Abstract
Equine laminitis is a debilitating disease affecting the digital laminae that suspends the distal phalanx within the hoof. While the clinical progression of the disease has been well documented, the molecular events associated with its pathogenesis remain largely unknown. We have investigated the expression of genes coding for proteins containing a Disintegrin and Metalloprotease domain (ADAM), as well as genes encoding the natural inhibitors of these enzymes (Tissue Inhibitor of MetalloProtease; TIMP) in horses with naturally acquired (acute, chronic and aggravated chronic cases collected in clinic) or experimentally-induced (black walnut extract and starch gruel models) laminitis using real time quantitative RT-PCR. Changes in expression of these enzymes and regulators may underlie the pathologic remodeling of lamellar tissue in laminitis. Genes encoding ADAMs involved in inflammation (ADAM-10 and ADAM-17), as well as those implicated in arthritis (ADAMTS-1, ADAMTS-4 and ADAMTS-5) were cloned, and the sequences used to generate specific oligonucleotide primers for the RT-qPCR experiments. Our results show that genes encoding ADAM-10 and 17 were not induced in most laminitic animals whereas ADAMTS-4 gene expression was strongly upregulated in practically all cases of experimentally induced and naturally acquired laminitis. The expression of MMP-9 and ADAMTS-5 was also increased in many of the laminitic horses. In addition, TIMP-2 gene expression was decreased in most laminitic horses, whereas expression of genes encoding other TIMPs, namely TIMP-1 and TIMP-3 was randomly increased or decreased in the various models. We conclude that elevated expression of lamellar ADAMTS-4 is a common feature of laminitis consistent with a central role of the gene product in the pathophysiology of laminitis.
Introduction
Equine laminitis is a debilitating disease that causes acute and often chronic lameness. The disease compromises the integrity of the digital laminae that suspend the horse’s axial skeleton within the hoof. The digital laminae are comprised of a dermal layer attached to the distal phalanx and an epidermal layer connected to the inner surface of the hoof wall. At the interface of the interdigitating layers is a basement membrane that arises from the basal epithelial cells of the epidermal lamellae (Grosenbaugh et al., 1999; Sloet van Oldruitenborgh-Oosterbaan, 1999). A number of events have been characterized that likely contribute to the loss of integrity of the hoof-lamellar attachment interface during laminitis, including a loss of hemidesmosomes, upregulation of proteases, and inflammation associated with systemic leukocyte activation and laminar infiltration (Belknap et al., 2007; Black et al., 2006; Blikslager et al., 2006; Fontaine et al., 2001; French and Pollitt, 2004; Grosenbaugh et al., 1999; Hurley et al., 2006; Johnson et al., 1998; Loftus et al., 2006; Loftus et al., 2007b; Mungall and Pollitt, 1999; Sloet van Oldruitenborgh-Oosterbaan, 1999).
Metalloproteases are the main family of enzymes that control the extracellular environment (Stamenkovic, 2003). They are involved in the processing of growth factors and cytokines by cleaving pro forms from the cell surface as well as through proteolytic modification that can either potentiate or mitigate the bioactivity of these substrates, thus mediating both inflammation and chemotaxis (Blobel, 2005; Garton et al., 2001; Ludwig and Weber, 2007; Schlondorff and Blobel, 1999). Metalloproteases also bind and cleave proteins that constitute the extracellular matrix (ECM). Remodeling of the ECM occurs under normal physiological conditions, for example during embryonic development or vasculogenesis, but also contributes to pathological conditions such as cancer invasion and osteoarthritis (Kahari and Saarialho-Kere, 1999; Ortega et al., 2003; Ravanti and Kahari, 2000; Rundhaug, 2005; Werb and Chin, 1998). The involvement of metalloproteases in tissue pathology has made these enzymes attractive targets for the pharmaceutical industry. However, many clinical trials have shown that broad-spectrum inhibition of metalloproteases can be detrimental to the subject (Clegg et al., 1998; Han, 2006; Hu et al., 2007; Hudson et al., 2006; Malemud, 2006; Planting et al., 2005; Wojtowicz-Praga, 1999; Xue et al., 2006). These results are not unexpected, because while some metalloproteases destroy the ECM proteins, others modulate positive signals that control inflammation as well as collagen deposition (Monaco et al., 2006). Consequently, it is critical to have a complete view of metalloprotease activation during a specific pathology to identify the enzymes that are beneficial versus those that may contribute to pathology. Not only would it be desirable to provide pharmacological inhibition to only the pathologically relevant proteases, it would also be logical to restrict the extent of that inhibition to the affected tissue.
Here we investigate the expression of genes encoding three classes of metalloproteases and their endogenous inhibitors in two experimental models of laminitis, as well as in clinical cases. The first class corresponds to transmembrane metalloproteases of the ADAM family known to process cytokines critical for inflammation (ADAM-10 and 17) (Black et al., 1997; Moss and Lambert, 2002; Sahin et al., 2004; Tanaka et al., 1998). The second class of metalloproteases corresponds to secreted metalloproteases containing thrombospondin repeats (ADAMTS). ADAMTS are responsible for the degradation of proteoglycan in cartilage and contribute to osteoarthritis (Malfait et al., 2002; Nagase and Kashiwagi, 2003; Sugimoto et al., 1999; Tortorella et al., 2001). The last class of metalloproteases is the classical matrix metalloproteases (MMP), which are also secreted and cleave collagen not only during collagen deposition, but also during its removal from the extracellular environment (Little et al., 2002; Stamenkovic, 2003). All of these metalloproteases are inhibited in vivo by Tissue specific Inhibitors of Metalloproteases (TIMP). TIMPs are produced endogenously and maintain the homeostasis of proteolytic activity of a tissue (Amour et al., 2000; Amour et al., 1998; Cross et al., 2005; Hashimoto et al., 2001; Kashiwagi et al., 2001). The metalloproteases and their inhibitors form a dynamic network in which modification of any of the partners is likely to provoke an imbalance resulting in pathological consequences.
Recent work has identified two members of the MMP family, MMP-2 and MMP-9 that are upregulated in laminitis (Johnson et al., 1998; Loftus et al., 2006; Mungall and Pollitt, 1999; Pollitt and Daradka, 1998). The increase of MMP-9 in the BWE model correlates with migration of neutrophils into the laminae (Loftus et al., 2006). Neutrophils store the enzyme in their tertiary granules (Chakrabarti and Patel, 2005) and as such MMP-9 transcription is not necessarily required for the accumulation of this enzyme in tissues harboring migrant neutrophils. In cases of laminitis arising from the carbohydrate overload model and natural disease, MMP-2 is induced and activated in concert with variable amounts of MMP-9 accumulation (Johnson et al., 1998; Mungall and Pollitt, 1999).
To study the relative levels of expression of genes encoding the metalloproteases and their corresponding inhibitors in the digital lamellae during laminitis we designed minimally degenerate primers, cloned and sequenced equine ADAM-10 and 17, as well as ADAMTS-4 and 5 and developed specific primers based on these sequences. We have used samples generated from the laminae of horses treated with black walnut extract (BWE), which promotes a reversible form of laminitis, as well as a model of carbohydrate overload (CHO) that promotes the irreversible form of the disease (Galey et al., 1990; Garner et al., 1975; Thomsen et al., 2000; Uhlinger, 1989). These results were compared to clinical cases of laminitis.
