Abstract
Infectious complications following allogeneic hematopoietic cell transplantation (HCT) from unrelated donors (URD) result in significant morbidity. We hypothesized that recipients of an URD with an activating natural killer cell immunoglobulin-like receptor (KIR) (B/x) genotype would have decreased infectious complications due to enhanced NK cell function. We compared the infectious complications in 116 recipients of a graft from a donor with an A/A KIR (n = 44) genotype and a B/x KIR (n = 72) genotype. All recipients participated in the prospective NMDP infection project collecting infection data from conditioning until six months post-transplant. The cohort with a B/x donor had fewer initial bacterial infections by day 180 (A/A: 86% [95% CI, 75 – 95]; B/x: 68% [95% CI, 57 – 78]; p=0.02). There was no difference in the incidence of viral or fungal infections. When accounting for multiple infections, fewer bacterial infections were seen in the B/x cohort (A/A: 3.55/patient; B/x: 2.63/patient; p = 0.09). During the study period, only 19 patients had no infections; of these, 15 had received cells from a B/x KIR donor. The role of donor KIR genotype on infection complications is intriguing and warrants further investigation.
INTRODUCTION
Infections remain a common complication following allogeneic transplantation (HCT). In fact, almost 20% of recipients of hematopoietic cell grafts from unrelated donors (URD) report infection as the primary cause of death (1). Natural killer (NK) cells, a subset of lymphocytes, act as cytotoxic effectors and/or cytokine producers and regulators, engaging with a wide range of immunologic effector cells to control the immune response. NK activity is controlled by several families of cell surface inhibitory and/or stimulatory receptors including the killer immunoglobulin-like receptors (KIR); inhibitory KIR use HLA class I as their cognate ligands (2). KIR are genetically diverse, but can be broadly categorized into two haplotypes (3). The predominately inhibitory haplotype “A”, has at most only a single activating gene, KIR2DS4, although some A haplotypes lack even this stimulatory gene (4, 5) Conversely, the B haplotype has, in addition to KIR2DS4 which is found in nearly all individuals, at least one additional activating gene (e.g. KIR2DS2, KIRDS3, KIRDS5, KIR3DS1). An activating KIR genotype must have at least one B haplotype, either as homozygous B/B or heterozygous B/A (noted as B/x). In a Caucasian population, it is expected that approximately 2/3 of people will have an activating KIR genotype (6, 7).
An individual’s KIR genotype may alter their response to specific infections. For example, HIV-infected individuals with the activating KIR gene KIR3DS1 in concert with HLA-BW4 demonstrate delayed progression to AIDS (8–10). Furthermore, clearance of chronic hepatitis B and C may be in part regulated by the KIR genotype (4, 11, 12).
Following allogeneic transplantation, NK cells recover most quickly though their specific contribution to either favorable or adverse post-HCT outcomes is uncertain (13). In addition, we recently reported that the donor KIR genotype significantly improves post-transplant survival in transplants for acute myelogenous leukemia (AML) (14). However, data examining donor KIR and post-transplant infectious complications have been mixed and studied primarily following allogeneic sibling HCT (15, 16).
We hypothesized that, following an URD allogeneic HCT, recipients of cells from donors with the activating KIR genotype (B/x) will have fewer infections. Therefore, we designed a study to analyze infectious complications in relation to donor KIR genotypes.
PATIENTS MATERIALS AND METHODS
Patients
The National Marrow Donor Program (NMDP) conducted a project to assess infectious complications following allogeneic URD HCT. All patients were transplanted in 2002. A total of 211 recipients at 27 transplant centers were enrolled. All data were prospectively collected and extensively audited to confirm accuracy of the infectious complications reported. A subset of recipients (n=116) had DNA samples available for donor KIR genotyping through the NMDP Research Repository, and their infectious outcomes are reported here. The KIR genotyping was performed utilizing MALDI-TOF mass spectrometry (17).
