Summary
Controlled expansion and contraction of lymphocytes both during and after an adaptive immune response is imperative to sustaining a healthy immune system. Both extrinsic and intrinsic pathways of lymphocyte apoptosis are programmed to eliminate cells at the proper time to ensure immune homeostasis. Genetic disorders of apoptosis described in mice and humans have established Fas and Bim as critical pro-apoptotic molecules responsible for T-cell death in response to T-cell receptor restimulation and cytokine withdrawal, respectively. Emerging evidence prompts revision of this classic paradigm, especially for our understanding of restimulation-induced cell death (RICD) and its physiological purpose. Recent work indicates that RICD employs both Fas and Bim for T-cell deletion, dispelling the notion that these molecules are assigned to mutually exclusive apoptotic pathways. Furthermore, new mouse model data combined with our discovery of defective RICD in X-linked lymphoproliferative disease (XLP) patient T cells suggest RICD is essential for precluding excess T-cell accumulation and associated immunopathology during the course of certain infections. Here we review how these advances offer a refreshing new perspective on the phenomenon of T-cell apoptosis induced through antigen restimulation, including its relevance to immune homeostasis and potential for therapeutic interventions.
Keywords: T cells, T cell receptors, apoptosis, signal transduction, autoimmunity
Introduction
Clonal expansion of activated T cells represents the engine that propels an effective adaptive immune response leading to effector cytokine secretion, B-cell help for optimal antibody production, and/or cytotoxic elimination of infected target cells. The astonishing proliferative capacity of resting T cells [estimated at 109-1011-fold increase in cell number given optimal stimulation (1)], coupled with the potency of their acquired effector functions, requires several checkpoints to properly constrain and eventually curtail the response to prevent collateral damage. Among multiple layers of regulation capable of suppressing lymphocyte growth and function, programmed cell death via apoptosis remains the essential process for disposing excess lymphocytes reacting to either foreign or self-antigens. Without this barrier, prolonged persistence and/or unchecked accumulation of activated lymphocytes can result in immunopathology, autoimmunity, and lymphoid cancers. In this review, we explore the mechanistic details of ‘hard-wired’ apoptosis pathways that control the size and scope of the effector T-cell response at various stages, from initial antigen-driven proliferation through contraction and memory T-cell selection. We also discuss genetic disorders of impaired lymphocyte homeostasis. These disorders reveal the biological significance of these apoptotic pathways and provide new insights from lymphoproliferative diseases that were not previously thought to encompass T-cell death defects. Finally, we prognosticate on how autoregulatory T-cell apoptosis may be exploited therapeutically for peripheral tolerance induction via clonal deletion.
Two major pathways for T-cell apoptosis: RICD and CWID
Primary stimulation of T cells occurs upon prolonged encounter with antigen-presenting cells (APCs). Optimal T-cell activation is achieved via recognition of antigen (Ag)-loaded MHC complexes through a specific T cell receptor (TCR), and concomitant engagement of CD28, inducible costimulator (ICOS), and/or other costimulatory molecules on the APC surface. This interaction triggers blastogenesis, rapid production of interleukin 2 (IL-2), and upregulation of the high affinity IL-2 receptor CD25. IL-2 is a key growth and survival cytokine for activated T cells. Its uptake via CD25 binding comprises an autocrine signaling loop that drives responding T cells into cycle for several rounds of proliferation. Other cytokines and signaling receptors help to program specific effector functions for these responding T cells. However, it is the relative abundance of Ag and IL-2 in the surrounding milieu that ultimately dictates how and when the vast majority of effector T cells will proliferate or succumb to apoptosis (2) (Fig. 1). Indeed, emerging evidence suggests that relative sensitivity to IL-2 delineates CD25hi ‘terminally differentiated’ effector T cells that are more prone to apoptosis from CD25lo cells that give rise to long-lived memory T cells (3).
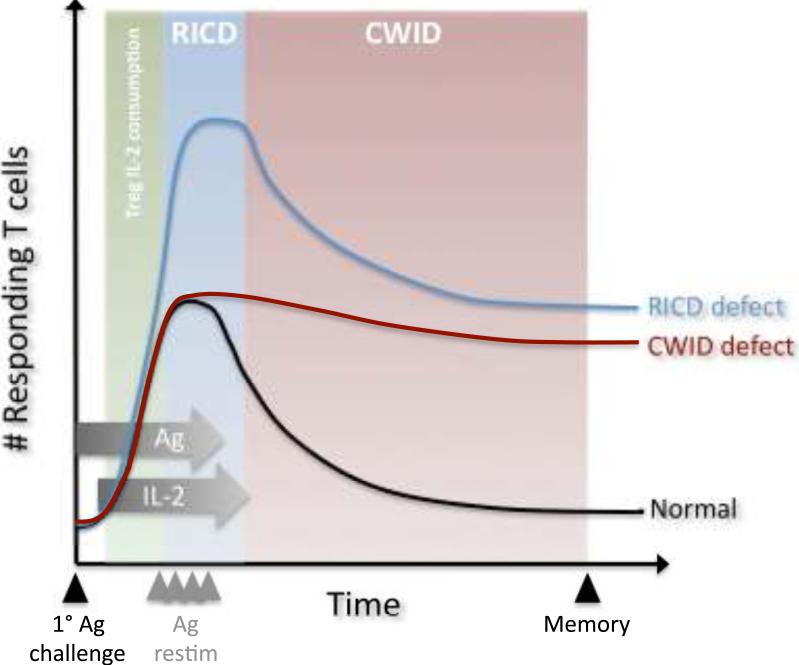
Fig. 1. Enforcement of T-cell homeostasis via apoptosis.
Primary Ag challenge induces clonal proliferation of T cells, IL-2 production, and acquisition of effector functions. For a typical immune response (black line), T-cell fate is determined by the relative abundance of Ag and IL-2 (horizontal gray arrows). TCR restimulation upon secondary encounter with Ag (Ag restim, gray arrowheads) can induce RICD in the presence of IL-2 (blue zone), setting an upper limit on T-cell expansion. When Ag is cleared and IL-2 levels decrease, CWID controls contraction of the effector T-cell pool (red zone), sparing a small pool of surviving memory T cells. In certain situations, consumption of IL-2 by Treg cells may suppress T-cell expansion by inducing CWID in freshly activated T cells (green zone). Defects in RICD (blue line) or CWID (red line) contribute to excess accumulation of T cells at different stages of the response, perturbing immune homeostasis.
When Ag and IL-2 are abundant, TCR re-engagement of an activated, cycling T cell can trigger an autoregulatory form of apoptosis we refer to herein as ‘restimulation-induced cell death’ (RICD). This phenomenon of ‘cell suicide’ was first described over 20 years ago in murine T-cell hybridomas and clones upon exposure to Ag-loaded APCs or agonistic anti-TCR Abs (4, 5). After subsequent work had demonstrated that TCR ligation was only connected to this built-in ‘death program’ in activated T cells that were proliferating after recent primary Ag stimulation (6, 7), this pathway became commonly referred to as ‘activation-induced cell death’ (AICD), a mellifluous but confusing term. As recent reviews have pointed out, AICD can encompass cell death triggered by antigen in any context (e.g. immature thymocyte deletion in the thymus), thus obscuring mechanistic differences (8-10). Moreover, the term AICD superficially and perhaps unintentionally equates the process of naive T-cell ‘activation’, synonymous with expansion and differentiation of apoptosis-resistant naive T cells, with TCR restimulation of activated, cycling T cells in the presence of IL-2 that promotes T-cell death. Because it is difficult to understand how an immune response could be launched if activation generally induced cell death, we demarcate these two processes and use RICD in reference to TCR-mediated apoptosis of mature T cells. In retrospect, it is now clear how a defined window of apoptosis susceptibility for non-transformed T cells, coupled to cell cycle progression, could explain why immortalized, continually cycling T-cell hybridomas are exquisitely sensitive to RICD without IL-2 (11, 12).