Material and Methods
BWE Samples
Archived samples of laminar tissue from previous studies (Black et al., 2006; Waguespack et al., 2004a; Waguespack et al., 2004b) were used. Briefly, healthy horses were administered either 6 L of water as a control (n = 10) or BWE (n = 10) (2 g heartwood/kg body weight, prepared as described; Eaton et al., 1995) via nasogastric intubation. Horses were euthanized at 1.5 hours post-induction, after a 30 % drop in the baseline blood leukocyte count (3 to 5 hours after BWE), or at the onset of Obel grade 1 lameness (typically around 10–12 hours after BWE). All animal protocols were approved by the Institutional Animal Care and Use Committees (IACUCs) of the Ohio State University or Auburn University.
CHO Samples
Archived samples of lamellae from a previous study (Johnson et al., 2000) were used. Briefly, healthy horses were administered starch at a concentration of 17.6 grams of starch per kg of body weight via a nasogastric tube as described, or water as a control (Johnson et al., 2000). Horses were euthanized when they developed Obel grade 3 lameness (experimental group) typically 36 to 48 hours after administration of starch, or at equivalent time points for control animals. All animal protocols were approved by the IACUC of the University of Missouri, College of Veterinary Medicine Equine Hospital.
Clinical Samples
These were obtained from horses euthanized at the University of Missouri, School of Veterinary Medicine Equine Clinical Center. Animals were grouped as follows: Those presenting “acute clinical laminitis” had not previously shown signs of this disease; those presenting “chronic clinical laminitis” had a history of the condition and were chronically lame but not in an acute episode; those presenting “chronic aggravated laminitis” had a history of the condition and were experiencing a debilitating episode. Control clinical samples were from horses euthanized for a variety of conditions unrelated to laminitis and had no history of laminitis.
Acquisition of Hoof Lamellar Tissues
This procedure has been described (Johnson et al., 2000; Loftus et al., 2006). For BWE and starch-induced laminitis, horses were anesthetized (Loftus et al., 2006; Loftus et al., 2007a), the distal aspect of the right fore limb disarticulated from each horse at the level of the metacarpophalangeal joint and acquisition of lamellar tissue from each hoof accomplished within 5 minutes using a band saw. Horses were euthanized immediately after removal of the hoof. For clinical samples, lamellae were isolated with 10 minutes of euthanasia. Blocks of lamellar tissue (approximately 5 × 5 × 5 mm) were obtained by sharp dissection and either placed in 10% formalin or immediately frozen by submersion in liquid nitrogen and stored at −80° C until processed. In all cases, blocks of lamellar tissue were obtained from the mid-point between the level of the coronary band and the ground-bearing surface on the dorsal aspect of the hoof.
RNA Isolation and cDNA synthesis
Laminar samples from various treatment groups were pulverized using a tissue homogenizer. Total RNA was then extracted from the tissue using the guanidinium isothiocyanate method as described previously (Alfandari et al., 1995). RNA was quantified at OD260 and 1 μg of each sample was run on a 1% agarose gel with 0.1 μg/ml ethidium bromide to ensure RNA integrity. Using 0.5 g of frozen laminar tissue and 10 ml of guanidinium isothiocyanate solution we obtained 25–160 μg of RNA depending on the sample. Ten μg of this RNA was treated by RNAse free DNAse 1 (Promega) prior to cDNA synthesis reaction using oligo dT and MMLV reverse transcriptase in a 50 μl reaction volume (Promega). Before addition of the MMLV, 10 μl of the reaction mix was removed and saved for the no-RT control PCR and to control for complete genomic DNA digestion. This cDNA was then diluted (1:10) in sterile H2O and 1 μl of this dilution was used for each 20 μl RT-qPCR reaction. Total RNA was also extracted from frozen equine testis tissue using a tissue homogenizer. Using 100 mg of tissue we were able to extract approximately 70 μg of total RNA. cDNA synthesis was performed as described for the BWE treated animals. The cDNA for the clinical and carbohydrate overload samples were obtained as previously described (Waguespack et al., 2004a,b).
RT-qPCR and data processing
The quantitative PCR data were obtained using the Roche Lightcycler 2.0, with the Takara SybrGreen mix. Each primer set (Table 1) was tested in order to determine the melting curve of the amplification product, which was then used to determine the optimal fluorescence reading temperature. The reading temperature for each primer set was selected at 2–4°C before complete dissociation of the specific amplification product (Fig. 1). The efficiency of each primer set was measured using a titration curve with serial dilution of a testis cDNA (Table 2). Following each reaction, PCR products were run on 2% agarose gels with 0.1 μg/ml ethidium bromide to confirm that the proper size fragment was amplified for each product. Cycling conditions were as follows: 1 cycle at 95°C for 5 minutes, 45 cycles of 60°C for 30s, 72°C for 30s, a single fluorescence reading at a predetermined temperature, 95° C for 15s, 1 cycle for the melting curve using a stepwise increase of temperature from 65°C to 95°C with a constant fluorescence reading. In order to determine the most stable control gene for comparison using the ΔΔCT method, we used the GeNorm computer program (Ghent University). We tested the expression patterns of glyceraldeyhde 3-phosphate dehydrogenase (GAPDH), β-actin, and β2-microglobulin and the most stable control gene in both the BWE and CHO/Clinical laminitis sets was determined to be GAPDH and was used as our control. Each sample was then run in triplicate and CT values were averaged and analyzed using the ΔΔCT method of analysis (Livak and Schmittgen, 2001). The results for the control animals were averaged separately for animals from the BWE subset (n=3) and animals from the CHO/Clinical laminitis subsets (n=4). ΔΔCT=(CTgeneX − CTGAPDH)Laminitis − (CTgeneX − CTGAPDH)Control.
Table 1.
