Abstract
Electrodermal screening (EDS) is based on three commonly held assumptions: acupuncture points (APs) have lower electrical resistance than non-APs; resistance at APs varies with health and disease; and effective acupuncture treatments are associated with normalization of resistance at APs. Although evidence confirming these assumptions is limited, EDS is frequently practiced worldwide. Researchers are also beginning to assess EDS' utility as an outcome measure in acupuncture trials. Fundamental in developing EDS as a research tool is the need for an accurate and reliable measurement. We developed an automated multichannel prototype system, the Octopus, and recorded electrical resistance and capacitance at eight skin sites in 33 healthy participants over 2 hours. The Octopus accurately measured against known resistors (within 2.5% of the mean value) and capacitors (within 10% of the mean value), and yielded repeatable readings at all eight skin sites: LR 1 (r = 0.79), SP 1 (r = 0.79), toe non-AP (r = 0.77), LU 9 (r = 0.97), PC 6 (r = 0.96), wrist non-APs (r = 0.97), SP 6 (r = 0.96), and leg non-APs (r = 0.97). Resistance at APs was significantly lower than the nearby non-APs in one out of three comparisons.
Keywords: capacitance, physiological marker, skin resistance, step voltage response
1. Introduction
Electrodermal screening (EDS), based on the pioneering work of Nakatani [1], Niboyet [2], and others [3–5] employs measurements of skin resistance (or conductance) at acupuncture points (APs) as diagnostic aids to determine energetic imbalances in the meridians and to guide treatment strategies. This practice assumes that APs have lower electrical resistance than nearby non-acupuncture sites (non-APs) [3 – 5], that skin resistance at APs differs in health and disease [6–8] and that effective acupuncture treatments may be associated with normalization of skin resistance at APs [9–11].
Reports looking at whether APs have different electrical properties from non-APs are limited in number and conflicting [4,12–16]. Nonetheless, a recent systematic review published by Ahn et al found evidence to support the contention that meridians and APs are electrically distinguishable from surrounding tissue [17]. Several factors may account for the controversial findings, including technical challenges such as: different types of recording instruments (alternating current vs. direct current); different types of electrodes; the pressure, angle, and duration of electrode probe application and; the skin-electrode interface [18]. Also influencing skin resistance and capacitance recordings is the condition of the underlying skin and the inherent fluctuating nature of the physiological phenomenon being measured. Further confounding reported results, the devices used to measure skin resis tance at APs have only rarely been tested for accuracy and reliability [19–23]. A final significant, but less often cited confounding factor, is that the target of the measurements – the APs and non-APs – are inadequately demarcated in terms of their supposed topographical size, shape and anatomical location.
Before EDS can justifiably be used in acupuncture research, the long-held assumptions about skin resistance at APs must be tested and validated. A measuring system that not only minimizes technical confounders but also allows simultaneous data collection at multiple skin sites over extended periods of time is needed.
The objectives of this research project were to: (1) develop an 8-channel prototype skin resistance and capacitance measuring system, the Octopus, that can record consistent measurements against known resistors and capacitors; (2) evaluate test-retest reliability of resistance and capacitance measurements at eight skin sites in healthy subjects over 2 hours and; (3) compare skin resistance and capacitance at select APs and nearby non-APs in a preliminary manner.
2. Materials and Methods
2.1. Participants
Thirty-three healthy male and female volunteers, aged ≥ 18 years, were recruited from the National College of Natural Medicine (NCNM). Our single inclusion criterion was general good health (by the participant's own assessment). Individuals who smoked or had any chronic or acute illness were excluded. Ethics approval of this study was obtained from the NCNM Institutional Review Board. All participants provided written informed consent.
2.2. Experimental setting
The study was conducted between December 1, 2008 and February 5, 2009 at the Psychophysiology Laboratory of Helfgott Research Institute at NCNM. Each participant was given $20 for their 2.5 hour involvement in the study.
2.3. Experimental equipment
The Octopus, a prototype for a future 32-channel system, was developed to measure skin resis tance and capacitance in a near-continuous manner over several hours. The design considerations that went into developing this prototype is described [24]. A parallel resistance and capacitance network model is used as an equivalent circuit for electrical skin properties. The Octopus inputs repeated step responses from 0 volt to a specified voltage to make its measurements. Resistance and capacitance are then derived from the transient step responses. When a measurement is complete, the input signal is zeroed out. Consecutive measurements are recorded in similar fashion in each of the eight channels. Unlike typical DC measuring systems that use a steady state value and derive only skin resistance, the Octopus derives both resistance and capacitance values by measuring the time response to a voltage step. Using an R//C + R (resistance in parallel with capacitance, then in series with resistance) network, Reichmanis et al applied the same principle of inputting voltage step perturbations to study the impedance (a parameter that describes resistance plus capacitance) of a representative portion of the acupuncture system [25].