For primary T cells, competency to die through TCR restimulation is conferred by the presence of IL-2, although additional cytokines (e.g. IL-4) can participate by driving T cells into cycle (13). These requirements permit the conceptualization of RICD as a self-limiting negative feedback mechanism for controlling T-cell expansion during an ongoing immune response (2). The paradoxical buildup of activated T cells and autoantibodies in mice lacking IL-2 or IL-2 receptor subunits reflects the loss of this clonal deletion mechanism (14-16), which is exacerbated by a failure of polyclonal deletion by Treg cells (21). Through Ag-specific deletion in the periphery, RICD helps to limit immunopathologic or autoimmune manifestations that may arise from excessive effector T-cell expansion. This process also maintains adequate ‘space’ for other T-cell clones to respond to different antigenic challenges. Thus, RICD sets the upper boundary for T-cell expansion in the presence of persistent Ag and is therefore considered a ‘propriocidal’ form of cell death that constrains the size of the effector T-cell pool (2, 8).
Historically, RICD has primarily been studied in well-defined experimental systems, usually in vitro. Understanding its physiological relevance in vivo has required increasingly more sophisticated experimental approaches. There is also a paradox inherent in destroying effector T cells through Ag restimulation during an ongoing immune response. However, we posit that RICD establishes a propriocidal threshold for responding T cells, based on the quantity and quality of the antigenic stimulus, growth cytokines, and other environmental signals. The rapid reproduction of most microbial pathogens requires an explosive lymphocyte response which requires tempering. Self-regulation by feedback could minimize immune-mediated tissue damage during the unpredictable evolution of an infection. This balance between pathogen clearance and T-cell regulation is perturbed if normal RICD is impaired. For instance, a profound RICD defect in patients with X-linked lymphoproliferative disease (XLP) explains the acute bouts of lymphocyte hyperproliferation spurred by recurrent Ag encounters associated with persistent viral infections (17). As discussed later, XLP reveals the clinical significance of RICD in T-cell homeostasis.
Most effector T cells reacting to an invading pathogen are purged as the infection is cleared and IL-2 levels dwindle at the conclusion of a successful immune response. During this contraction phase, an intrinsic pathway of apoptosis is triggered by withdrawal of IL-2 and other cytokines, i.e. cytokine withdrawal-induced death (CWID). CWID culls the vast majority of effector T cells, save a select few that survive as memory T cells. Like RICD, the connection between apoptosis induction and IL-2 withdrawal was first established in mouse T-cell lines (18, 19). Kuroda and colleagues (20) demonstrated the physiological relevance of CWID in observing prolonged survival of staphylococcal enterotoxin A (SEA)-activated T cells in vivo in mice implanted with slow-release IL-2 osmotic pumps. Indeed, the decline of effector T-cell numbers under normal physiological conditions correlates well with waning IL-2 concentration in the surrounding environment. Consequently, CD25 expressed on the cell surface also decreases. This relationship is also exploited by IL-2-dependent, FoxP3+ regulatory T cells (Tregs) that can ‘steal’ survival cytokines from competing conventional T cells, ostensibly through constitutive expression of CD25 (21). Hence Tregs can suppress T-cell responses by accelerating the natural process of CWID via IL-2 consumption, examined in detail in the next section.
CWID and RICD operate at different phases of the immune response as hard-wired feedback response programs, influenced by the dynamic localization of cells, antigen, and cytokine. Both processes are exquisitely regulated by the availability of Ag and IL-2 as well as other growth/survival cytokines. Mechanistically, the prevailing dogma suggests that these two processes eliminate T cells through distinct biochemical mechanisms of apoptosis, known as the intrinsic and extrinsic pathways (2). The intrinsic pathway is controlled by relative expression of Bcl-2 family proteins that regulate mitochondrial outer membrane potential (MOMP). When mitochondria are depolarized, cytochrome c release catalyzes the cleavage and activation of procaspase 9. Extrinsic apoptosis is signaled principally through death receptors (DRs) of the tumor necrosis factor receptor (TNFR) superfamily, such as Fas. Ligand binding triggers the nucleation of death-inducing signaling complexes (DISCs) at the DR cytoplasmic tail, which serve as platforms for recruitment and autocatalytic cleavage and activation of procaspases 8 and 10. Both pathways converge at the activation of downstream caspases (i.e. caspase 3/6/7). In certain contexts, extrinsic apoptosis signals also trigger the intrinsic pathway. For example, cleavage of Bid by caspase 8 generates an active, truncated form (tBid) capable of inducing mitochondrial depolarization and magnifying downstream caspase activity (22). Dependence on this amplification loop for Fas-induced apoptosis distinguishes Type II from Type I cells; indeed, IL-2 may facilitate RICD in part by converting activated T cells to a Type I phenotype (23, 24).
CWID induces intrinsic apoptosis. Withdrawal of IL-2 or other γ-chain cytokines specifically upregulates and activates Bim, a key pro-apoptotic protein that antagonizes the function of anti-apoptotic Bcl-2 family proteins (e.g. Bcl-2, Bcl-xL, and Mcl-1) and activates Bax, which causes mitochondrial permeabilization (25). RICD is often wholly attributed to an extrinsic apoptosis signal through Fas, which may be stimulated in cis or in trans by membrane-anchored FasL exposed on the surface of restimulated T cells. The involvement of Bim and Fas in CWID and RICD, respectively, was unveiled in studies of mutant and knockout mouse models. Subsequently, genetic studies demonstrated the importance of Bim- and Fas-mediated apoptosis in human diseases (8, 26). However, as we discuss later, growing evidence forces us to reconsider how Fas and Bim may actually cooperate in governing T-cell homeostasis.
Regulatory T cells in CWID
Maintenance of immune homeostasis also encompasses a ‘dominant tolerance’ mechanism conferred by a population of CD4+CD25+ Tregs. Immune cells with ‘regulatory potential’ were first described as a subset of thymocytes that could suppress other helper T-cell activities (27). The existence of ‘suppressor’ T cells was doubted for several decades until two groups demonstrated that a naturally occurring population of CD4+CD25+ Tregs could inhibit Ag-induced expansion of CD4+ and CD8+ T cells (28, 29). Tregs can be specifically identified by the expression of a master regulatory protein called forkhead box protein 3 (Foxp3), which is absolutely required for Treg function (30). The biological importance of this subset emerged from seminal experiments showing that day 3 thymectomized mice and mice depleted of CD25+ Tregs succumbed to systemic autoimmunity (31-33). In humans, patients with immunodysregulation polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome suffer from severe atopic autoimmunity in the absence of functional Tregs, resulting from mutations in FOXP3 (34, 35). Despite a deluge of research on this fascinating cell subset, a number of incongruities have emerged regarding the mode of action of Tregs.
Early work conceived that Tregs suppress conventional T-cell responses by direct cell-cell contact through an ill-defined suppressive mechanism (29-33). Suggestions that Tregs blocked IL-2 production by T-effector cells, and thereby initial proliferation, failed to stand up to further scrutiny (21, 42). Some suggested that Treg:effector T-cell contact caused the death of target T cells (36). We and others have found that natural Tregs do not kill target T cells by active Fas or contact-dependent, granzyme-mediated mechanisms typically associated with CD8+ cytotoxic T lymphocytes (CTLs) (21, 37). A mechanism involving direct cytolysis, which typically occurs within hours, is incompatible with the relatively slow kinetics of Treg suppression (21). However, there is general consensus that Treg cells require IL-2 and close contact with target cells in vitro (38, 39). In 2005, Scheffold's and Stockinger's groups (40, 41) demonstrated that consumption of IL-2 by Tregs contributed to suppressive function. In line with these observations, we discovered that Tregs can deplete IL-2 from the microenvironment of stimulated Th0 cells, inducing intrinsic apoptosis of the latter (21). Because optimal IL-2 production by activated Th0 cells relies on a positive-feedback loop signals from available IL-2 in the local surroundings, early consumption of IL-2 by Tregs can perturb this feed-forward loop and exacerbate the effect of IL-2 deprivation. Thus, IL-2 consumption by Tregs induces early deletion of effector T cells via CWID. This deletional regulation is polyclonal, because cytokine deprivation in theory does not require specific antigen involvement. IL-2 consumption by Tregs also contributes to a lack of functional competency and cytokine production by lingering effector T cells at later stages of the T-cell response. Whether CWID is triggered solely by consumption of cytokines or in combination with transforming growth factor-β (TGF-β) or other environmental factors in vivo remains to be investigated. Nonetheless, polyclonal deletion is a form of fratricide that may be best exemplified by Tregs but also induced by other cytokine-dependent lymphocyte populations (21).