Infection Data
Prospective data regarding infectious complications as well as antimicrobial prophylaxis were collected weekly from the start of conditioning through day 90 following HCT and then every two weeks until day 180. Infections were included in the specific microbial categories only when a culture was identified. A subset of patients had clinical infectious syndromes without a specific organism identified but therapy was initiated, such as for probable fungal infections. Fevers of unknown origin or neutropenic fever without an identified organism were excluded. Recurrent infection was based on intervals from the time of first infection to the next reported episode of the same infection. A recurrent bacterial infection was one that followed a 7 day window of negative cultures except for Clostridium difficile infection which required a 30-day culture negative period. The following viral infections were considered recurrent after 14 days: varicella zoster (VZV), adenovirus, enterovirus, influenza, parainfluenza, and rhinovirus. However, a 60 day window was required to score recurrent cytomegalovirus (CMV), herpes simplex virus (HSV), and polyomavirus infections. CMV reactivation was determined by antigenemia or PCR positive surveillance as defined by institutional guidelines and subsequently resulted in an initiation or change of anti-CMV therapy. Fungal infections with yeast or Cryptococcus could be recurrent after a 14-day window, but molds (e.g., Aspergillus, Fusarium, Mucor, etc.) required a 90 day window.
Statistical Analysis
The primary objective of this study was to compare infectious complications in HCT recipients with donors having an A/A KIR genotype versus those with B/x KIR genotype. Outcomes of interest included infection density, defined as the number of infections per patient days at risk; cumulative incidence of bacterial, viral, fungal, and overall infectious complications at day 180; incidence of grade II to IV acute graft-versus-host disease (GvHD) (18); and the incidence of chronic GvHD. In addition, we analyzed treatment related mortality (TRM) at day 100 and overall survival (OS) at one year.
Patient-, infection-, and treatment-related factors were compared between the two KIR genotype cohorts using Chi square testing for categorical and Mann-Whitney testing for continuous variables. Models were fit for the infection density at day 180 by using a stepwise Poisson regression model with an offset for the patient’s on study time. Factors tested in the model included, in addition to the KIR genotype, patient age, stem cell source (marrow versus peripheral blood), diagnosis and disease status, HLA matching as defined by Weisdorf et al (19), and cytomegalovirus (CMV) serostatus of donor and recipient. Because patients develop multiple infections over the 6 month period of observation, modeling of variables in relation to infection density were tested for both overall infection as well as bacterial, fungal and viral groupings. We also examined, using Cox regression models, the time to first infection of a given type. Here we included acute GVHD as a time dependent covariate. The probability of overall survival (OS) was calculated using the Kaplan-Meier estimate and the survival compared for each KIR genotype group at 180 and 360 days using a stepwise pseudo-value regression model (20).