The measurement circuit is time multiplexed across the eight channels at the rate of two measurements per second, providing a complete set of measurements every 4 seconds. To accommodate eight channels of multiplexed measurements with a multiplexing rate of 2 Hz, the Octopus board, shown in Figure 1 was constructed and interfaced to a National Instruments PCI-6251 acquisition board shown in Figure 2.
Figure 1.
Block diagram of the eight channel Octopus custom board.
Figure 2.

The Octopus system. The National Instruments SCC-68 I/O screw terminal board with cable interface to the PCI card inside a desktop computer is shown on the left. The custom designed Octopus board on the right (depicted in Figure 1), with a white SCC box cover. The eight leads connecting to skin electrodes and the ground connector on the right are shown on top of the Octopus board.
During each measurement, the input step is initially 0 V for 20 ms, as indicated in Figure 2. This helps discharge any static charge on the skin. The appropriate voltage either 2 V (for low skin resistance) or 5 V is applied for 450 ms, after which time the input returns to 0 V and the circuit switches to the next channel, leaving the previous channel floating until its turn to be mea sured again 4 seconds later.
2.4. Calibration of instrument
Prior to the testing, a 50-hour test was run to assess instrument consistency in measuring known resistors and capacitors. Consistency, reported as the range of resistance measurements, was within 2.5% of the mean value. The range of the capacitance measurements was within 10% of the mean value. Our measurements were consistent, with standard deviations of less than 0.5%, making the Octopus more than adequate for trend measurements, our goal at this stage of experimentation.
2.5. Electrodes
The silver-silver chloride unshielded cup electrode (Ag-AgCl 4mm TP electrode EL254; Biopac Systems Inc., Goleta, CA) was soldered to an isolated lead and enclosed by a cylindrical plastic chamber. The lead wires terminated in standard touch-proof connectors. The 4 mm diameter size was selected to optimize mea surements of the presumed 1–10 mm size APs [26,27]. The electrodes were coupled with the skin surface using the recommended electrode gel (Sigma Gel; Biopac Systems). The electrode cup was filled with gel and the surplus was removed with a spatula. Our goal was to disturb the electrolyte skin system as little as possible to minimize interaction between skin and electrolytes. The reference electrode (Kendall MediTrace 530 ECG conductive electrode with a 49.06 mm2 active surface area) was placed on the volar surface of the wrist, just proximal to AP PC 6. We pre-tested the electrodes in a preliminary assessment and achieved a good contact when light compression was applied with an elastic adhesive or cloth wrist band.
2.6. APs and non-APs
Three distinct anatomical skin regions (the wrist, the great toe and the leg) were included for measurement. Resistance and capacitance recordings were made at five APs and three nearby non-APs (Figure 3A, 3B and 3C) including right Lung (LU 9), Pericardium (PC 6), and a non-APs located midway between these two APs (Figure 3A); right Spleen (SP 1) and Liver (LR 1) and a non-AP located midway at 4 mm proximal to these points (Figure 3B) and; SP 6, and a non-AP located on the border of the tibia 3.5 cm proximal to SP 6 that was not on the Liver or Kidney meridians (Figure 3C). These specific APs were chosen because of their clinical importance, their use in previous trials and to include APs with different underlying anatomy [5,28–31].
Figure 3.
Biopac electrodes on acupuncture points (APs) and nearby non-AP sites, including (A) right Lung (LU 9), Pericardium (PC 6), and a non-AP site located midway; (B) right Spleen (SP 1), Liver (LR 1) and a non-AP site located midway; and (C) SP 6 and a non-AP site located on the border of the tibia.
AP location and electrode application were performed by one of two acupuncturists who had 8 and 2 years experience, respectively. Their point location techniques entailed use of anatomical charts, standard cun measurements (1 cun ~ 2 cm), palpation and what the acupuncturists described as an “energetic felt sense” that was present at APs but not at non-AP sites. The skin surfaces were first inspected for level of moisture, dryness, moles, tattoos and other lesions. If any lesions were noted, similar sites were inspected on the alternative limb. The skin was cleansed with isopropyl alcohol and allowed to air dry. The acupuncturists affixed the electrodes to the skin with paper tape. To maintain optimal skin-electrode contact, the cup electrodes were held in place with a gently compressive athletic wrist band applied around the wrist and leg or a self-adhering elastic bandage around the large toe.