Curiously, close proximity but not direct physical contact is likely sufficient for Treg cells to compete effectively for cytokines produced by activated T cells or APCs and block the autocrine/paracrine loops essential for effector T-cell survival, proliferation, and cytokine production (41). This possibility was not considered in former interpretations of transwell experiments involving Tregs. Shimpl et al. (42) made the intriguing observation that Treg cells can block the autoimmune disease that develops in IL-2-deficient mice. Treg cells actually kill IL-2 knockout (KO) T cells in vitro, suggesting the possibility that Treg cells can cause apoptosis by consumption of cytokines other than IL-2 (21). Because Treg cells are themselves heavily dependent on common γ (γc)-chain cytokines, they will subjugate activated cells in the absence of IL-2. Indeed, we found that the addition of other γc-chain cytokines (e.g. IL-4, IL-7, IL-15, IL-21) to the Treg/Tresp co-culture completely rescues cells from apoptosis (21, 43).
Our discovery of Treg-mediated CWID suggests that their suppressive property depends mainly on cytokine availability, confirming this process is not antigen specific (44). Notably, Treg cells suppress experimental inflammatory bowel disease (IBD), in which local inflammatory pathology is partially driven by cytokine-dependent homeostatic proliferation of naive CD4+ cells in lymphopenic mice and not Ag-driven expansion (21). The diversity of autoimmune pathology noted in scurfy (Foxp3 mutant) mice and IPEX patients also attests to the significance of Treg suppression via CWID irrespective of target Ags (45). In both disease settings, general lymphocytosis and extensive T-cell infiltration in multiple tissues results in autoimmune-mediated damage to the small bowel, pancreas, thyroid, skin, and hematopoietic system.
Accelerated CWID by Tregs may exert suppression differently depending on the Treg:effector T-cell balance. In initial phases of the immune response, the effector T cell:Treg ratio is higher because effector T cells are proliferating and Tregs are not. However, Tregs then consume IL-2 from effector T cells, inducing their own proliferation and causing CWID of the IL-2 producers. Expanding Tregs can also produce suppressive mediators or trigger other cells to produce them under cytokine-deprived conditions. As the effector T cell:Treg ratio decreases, the remaining effector T cells could become more susceptible to functional suppression via TGF-β, IL-10, CTLA-4, etc. The ability of Tregs to accelerate CWID reduces the intensity of an immune response, which could prevent autoimmunity and maintain homeostasis of both conventional and regulatory T-cell subsets. Indeed, IPEX appears to be a genetic disorder of impaired polyclonal T-cell apoptosis in part, given some of the phenotypic similarities with other murine and human diseases we discuss in the next section.
The players and the consequences: genetic disorders of impaired lymphocyte apoptosis
CWID: Bim is in the driver's seat
The seminal role of Bim in the execution of CWID has been well established since the first description of Bim-deficient mice (46). Lymphocytes from these mice are refractory to apoptosis induced by IL-2 deprivation, which manifests phenotypically as lymphoid and myeloid hyperplasia, aberrant T-cell development, and autoimmune kidney disease. Cytokine withdrawal increases Bim expression, which tips the balance of pro- and anti-apoptotic Bcl-2 family protein expression enough to induce mitochondrial depolarization and apoptosis. Strasser's group and others (46-52) have implicated Bim in multiple immunological processes, including T-cell contraction following challenge with superantigen or viral infection, CD8+ T-cell cross-tolerance, negative selection of thymocytes, and regulation of antigen-presenting cells (APCs) including B cells and dendritic cells (DCs). Puma (p53-upregulated mediator of apoptosis), another pro-apoptotic Bcl-2 family protein, is upregulated as cytokine levels decline and can also participate in CWID and lymphocyte contraction, albeit to a lesser extent than Bim based on comparative KO mouse experiments (53, 54). Not surprisingly, Bim KO Tresps are resistant to death induction by Tregs (21). Treg cells can suppress IBD caused by CD4 cells derived from wildtype but not Bim KO mice, substantiating the connection between cytokine withdrawal and Bim-dependent apoptosis in vitro and in vivo (21).
The potency of Bim pro-apoptotic function is carefully regulated. For example, IL-2 signaling through Ras/extracellular signal-regulated kinase (ERK) activation promotes Bim protein degradation as well as destabilization of Bim mRNA (55, 56). We recently discovered a new form of autoimmune lymphoproliferative syndrome (ALPS) (discussed in further detail below) attributed to abnormal suppression of Bim expression in a patient harboring a gain-of-function NRAS mutation (26). Constitutive Ras/ERK signaling even after IL-2 withdrawal restrained Bim expression at abnormally low levels in the patient's lymphocytes, resulting in splenomegaly, lymphadenopathy, autoimmune cytopenias, and lymphoid malignancies. Unexpectedly, we also found partial resistance to RICD for this patient's T cells in a later report (57). Bim may therefore play a broader role in regulating T-cell apoptosis than first appreciated. Nevertheless, the importance of Bim for CWID in both mice and humans remains indisputable.
RICD: Is Fas the key?
The contribution of Fas/FasL interactions to RICD was first appreciated in the characterization of two spontaneous mouse mutants phenotypically described as lymphoproliferation (lpr) and generalized lymphoproliferative disease (gld). Both strains featured mild autoimmune manifestations akin to systemic lupus erythematosus (SLE) as well as lymphadenopathy and splenomegaly explained in part by accumulation of atypical CD4−CD8− double-negative T cells (DNT). Homozygous recessive mutations found in the genes encoding Fas (lpr) and FasL (gld) explained why the Fas-induced apoptosis signal was defective in T cells from these mice (58, 59). Activated T cells from lpr or gld mice also showed defective Ag-induced cell suicide, suggesting Fas and its ligand were directly involved in the implementation of RICD (60, 61). Fas/FasL signaling was inextricably linked to RICD in a series of studies that showed TCR crosslinking on T-cell hybridomas or CD4+ T-cell clones induced FasL on the cell surface and that blocking Fas/FasL interactions rescued cells from RICD (62-65). Later work demonstrated that TCR restimulation induces FasL expression in an IL-2-dependent manner, enhanced DISC assembly following Fas engagement, and ultimately sensitized Fas-bearing clones in an Ag-specific manner, sparing bystander cells from apoptosis despite their proximity to adjacent FasL+ restimulated cells (13, 24, 66).
Fas-mediated apoptosis was shown to be important in maintaining lymphocyte homeostasis in humans through the discovery of ALPS. Features of ALPS include an enlarged spleen and lymph nodes containing an abnormally high DNT population, autoimmune cytopenias driven by autoantibody production, and increased susceptibility to lymphoma development (67). Although similar patients had been documented decades earlier, the genetic etiology of this disease remained unknown until similarities to the lpr/gld mouse models were recognized, spurring the discovery of deleterious mutations in FAS and FASL in humans (68, 69). ALPS usually results from heterozygous germline FAS mutations that produce a non-functional receptor capable of ‘dominantly interfering’ with signaling from wildtype (WT) FAS molecules (68). Most ALPS patients fall into this category (termed ALPS Type Ia), although mutations in FASL (ALPS Type Ib) and caspase 10 (ALPS Type II), as well as somatic Fas mutations (ALPS Type Im) have also been uncovered (70-72).