Primer Pairs used for specific gene amplification and for qRT-PCR
| Forward | Reverse | Size bp | |
|---|---|---|---|
| ADAM10 | CCGTTTCACTCTGTTATTTATCAT | AGGGATTTGTAGGGTCTTTCTCAT | 397 |
| ADAM17 | TGGCAGGACTTCTTCAGCGGACAC | TTTCTTCATTTGGATAACTTTTTG | 571 |
| ADAM-TS1 | TGAAAAGCAGGAAAAAGATGAGAAT | AAGGAGGAACGAATGGTAGGAGGTA | 436 |
| ADAM-TS4 | GCTGTGCTATTGTGGAGGATGATGG | CCAGGGAAAGTCACAGGCAGATG | 507 |
| ADAM-TS5 | ACAGAAGAGAAGCGGCTTAATGTCTTCCA | CCCTCTTTCCTGTGCAGTAGCGGCCATT | 504 |
| TIMP1 | ACCTTACAGCGGCGTTATGAGAT | ATAGGAATGGGAAAGAGGGTGAA | 495 |
| TIMP2 | AGGTGGACTCTGGGAACGACATC | GCTCTTCTTCTGGGTGGTGCTCA | 246 |
| TIMP3 | CCCTTTGGCACACTGGTCTACAC | GAGAAGAATGGCAAAAGCAGGAG | 864 |
| MMP9 | CGCCCCCTGCCACTTCCCCTTCACC | GAGGCGCCCATCACTGCGGCCCTCT | 408 |
| GapDH | TTGTCATCAACGGAAAGGCCATCA | ACGGAAGGCCATGCCAGTGAGCTT | 456 |
| β2-Microglobulin | CAGGTTTACTCACGTCACCC | CTGGTTAGAGGTCTCGATCCC | 240 |
| β-Actin | GGGAAATCGTGCGTGACAT | AGCACTGTGTTGGCGT | 616 |
| qPCR | |||
| ADAM10 | CCGTTTCACTCTGTTATTTATCAT | TCTTCAGGAGTTCTGGACCATTA | 191 |
| ADAM17 | CAGACCATCGCTTTTACAGACAC | TTTCTTCATTTGGATAACTTTTTG | 252 |
| ADAM-TS1 | GGTGCAAGCTCATCTGTCAA | TCCATTTCCTCCGCAAATAC | 194 |
| ADAM-TS4 | GCCTTTGGGGAGACGCTGCTACTA | GATGTGAGCCCCAGGTCCCCCAGC | 282 |
| ADAM-TS5 | AACTGGGGGTCCTGGGGGTCCTGG | CATTTCTTGCCTCACACTGCTCAT | 158 |
| TIMP1 | GTCTCCGGCATTCTGTTGTT | TAGCGGGGGTGTAGACAAAC | 244 |
| TIMP3 | CCCTTTGGCACACTGGTCTACAC | GTTGCAGAGTCCTGTGTACATCTT | 192 |
| MMP9 | CGCCCCCTGCCACTTCCCCTTCACC | CCGTCCTGGGTGTAGAGTTTCTC | 208 |
| β-Actin | CGACATCCGTAAGGACCTGT | GTGGACAATGAGGCCAGAAT | 192 |
| GapDH | GATTGTCAGCAATGCCTCCT | AAGCAGGGATGATGTTCTGG | 194 |
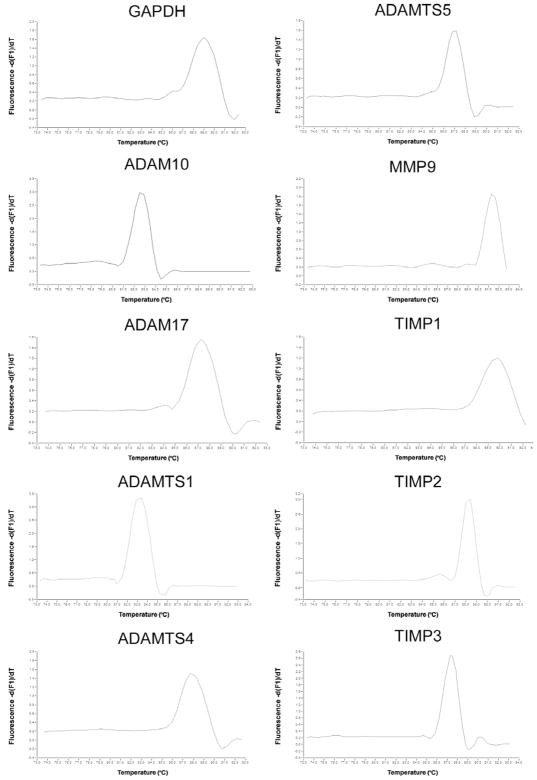
Figure 1. Dissociation curves of the qPCR products.
The graphs present the melting curves of each primer set. The presence of a single peak indicates that a single DNA fragment was amplified. The temperature of melting for each product depends on the size and GC contents. The fluorescence reading is performed 2 to 4 degrees lower than the melting temperature to avoid noise due to primer hybridization.
Table 2.
PCR Amplification Efficiencies
| Efficiency | Slope | R2 | Size | Melting Temp | |
|---|---|---|---|---|---|
| ADAM10 | 99.5 | −3.33 | .923 | 191 | 80 |
| ADAM17 | 89.9 | −3.59 | .884 | 252 | 80 |
| ADAMTS1 | 106.8 | −3.172 | .964 | 194 | 81 |
| ADAMTS4 | 93.7 | −3.482 | .972 | 282 | 86 |
| ADAMTS5 | 102.1 | −3.273 | .903 | 158 | 85 |
| TIMP1 | 89.1 | −3.914 | .926 | 244 | 88 |
| TIMP2 | 88.1 | −3.644 | .991 | 246 | 86 |
| TIMP3 | 91.5 | −3.542 | .972 | 192 | 82 |
| MMP9 | 90.9 | −3.561 | .93 | 208 | 88 |
| GAPDH | 92.9 | −3.505 | .911 | 194 | 87 |
Testis cDNA was diluted in 2 fold increments in sterile H2O from 1:1 to a 1:10 dilution to create templates to use for the generation of a standard curve for each gene tested. Efficiencies between 85–110% along with a slope between −3.0 to −4.0 and an R2 value between 0.90 and 1 were acceptable as being equal in efficiency.
Cloning of ADAMs
Testis cDNA was used as template to amplify ADAM related metalloproteases, as well as TIMPs, by RT-PCR using the minimally degenerate oligonucleotides listed in table II. Cycling conditions for all genes were: 1 cycle at 95°C for 4 minutes, 35 cycles of 94°C 30 seconds, 55°C 30 seconds, 72°C 1 minute, 1 cycle 72°C for 5 minutes. Using equine testis cDNA as a template each gene’s correct product size was amplified. These PCR products were cloned into pCR4-TOPO (Invitrogen) and sequenced either by the University of Massachusetts Amherst genomic facility or the GeneWiz company. These sequences were then analyzed using BLAST and, upon confirmation of each gene’s identity, specific primers were designed for real time quantitative RT-PCR. Degenerate oligonucleotides were also employed to clone full-length constructs of both MMP-9 and ADAMTS-4.
Results
Cloning of ADAM related metalloproteases
ADAMs are a group of cell surface proteins that are known to be involved in inflammatory diseases, cartilage degradation, and embryo development (Alfandari et al., 2001; Cousin et al., 2000; Moss and Lambert, 2002; Nagase and Kashiwagi, 2003). In order to study their potential role in equine laminitis, the equine orthologs of ADAM-10, ADAM-17, ADAMTS-4, and ADAMTS-5 expressed genes were cloned. We performed a multiple sequence alignment for each gene between several mammalian species in order to determine regions of homology at the protein level. Minimally degenerate oligonucleotide primers were then designed for the most homologous regions of each gene. In order to clone these genes, cDNA was made from equine testis tissue as most ADAM identified at present are expressed in this tissue (Kim et al., 2006; Wolfsberg et al., 1995). Each primer set amplified their expected fragments, which were cloned into pCR4-TOPO (Invitrogen) for sequencing. The complete ADAMTS-4 sequence was obtained. The expressed gene fragments obtained for ADAM-10 and 17 correspond to part of the propeptide and metalloprotease domains, but do not include the active site. The expressed gene fragment obtained for ADAMTS-5 includes part of the metalloprotease domain with the active site, the disintegrin and the first thombospondin repeat (TSP1). A comparison of obtained sequences with their human counterparts showed significant homology i.e., 95% homology for equine ADAM-10, 91% homology for equine ADAM-17, 90.8% homology for ADAMTS-4, and 91% homology for ADAMTS-5. Specific primers were then designed to analyze gene expression in the lamellae, and their specific fragments are shown in figure 2. The positions of the degenerate and specific primers are indicated in Tables 1 and 3 in the material and methods. For each primer set, PCR amplification was performed on no-RT controls, genomic DNA (not shown) and cDNA samples to assure that no interference with genomic DNA could influence the quantification results. A typical result of these controls is presented in figure 2 obtained from a sample from the 12h time point in the BWE model. All of these sequences are now available on NCBI and their accession numbers are: ADAMTS-4; EU025848, ADAMTS-5; EU025851, ADAM-10; EU025849, ADAM-17; EU025850, MMP-9; EU025852. In addition, we used sequences already deposited in the NCBI library for TIMP-1 (U95039), TIMP-2 (EF077283), TIMP-3 (NM001081870), and ADAMTS-1 (XM001496488).