To minimize artifact and noise in the measurements, participants were asked to sit quietly in a recliner chair in a quiet room for the 2-hour data collection period. The duration of 2 hours for measurements was chosen because we anticipated this was the maximum time participants could sit quietly to give us at least 1 continuous hour of usable data. Participants were allowed to read materials of their choice. The study coordinator turned on the Octopus instrument which then automatically recorded resistance and capacitance in a sequential manner at the eight sites in the following order: LU 9, PC 6, non-APs on wrist, SP 1, LR 1, non-APs on large toe, SP 6 and non-APs on leg. The non-APs were tested under the same experimental conditions as the APs. The study coordinator checked on the participant, the instrument and the computer screen at 15 minute intervals to assure participant comfort, proper equipment functioning and accurate data acquisition.
Resistance and capacitance measurements were recorded sequentially every 0.5 seconds at each of the eight skin sites during the 2-hour test period. An average total of 1883 recordings per site were collected. Our rationale for a fixed rather than a random sequence of data acquisition was that we primarily wanted to test whether measurements at the same skin sites, under the same conditions, were repeatable over time or if a bias (a drift in values) would occur. Data analysis was performed on the middle 70 minutes of the 120-minute recording period to allow for up to an initial 30 minutes for skin-electrode interface stabilization and elimination of artifact and noise in the last 20 minutes. The approximate 1094 recordings acquired during this time period were averaged into a single mean resistance measure for each of the eight recording sites.
2.7. Statistical analysis
Statistical analysis was performed using SAS version 9.1 (SAS Institue, Cary, NC, USA). The reliability of the skin measurements taken with each electrode was estimated as the ratio of the between-person variance to the total variance. The between and within-components of variance were computed across the 33 participants using a standard method [32]. Comparisons of resistance for APs and non-APs were performed using the Wilcoxon paired signed rank test. To minimize the confounding introduced by comparing more than one AP to a given non-AP, the two APs on the toe (LR 1 + SP 1) and the two APs on the wrist (LU 9 + PC 6) were averaged. Both Kolmogorov-Smirnov and Shapiro-Wilk tests of normality were performed on each of the eight mean resistance measures. All eight mean were non-normally distributed so the non-parametric Wilcoxon paired sign test was used to compare the three APs mean resistance measures to the corresponding non-APs mean resistance measures.
3. Results
3.1. Reliability
The Octopus recordings were reliable at all eight skin sites, LR 1 (r = 0.79), SP 1 (r = 0.79), toe non-AP (r = 0.77), LU 9 (r = 0.97), PC 6 (r = 0.96), wrist non-AP (r = 0.97), SP 6 (r = 0.96), and leg non-AP (r = 0.97).
3.2. APs to non-APs comparison
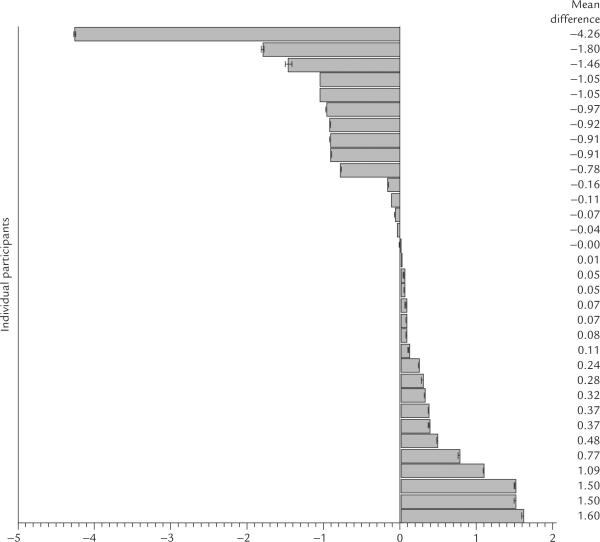
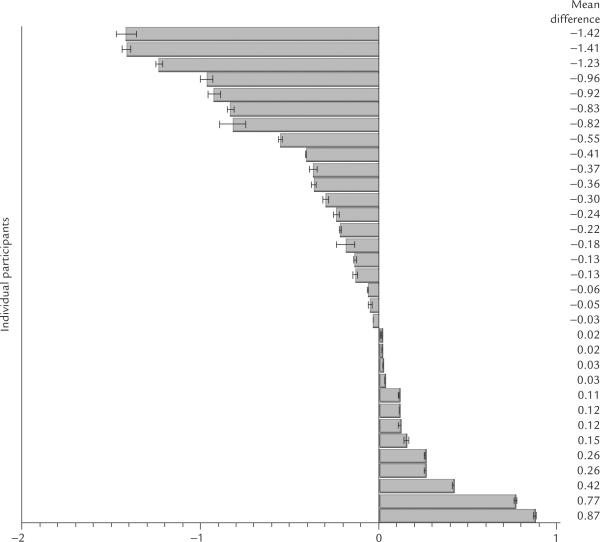
The averaged toe (LR 1 + SP 1) mean resistance measure was the only AP pairs to differ significantly from its non-AP counterpart (Z = 2.1, p < 0.03). The resis tance of the averaged pairs of toe APs was lower than the resistance at the nearby non-APs. The differences in resistance (AP resistance minus non-APs resistance) for all three comparisons are depicted in Figure 4. Individual participants had lower or higher resis tance for the averaged APs compared with the corresponding non-APs. The mean resistance differences in each of the 33 individual participants, for the three comparisons, are depicted in Figures 5, 6, and 7.