Fisher et al. (68) observed an apoptosis defect in ALPS Ia patient T cells restimulated with agonistic anti-CD3 antibodies. These in vitro data using murine and human T cells collectively suggested that RICD employed Fas. Indeed, RICD has often been equated with Fas-induced death in the literature. Multiple studies since that time have challenged this notion and revealed greater intricacies in the molecular regulation of RICD. Most of the original experiments mentioned above focused on CD4+ T cells. Fas is undoubtedly a major contributor to RICD of CD4+ T cells, with variable sensitivity noted for certain differentiated subsets. To wit, pro-inflammatory Th17 cells are less susceptible due to high expression of c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein (cFLIP), which directly antagonizes Fas-mediated capsase 8/10 activation (73, 74).
Fas regulation of CD8+ T cell RICD appears, however, to be more tenuous. CD8+ T-cell clearance following lymphocytic choriomeningitis virus (LCMV) infection apparently proceeds normally in lpr and gld mice (75, 76). In fact, subsequent work suggests that Fas only plays a minor role in killing isolated activated CD8+ T cells following TCR re-engagement (57, 77). Instead, propriocidal death of CD8+ T cells probably relies on several different molecules for optimal RICD in different contexts. Under certain circumstances, other DR ligands including TNF and TNF-related apoptosis inducing ligand (TRAIL) have been implicated in CD8+ T-cell RICD (77, 78). However, these molecules may be minor players in CD8+ T-cell deletion, considering no severe defects in lymphocyte homeostasis are detected in mice deficient in TNF, TRAIL, or their respective DRs. Cytolytic granule exocytosis, utilized by CD8+ CTLs to kill virally infected target cells, also participates in self-regulatory apoptosis through the action of perforin and granzymes, particularly for ALPS patient T cells (60, 79). In fact, granzyme B may preferentially drive RICD in T helper 2 (Th2) CD4+ T cells, which are less sensitive to Fas-mediated apoptosis following TCR ligation than Th1 cells (80). Finally, we recently showed that TCR-induced upregulation of BIM expression figures prominently in human CD8+ T-cell RICD and that elevated BIM levels in activated T cells from ALPS patients correlated with normal or enhanced sensitivity to RICD in vitro (57). Increased BIM expression also accounts for deletion of Ag-specific CTLs in patients with chronic, uncontrolled hepatitis B virus infection (81). Viral infections featuring the persistent presence of antigen may represent the most physiologically relevant scenario for examining RICD in vivo. Taken together, these results belie the generalization that TCR- and Fas-induced apoptosis are one and the same.
Some of the most compelling insights into the role of Fas in RICD come from an elegant study using conditional knockout mice in which Fas was selectively ablated from T cells (82). Their Fas-deficient effector T cells displayed comparable expansion and contraction relative to WT counterparts following strong, repeated Ag-challenges in vivo, implying that RICD remained functional during peak responses in these mice. In contrast, T-cell-restricted loss of Fas still gave rise to autoantibody production and an expanded DNT population, likely derived from the extended survival of chronically stimulated autoreactive T cells. Thus, Fas may be responsible for deletion of autoreactive T-cell clones but expendable for RICD of normal effector T cells.
So what can be said with confidence about Fas signaling in immune homeostasis? Since the original characterization of lpr and gld mice, more sophisticated mouse models reveal Fas to be an important safeguard against autoimmunity through elimination of autoreactive T cells as well as APCs. Selective ablation of Fas expression in B cells or dendritic cells (DCs) also results in ALPS-like lymphoproliferation and systemic autoimmunity (82). Similarly, when FasL is specifically removed from T cells, mild autoimmunity develops concomitantly with the extended survival and accumulation of DNTs and APCs, including B cells and splenic CD11chi DCs (83). Disruption of germinal center (GC) architecture and reduced T-cell dependent antibody production also occur, which could affect the selection of memory B cells and plasma cells. High Fas expression on GC B cells makes them sensitive to apoptosis until T-cell help is conveyed through CD40 engagement, establishing a competitive clonal selection process. In fact, excising Fas from germinal center B cells alone causes fatal T and B-cell overproliferation (84). In summation, these findings redefine Fas as an important regulator of self-tolerance in the periphery through clearance of excess APCs and autoreactive T and B-cell clones, especially in GC reactions. This regulatory function naturally extends to tumor suppression, explaining the variety of B-cell lymphomas that appear in humans with impaired Fas-FasL signaling (85). Interestingly, FasL deficiency in B cells also gives rise to autoimmunity, including excessive T-cell accumulation in aged mice (83). More work is required to understand how Fas-FasL interactions between T and B cells may be cell-autonomous or interdependent or in maintaining lymphocyte homeostasis. Nevertheless, the role of Fas in regulating diverse immune cell types profoundly influences peripheral tolerance.
Compensatory cooperation between Fas and Bim in driving RICD
The former paradigm proposing two mutually exclusive apoptotic pathways for T-cell deletion, Fas-driven RICD versus Bim-mediated CWID, now appears too simplistic. We appreciate that RICD features hallmarks of the intrinsic apoptosis pathway, including Fas-independent mitochondrial depolarization and caspase 9 activation (57). Moreover, changes in the balance of pro- and anti-apoptotic Bcl-2 family members modulate RICD sensitivity. For example, caspase-mediated cleavage of hematopoietic progenitor kinase 1 (HPK1) after primary Ag stimulation sensitizes effector T cells to RICD; the HPK1-C cleavage fragment selectively suppresses inhibitor of NF-κB (IκB) kinase (IKK) activity upon TCR re-engagement, thereby precluding NF-κB-mediated upregulation of anti-apoptotic proteins like Bcl-2A1 (86). Surprisingly, TCR restimulation triggers robust Bim upregulation in activated T cells in the presence of IL-2, apparently superseding suppressive signals associated with IL-2 signaling (87). Further analysis revealed this induction was required for full RICD sensitivity. Knockdown of Bim expression using small interfering (RNA) siRNA partially rescued primary T cells following restimulation, particularly in the CD8+ compartment. This may explain why more activated CD8+ T cells accrue in Bim−/− mice compared to WT immediately following infection with LCMV and HSV-1 (51, 52). Enhanced expansion of Bim−/− CD8+ T cells is unchecked due to impaired RICD in the presence of plentiful viral antigens. This defect is conceptually different from defective T-cell contraction in the absence of Bim once the infection is cleared and IL-2 levels dissipate. Thus, Fas- and Bim-induced apoptotic signals apparently collaborate to optimize RICD. Adaptability may derive from the preferential use of these extrinsic or intrinsic pathways for different antigens and T-cell lineages.
The generation and characterization of Bim-deficient lpr mice, reported by three independent groups in 2008 (88-90), provides ample evidence that Fas and Bim are likely the two essential partners involved in ensuring T-cell homeostasis, at least in rodents. Compared with single mutant mice, loss of both Fas and Bim function markedly accelerates lymphocytosis with dramatic DNT expansion, lymphadenopathy and splenomegaly, and early-onset, fatal SLE-like autoimmune disease. This striking phenotype recapitulates previous studies in which transgenic Bcl-2 overexpression greatly exacerbated lymphocyte accumulation (particularly DNTs) in lpr mice – consistent with the assertion that Bim affects the balance of Bcl-2-related proteins at the mitochondrion (91, 92). The severity of immunopathology in Bim−/− lpr mice, compared with relatively mild autoimmunity noted on the C57BL/6 background for either single mutant mouse, indicates that Fas and Bim apparently synergize in downsizing immune responses. Experiments in which single and double mutant mice with different viral infections shed light on the kinetics Ag-specific CD8+ T-cell contraction. Hughes et al. (88) found that clonal CD8+ T-cell deletion following acute HSV-1 infection was largely Bim-dependent. In contrast, both Fas and Bim were necessary for clonal deletion during chronic murine herpesvirus-68 (MHV-68) infection, which is thought to resemble EBV infection in humans (88). Also, Weant et al. (90) observed that elimination of three clonal CD8+ T-cell populations responding to acute LCMV infection also required both Fas and Bim, with variable persistence of expanded clones documented for different antigen specificities.