Figure 2. PCR amplification using real time oligonucleotide primers.
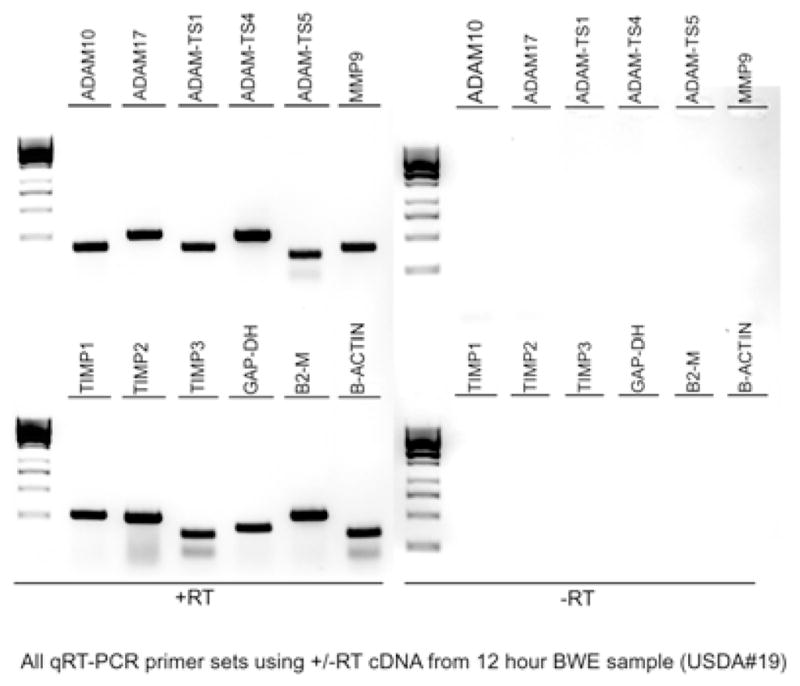
Total RNA from the laminae of one horse treated with the BWE for 12h (USDA#19) was used to generate cDNA. Specific primers designed for the Real Time PCR experiments were tested for their ability to amplify the expected size fragments in 35 cycles. A representative image of a 2% gel is shown. Similar amplification experiments were also performed on total RNA processed identically but without the addition of reverse transcriptase (−RT) and was performed for each set of cDNA.
Table 3.
Cloning Primers
| Forward | Reverse | Size in bp | |
|---|---|---|---|
| ADAM10 | TAAATAAATACATTAGACATTATGAAGGAT | GAGAGSCCATAGTTCTGAACAGTRATRATT | 1032 |
| ADAM 17 | TCAGCYYTGMAAAGGCAYTTTAAATTATACTT | TAAGCYARTCCAAGWGTTCCCATATCAAAATC | 805 |
| ADAM-TS4 | CACCCTTGGGKATGGCWGAYGTKGGCACMRT | TTGCATYGGTCTCGRGGGGMCACWCCTGWR | 868 |
| ADAM-TS5 | ATTTATGTGGGCATCATTCATGTGACYACY | AGACTGATARCCATTYYTKGCYTTCACACTG | 817 |
| FL MMP9 deg | ATGAGCCYCYKGCAGCCCYTGGT | CTAGTCCTCAGGGCACTMCAGGA | 2288 |
| FL MMP9 spec. | ATGAGCCCCTGGCAGCCCTTGGTCC | GACCCCTAGTCCTCAGGGCACT | 2288 |
| FL ADAM-TS4 deg | ATGTCCCASAYRGRCTCGCATCC | GCCGGGATTGTGAGGTTATTTCC | 2514 |
| FL ADAM-TS4 spec. | ATGTCCCAGATAGGCTCGCATCCC | GCCGGGATTGTGAGGTTATTTCC | 2514 |
Gene Expression
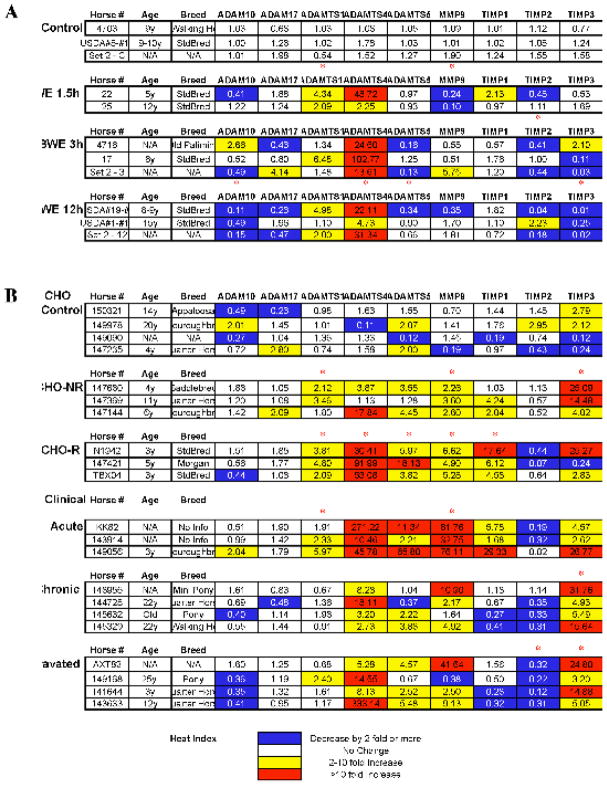
Laminar samples were obtained, as described in the materials and methods, from black walnut extract (BWE) treated (Belknap et al., 2007; Loftus et al., 2006) as well as carbohydrate (CHO) treated horses (Johnson et al., 2000). Both treatments have previously been shown to induce laminitis (Galey et al., 1991; Garner et al., 1975; Thomsen et al., 2000; Uhlinger, 1989). We also obtained samples from clinical cases of laminitis that are classified as acute, chronic mild or aggravated chronic laminitis. Selected gene expression was tested by real time quantitative RT-PCR using GAPDH, β-actin and β2 microglobulin as control for mRNA quantity and integrity. Because the number of horses for each treatment is low (2 to 4), and the expression of each gene can vary significantly, we have chosen to present the results of individual horses (Fig. 3) as well as the average for each treatment (Fig. 4). In figure 3, each line represents a single horse, and horses with similar classifications are grouped. Each cell in the figure corresponds to the mean of triplicates and represents the fold change for each gene when compared to mean values from control horses for the corresponding gene (see methods). The housekeeping gene GAPDH was found to be the most constant across the various treatments and was therefore used to normalize our data (geNorm, Ghent University). To facilitate the interpretation of the results, decreases of 2 fold or more (0.5 or less) are indicated by a blue background, increases of 2 to 10 fold are indicated by a yellow background, while increases of more than 10 fold are in red. For this study we have considered that an increase, or decrease, of less than two fold is not significant. Also, due to the large variation within a treatment group and the small number of horses, many of the observed trends are not found to be significant in a t test. For example, ADAMTS4 expression after 3 hours of BWE treatment is increased between 13.61 and 102.77 fold, thus appearing as not statistically significant. Finally, it is important to note that in general we found that for equal amounts of total RNA, as measured by the Optical Density at 260 nm, PCR amplification for all genes was increased in laminae from treated horses when compared to controls suggesting that the proportion of mRNA may be increased in these samples. These variations were eliminated by the use of the normalizing gene GAPDH.