Figure 4.
Differences in resistance between acupuncture points (AP) and non-acupuncture sites (non-APs) appear to be associated with the distance between the two sites.
Figure 5.
Mean resistance differences between the average of the AP pair (LU 9 + PC 6) and the nearby non-AP comparison site in 33 individual participants.
Figure 6.
Mean resistance differences between the average of the AP pair (LR 1 + SP 1) and the nearby comparison site in 33 participants.
Figure 7.
Mean resistance differences between SP 6 and its nearby non-AP comparison site in 33 participants.
Because the likelihood of finding lower resistance at the APs than the non-APs appeared to be inversely related to the distance the AP from its comparison non-APs, we performed a simple within-subjects linear trend test as an exploratory analysis. The result was statistically significant [F(1,31) = 28.6, p < 0.001]. The LR 1 and SP 1 APs, which were approximately 4 mm from their comparison non-APs, had significantly lower resistance than the non-AP, in contrast to SP 6 and its comparison non-AP. SP 6 was 3.5 cm away from its comparison non-AP and had overall higher resis tances than the non-AP (Figure 4). The non-AP comparison site for SP 6 was chosen to avoid a site on the Spleen, Kidney or Liver meridians.
3.3. Exploratory analyses
Analyses were performed to determine if gender or age was related to the difference in resistance. Spearman's rank correlation revealed no statistically significant differences between gender or age (p > 0.15).
4. Discussion
Our objectives for this research project were to develop an 8-channel prototype instrument, test its accuracy in measuring external standards and assess its reliability in recording near-continuous skin resistance and capacitance on human skin over several hours. We also sought to make preliminary comparisons between skin resistance and capacitance at APs and the nearby non-APs. We found that the Octopus performs consistent measurements of known resistors and capacitors and records repeatable skin resistance values after initial stabilization of the skin electrode interface. Due to a software malfunction, we were unable to interpret the capacitance recordings obtained. This software problem will be fixed in future measurements.
Our comparison of skin resistance at three independent pairs of APs to nearby non-APs yielded mixed results. Only one of the three independent pairs, the Liver and Spleen APs (on the large toe), had a lower mean resistance than the nearby comparison site (4 mm away). The two other comparisons (1 cm and 3.5 cm from their respective control non-APs) showed no significant differences. Our mixed findings reflected the overall contradictory results reported in the literature [17]. Several reasons may account for not finding consistently lower resistances at the APs compared with the non-APs: (1) actual differences in resistance between APs and non-APs may not exist; (2) differences may exist but are undetectable because of technical challenges related to the methodology employed; (3) the choice of APs versus non-APs was inappropriate; or (4) the size, shape and boundaries of the APs and non-APs were inaccurately demarcated.