RESULTS
Patients
Forty-four (38%) patients received cells from a donor with a KIR A/A genotype and 72 (62%) from a donor with the activating KIR B/x genotype. The demographic and transplant characteristics in each group are shown in Table 1. Patient characteristics including median age of patients, gender and Karnofsky performance status were similar. Nearly 3/4 of patients in each cohort had acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). The median age of the donors was similar between the two groups: A/A donors, 34 years (range 21–59 years); B/x donors, 34 years (range 19–56 years). The median time from diagnosis to HCT was similar in the two cohorts at 11 months (range 1–159 months) for A/A donor recipients and 14 months (range 0.6 to 157 months) for those receiving cells from a B/x donor. The conditioning intensity was similar between the two groups. The median follow-up of survivors is 48 months for the A/A donor cohort (range 27 – 52 months) and 47 months for the B/x donors (28 – 53 months).
Table 1.
Characteristics of patients defined by KIR Genotype
| Donor KIR Genotype A/A | Donor KIR Genotype B/x | P-value | |
|---|---|---|---|
| N=44 | N=72 | ||
| Variable: | N (%) | N (%) | |
| Recipient Age, years Median (range) |
39 (2 – 67) | 40 (1 – 66) | 0.66 |
| Donor Age, years Median (range) |
34 (21 – 59) | 34 (19 – 56) | 0.30 |
| Male sex | 25 (57) | 44 (61) | 0.65 |
| Karnofsky prior to transplant ≥ | 21 (57) | 43 (68) | |
| 90% | 0.45 | ||
| Disease | 0.68 | ||
| Acute Leukemia/MDS | 32 (73) | 52 (72) | |
| CML | 4 (9) | 6 (8) | |
| Lymphoma | 7 (16) | 10 (14) | |
| Non-Malignant Disorders | 1 (2) | 4 (6) | |
| HLA-A,B,C & DRB1 matching | 0.43 | ||
| Well-matched | 24 (54) | 41 (57) | |
| Partially matched | 10 (23) | 21 (29) | |
| Mismatched | 10 (23) | 10 (14) | |
| Conditioning regimen | 0.30 | ||
| Myeloablative | 33 (75) | 40 (55) | |
| Reduced intensity | 8 (18) | 22 (31) | |
| Non-myeloablative | 3 (7) | 9 (13) | |
| Unknown | 0 | 1 (1) | |
| Donor/recipient sex match | 0.53 | ||
| Male/Male | 15 (34) | 22 (31) | |
| Male/Female | 9 (20) | 18 (25) | |
| Female/Male | 10 (23) | 22 (31) | |
| Female/Female | 10 (23) | 10 (14) | |
| Donor/recipient CMV match | 0.51 | ||
| Negative/Negative | 8 (18) | 21 (29) | |
| Negative/Positive | 16 (36) | 27 (38) | |
| Positive/Negative | 11 (25) | 10 (14) | |
| Positive/Positive | 8 (18) | 13 (18) | |
| Unknown | 1 (2) | 1 (1) | |
| Graft type | 0.97 | ||
| Bone marrow | 24 (55) | 39 (54) | |
| Peripheral blood stem cells | 20 (45) | 33 (46) | |
| GVHD Prophylaxis | 0.68 | ||