Figure 3. Real time RT-PCR data analysis.
Real time RT-PCR was performed on samples of cDNA from 32 horses. Each gene-specific oligonucleotide pair was run in triplicate on three different days on a ROCHE light cycler 2.0. The relative value for each sample was obtained using the ΔΔCT method, which normalizes both to the control sample and a selected control gene (GAPDH). Cells highlighted in yellow correspond to a 2 to 10 fold increase. Cells with values greater than 10 are in red and correspond to an increase of more than 10 fold. Cells with values inferior to 0.5 are highlighted in blue and correspond to a 2 fold or more decrease. The horse number, age and breed, as well as the results for each of the selected genes are presented. A red asterisk above a specific treatment indicates statistical significance (t test p<0.05). Many variations that appear biologically relevant (All horses with a large increase) may not appear statistically significant because of the amplitude of variation within a treatment. BWE; Black Walnut Extract, CHO; Carbohybdrate Overload.
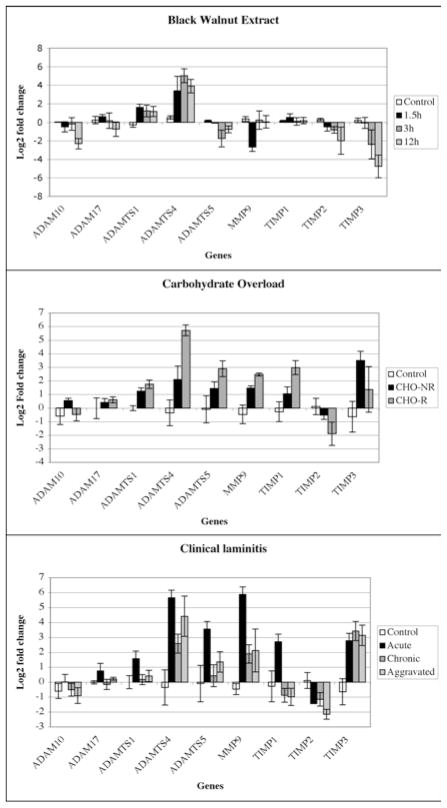
Figure 4. Average gene expression.
The relative gene expression data from individual horses were averaged and are presented in the histogram as the Log2 fold change. A value of zero corresponds to no change. Negative values represent a decrease and positive values an increase. The error bars correspond to the standard error calculated from the standard deviation of the mean.
General observations
The results show that expression of genes encoding ADAMs associated with inflammation, such as ADAM-10 and ADAM-17 (TNF-α Converting Enzyme; TACE), is not substantially increased in horses with laminitis independent of the mode of induction and the extent of the disease (n=25 horses). In fact, in several cases we observed a decrease of ADAM-10 gene expression with or without a similar decrease in ADAM-17 expression.
At the other end of the spectrum, our results show that in most horses with laminitis, ADAMTS-4 gene expression was dramatically increased (n=24 out of 25). The level of gene induction varied widely from a 2 to 333 fold increase. The most consistent and important increase (over 10 fold) was observed in both CHO-induced laminitis with response (CHO-R), and clinical acute cases. While elevated ADAMTS-4 gene expression is associated with advanced stages of laminitis in these horses, increased expression of this ADAM was also observed in horses treated with BWE after only 1.5 hours, suggesting that ADAMTS-4 gene expression is induced before any obvious lameness can be observed and is maintained throughout the progression of the disease. Another general finding is that TIMP-2 gene expression was decreased in 17 out of 25 horses with laminitis. The association is even greater in horses with signs of lameness (BWE 12h, CHO-Reactive, all clinical). In this case, decreased expression was found in 13 out of 17 horses. In contrast, TIMP-1 and TIMP-3 gene expression were found randomly increased or decreased in the various models. Significantly, the CHO treated horse samples were tested for the presence of MMP-9 protein by zymography (Loftus et al., submitted) and these results corroborate our results on mRNA expression (Fig. 3, CHO) showing an association between gene and protein expression in this instance.
Specific findings
In the BWE model (Fig. 3 and 4, BWE), ADAMTS-5 gene expression was decreased rather than increased (n=8, 3 decreased, 4 level, 0 increased). In fact for this model, ADAMTS-4 was the only gene that was consistently induced. Interestingly, following BWE treatment, MMP-9 gene expression was initially slightly reduced (1.5), while TIMP-2 was reduced in 5 out of 8 horses at all time points. TIMP-3 was also reduced in 5 out of 8 horses at the onset of lameness.
In the CHO model of laminitis (Fig. 3 and 4, CHO), we found two types of responses based on whether or not lameness was induced. Three horses presented no signs of lameness (CHO-NR), while the other three tested had developed acute laminitis (CHO-R). The lame horses showed elevated levels of ADAMTS-1, 4, 5, MMP-9 and TIMP-1 and a decrease in TIMP-2 gene expression. In contrast, CHO-treated horses without lameness, exhibited levels of MMP-9 gene expression that were comparable to responding animals, however, ADAMTS-4 was not induced as strongly and TIMP-3 was increased in all three CHO-NR horses. These results suggest that elevated expression of genes encoding both MMP-9 and ADAMTSs, particularly ADAMTS-4, may be linked to the development of severe laminitis. While expression of genes encoding TIMP-1 and TIMP-3 were either unaffected or increased in both CHO and natural laminitis, expression of the gene encoding TIMP-2 was often reduced (n=11 out of 17). In addition, the CHO non-responder horses did not show a significant decrease in lamellar TIMP-2 gene expression.
The clinical cases of laminitis (Fig. 3 and 4, Clinical Acute) share the best similarity of gene expression with the CHO model. Specifically, all horses showed elevated expression of genes encoding ADAMTS-4, ADAMTS-5 and MMP-9. From the clinical cases two more categories were made from horses with chronic lameness and either a chronic (4 horses) or chronic aggravated (3 horses) affliction. In the aggravated category, all horses showed an elevated expression of the gene encoding ADAMTS-4 and a decreased expression of the TIMP-2 gene. Similar to the Clinical acute, expression of both the ADAMTS-5 and MMP-9 genes was significantly increased in 3 out of the 4 horses (the same horse lacked increased expression of both genes). Interestingly in the Chronic group, the only horse that showed no decrease in TIMP-2 gene expression had an 11-fold increase in MMP-9 gene expression, suggesting that increased MMP-9 activity achieved either by elevated expression or reduced inhibition may participate in the development of laminitis.