We believe that our methodology overcame many technical confounders associated with skin resistance measurements. We were able to maintain constant electrode pressure using cup electrodes filled with an incompressible gel and securing them to the skin. We minimized difficulties associated with contact resistance and electrode polarization by using Ag/AgCl electrodes. We addressed potential problems at the skin level by avoiding scars, cuts, areas of hyperkeratosis or skin that had a high density of sweat glands. We accounted for physiological fluctuations in skin resistance by recording over 2 hours with the electrodes undisturbed during the test period. In addition, we believe our choice of APs was appropriate as the LR 1 and SP 1 are regularly measured in EDS [20,21,33]; the Lung and Pericardium meridians [13,29–31] and the SP 6 [23,31] have all been studied in previous research. One issue, however, that neither we nor the majority of other researchers have accounted for, is specifying the exact dimensions of our “targets”, the APs and the non-APs. In other words, since we do not know the exact size of any AP, we cannot define what constitutes the outside “borders” of the APs. Which exact area of skin surface constitutes an AP versus a non-AP? Where does an AP end and a non-AP begin?
Without the knowledge of the exact sizes of the APs tested in this study, we can only say that the 4 mm diameter area centered over what was defined by the acupuncturist as an AP, had lower, higher or the same resistance as a 4 mm diameter area a few centimeters away from that presumed AP. We believe that a better way to test the hypothesis that APs have lower resistance than non-APs would be to measure the skin immediately surrounding the 4 mm area defined as the AP and then determine if a given AP has relatively lower resistance than the immediately adjacent skin. The most compelling evidence for APs having relatively lower skin resistance compared with nearby non-APs is seen in the topographic maps produced by Becker et al [4]. In their carefully conducted experiment, researchers plotted electrical skin conductance at two separate APs [Large Intestine (LI 4) and Triple Energizer (TE 4)] using a 36-electrode probe that covered a 2.5 cm2 area. The probe was placed on the skin, centered over each of the APs. Conductance plots recorded in this manner showed lines of equal conductance surrounding a central point with the conductance decreasing with distance from the center. This led the authors to conclude that APs are loci of relative, not absolute, high conductance (low resistance) compared with surrounding skin. In addition, the appearance of their conductance plots suggests that the particular APs tested were approximately 3–5 mm in diameter.
A recent publication by Kramer et al used a similar study design to map skin resistance [16], using a flexible plastic foil with 64 probes. The probes were spaced 8 mm apart, recorded and plotted skin resistance in a 64 square grid, each square covering 2.25 cm2. The foil was placed over a 6 × 6 cm anatomical area that included both APs and non-APs. After measurements were recorded, an acupuncturist, blinded to the readings, clinically identified AP locations within the overall 6 × 6 cm area and marked the skin at the AP with an “X”. The 2.25 cm2 area surrounding the “X” defined the AP. The resistance in that 2.25 cm2 area was then compared with the resistance in the overall 6 × 6 cm2 area. No significant differences between the 2.25 cm2 APs and a larger skin area were detected in 62.8% of 631 measurements. Although this topographical mapping is similar to that of Becker et al, the spatial resolution in Becker's study was greater, with measurements recorded at distances of approximately 1.5 mm apart as compared with 8 mm apart in the study of Kramer et al. Within their overall 2.5 cm2 area, Becker's group identified a discrete central area of higher conductance. If a typical AP measures only 3–5 mm in diameter, it may not be surprising that the probes of Kramer et al, placed 8 mm apart, were unable to distinguish an AP from a non-APs.
Kramer et al acknowledged that the actual AP area of resistance might be too small to be registered by their 6 × 6 cm array. We agreed and recognized that our APs and comparison sites might have been too far apart to detect local, relative (rather than absolute) differences. Our study utilized 4 mm diameter cup electrodes that were placed respectively 0.4 cm, 1 cm and 3.5 cm away from the nearby comparison non-APs. The only AP pair (LR 1 & SP 1) that measured significantly lower resistance was within 0.4 cm of the nearby control site. In the comparison sites that were 3.5 cm apart, the resis tance at the AP (SP 6) in the majority of participants was actually higher than at the non-AP comparison site. In conclusion, a prototype 8-channel skin resistance measuring system was developed for this project and was shown to accurately measure resistance and capacitance against external standards. The device also performed repeatable measurements of skin resistance at APs and non-APs over 2 hours in healthy participants. Comparisons of skin resistance at selected APs to non-APs showed lower skin resistance at the APs in only one out of the three comparisons. For future studies, we recommend when comparing AP to non-APs, the measuring electrodes should be placed as close to each other as possible to test the hypothesis that APs have lower resistance than non-APs more accurately.