These results yield several important insights into the cooperative function of Fas and Bim in terminating T-cell responses, especially for RICD in CD8+ T cells. First, enhanced expansion and greater absolute numbers of Ag-specific populations in Bim−/− lpr mice was noted for each viral infection tested relative to Bim−/− or lpr mice alone, although the timing and magnitude of these differences varied. Second, the dual requirement for Fas- and Bim-driven clonal deletion was more apparent with chronic infection, during which repeated TCR stimulations with persistent Ags is probable. Differences in the acute infection models used were also telling: acute LCMV infection relays systemic inflammatory signals via replication in multiple lymphoid and non-lymphoid tissues, whereas HSV-1 infection is usually limited to the site of infection (i.e. hind footpads). A greater and more widely disseminated Ag burden associated with acute LCMV infection may require RICD-mediated T-cell regulation, explaining why both Fas and Bim are necessary in this scenario. Third, T-cell accumulation varied with genotype in different lymphoid tissues; significant differences in LCMV- and MHV68-specific CD8+ T-cell numbers measured for Bim−/− lpr mice versus single mutants were more pronounced in the lymph nodes. More research is required to ascertain whether Fas- and Bim-dependent RICD is more relevant to T-cell regulation in different secondary lymphoid compartments, perhaps related to Ag abundance or presentation by varying assortments of local APCs. Finally, distinct antigenic determinants elicited variable expansion and contraction profiles, as illustrated by the responses of CD8+ T-cell populations specific for three different LCMV peptides. These differences could reflect variation in the strength of TCR signaling, which is known to directly affect RICD sensitivity.
These studies illustrate how Fas and Bim can compensate for one another in vivo in regulating T-cell homeostasis (9), fitting nicely with independent findings in human T cells (57). This ‘counterbalance’ effect is particularly relevant to the process of RICD in CD8+ T cells. Bim compensation for the loss of Fas in this situation is likely established only from early development, explaining why short-term blockade or knockdown of either molecule in vitro results in a partial, readily detectable reduction in RICD. Conversely, CD8+ T cells from ALPS Ia patients display normal RICD sensitivity and higher steady-state Bim expression than normal controls, suggesting they could be ‘primed’ for deletion in this manner (57). RICD is therefore a key physiological governor of T-cell responses and peripheral tolerance. Moreover, Fas- and Bim-mediated apoptosis pathways are distinct but not mutually exclusive, as both can cooperate in propriocidal T-cell death of effector T cells.
SAP, NTB-A, the ‘threshold’ model of RICD
No ALPS patients have been described with lymphoproliferative disease (LPD) comparable in severity to that found in Bim−/− lpr mice. Loss of Fas signaling results in the accumulation of relatively anergic DNTs, and apparently permits the survival of autoreactive lymphocytes that likely experience weak, chronic restimulation with circulating self-antigens. Stronger TCR signals delivered through direct CD3 crosslinking, or perhaps in the context of certain infections, coordinate several apoptotic mediators for RICD. The studies summarized above tell us that Fas and Bim are the two most potent weapons for killing T cells upon secondary TCR engagement. Regardless of whether the primed T cell encounters self- or foreign Ag upon restimulation, the signal must reach a certain ‘threshold’ to trigger RICD. This threshold model of RICD fits with a demonstrated correlation between RICD sensitivity and the extent of TCR-induced tyrosine phosphorylation on the CD3ζ chain – a well-established barometer of TCR signal strength. Although the first of three immunoreceptor tyrosine-based activation motifs (ITAMs) found in the CD3ζ chain is the most potent apoptosis stimulator, phosphorylation of multiple ITAMs on CD3ζ and single ITAMs on the CD3 ε, γ, or δ chains amplifies the restimulation signal for optimal RICD (93). Consequently, the intensity of RICD is also linked to the amount of TCR expression; the aggregation of more active CD3 complexes generates a stronger, sustained signal (94). Hence the threshold model of RICD resembles the negative selection system designed to eliminate self-reactive clones in the thymus, whereby T cells that receive too strong a signal are deleted through a Bim-dependent mechanism.
Lymphocyte stimulation is not conveyed through antigen receptor signaling in isolation. Undoubtedly, a combination of costimulatory and inhibitory molecules also affect RICD upon secondary encounter with an APC. Unfortunately, our understanding of how such signals modify the RICD threshold in different situations is incomplete. For example, the effect of CD28 signaling on RICD remains nebulous. Initial data suggested that CD28 costimulation did not affect RICD (95). Since then, conflicting data on how CD28 can positively or negatively regulate RICD sensitivity has emerged under disparate experimental conditions. Perhaps even less is understood about exactly what makes the internal biochemistry of T-cell stimulation distinct in naive versus effector T cells, fating the latter population to die upon TCR ligation.
Our recent discovery regarding impaired RICD for T cells derived from XLP patients reveals a clue to this conundrum (17). XLP is a rare disease affecting 2-3 per million boys that typically remains undiagnosed prior to infection with the Epstein-Barr virus (EBV). The unique vulnerability of XLP patients to acute EBV infection was appreciated in Purtilo's first description of the disease (96, 97). Classic XLP1 patients harbor null mutations in SH2D1A, the gene encoding a small SH2-adapter protein known as SLAM (signaling lymphocyte activation molecule)-associated protein (SAP). Loss of SAP expression results in impaired humoral responses stemming from poor T-cell help to B cells, defective natural killer (NK) and CTL-mediated cytotoxicity, and failed development of specialized natural killer T (NKT) cells (98). These phenotypes are largely recapitulated in SAP knockout mice, suggesting SAP participates in several signaling processes in murine and human lymphocytes. Our new findings ascribe a pro-apoptotic function for SAP in potentiating TCR signal strength for optimal RICD. We found that SAP deficiency results in a specific, profound defect in RICD but not activation in XLP patient T cells. RNA interference-mediated silencing of SAP expression in control T cells recapitulated this defect, which was more prominent in CD8+ T cells. The absence of SAP impairs TCR-induced upregulation of FasL, Bim, and other pro-apoptotic mediators required for RICD. However, RICD was rescued by bypassing proximal TCR signaling with phorbol ester/ionomycin treatment or directly enhancing TCR signal strength using more powerful anti-CD3 agonistic antibodies and extensive crosslinking. Hence, SAP plays an integral part in boosting proximal TCR signal strength to surpass the RICD threshold and commit the cell to death.
SAP was originally discovered based on its association with SLAM, the founding member of an immunomodulatory co-receptor family expressed on hematopoietic cells (99). In general, homotypic association of SLAM family receptors induces SAP to dock with phosphorylated ITAMs in their cytoplasmic tails. SAP binding displaces pre-bound inhibitory phosphatases SHP1 or SHP2 and recruits Src family kinases like FYN and LCK that propagate further signals through downstream substrate phosphorylation. In this fashion, SAP facilitates the costimulatory functions of several SLAM family members expressed on T cells and NK cells. In its absence, these receptors transmit inhibitory signals through SHP1 and SHP2 that antagonize activating receptors, including the TCR. Thus, SAP is a molecular switch governing SLAM receptor-derived signals linked to multiple immune functions (100).
We also explored the biochemical link between SAP and TCR signal transduction. An RNA interference-based screen of SLAM receptors revealed that specific knockdown of SLAMF6, also known as NK, T, and B-cell antigen (NTB-A), diminished RICD sensitivity. Additionally, TCR restimulation increases SAP binding to NTB-A as SHP-1 association is reduced. NTB-A receptors colocalize with CD3 clusters on the cell surface, where SAP-bound NTB-A can presumably enhance TCR signaling cascades via exclusion of SHP1 and/or recruitment of additional kinases. Although we found that FYN is dispensable for RICD, our current data implicate other Src-family kinases in this process (A.L. Snow, unpublished observations). As predicted by the ‘molecular switch’ model of SAP function, NTB-A relays a weak SHP1-mediated inhibitory signal in XLP patient T cells upon TCR engagement, such that knockdown of either NTB-A or SHP1 partially restores RICD sensitivity in the absence of SAP. The same biochemical mechanism dictates activating or inhibitory NTB-A signals for NK cell cytotoxic function in normal versus XLP patients, respectively (101).
