. As a matter of fact, however, the lack of a relationship between prostate size and LUTS is well established, and BOO was reported to be only a minor determinant of LUTS severity [2,3]. It is therefore presumed that factors beside prostate enlargement and BOO contribute to the development and severity of LUTS.
The pathogenesis of LUTS is considered to be multifactorial, in which age-related changes of bladder structure and function seem to play a central role [12-16]. Vascular diseases such as artherosclerosis and endothelial dysfunction in the pelvic vascular system are one of the possible mechanisms contributing to bladder dysfunction with age [4].
In recent years, many studies have reported that LUTS are closely associated with erectile dysfunction and most of these studies have also presumed that vascular alteration is a cause of the correlation [10,17,18].
Some animal studies have reported that pelvic ischemia causes the structural and functional alteration of the bladder [5-7]. Clinically, hypertension, DM, hyperlipidemia, and smoking are representative risk factors for vascular diseases. These risk factors are known to increase the risk of developing artherosclerosis by 1.8-4.5 times [19,20]. To date, several studies have investigated the correlation between LUTS and these risk factors. Michel et al found a significant age-independent association between the severity of LUTS and the presence of hypertension [21], and Joseph et al conducted a population-based study (Flint Men's Health Study) in 708 men aged 40 years or older. These authors reported that hypertension was a risk factor for LUTS [22]. Unlike these reports, however, there are also some reports that hypertension is not associated with LUTS [23,24]. Also, in our series, there was a closer relationship between moderate/severe LUTS and hypertension than with other vascular risk factors such as DM, hyperlipidemia, and smoking, but these results did not reach a statistical significance (p=0.05). Both DM and smoking were well known risk factors of vascular diseases. Joseph et al and Seim et al reported that these two risk factors were closely associated with LUTS [22,25]. According to Rohrmann et al, however, following the Third National Health and Nutrition Examination Survey (NHANES III) which was conducted in 2,797 men aged 60 years or older, there was no significant correlation between smoking and LUTS [26]. Besides, Ponholzer et al also reported that vascular risk factors such as DM and smoking had no significant correlation with IPSS [27]. In our study, none of the four vascular risk factors was shown to be independently correlated with LUTS. As described here, according to studies on the association between a single vascular risk factor and LUTS, no consistent results have been reported, and based on these results, it is presumed that a single vascular risk factor may be somewhat insufficient to influence LUTS. For this reason, the current study attempted to investigate the association between not only a single vascular risk factor but also the combination of these factors and LUTS.
To date, as shown in the current study, two studies have been conducted to investigate the association between the number of vascular risk factors and LUTS. In association with this, Gibbons et al retrospectively analyzed 237 patients with prostate cancer who underwent radical prostatectomy and thereby evaluated the correlation between the number of vascular risk factors and IPSS. These authors reported that IPSS increased significantly as the number of vascular risk factors increased [28]. According to a recent study examining the correlation between vascular risk factors and LUTS in 1,724 men and 812 women, women with 1 or more vascular risk factors had a higher IPSS than those without a risk factor. In men, however, the IPSS was identical in those with no and 1 vascular risk factor, and increased significantly in those with 2 or more risk factors [27]. These results were similar to our finding that IPSS was significantly higher in men with 3 or more vascular risk factors than those without a risk factor. As described here, the impact of vascular risk factors on LUTS in men was only present in those with more than 2 or 3 risk factors; this might be because BPH plays a important role in the development of LUTS, and this observation might explain the fact that the results of numerous studies assessing the role of single vascular risk factors such as hypertension or smoking status on LUTS in men were contradictory.