Acknowledgments
This study was funded by the National Institutes of Health (NIH)/National Center for Complementary and Alternative Medicine (NCCAM) AT004134. We thank Jinkook Yun and Ken Luchini for their engineering advice and their assistance with the preliminary studies that led to the NCCAM funding of this project.
Footnotes
Conflicts of interest Miridia Technology Inc., Idaho, USA.
References
- 1.Nakatani Y. On the nature of the acupuncture points and meridians. J Japan Orient Med. 1953;3:39–49. [Google Scholar]
- 2.Niboyet JEH, Bourdiol RJ, Regard PG. Traité D'Acupuncture. Maisonneuve; Paris: 1963. La moindre résistance a l'électricité de surfaces punctiformes et de trajects cutanés concordant avec les points et meridiens, bases de l'acupuncture. In French. [Google Scholar]
- 3.Voll R. Twenty Years of Electroacupuncture Diagnosis in Germany. A progress report. Am J Acupunct. 1975;3:7–17. [Google Scholar]
- 4.Becker R, Reichmanis M, Marino A, Spadaro J. Electrophysiological correlates of acupuncture points and meridians. Psychoenergetic Syst. 1976;1:105–12. [Google Scholar]
- 5.Reichmanis M, Marino AA, Becker RO. Electrical correlates of acupuncture points. IEEE Trans Biomed Eng. 1975;22:533–5. doi: 10.1109/tbme.1975.324477. [DOI] [PubMed] [Google Scholar]
- 6.Saku K, Mukaino Y, Ying H, Arakawa K. Characteristics of reactive electropermeable points on the auricles of coronary heart disease patients. Clin Cardiol. 1993;16:415–9. doi: 10.1002/clc.4960160509. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan SG, Eggleston DW, Martinoff JT, Kroening RJ. Evoked electrical conductivity on the Lung acupuncture points in healthy individuals and confirmed lung cancer patients. Am J Acupuncture. 1985;13:261–6. [Google Scholar]
- 8.Tsuei JJ, Lam FMK, Jr, Mi M, Zhao Z. Study on bioenergy in Diabetes Mellitus patients. Am J Acupunct. 1989;17:31–8. [Google Scholar]
- 9.Matsumoto T, Hayes MF., Jr Acupuncture, electric phenomenon of the skin, and postvagotomy gastrointestinal atony. Am J Surg. 1973;125:176–80. doi: 10.1016/0002-9610(73)90023-8. [DOI] [PubMed] [Google Scholar]
- 10.Kawakita K, Kawamura H, Keino H, Hongo T, Kitakohji H. Development of the low impedance points in the auricular skin of experimental peritonitis rats. Am J Chin Med. 1991;19:199–205. doi: 10.1142/S0192415X91000272. [DOI] [PubMed] [Google Scholar]
- 11.Ahn A, Schnyer R, Conboy L, Laufer M, Wayne P. Electrodermal measures of Jing-Well points and their clinical relevance in endometriosis-related chronic pelvic pain. J Altern Complement Med. 2009 doi: 10.1089/acm.2008.0597. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarroll GD, Rowley BA. An investigation of the existence of electrically located acupuncture points. IEEE Trans Biomed Eng. 1979;26:177–81. doi: 10.1109/tbme.1979.326392. [DOI] [PubMed] [Google Scholar]
- 13.Martinsen OG, Grimnes S, Morkrid L, Hareide M. Line patterns in the mosaic electrical properties of human skin–a cross-correlation study. IEEE Trans Biomed Eng. 2001;48:731–4. doi: 10.1109/10.923791. [DOI] [PubMed] [Google Scholar]
- 14.Noordergraaf A, Silage D. Electroacupuncture. IEEE Trans Biomed Eng. 1973;20:364–6. doi: 10.1109/TBME.1973.324289. [DOI] [PubMed] [Google Scholar]
- 15.Kramer S, Zaps D, Wiegele B, Irnich D. Changes in electrical skin resistance at Gallbladder 34 (GB34) J Acupunct Meridian Stud. 2008;1:91–6. doi: 10.1016/S2005-2901(09)60028-5. [DOI] [PubMed] [Google Scholar]
- 16.Kramer S, Winterhalter K, Schober G, Becker U, Wiegele B, Kutz K, et al. Characteristics of electrical skin resistance at acupuncture points in healthy humans. J Altern Complement Med. 2009;15:1–6. doi: 10.1089/acm.2008.0331. [DOI] [PubMed] [Google Scholar]