| Cyclosporine +/− Other | 11 (25) | 22 (31) | |
| Tacrolimus +/− Other | 23 (52) | 39 (54) | |
| T-Cell Depletion | 7 (16) | 9 (13) | |
| Other | 3 (7) | 2 (3) |
Abbreviations: CML = chronic myelogenous leukemia; CMV = cytomegalovirus; GVHD = graft versus host disease
Bacterial Infections
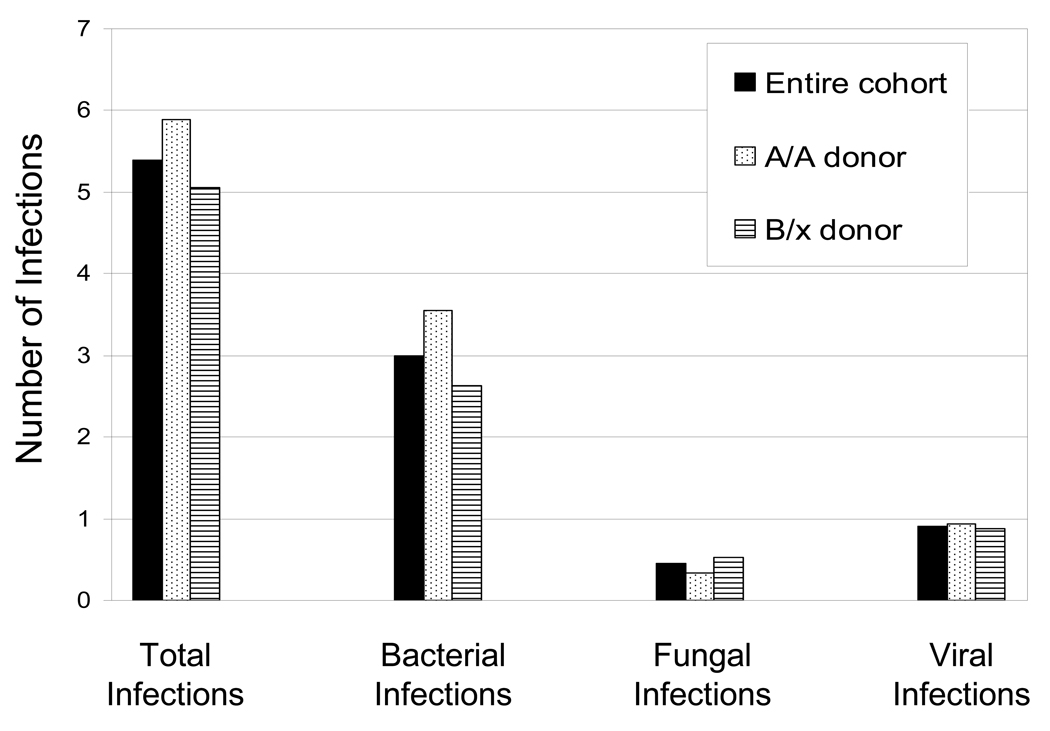
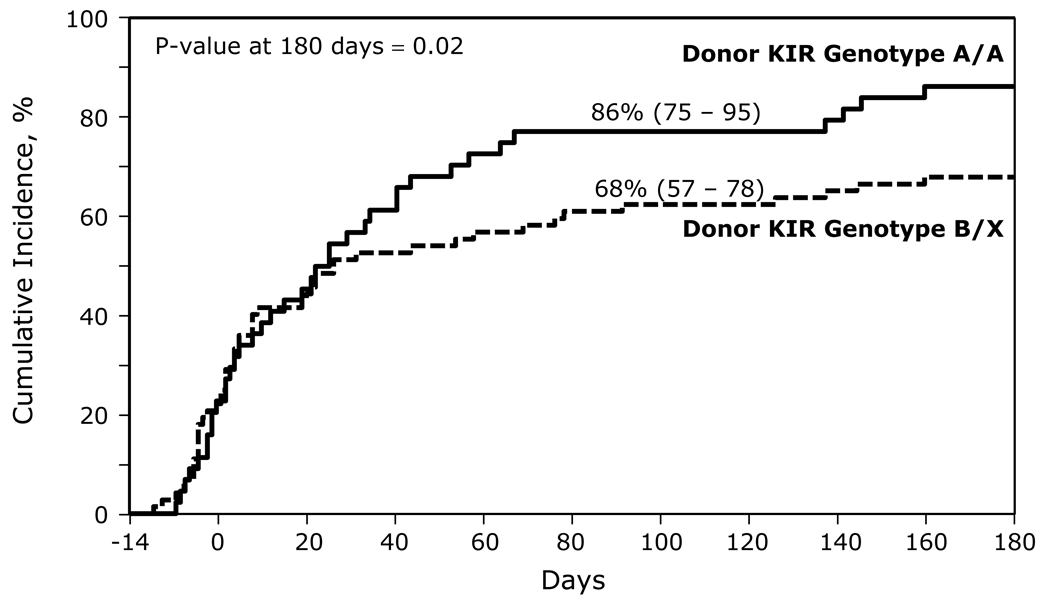
As expected, bacterial infections were the most common type of infection in the first 180 days post-transplant. For the entire cohort of patients, the mean number of bacterial infections was 2.99 per patient in the first 180 days following HCT (Figure 1). With an A/A donor versus a B/x donor, the mean number of bacterial infections was 3.55/patient versus 2.63/patient, respectively, (p= 0.09). In the first 180 days, the cumulative incidence of bacterial infections for A/A donor HCT was 86% (95% confidence interval [CI], 75 – 95) compared to only 68% (95% CI, 57 – 78) for B/x donor (p=0.02, Figure 2).
Figure 1.
Mean number of infections during the first 180 days following HCT. The mean number of infections is shown as the infection density defined as the number of infections per patient per days at risk and normalized over the 180 study period.
Figure 2.
The cumulative incidence of bacterial infections in recipients of a KIR A/A genotype and a KIR B/x genotype unrelated donor graft.
Twenty-nine patients had no bacterial infections (A/A: n = 6 [14%]; B/x: n = 23 [32%]), while the entire cohort of patients experienced 253 episodes of bacterial infection during the study period. The predominant bacterial infections were Staphylococcus epidermidis, which accounted for 90 separate infectious episodes. Other common bacterial infections included gram negative bacteria accounting for 51 episodes (20%), Enterococcus infections (45 episodes, 18%), and Clostridium difficile (18 episodes, 7%).
Antibacterial prophylaxis data was captured from weeks 5 – 24 of the study period. Nearly half the patients did not receive any bacterial prophylaxis during this time period. The predominant antibacterial utilized was penicillin which was given to 6 patients each in the A/A and B/x cohort between 5 and 12 weeks post-transplant, and 7 and 8 patients in the respective cohorts from weeks 13 to 24.
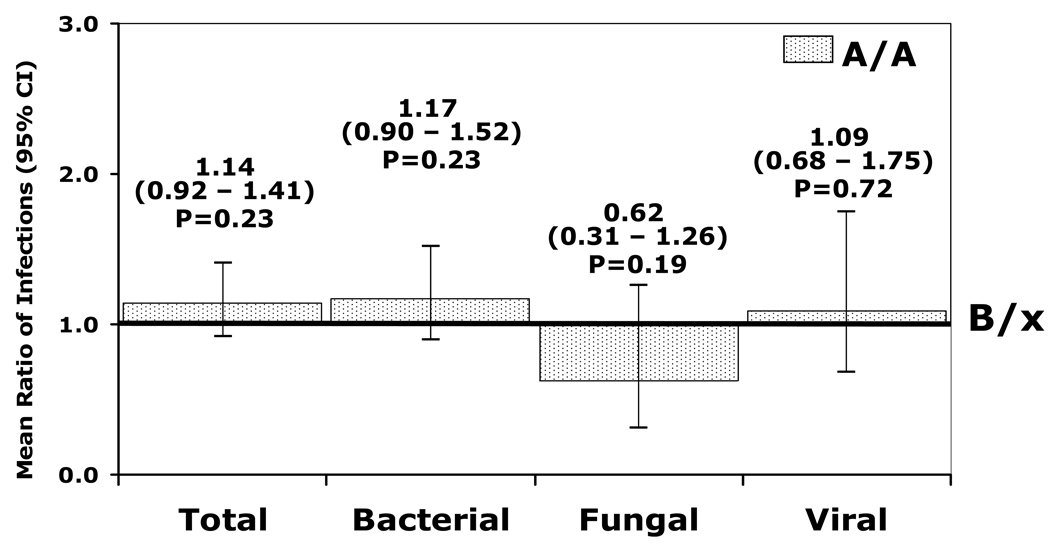
The Poisson adjusted mean number of bacterial infections is shown in Figure 3. Although not reaching statistical significance, the ratio of the adjusted mean number of bacterial infections in the A/A cohort was higher (1.17 [(95% CI, 0.90 to 1.52]; p=0.23) compared to recipients of a B/x donor (reference group rate). Factors associated with an increased risk of bacterial infections are shown in Table 2. Greater HLA mismatch was associated with more bacterial infections and, while marrow versus blood as a stem cell source was associated with fewer bacterial infections, neither KIR genotype nor other factors were found to be significant in the Poisson regression model.