These data provide a coherent concept for how SAP-NTB-A signaling promotes RICD by augmenting TCR signaling. Molecular refinement of this idea may shed light on how co-receptor molecules may adjust TCR signals to achieve RICD thresholds in different T-cell subsets in varying antigenic environments. Indeed, SAP in combination with NTB-A and other SLAM receptors may assist in ‘fine tuning’ TCR signal strength upon restimulation to influence lymphocyte apoptosis sensitivity during immune responses. These observations may also portend novel treatment strategies for XLP patients, since several new targets seem possible. For example, selective SHP-1 or NTB-A inhibitors may lower the RICD threshold for SAP-deficient T cells and help rein in excessive CD8+ T-cell proliferation coincident with certain viral infections (see below). Conversely, SAP mimetics that block recruitment of SHP-1 molecules near the engaged TCR complex could boost signaling over the RICD threshold.
Disorders of defective RICD: XLP vs. ALPS
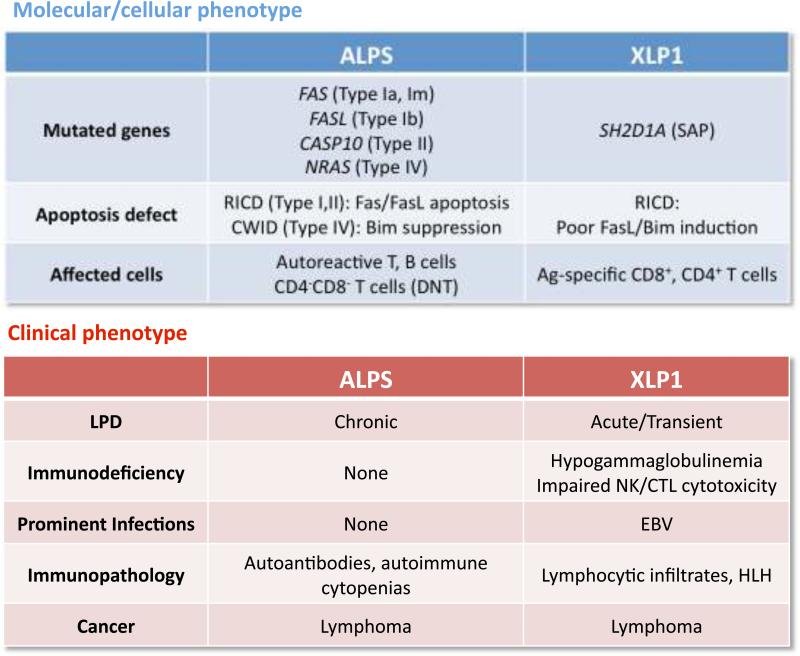
Our investigation of apoptosis sensitivity in XLP patient T cells was based on the hypothesis that lymphocyte accumulation associated with loss of SAP expression might be related to LPD characteristic of other congenital disorders of programmed cell death such as ALPS. Loss of Fas signaling alone in ALPS Ia patients creates a unique accumulation of relatively anergic DNTs and biases towards the survival of autoreactive lymphocytes that likely experience weak, chronic restimulation with circulating self-antigens. We now appreciate that stronger TCR signals, perhaps in the context of certain infections, rely on the cooperative action of several apoptotic mediators for RICD, including Fas/FasL and Bim. When proximal signaling is dampened by the absence of SAP, TCR restimulation resembles partial agonist signaling susceptible to negative feedback regulation through SHP-1 (102). This directly diminishes the upregulation of both FasL and Bim. These biochemical differences explain why ALPS and XLP are distinct immune disorders, despite their shared connection to apoptosis defects (Fig. 2). The data suggest that disruption of Fas-mediated apoptosis in ALPS applies chiefly to peripheral tolerance. Autoreactive B cells escape germinal center selection and secrete autoantibodies, as self-reactive T cells survive repeated encounters with self-Ags in secondary lymphoid organs. By contrast, genetic deficiency of SAP impairs TCR signaling and thus applies more specifically to RICD of effector cells, particularly for CD8+ T cells. SAP-deficient T cells are routinely killed in response to direct Fas ligation or IL-2 withdrawal. If foreign Ag is cleared properly after an infection, therefore, it stands to reason that T-cell contraction via CWID proceeds normally in XLP patients, explaining why they lack the chronic splenomegaly or lymphadenopathy seen in ALPS. Because SAP-deficient T cells do not provide adequate B-cell help, autoantibody production is limited by generally poor humoral immunity(103). However, both ALPS and XLP patients share an increased likelihood of B-cell lymphoma development, regardless of EBV exposure (85, 97). This increased susceptibility probably stems from a common failure of B-cell homeostasis. Fas-mediated elimination of activated B cells could malfunction due to Fas pathway mutations (ALPS) or flawed induction of FasL on activated T cells (XLP).
Fig. 2.
Distinguishing features of two human genetic diseases of defective RICD: ALPS vs. XLP1.
Closer examination of XLP pathogenesis offers new insight into the physiological importance of RICD. Unlike ALPS, patients with XLP suffer from acute bouts of T-cell accumulation often associated with infection. Without SAP-dependent RICD in place to restrain expansion, activated effector T cells accumulate in lymph nodes, lungs, and other antigen-rich environments. The severity of acute LPD in these patients may correlate with Ag levels during infection, as well as the speed at which that infection is cleared by the adaptive immune response. Ag persistence during a chronic viral infection exacerbates LPD, especially CD8+ effector T cells. Also, participating APCs survive longer due to insufficient FasL expression on the T cells they continue to re-encounter. This vicious cycle can contribute to more severe phenotypes seen in some XLP patients, including vasculitis, macrophage activation syndrome (MAS), hemophagocytic lymphohistiocytosis (HLH), and fulminant infectious mononucleosis (FIM) associated with EBV infection. In these situations, XLP patients often succumb to extensive immune-mediated damage to host tissues following infiltration of activated lymphocytes into the liver, central nervous system (CNS), and other organs.
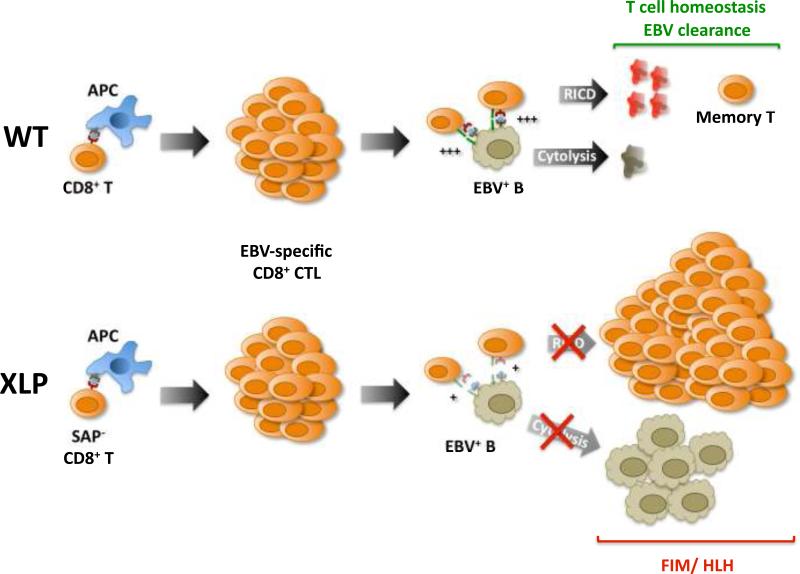
It is also interesting to reflect on why EBV is the most life-threatening infection that accompanies XLP. First, the first stages of lytic and latent EBV infection in the tonsil promote survival and proliferation of a large population of highly immunogenic EBV+ B cells. These B cells serve as APCs, triggering a vigorous immune response directed against multiple viral antigens. The majority of EBV-infected B cells are rapidly eliminated by CTLs and NK cells in normal individuals. This forces the virus into a benign latent state that persists in a small pool of memory B cells for the lifetime of the host (104). In XLP patients, the expansion of EBV+ B lymphoblasts is not controlled effectively for two reasons. First, recent data indicate SAP is required to form long-lasting T-B cell conjugates, explaining why B-cell help is not delivered effectively by SAP-deficient T cells (105). Second, SAP deficiency also spoils effector cell cytotoxic capability, leaving EBV-specific CTLs and NK cells unable to kill infected B-cell targets (106). Because EBV cannot be cleared, effector CD8+ T cells experience short-lived encounters with EBV+ B cells that weakly restimulate the TCR. Third, without SAP, TCR restimulation signals are intrinsically weaker and fail to push activated CD8+ T cells past the threshold for RICD. Thus, the unbridled accumulation of CD8+ T cells during EBV infection in XLP patients reflects multiple impairments of RICD, potentially constituting a ‘perfect storm’ of lethal EBV-induced FIM/HLH (Fig. 3).