- 17.Ahn AC, Colbert AP, Anderson BJ, Martinsen OG, Hammerschlag R, Cina S, et al. Electrical properties of acupuncture points and meridians: a systematic review. Bioelectromagnetics. 2008;29:245–56. doi: 10.1002/bem.20403. [DOI] [PubMed] [Google Scholar]
- 18.Ahn AC, Martinsen OG. Electrical characterization of acupuncture points: technical issues and challenges. J Altern Complement Med. 2007;13:817–24. doi: 10.1089/acm.2007.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treugut H, Gorner C, Ludtke R, Burghardt VV. Reliabilitat der energetischen Meridianmessung mit Prognos A(R) Forsch Komplementarmed. 1998;5:284–9. doi: 10.1159/000021160. In German. [DOI] [PubMed] [Google Scholar]
- 20.Colbert AP, Hammerschlag R, Aickin M, McNames J. Reliability of the Prognos electrodermal device for measurements of electrical skin resistance at acupuncture points. J Altern Complement Med. 2004;10:610–6. doi: 10.1089/acm.2004.10.610. [DOI] [PubMed] [Google Scholar]
- 21.Jessel-Kenyon J, Pfeiffer L, Brenton M. A statistical comparison of repeatability in three commonly used bioelectric devices: Kirlian photography, the segmental electrogram, and the AMI of Motoyama. Acupunct Med. 1998;16:40–2. [Google Scholar]
- 22.Wiegele B, Schober G, Kuder J, Kolb FP, Irnich D. A new sensor technique for measurements of electrical potential profiles of human skin at acupuncture points. Forsch Komplementmed. 2006;13:227–32. doi: 10.1159/000094704. In German. [DOI] [PubMed] [Google Scholar]
- 23.Cho SH, Chun SI. The basal electrical skin resistance of acupuncture points in normal subjects. Yonsei Med J. 1994;35:464–74. doi: 10.3349/ymj.1994.35.4.464. [DOI] [PubMed] [Google Scholar]
- 24.Thong T, Colbert A, Larsen A. An 8-channel skin impedance measurement system for acupuncture research. 31st Annual International IEEE EMBS conference; Minneapolis, Minnesota, USA. September 2–6, 2009; pp. 861–4. [DOI] [PubMed] [Google Scholar]
- 25.Reichmanis M, Marino AA, Becker RO. Laplace plane analysis of transient impedance between acupuncture points Li-4 and Li-12. IEEE Trans Biomed Eng. 1977;24:402–5. doi: 10.1109/TBME.1977.326154. [DOI] [PubMed] [Google Scholar]
- 26.Jakoubek B, Rohlicek V. Changes of electrodermal properties in the “acupuncture points” on men and rats. Physiol Bohemoslov. 1982;31:143–9. [PubMed] [Google Scholar]
- 27.Halek J, Opavsky J, Kolarova J. Problems of the skin resistance measuring in randomly chosen and so-called active points of the skin. Acta Univ Palacki Olomuc Fac Med. 1984;107:51–62. [PubMed] [Google Scholar]
- 28.Lee J, Kim D, Park I, Park H, Cho J. Characteristics of human skin impedance including at biological active points. IEICE Trans Fund Electron Commun Comput Sci. 2003;86:1476–9. [Google Scholar]
- 29.Colbert AP, Yun J, Larsen A, Edinger T, Gregory WL, Thong T. Skin impedance measurements for acupuncture research: development of a continuous recording system. Evid Based Complement Alternat Med. 2008;5:443–50. doi: 10.1093/ecam/nem060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon CS, Choy TT, Koide FT. A reliable method for locating electropermeable points on the skin surface. Am J Chin Med. 1980;8:283–9. doi: 10.1142/s0192415x80000256. [DOI] [PubMed] [Google Scholar]
- 31.Ahn AC, Wu J, Badger GJ, Hammerschlag R, Langevin HM. Electrical impedance along connective tissue planes associated with acupuncture meridians. BMC Complement Altern Med. 2005:5–10. doi: 10.1186/1472-6882-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winer B. Statistical principles in experimental design. 2nd ed. McGraw Hill; New York: 1962. [Google Scholar]
- 33.Colbert A, Hayes M, Aickin M, Hammerschlag R. Physiologic variability of electrical skin resistance measurements at the ting acupuncture points. Med Acupunct. 2006;17:12–9. [Google Scholar]