Discussion
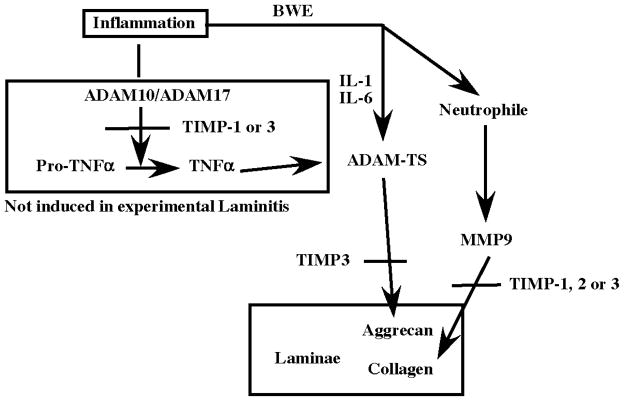
We have investigated the expression of genes encoding ADAM related metalloproteases and their natural inhibitors (TIMPs) in samples of digital lamellae collected from horses with experimentally-induced laminitis (BWE and CHO models) and from clinical cases. We have found that expression of genes encoding ADAM-10 and 17, which are known to participate in the inflammatory cascade by activating cytokines, was not induced. We also found that in practically all cases of laminitis, expression of the gene encoding aggrecanase, ADAMTS-4, was dramatically increased. Our results suggest that while ADAMTS-4 is likely necessary for development of laminitis, it is not sufficient to promote the full irreversible form of laminitis. In this regard, in horses with the most severe form of the disease, genes encoding ADAMTS-5 and MMP-9 were also induced, while that encoding the inhibitor TIMP-2 was reduced. We propose: i) that laminitis is primed by an excess of ADAMTS-4, which could release aggrecan or another target proteoglycan, thus, facilitating the access of MMPs to the basal lamina components and ii) when elevated expression of ADAMTS-4 is combined with increased MMP-9 and a decrease of TIMP-2, the proteolytic cascade results in the destruction of the laminae and the progression of the disease (Fig. 5).
Figure 5. Schematic diagram of ADAM, ADAMTS, MMP and TIMP potential interaction in Horse laminitis.
The experimental models of laminitis both appear to stimulate cytokines responsible for inflammation. This does not stimulate the production of TNFα by ADAM-10 or 17 sheddases. In contrast IL-1 and IL-6 are increased and are known to induce the production of aggrecanase (ADAMTS-4) in this case. ADAMTS-4 in turn cleaves aggrecan (or other proteoglycans), making the lamellae less resistant to load pressure. At the same time, leukocyte invasion is initiated and sustained by maintained inflammation due to the constant pressure produced by the weight of the horse. Leukocyte production of MMPs is then responsible for the destruction of the collagen previously exposed by the degradation of aggrecan. This model suggests that preventing ADAMTS-4 activation or function early may prevent the development of the disease. Once this first step is completed, inhibiting MMP should be able to reduce or stop disease progression. It would therefore be critical to inhibit ADAMTS-4 during the first hours of clinical signs.
Previous studies have shown that MMP-9 and MMP-2 are induced during the progression of laminitis. While only MMP-9 is consistently induced using BWE up to Obel grade 1 lameness (Loftus et al., 2006; Loftus et al., 2007b), both MMPs are increased in the CHO model (Kyaw-Tanner and Pollitt, 2004). Our results confirm elevated MMP-9 gene expression following CHO treatment, but are not as clear for the BWE model despite a significant elevation in lamellar MMP-9 protein in this condition as a result of neutrophil recruitment. Failure to detect elevated expression of the gene encoding MMP-9 in the lamellae of BWE-treated horses may be due to the choice of normalizing gene (GAPDH in our study compared to β2-microglobulin and β-actin in the study of (Loftus et al., 2006). Our method of normalization tends to reduce differences so that only the strongest variations are visible. Nevertheless, we have found that the results obtained for MMP-9 expression in the CHO model, using the same horses, are in complete agreement with the results obtained for the MMP-9 protein by Loftus and colleagues (Submitted), validating this method. Interestingly, in most horses, and independent of the mode of induction of laminitis, we find that TIMP-2 gene expression is reduced. TIMP-2 inhibits both MMP-2 and MMP-9 (Gomez et al., 1999; Nagase et al., 2006), consequently, even in the absence of a significant increase in MMP mRNA, the activity of the MMP may be increased.
We initially studied ADAM-10 and 17 gene expression because these metalloproteinases can process TNFα, a pro-inflammatory cytokine that could play a role in the initiation of laminitis. Surprisingly, our results show no increase in either ADAM-10 or ADAM-17 gene expression in the laminae of horses independent of the treatment. In addition, the increase of TIMP-1 and 3 gene expression in many of the horses with acute laminitis suggests that the overall activity of ADAM-10 and 17 is in fact likely reduced. Although unexpected, these results are in accordance with a previous study showing that while genes encoding many pro-inflammatory cytokines are increased in laminitis, expression of the gene encoding TNFα, is not increased (Belknap et al., 2007).
ADAMTS-4 is one of the main proteins involved in both physiological cartilage remodeling and also in pathology associated with osteoarthritis (Malfait et al., 2002; Song et al., 2007). In the cartilage, ADAMTS-4 and 5 cleave the proteoglycan aggrecan. Aggrecan functions to provide mechanical resistance to compressive loads. It does so by its ability to attract and retain water via its long glycosylated side chains. It is important to determine whether aggrecan is present in the horse lamellae and performs a similar function to that in cartilage. Interestingly, an earlier study using Periodic acid-Schiff (PAS) staining in the basal membrane of the laminae showed a significant decrease during CHO induced laminitis, suggesting that glycoproteins and proteoglycans may be lost during the progression of the disease (Pollitt, 1996). In cartilage, ADAMTS-4 is induced by several cytokines including IL-1, IL-6 and TNFα (Tortorella et al., 2001), as well as by fragments of fibronectin (Homandberg et al., 1992; Stanton et al., 2002). Both Il-1β and IL-6 genes are induced during BWE-induced and oligofructose-induced laminitis (Belknap et al., 2007), and thus, may contribute to the induction of ADAMTS-4 in this disease. Our results show a significant increase in ADAMTS-4 gene expression during both the early and late phases of laminitis, suggesting that it could be one of the early response genes activated following “stimuli” that promotes the disease. If so, it would be an attractive target for therapeutic intervention. The most effective endogenous inhibitor of ADAMTS-4 is TIMP-3, while other TIMPs have a greater affinity for the MMPs (Clegg et al., 1998; Cross et al., 2005; Kashiwagi et al., 2001). While there is some association in the BWE model between treated horses and a decrease of TIMP-3 gene expression (6/8 treated horse, 0/3 untreated), this is not found in either the CHO or the clinical laminitis cases where TIMP-3 gene expression is either stable or increased. Furthermore, the increase of TIMP-3 gene expression is not associated with a better prognosis for the affected horse.
While aggrecan protects collagen from degradation by the MMPs, other ECM proteins such as fibronectin and thrombospondin also interfere with ADAMTS-4 access and degradation of aggrecan (Hashimoto et al., 2004; Tortorella et al., 2000). Thus both ADAMTS-4 and MMPs may need to be activated to promote the full development of laminitis. Fortunately, small molecule inhibitors like hydroxamate are capable of inhibiting both ADAMTS and MMP (Noe et al., 2005a; Noe et al., 2005b; Pollitt et al., 1998; Sugimoto et al., 1999) and may therefore, if administered both locally and during a critical window, be able to prevent the development of laminitis. The study of the ADAMTS-4 protein will be a critical step to test whether the zymogen or the active protease is expressed and to measure increases at the protein level, rather than mRNA. Similarly, it may be interesting to detect the protein in situ to determine which structures it is associated with. Finally, the detection and measurement of aggrecan fragments may provide the best route to test ADAMTS-4 activity in horses with laminitis (Sugimoto et al., 1999).