Figure 3.
The adjusted relative infection density for a KIR A/A (dotted bar) donor recipient as compared to a KIR B/x (line) donor recipient during the first 180 days. A value above 1.0 indicates a higher mean ratio of infections in the KIR A/A cohort.
Table 2.
Other factors associated with risk of infections
| Any Infection | ||
|---|---|---|
| Variable | Relative Mean number of Infections (95% CI) |
p- value |
| Donor Age, years | ||
| 50 and older | 1.00 | 0.05§ |
| 40 – 49 | 0.88 (0.60 – 1.27) | |
| 30 – 39 | 0.84 (0.59 – 1.19) | 0.48* |
| 20 – 29 | 0.64 (0.45 – 0.91) | 0.32* |
| 0.01* | ||
| Donor/Recipient sex match | 0.03§ | |
| Female/Female | 1.00 | |
| Male/Male | 1.25 (0.93 – 1.68) | 0.13* |
| Female/Male | 1.10 (0.80 – 1.51) | 0.54* |
| Male/Female | 0.80 (0.56 – 1.14) | 0.21* |
| Donor/Recipient CMV Serostatus | 0.0005§ | |
| Positive/Positive | 1.00 | |
| Positive/Negative | 0.91 (0.64 – 1.29) | 0.59* |
| Negative/Positive | 1.48 (1.08 – 2.00) | 0.01* |
| Negative/Negative | 0.89 (0.61 – 1.30) | 0.56* |
| Bacterial Infections | ||
| Variable |
Ratio of Mean number of Infections* (95% CI) |
p- value |
| HLA Match | < 0.0001§ | |
| Well Matched | 1.00 | |
| Partially Matched | 1.65 (1.22 – 2.23) | 0.001* |
| Mismatched | 2.24 (1.59 – 3.13) | 0.0001* |
| Graft Type | 0.0007 | |
| PBSC | 1.00 | |
| Marrow | 0.61 (0.46 – 0.81) | |
| Viral Infections | ||
| Variable |
Ratio of Mean number of Infections* (95% CI) |
p- value |
| Donor/Recipient sex match | ||
| Female/Female | 1.00 | |
| Male/Male | 5.11 (1.78 – 14.42) | 0.002* |
| Female/Male | 3.31 (1.08 – 9.98) | 0.03* |
| Male/Female | 2.84 (0.90 – 8.80) | 0.07* |
| Donor/Recipient CMV Serostatus | ||
| Positive/Positive | 1.00 | |
| Positive/Negative | 1.13 (0.46 – 2.73) | 0.79* |
| Negative/Positive | 2.14 (1.03 – 4.40) | 0.04* |
| Negative/Negative | 0.30 (0.10 – 0.92) | 0.04* |
p-value of overall test
Compared to the reference group rate 1.0
Viral Infections
The mean estimated number of viral infections in the first 180 days post-transplant for the entire cohort was 0.90 per patient (Figure 1). The density of viral infection was similar. Recipients of an A/A donor had a mean of 0.93 viral infections per patient versus only 0.88 per patient per days at risk for a B/x donor (p=0.53). The cumulative incidence of viral infection was also similar (A/A donor: 45% [95% CI, 31 – 60]; B/x: 40% [95% CI, 29 to 52%], p=0.58).