Hypertension, DM, and smoking are well known risk factors for vascular diseases such as artherosclerosis. Via different paths from vascular diseases, however, they might also have an impact on LUTS. Increased sympathetic activity and/or α1-adrenoreceptor activity might be a common pathway for both hypertension and LUTS. Increased α1-adrenoreceptor activity might also explain the improvement in LUTS with the use of α1-adrenoreceptor antagonist, despite the lack of objective improvement in BOO. DM may also induce LUTS through detrusor underactivity or sensory neuropathy. Smoking increases sympathetic nervous system activity and may contribute to LUTS via an increase in the tone of the prostate. For this reason, to understand more exactly the potential role of vascular diseases in the development of LUTS, further studies are warranted to investigate the association between LUTS and the direct indicators for vascular diseases such as intima-media thickness of the carotid/iliac artery as well as vascular risk factors.
The current study is a population-based cohort study that was conducted by selecting study participants in accordance with the proportion of population in a relatively larger-scale community-based population. To date, most studies on the association between LUTS and vascular risk factors have also been conducted in a smaller number of patients or those who visited outpatient clinics for a regular medical check-up, which therefore discloses the limitation that their results cannot be generalized.
As described earlier, according to some studies reporting a correlation between LUTS and vascular risk factors, there is a close relationship between the two conditions. But most of these studies, including the current one, have a cross-sectional design based on the statistics collected at a certain point in time. These cross-sectional studies are not sufficient to clarify whether vascular risk factors induce LUTS or whether two different conditions simply occur as a result of the common etiology. That is, a causal relationship between the two conditions remains obscure. To date, no studies have investigated the time-dependent correlations between the two conditions in a single cohort. The current study was based on a cohort study, the Hallym Aging Study about the quality of life in Korean elderly people, which has been conducted by the Institute for Aging Studies at Hallym University from 2003 to the present. Such a longitudinal study will then disclose. This will then disclose a more definite causal relationship between LUTS and vascular risk factors.
CONCLUSIONS
In a population-based cohort study of aging men, men with risk factors for vascular disease are more likely to have moderate/severe LUTS than men with no vascular risk factors and these findings suggest that vascular risk factors play a role in the development of LUTS.
Footnotes
This research was supported by the Hallym University Research Fund, 2002 (HRF-2004-49).
The authors have nothing to disclose.
References
- 1.Kupelian V, Wei JT, O'Leary MP, Kusek JW, Litman HJ, Link CL, et al. BACH Survery Investigators. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381–2387. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology. 2001;58:5–16. doi: 10.1016/s0090-4295(01)01298-5. [DOI] [PubMed] [Google Scholar]
- 3.Barry MJ, Cockett AT, Holtgrewe HL, McConnell JD, Sihelnik SA, Winfield HN. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993;150:351–358. doi: 10.1016/s0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- 4.Tarcan T, Azadzoi KM, Siroky MB, Goldstein I, Krane RJ. Age-related erectile and voiding dysfunction: the role of arterial insufficiency. Br J Urol. 1998;82(Suppl 1):26–33. doi: 10.1046/j.1464-410x.1998.0820s1026.x. [DOI] [PubMed] [Google Scholar]
- 5.Azadzoi KM. Effect of chronic ischemia on bladder structure and function. Adv Exp Med Biol. 2003;539:271–280. doi: 10.1007/978-1-4419-8889-8_19. [DOI] [PubMed] [Google Scholar]
- 6.Shenfeld OZ, Meir KS, Yutkin V, Gofrit ON, Landau EH, Pode D. Do atherosclerosis and chronic bladder ischemia really play a role in detrusor dysfunction of old age? Urology. 2005;65:181–184. doi: 10.1016/j.urology.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 7.Gill HS, Monson FC, Wein AJ, Ruggieri MR, Levin RM. The effects of short-term in-vivo ischemia on the contractile function of the rabbit urinary bladder. J Urol. 1988;139:1350–1354. doi: 10.1016/s0022-5347(17)42917-x. [DOI] [PubMed] [Google Scholar]