Acknowledgments
This work was supported by the cooperative State Research Education Service, U.S. Department of Agriculture, Massachusetts Agricultural Experimental Station, under project No, MAS00907/MAS00904, the United States Public Health Service Grant DE016289, the USDA grant NRI-CSREES 2007-01350, the American Quarter Horse Association and the Animal Health Foundation of St. Louis.
References
- Alfandari D, Cousin H, Gaultier A, Smith K, White JM, Darribere T, DeSimone DW. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr Biol. 2001;11:918–930. doi: 10.1016/s0960-9822(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Whittaker CA, DeSimone DW, Darribere T. Integrin alpha v subunit is expressed on mesodermal cell surfaces during amphibian gastrulation. Dev Biol. 1995;170:249–261. doi: 10.1006/dbio.1995.1212. [DOI] [PubMed] [Google Scholar]
- Amour A, Knight CG, Webster A, Slocombe PM, Stephens PE, Knauper V, Docherty AJ, Murphy G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473:275–279. doi: 10.1016/s0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knauper V, Docherty AJ, Murphy G. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Giguere S, Pettigrew A, Cochran AM, Van Eps AW, Pollitt CC. Lamellar pro-inflammatory cytokine expression patterns in laminitis at the developmental stage and at the onset of lameness: innate vs. adaptive immune response. Equine Vet J. 2007;39:42–47. doi: 10.2746/042516407x155406. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Black SJ, Lunn DP, Yin C, Hwang M, Lenz SD, Belknap JK. Leukocyte emigration in the early stages of laminitis. Vet Immunol Immunopathol. 2006;109:161–166. doi: 10.1016/j.vetimm.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Blikslager AT, Yin C, Cochran AM, Wooten JG, Pettigrew A, Belknap JK. Cyclooxygenase expression in the early stages of equine laminitis: a cytologic study. J Vet Intern Med. 2006;20:1191–1196. doi: 10.1892/0891-6640(2006)20[1191:ceites]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol. 2005;78:279–288. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- Clegg PD, Jones MD, Carter SD. The effect of drugs commonly used in the treatment of equine articular disorders on the activity of equine matrix metalloproteinase-2 and 9. J Vet Pharmacol Ther. 1998;21:406–413. doi: 10.1046/j.1365-2885.1998.00157.x. [DOI] [PubMed] [Google Scholar]
- Cousin H, Gaultier A, Bleux C, Darribere T, Alfandari D. PACSIN2 is a regulator of the metalloprotease/disintegrin ADAM13. Dev Biol. 2000;227:197–210. doi: 10.1006/dbio.2000.9871. [DOI] [PubMed] [Google Scholar]
- Cross NA, Chandrasekharan S, Jokonya N, Fowles A, Hamdy FC, Buttle DJ, Eaton CL. The expression and regulation of ADAMTS-1, -4, -5, -9, and -15, and TIMP-3 by TGFbeta1 in prostate cells: relevance to the accumulation of versican. Prostate. 2005;63:269–275. doi: 10.1002/pros.20182. [DOI] [PubMed] [Google Scholar]
- Fontaine GL, Belknap JK, Allen D, Moore JN, Kroll DL. Expression of interleukin-1beta in the digital laminae of horses in the prodromal stage of experimentally induced laminitis. Am J Vet Res. 2001;62:714–720. doi: 10.2460/ajvr.2001.62.714. [DOI] [PubMed] [Google Scholar]
- French KR, Pollitt CC. Equine laminitis: loss of hemidesmosomes in hoof secondary epidermal lamellae correlates to dose in an oligofructose induction model: an ultrastructural study. Equine Vet J. 2004;36:230–235. doi: 10.2746/0425164044877125. [DOI] [PubMed] [Google Scholar]
- Galey FD, Beasley VR, Schaeffer D, Davis LE. Effect of an aqueous extract of black walnut (Juglans nigra) on isolated equine digital vessels. Am J Vet Res. 1990;51:83–88. [PubMed] [Google Scholar]
- Galey FD, Whiteley HE, Goetz TE, Kuenstler AR, Davis CA, Beasley VR. Black walnut (Juglans nigra) toxicosis: a model for equine laminitis. J Comp Pathol. 1991;104:313–326. doi: 10.1016/s0021-9975(08)80043-6. [DOI] [PubMed] [Google Scholar]
- Garner HE, Coffman JR, Hahn AW, Hutcheson DP, Tumbleson ME. Equine laminitis of alimentary origin: an experimental model. Am J Vet Res. 1975;36:441–444. [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- Gomez DE, De Lorenzo MS, Alonso DF, Andrade ZA. Expression of metalloproteinases (MMP-1, MMP-2, and MMP-9) and their inhibitors (TIMP-1 and TIMP-2) in schistosomal portal fibrosis. Am J Trop Med Hyg. 1999;61:9–13. doi: 10.4269/ajtmh.1999.61.9. [DOI] [PubMed] [Google Scholar]
- Grosenbaugh DA, Morgan SJ, Hood DM. The digital pathologies of chronic laminitis. Vet Clin North Am Equine Pract. 1999;15:419–436. doi: 10.1016/s0749-0739(17)30153-0. [DOI] [PubMed] [Google Scholar]
- Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S88–91. doi: 10.1111/j.1440-1746.2006.04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto G, Aoki T, Nakamura H, Tanzawa K, Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4) FEBS Lett. 2001;494:192–195. doi: 10.1016/s0014-5793(01)02323-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Shimoda M, Okada Y. ADAMTS4 (aggrecanase-1) interaction with the C-terminal domain of fibronectin inhibits proteolysis of aggrecan. J Biol Chem. 2004;279:32483–32491. doi: 10.1074/jbc.M314216200. [DOI] [PubMed] [Google Scholar]
- Homandberg GA, Meyers R, Xie DL. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J Biol Chem. 1992;267:3597–3604. [PubMed] [Google Scholar]
- Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- Hurley DJ, Parks RJ, Reber AJ, Donovan DC, Okinaga T, Vandenplas ML, Peroni JF, Moore JN. Dynamic changes in circulating leukocytes during the induction of equine laminitis with black walnut extract. Vet Immunol Immunopathol. 2006;110:195–206. doi: 10.1016/j.vetimm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Kreeger JM, Keeler M, Ganjam VK, Messer NT. Serum markers of lamellar basement membrane degradation and lamellar histopathological changes in horses affected with laminitis. Equine Vet J. 2000;32:462–468. doi: 10.2746/042516400777584695. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Tyagi SC, Katwa LC, Ganjam VK, Moore LA, Kreeger JM, Messer NT. Activation of extracellular matrix metalloproteinases in equine laminitis. Vet Rec. 1998;142:392–396. doi: 10.1136/vr.142.15.392. [DOI] [PubMed] [Google Scholar]
- Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Kim T, Oh J, Woo JM, Choi E, Im SH, Yoo YJ, Kim DH, Nishimura H, Cho C. Expression and relationship of male reproductive ADAMs in mouse. Biol Reprod. 2006;74:744–750. doi: 10.1095/biolreprod.105.048892. [DOI] [PubMed] [Google Scholar]