More than half of all patients experienced no viral infections during the study period (A/A: n=24 [55%]; (B/x: n=43 [60%]). As expected, the predominant viral organism reported was CMV. There were 52 episodes of CMV reactivation during the study with 27 of these episodes occurring between weeks 5 and 12 following HCT. Other viral organisms reported included respiratory viruses (9 episodes) and HSV or VZV (14 episodes). Anti-viral prophylaxis post-HCT was similar between the two groups and consisted of predominantly either acyclovir or valacyclovir between day 0 and 4 weeks following HCT [A/A: n = 38 (86%); B/x: n = 62 (85%)]. This was continued through twelve weeks post-transplant in 59% of patients [A/A: n = 26(59%); B/x: n = 42 (58%)].
By Poisson regression analysis, the adjusted viral infection density (Figure 3) found that recipients of an A/A donor had a ratio of 1.09 compared to recipients of a B/x donor (95% CI, 0.68 – 1.75; p = 0.72). Donor-recipient sex match was associated with a higher ratio of viral infections in recipients of male donors regardless of recipient sex and with male recipients of female donors. Similarly, CMV serostatus was also significant such that a CMV seronegative recipient with a negative donor had a lower ratio of mean number of infections (0.3 [95% CI, 0.10 – 0.92], p = 0.04). Conversely, CMV seropositive recipients with a seronegative donor had a ratio of 2.14 (95% CI, 1.03– 4.40; p = 0.04) compared to the baseline group of seropositive donor and seropositive recipient. No other factors were significant in determining risks of viral infection.
Fungal Infections
Fungal infections were uncommon during the study period and included both yeast and mold infectious episodes. Only 26 fungal infection episodes were documented with 9 in the A/A group and 17 in the B/x group during the 180 days following HCT. Six of these were mold infections; 5 in the B/x cohort. Thirty-five patients in the A/A cohort and 54 patients in the B/x cohort had no fungal infections. Infection density analysis found that the mean estimated number of fungal infections was only 0.45 per patient (Figure 1). HCT from an A/A donor had a fungal infection density score of 0.33 compared to 0.53 for recipients of a B/x donor (p = 0.39). The cumulative incidence of fungal infection by day 180 in the A/A and the B/x cohorts were 20% (95% CI, 10 – 34) and 25% (16 – 36), respectively (p = 0.57). Over one-third of patients in each group received fluconazole for anti-fungal prophylaxis, particularly in the initial 4 weeks post-HCT.
When the fungal infection density was analyzed for other potential risk factors, patients with an A/A donor had a lower mean ratio of fungal infections at 0.62 (0.31–1.26) versus a B/x donor (p=0.19) (Figure 3). There were no other factors associated with altered risks of fungal infections.
All Infections
Over the study period only 19 (16%) patients had no infections. Of these, 15 were in the B/x cohort. Of these 19 patients, 7 received myeloablative conditioning (A/A = 2; B/x = 5), 8 received reduced intensity conditioning (A/A = 1; B/x = 7), and 4 received non-myeloablative conditioning (A/A = 1; B/x = 3). The mean number of all infections was 5.39 per patient per day at risk (Figure 1). The cumulative incidence of any infection by day 180 post-transplant was 93% (95% CI, 84 – 99) for A/A donor HCT compared to 88% (95% CI, 79 – 94) for a B/x donor(p = 0.30).
In the adjusted infection density analysis, recipients of an A/A donor had a mean ratio of infections of 1.14 (0.92 – 1.41; p = 0.23) compared to those with a B/x donor (Figure 3). Older donor age and CMV seropositive recipients were also associated with a higher ratio of infections.
Other Transplant Outcomes
Treatment related mortality (TRM) did not differ by KIR genotype. Recipients of an A/A donor compared to a B/x donor had 100-day TRM of 25% (95% CI, 12 – 40) versus 26% (95% CI, 16 – 39; p = 0.88). The cumulative incidence of grades II–IV acute GvHD at 100 days was also similar (A/A: 39% [95% CI, 25 – 53]; B/x: 32% [95% CI, 22 – 43], p=0.47). Survival at one year and chronic GvHD were also similar between the groups. Recipients of an A/A versus a B/x donor had a one year survival of 34% (95% CI, 21 – 49) versus 29% (95% CI, 19 – 40) and a cumulative incidence of chronic GvHD at one year of 34% (95% CI, 21 – 49) versus 36% (95% CI, 26 – 48).