Fig. 3. Impaired RICD of SAP-deficient CD8+ T cells contributes to EBV-induced FIM in XLP patients.
In normal individuals (WT), primary EBV exposure primes a robust CD8+ T-cell response to viral antigens. EBV-specific CTLs can strongly conjugate with and lyse EBV+ B lymphoblasts, helping to clear almost all virally infected cells. The relatively strong TCR restimulation signal (+++) received from this encounter triggers RICD and helps maintain T-cell homeostasis. In XLP patients, CTL generation proceeds normally, but ablation of SAP results in poor T-B conjugation, impaired cytotoxic elimination of EBV-infected B cells, and weak TCR restimulation (+) that fails to induce RICD. Consequently, unchecked expansion of both EBV+ B lymphoblasts and activated CD8+ T cells can give rise to fatal EBV-induced FIM/HLH.
This unique pathology in XLP patients illustrates how RICD maintains T-cell homeostasis during widely disseminated or chronic viral infections. Similar to LCMV or MHV-68 infection in Bim−/− lpr mice, EBV and other persistent infections in XLP patients highlight the consequences of defective RICD for an ongoing immune reaction with abundant Ag still prevailing. SAP-deficient mice, which mirror many of the immune anomalies in XLP patients, also show enhanced clonotypic expansion of activated CD8+ T cells following LCMV and MHV-68 infection (107-109). Furthermore, Chen and colleagues (110) observed that naive SAP−/− T cells expressing the OVA-specific transgenic OT-I die slightly less readily in the first three rounds of proliferation following OVA challenge – notably similar to the pronounced RICD defect we observed in activated CD8+ T cells from XLP patients. Interestingly, a pro-apoptotic role for SAP may extend to death pathways exercised in response to c-Myc translocation or DNA damage in premalignant lymphocytes (111, 112).
Exploiting RICD for tolerance induction
As we understand more about the intricacies of RICD for T-cell homeostasis, opportunities for new therapeutic strategies can be developed. For example, the process of RICD could be exploited for achieving tolerance in certain autoimmune disease settings via induced clonal deletion. The feasibility of this approach for ameliorating disease in experimental autoimmune encephalitis (EAE), a mouse model of multiple sclerosis (MS), has been long known (111). Repeated high-dose delivery of myelin basic protein (MBP) specifically deleted pathologic T cells expressing a transgenic TCR specific for an immunogenic MBP peptide (Ac1-11). This treatment protected the myelin sheath surrounding CNS neurons from autoimmune-mediated damage (113). Therefore, RICD provides one explanation for the paradoxical suppression of immune responses after high dose Ag administration observed long ago (114). This principle likely extends to additional settings, most notably solid organ transplantation. For instance, deletional tolerance of activated CD8+ T cells is now recognized as a contributor in improving the acceptance of allogeneic grafts with concomitant transfer of donor bone marrow cells (115). Antigen-directed clonal deletion can thereby be contemplated as a therapy for autoimmune diseases, allergic disorders, and allograft rejection.
In principle, this approach has advantages over current concepts to adoptively transfer Treg cells, which would cause polyclonal deletion of activated, cytokine-dependent T cells. By contrast, methods for inducing T-cell anergy or non-deletional exhaustion never rid the body of pathologic lymphocytes. This raises issues of therapeutic efficacy, since these inactive states of T cells are potentially reversible. However, selective deletion of autoreactive T cells offers perhaps the best path for inducing peripheral tolerance. CD4+ T cell deletion, moreover, could potentially also abrogate autoantibody production by depriving self-reactive B cells of adequate T-cell help. If this postulate is validated, utilization of RICD induced by high-dose auto-Ag administration could be extended to several autoimmune diseases that involve autoantibody-mediated destruction of self-tissues, including myasthenia gravis, autoimmune cytopenias, and others.
Several parameters must be carefully considered in the application of this therapeutic technique. Identification and recombinant production of immunodominant epitopes representing the most common and/or disease-causing Ags must be a priority. The timing, dosage, and route of Ag delivery must also be calibrated for each disease to avoid the unintended expansion of self-reactive T cells that might ensue from a typical prime-boost immunization protocol. Ideally, high-dose Ag should be given shortly after priming, when T cells are still in cycle and IL-2 is plentiful – two preconditions required for optimal RICD. Additional approaches for tipping the balance towards RICD and away from memory T-cell generation can also be contemplated. Possible interventions include neutralization of survival cytokines like IL-7 and IL-15 or forced costimulation through NTB-A or other receptors to artificially lower the RICD threshold. Finally, the lineage and maturation status of APC populations tasked for Ag presentation to targeted T cells could also be manipulated to induce deletion upon restimulation. Although we have much to learn in harnessing RICD for therapeutic purposes, this could be an exciting strategy for tolerance induction in the treatment of autoimmune diseases.
Conclusion
The immunological role of programmed cell death was appreciated first in the thymus and later for equilibrating and terminating peripheral lymphocyte responses. New studies are giving us a more thorough and nuanced understanding of how RICD contributes to immune system regulation. Studies in both murine and human cells have highlighted both Fas/FasL and Bim as the key controllers of effector T-cell expansion via RICD and contracting excess cells via CWID. Rare genetic disorders, such as ALPS, XLP, and related diseases, illustrate how defective RICD gives rise to deadly LPD. We continue to study unique patients with unexplained LPD and T-cell apoptosis defects, with the hope of uncovering mutations in novel genes that participate in RICD (A.L. Snow, unpublished data). The unforeseen role for SAP and NTB-A in RICD is a testament to the value of this approach and a sobering reminder of how incompletely we understand this process after two decades of research. Detailed analyses of genomic signatures and biochemical pathways that correspond to survival or death after TCR ligation are also beginning to provide valuable information (116). The interpretation of intriguing new data reviewed here and the reevaluation of older observations it prompts have revised outdated paradigms of lymphocyte homeostasis and revitalized our appreciation for RICD in normal and pathologic physiology. It also reminds us to avoid falling prey to intellectually appealing ‘either-or’ models of regulation at the expense of appreciating nature's unfailing ability to evolve new and more wondrous means to control complex processes with a limited protein set.
Acknowledgements
We thank all of the patients and their families for their participation in our research. We thank our collaborators at NIH (Helen Su, Joao Bosco Oliveira, Tom Fleisher, Koneti Rao) for past and present contributions to ALPS-related research. We also thank our colleagues at Cincinnati Children's Hospital (Rebecca Marsh, Lisa Filipovich, Jack Bleesing) for their continued collaboration and critical reading of this manuscript. Research in our laboratory is supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH. A.L. Snow holds a Pharmacology Research Associate Training Program (PRAT) Fellowship from the National Institute of General Medical Sciences.