- 8.Barry MJ, Fowler FJ, Jr, OLeary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The Measurement Committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 9.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Cho KS, Jo MK, Lim D, Son H, Park SK, Yoo KY, et al. Epidemiologic survey using international prostate symptom score (I-PSS) of lower urinary tract symptoms (LUTS) in elderly men above 40 years old in Seoul area. Korean J Urol. 2001;42:840–848. [Google Scholar]
- 12.Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology. 2003;62:3–10. doi: 10.1016/j.urology.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Elbadawi A, Diokno AC, Millard RJ. The aging bladder: morphology and urodynamics. World J Urol. 1998;16(Suppl 1):S10–S34. doi: 10.1007/pl00014134. [DOI] [PubMed] [Google Scholar]
- 14.Madersbacher S, Pycha A, Schatzl G, Mian C, Klingler CH, Marberger M. The aging lower urinary tract: a comparative urodynamic study of men and women. Urology. 1998;51:206–212. doi: 10.1016/s0090-4295(97)00616-x. [DOI] [PubMed] [Google Scholar]
- 15.Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs FD, Fourcade R, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409–414. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 16.Araki I, Zakoji H, Komuro M, Furuya Y, Fukasawa M, Takihana Y, et al. Lower urinary tract symptoms in men and women without underlying disease causing micturition disorder: a cross-sectional study assessing the natural history of bladder function. J Urol. 2003;170:1901–1904. doi: 10.1097/01.ju.0000092942.87643.27. [DOI] [PubMed] [Google Scholar]
- 17.Ponholzer A, Temml C, Obermayr R, Madersbacher S. Association between lower urinary tract symptoms and erectile dysfunction. Urology. 2004;64:772–776. doi: 10.1016/j.urology.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Ko DS, Jeong JY, Jang SN, Choi YJ, Kim DH, Kim JB, et al. Association between lower urinary tract symptoms and erectile dysfunction in aging men: Hallym Aging Study. Korean J Urol. 2008;49:633–640. [Google Scholar]
- 19.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 20.Murabito JM, D'Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 21.Michel MC, Heemann U, Schumacher H, Mehlburger L, Goepel M. Association of hypertension with symptoms of benign prostatic hyperplasia. J Urol. 2004;172:1390–1393. doi: 10.1097/01.ju.0000139995.85780.d8. [DOI] [PubMed] [Google Scholar]
- 22.Joseph MA, Harlow SD, Wei JT, Sarma AV, Dunn RL, Taylor JM, et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol. 2003;157:906–914. doi: 10.1093/aje/kwg051. [DOI] [PubMed] [Google Scholar]
- 23.Sidney S, Quesenberry C, Jr, Sadler MC, Lydick EG, Guess HA, Cattolica EV. Risk factors for surgically treated benign prostatic hyperplasia in a prepaid health care plan. Urology. 1991;38:13–19. doi: 10.1016/0090-4295(91)80193-b. [DOI] [PubMed] [Google Scholar]
- 24.Glynn RJ, Campion EW, Bouchard GR, Silbert JE. The development of benign prostatic hyperplasia among volunteers in the Normative Aging Study. Am J Epidemiol. 1985;121:78–90. [PubMed] [Google Scholar]
- 25.Seim A, Hoyo C, Ostbye T, Vatten L. The prevalence and correlates of urinary tract symptoms in Norwegian men: the HUNT study. BJU Int. 2005;96:88–92. doi: 10.1111/j.1464-410X.2005.05573.x. [DOI] [PubMed] [Google Scholar]
- 26.Rohrmann S, Crespo CJ, Weber JR, Smit E, Giovannucci E, Platz EA. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: findings from the third National Health And Nutrition Examination Survey. BJU Int. 2005;96:77–82. doi: 10.1111/j.1464-410X.2005.05571.x. [DOI] [PubMed] [Google Scholar]
- 27.Ponholzer A, Temml C, Wehrberger C, Marszalek M, Madersbacher S. The association between vascular risk factors and lower urinary tract symptoms in both sexes. Eur Urol. 2006;50:581–586. doi: 10.1016/j.eururo.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Gibbons EP, Colen J, Nelson JB, Benoit RM. Correlation between risk factors for vascular disease and the American Urological Association Symptom Score. BJU Int. 2007;99:97–100. doi: 10.1111/j.1464-410X.2007.06548.x. [DOI] [PubMed] [Google Scholar]