In multivariate regression analysis, the donor KIR genotype had no impact on the relative risk of acute GVHD grades II-IV, chronic GVHD, or one year survival. Only marrow as the stem cell source decreased the risk of chronic GVHD (RR: PBSC =1.0, marrow = 0.39 (95% CI, 0.20 – 0.76); p=0.005).
Discussion
HCT from an unrelated donor with a B/x KIR genotype resulted in a lower cumulative incidence of bacterial infections by 6 months post transplant, although analyses of specific other infectious complications including viral, fungal and overall infections were similar. Furthermore, after adjusting for multiple infections and other factors including patient age, stem cell source, disease, HLA match and CMV serostatus, there is a modestly but not significantly lower number of bacterial infections using a KIR B/x unrelated donor. The use of a KIR B/x unrelated donor had no impact on any other transplant outcomes including acute and chronic GVHD, TRM and one year survival in this small cohort.
All infection data for our cohort of 116 patients were collected prospectively during the study period with detailed auditing and review for completeness. We also analyzed multiple classes of infection including bacterial, fungal, viral, and overall infectious syndromes. Several other groups have reported on the impact of NK cells and donor KIR—either KIR ligand match status or KIR genotype—on infectious complications following allogeneic HCT (15, 16, 21–24). Only the study by Kim et al. reported on multiple infectious complications, including CMV reactivation, overall infectious events, bacterial infections, viral infections and fungal infections (24). However, their study analyzed the graft dose of NK cells infused, rather than the KIR genotype, from 61 HLA identical sibling donors. They observed that patients infused with >5×107 NK cells/ kg had fewer bacterial infections and fewer overall infections.
Only two studies have included unrelated donor HCT and infectious complications in relation to NK cells and KIR (15, 23). Schaffer et al reported outcomes in 190 unrelated donor transplants who received anti-thymocyte globulin (23). They reported a higher TRM in patients receiving KIR ligand mismatched donor grafts (n=23), apparently due to increased infectious complications, but the details of the infections were not reported. Cook et al analyzed HCT infections based on the donor A/A and B/x genotype in 145 HLA identical siblings, 65 URD and 24 haploidentical donor grafts (12). In the subset of CMV seropositive patients receiving myeloablative conditioning regimen and a CMV seropositive HLA identical sibling graft, they observed a decreased risk of CMV reactivation if the donor had a KIR B/x genotype. This differed from our data and studies by others (16, 22).
In recipients of HLA identical sibling T-replete transplants, Chen reported that reactivation of CMV was similar in recipients of an A/A versus a B/x donor (16). However, if the donor had a higher number of activating KIR genes compared to the recipient, there was a decreased risk of CMV reactivation. In 43 HLA identical sibling transplants, Clausen found no impact of the KIR genotype on CMV reactivation, but did find higher non-relapse mortality in recipients with donors who had 3 or more activating KIR genes (22).
Taken together, these data highlight the complexity and the uncertainties of the NK cell and KIR effect on infectious complications following allogeneic HCT. Based upon our cohort size and the number of infectious events we could not fully address how diagnosis, conditioning intensity, use of T cell depletion and HLA matching or donor-recipient KIR ligand matching might interact with the donor A/A versus B/x KIR genotype. Nonetheless, we found a lower cumulative incidence of bacterial infections in recipients with a B/x donor although we were unable to analyze the NK cell dose as reported by Kim (24). While not reaching statistical significance, it is notable that 21% of recipients of a B/x donor had no infectious complications between day 0 and six months post transplant, compared to only 9% of patients receiving cells from an A/A donor. Larger cohorts, best studied prospectively, will be needed to analyze these infectious risks more fully.
Our findings that recipients of B/x unrelated donor grafts have fewer bacterial infections are intriguing. This could be due to indirect interactions between NK cells and other components of the innate immune system. Alternatively, a ubiquitous bacterial component may more effectively trigger NK cells with an activating KIR genotype. Finally, it is also possible that the alloreactive effects of NK cells may be permissive or facilitative for subsequent immune reconstitution and thus further decrease the risk for later infectious complications.
In conclusion, the role of donor KIR genotype on the development of infections post-transplant is potentially important, but may be modified by multiple factors including T cell depletion, the donor source (related versus unrelated versus haploidentical), and possibly, the KIR ligand match status. Analysis of a larger cohort may more fully determine the impact of NK cells and KIR genotype on infectious outcomes post-transplantation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.CIBMTR Summary Slides. 2009 http://www.cibmtr.org/SERVICES/summary_slides.html. 2009.