- Kyaw-Tanner M, Pollitt CC. Equine laminitis: increased transcription of matrix metalloproteinase-2 (MMP-2) occurs during the developmental phase. Equine Vet J. 2004;36:221–225. doi: 10.2746/0425164044877242. [DOI] [PubMed] [Google Scholar]
- Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery CR, Caterson B. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271–288. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loftus JP, Belknap JK, Black SJ. Matrix metalloproteinase-9 in laminae of black walnut extract treated horses correlates with neutrophil abundance. Vet Immunol Immunopathol. 2006;113:267–276. doi: 10.1016/j.vetimm.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Loftus JP, Belknap JK, Stankiewicz KM, Black SJ. Laminar xanthine oxidase, superoxide dismutase and catalase activities in the prodromal stage of black-walnut induced equine laminitis. Equine Vet J. 2007a;39:48–53. doi: 10.2746/042516406x151320. [DOI] [PubMed] [Google Scholar]
- Loftus JP, Black SJ, Pettigrew A, Abrahamsen EJ, Belknap JK. Early laminar events involving endothelial activation in horses with black walnut- induced laminitis. Am J Vet Res. 2007b;68:1205–1211. doi: 10.2460/ajvr.68.11.1205. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Weber C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007;97:694–703. [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- Monaco S, Sparano V, Gioia M, Sbardella D, Di Pierro D, Marini S, Coletta M. Enzymatic processing of collagen IV by MMP-2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci. 2006;15:2805–2815. doi: 10.1110/ps.062430706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss ML, Lambert MH. Shedding of membrane proteins by ADAM family proteases. Essays Biochem. 2002;38:141–153. doi: 10.1042/bse0380141. [DOI] [PubMed] [Google Scholar]
- Mungall BA, Pollitt CC. Zymographic analysis of equine laminitis. Histochem Cell Biol. 1999;112:467–472. doi: 10.1007/s004180050430. [DOI] [PubMed] [Google Scholar]
- Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Noe MC, Natarajan V, Snow SL, Mitchell PG, Lopresti-Morrow L, Reeves LM, Yocum SA, Carty TJ, Barberia JA, Sweeney FJ, Liras JL, Vaughn M, Hardink JR, Hawkins JM, Tokar C. Discovery of 3,3-dimethyl-5-hydroxypipecolic hydroxamate-based inhibitors of aggrecanase and MMP-13. Bioorg Med Chem Lett. 2005a;15:2808–2811. doi: 10.1016/j.bmcl.2005.03.105. [DOI] [PubMed] [Google Scholar]
- Noe MC, Natarajan V, Snow SL, Wolf-Gouveia LA, Mitchell PG, Lopresti-Morrow L, Reeves LM, Yocum SA, Otterness I, Bliven MA, Carty TJ, Barberia JT, Sweeney FJ, Liras JL, Vaughn M. Discovery of 3-OH-3-methylpipecolic hydroxamates: potent orally active inhibitors of aggrecanase and MMP-13. Bioorg Med Chem Lett. 2005b;15:3385–3388. doi: 10.1016/j.bmcl.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Ortega N, Behonick D, Stickens D, Werb Z. How proteases regulate bone morphogenesis. Ann N Y Acad Sci. 2003;995:109–116. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Planting A, van der Gaast A, Schoffski P, Bartkowski M, Verheij C, Noe D, Ferrante K, Verweij J. A phase I and pharmacologic study of the matrix metalloproteinase inhibitor CP-471,358 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2005;55:136–142. doi: 10.1007/s00280-004-0905-z. [DOI] [PubMed] [Google Scholar]
- Pollitt CC. Basement membrane pathology: a feature of acute equine laminitis. Equine Vet J. 1996;28:38–46. doi: 10.1111/j.2042-3306.1996.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Pollitt CC, Daradka M. Equine laminitis basement membrane pathology: loss of type IV collagen, type VII collagen and laminin immunostaining. Equine Vet J Suppl. 1998:139–144. doi: 10.1111/j.2042-3306.1998.tb05133.x. [DOI] [PubMed] [Google Scholar]
- Pollitt CC, Pass MA, Pollitt S. Batimastat (BB-94) inhibits matrix metalloproteinases of equine laminitis. Equine Vet J Suppl. 1998:119–124. doi: 10.1111/j.2042-3306.1998.tb05130.x. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112 (Pt 21):3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- Sloet van Oldruitenborgh-Oosterbaan MM. Laminitis in the horse: a review. Vet Q. 1999;21:121–127. doi: 10.1080/01652176.1999.9695006. [DOI] [PubMed] [Google Scholar]
- Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- Stanton H, Ung L, Fosang AJ. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem J. 2002;364:181–190. doi: 10.1042/bj3640181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Takahashi M, Yamamoto Y, Shimada K, Tanzawa K. Identification of aggrecanase activity in medium of cartilage culture. J Biochem (Tokyo) 1999;126:449–455. doi: 10.1093/oxfordjournals.jbchem.a022471. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin Arthritis Rheum. 1998;27:392–399. doi: 10.1016/s0049-0172(98)80019-x. [DOI] [PubMed] [Google Scholar]
- Thomsen ME, Davis EG, Rush BR. Black walnut induced laminitis. Vet Hum Toxicol. 2000;42:8–11. [PubMed] [Google Scholar]
- Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, Arner E. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem. 2000;275:25791–25797. doi: 10.1074/jbc.M001065200. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- Uhlinger C. Black walnut toxicosis in ten horses. J Am Vet Med Assoc. 1989;195:343–344. [PubMed] [Google Scholar]
- Waguespack RW, Cochran A, Belknap JK. Expression of the cyclooxygenase isoforms in the prodromal stage of black walnut-induced laminitis in horses. Am J Vet Res. 2004a;65:1724–1729. doi: 10.2460/ajvr.2004.65.1724. [DOI] [PubMed] [Google Scholar]
- Waguespack RW, Kemppainen RJ, Cochran A, Lin HC, Belknap JK. Increased expression of MAIL, a cytokine-associated nuclear protein, in the prodromal stage of black walnut-induced laminitis. Equine Vet J. 2004b;36:285–291. doi: 10.2746/0425164044877099. [DOI] [PubMed] [Google Scholar]
- Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann N Y Acad Sci. 1998;857:110–118. doi: 10.1111/j.1749-6632.1998.tb10111.x. [DOI] [PubMed] [Google Scholar]
- Wojtowicz-Praga S. Clinical potential of matrix metalloprotease inhibitors. Drugs R D. 1999;1:117–129. doi: 10.2165/00126839-199901020-00001. [DOI] [PubMed] [Google Scholar]
- Wolfsberg TG, Straight PD, Gerena RL, Huovila AP, Primakoff P, Myles DG, White JM. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev Biol. 1995;169:378–383. doi: 10.1006/dbio.1995.1152. [DOI] [PubMed] [Google Scholar]
- Xue M, Le NT, Jackson CJ. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets. 2006;10:143–155. doi: 10.1517/14728222.10.1.143. [DOI] [PubMed] [Google Scholar]