- 2.Abi-Rached L, Parham P. Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. The Journal of Experimental Medicine. 2005;201:1913–1932. doi: 10.1084/jem.20042558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhrberg M, Valiante NM, Shum BP, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 4.Lu Z, Zhang B, Chen S, et al. Association of KIR genotypes and haplotypes with susceptibility to chronic hepatitis B virus infection in Chinese Han population. Cell Mol Immunol. 2008;5:457–463. doi: 10.1038/cmi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genetics and the Immune Response. Tissue antigens; Abstracts of the 35th Annual Scientific Meeting of the Australasian Society for Immunology and the 14th International HLA and Immunogenetics Workshop; 4–8 Dec 2005; Melbourne, Australia. pp. 343–640. [DOI] [PubMed] [Google Scholar]
- 6.Hsu KC, Liu XR, Selvakumar A, Mickelson E, O'Reilly RJ, Dupont B. Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol. 2002;169:5118–5129. doi: 10.4049/jimmunol.169.9.5118. [DOI] [PubMed] [Google Scholar]
- 7.Uhrberg M, Parham P, Wernet P. Definition of gene content for nine common group B haplotypes of the Caucasoid population: KIR haplotypes contain between seven and eleven KIR genes. Immunogenetics. 2002;54:221–229. doi: 10.1007/s00251-002-0463-7. [DOI] [PubMed] [Google Scholar]
- 8.Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- 9.Gaudieri S, DeSantis D, McKinnon E, et al. Killer immunoglobulin-like receptors and HLA act both independently and synergistically to modify HIV disease progression. Genes Immun. 2005;6:683–690. doi: 10.1038/sj.gene.6364256. [DOI] [PubMed] [Google Scholar]
- 10.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 11.Rauch A, Laird R, McKinnon E, et al. Influence of inhibitory killer immunoglobulin-like receptors and their HLA-C ligands on resolving hepatitis C virus infection. Tissue Antigens. 2007;69 Suppl 1:237–240. doi: 10.1111/j.1399-0039.2006.773_4.x. [DOI] [PubMed] [Google Scholar]
- 12.de Arias AE, Haworth SE, Belli LS, et al. Killer cell immunoglobulin-like receptor genotype and killer cell immunoglobulin-like receptor-human leukocyte antigen C ligand compatibility affect the severity of hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2009;15:390–399. doi: 10.1002/lt.21673. [DOI] [PubMed] [Google Scholar]
- 13.Shilling HG, McQueen KL, Cheng NW, Shizuru JA, Negrin RS, Parham P. Reconstitution of NK cell receptor repertoire following HLA-matched hematopoietic cell transplantation. Blood. 2003;101:3730–3740. doi: 10.1182/blood-2002-08-2568. [DOI] [PubMed] [Google Scholar]
- 14.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook M, Briggs D, Craddock C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006;107:1230–1232. doi: 10.1182/blood-2005-03-1039. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Busson M, Rocha V, et al. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006;38:437–444. doi: 10.1038/sj.bmt.1705468. [DOI] [PubMed] [Google Scholar]
- 17.Houtchens KA, Nichols RJ, Ladner MB, et al. High-throughput killer cell immunoglobulin-like receptor genotyping by MALDI-TOF mass spectrometry with discovery of novel alleles. Immunogenetics. 2007;59:525–537. doi: 10.1007/s00251-007-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26:4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 21.Mancusi A, Ruggeri L, McQueen K. Donor Activating KIR Genes and control of infections after haploidentical haematopoietic transplantation. Bone Marrow Transplantation. 2006;37:3. [Google Scholar]
- 22.Clausen J, Wolf D, Petzer AL, et al. Impact of natural killer cell dose and donor killer-cell immunoglobulin-like receptor (KIR) genotype on outcome following human leucocyte antigen-identical haematopoietic stem cell transplantation. Clin Exp Immunol. 2007;148:520–528. doi: 10.1111/j.1365-2249.2007.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaffer M, Malmberg KJ, Ringden O, Ljunggren HG, Remberger M. Increased infection-related mortality in KIR-ligand-mismatched unrelated allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78:1081–1085. doi: 10.1097/01.tp.0000137103.19717.86. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Sohn SK, Lee NY, et al. Transplantation with higher dose of natural killer cells associated with better outcomes in terms of non-relapse mortality and infectious events after allogeneic peripheral blood stem cell transplantation from HLA-matched sibling donors. Eur J Haematol. 2005;75:299–308. doi: 10.1111/j.1600-0609.2005.00514.x. [DOI] [PubMed] [Google Scholar]