References
- 1.Levine BL, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 2.Lenardo M, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 3.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8(+) T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Ashwell JD, Cunningham RE, Noguchi PD, Hernandez D. Cell growth cycle block of T cell hybridomas upon activation with antigen. J Exp Med. 1987;165:173–194. doi: 10.1084/jem.165.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashwell JD, Longo DL, Bridges SH. T-cell tumor elimination as a result of T-cell receptor-mediated activation. Science. 1987;237:61–64. doi: 10.1126/science.3037698. [DOI] [PubMed] [Google Scholar]
- 6.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 7.Russell JH, White CL, Loh DY, Meleedy-Rey P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc Natl Acad Sci USA. 1991;88:2151–2155. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidere N, Su HC, Lenardo MJ. Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol. 2006;24:321–352. doi: 10.1146/annurev.immunol.24.021605.090513. [DOI] [PubMed] [Google Scholar]
- 9.Bouillet P, O'Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–519. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 10.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehme SA, Lenardo MJ. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol. 1993;23:1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 12.Shi YF, Sahai BM, Green DR. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989;339:625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- 13.Zheng L, Trageser CL, Willerford DM, Lenardo MJ. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J Immunol. 1998;160:763–769. [PubMed] [Google Scholar]
- 14.Kneitz B, Herrmann T, Yonehara S, Schimpl A. Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2-deficient mice. Eur J Immunol. 1995;25:2572–2577. doi: 10.1002/eji.1830250925. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 16.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 17.Snow AL, et al. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duke RC, Cohen JJ. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 19.Rodriguez-Tarduchy G, Lopez-Rivas A. Phorbol esters inhibit apoptosis in IL-2-dependent T lymphocytes. Biochem Biophys Res Commun. 1989;164:1069–1075. doi: 10.1016/0006-291x(89)91778-6. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda K, et al. Implantation of IL-2-containing osmotic pump prolongs the survival of superantigen-reactive T cells expanded in mice injected with bacterial superantigen. J Immunol. 1996;157:1422–1431. [PubMed] [Google Scholar]
- 21.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 23.Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz I, Krueger A, Baumann S, Schulze-Bergkamen H, Krammer PH, Kirchhoff S. An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death. J Immunol. 2003;171:2930–2936. doi: 10.4049/jimmunol.171.6.2930. [DOI] [PubMed] [Google Scholar]
- 25.Merino D, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira JB, et al. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci USA. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–914. [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudensky A. Foxp3 and dominant tolerance. Philos Trans R Soc Lond B Biol Sci. 2005;360:1645–1646. doi: 10.1098/rstb.2005.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982;156:1565–1576. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunologic research. 2007;38:112–121. doi: 10.1007/s12026-007-0022-2. [DOI] [PubMed] [Google Scholar]
- 35.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 36.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 39.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 40.Barthlott T, et al. CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol. 2005;17:279–288. doi: 10.1093/intimm/dxh207. [DOI] [PubMed] [Google Scholar]
- 41.Scheffold A, Huhn J, Hofer T. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur J Immunol. 2005;35:1336–1341. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- 42.Schimpl A, et al. IL-2 and autoimmune disease. Cytokine Growth Factor Rev. 2002;13:369–378. doi: 10.1016/s1359-6101(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 43.Pandiyan P, Lenardo MJ. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct. 2008;3:6. doi: 10.1186/1745-6150-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 45.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–538. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 47.Hildeman DA, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 48.Chen M, Huang L, Wang J. Deficiency of Bim in dendritic cells contributes to overactivation of lymphocytes and autoimmunity. Blood. 2007;109:4360–4367. doi: 10.1182/blood-2006-11-056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davey GM, et al. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196:947–955. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the proapoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grayson JM, Weant AE, Holbrook BC, Hildeman D. Role of Bim in regulating CD8+ T-cell responses during chronic viral infection. J Virol. 2006;80:8627–8638. doi: 10.1128/JVI.00855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci U S A. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erlacher M, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischer SF, Belz GT, Strasser A. BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection. Proc Natl Acad Sci USA. 2008;105:3035–3040. doi: 10.1073/pnas.0706913105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 56.Matsui H, Asou H, Inaba T. Cytokines direct the regulation of Bim mRNA stability by heat-shock cognate protein 70. Mol Cell. 2007;25:99–112. doi: 10.1016/j.molcel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Snow AL, Oliveira JB, Zheng L, Dale JK, Fleisher TA, Lenardo MJ. Critical role for BIM in T cell receptor restimulation-induced death. Biol Direct. 2008;3:34. doi: 10.1186/1745-6150-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 60.Gillette-Ferguson I, Sidman CL. A specific intercellular pathway of apoptotic cell death is defective in the mature peripheral T cells of autoimmune lpr and gld mice. Eur J Immunol. 1994;24:1181–1185. doi: 10.1002/eji.1830240526. [DOI] [PubMed] [Google Scholar]
- 61.Russell JH, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alderson MR, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunner T, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 64.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 65.Ju ST, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 66.Hornung F, Zheng L, Lenardo MJ. Maintenance of clonotype specificity in CD95/Apo-1/Fas-mediated apoptosis of mature T lymphocytes. J Immunol. 1997;159:3816–3822. [PubMed] [Google Scholar]
- 67.Sneller MC, et al. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest. 1992;90:334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fisher GH, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 69.Rieux-Laucat F, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 70.Holzelova E, et al. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. N Engl J Med. 2004;351:1409–1418. doi: 10.1056/NEJMoa040036. [DOI] [PubMed] [Google Scholar]
- 71.Wang J, et al. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 72.Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98:1107–1113. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu Y, et al. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114:1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Xu G, Zhang L, Roberts AI, Shi Y. Th17 cells undergo Fas-mediated activation-induced cell death independent of IFN-gamma. J Immunol. 2008;181:190–196. doi: 10.4049/jimmunol.181.1.190. [DOI] [PubMed] [Google Scholar]
- 75.Lohman BL, Razvi ES, Welsh RM. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor-ligand interactions. J Virol. 1996;70:8199–8203. doi: 10.1128/jvi.70.11.8199-8203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmermann C, Rawiel M, Blaser C, Kaufmann M, Pircher H. Homeostatic regulation of CD8+ T cells after antigen challenge in the absence of Fas (CD95). Eur J Immunol. 1996;26:2903–2910. doi: 10.1002/eji.1830261215. [DOI] [PubMed] [Google Scholar]
- 77.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 78.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 79.Mateo V, et al. Perforin-dependent apoptosis functionally compensates Fas deficiency in activation-induced cell death of human T lymphocytes. Blood. 2007;110:4285–4292. doi: 10.1182/blood-2007-05-088286. [DOI] [PubMed] [Google Scholar]
- 80.Devadas S, et al. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 81.Lopes AR, et al. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118:1835–1845. doi: 10.1172/JCI33402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stranges PB, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mabrouk I, et al. Prevention of autoimmunity and control of recall response to exogenous antigen by Fas death receptor ligand expression on T cells. Immunity. 2008;29:922–933. doi: 10.1016/j.immuni.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 84.Hao Z, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Straus SE, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 86.Brenner D, et al. Caspase-cleaved HPK1 induces CD95L-independent activation-induced cell death in T and B lymphocytes. Blood. 2007;110:3968–3977. doi: 10.1182/blood-2007-01-071167. [DOI] [PubMed] [Google Scholar]
- 87.Sandalova E, Wei CH, Masucci MG, Levitsky V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc Natl Acad Sci USA. 2004;101:3011–3016. doi: 10.1073/pnas.0400005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hutcheson J, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 90.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8(+) T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 91.Reap EA, Felix NJ, Wolthusen PA, Kotzin BL, Cohen PL, Eisenberg RA. bcl-2 transgenic Lpr mice show profound enhancement of lymphadenopathy. J Immunol. 1995;155:5455–5462. [PubMed] [Google Scholar]
- 92.Strasser A, Harris AW, Huang DC, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Combadiere B, Freedman M, Chen L, Shores EW, Love P, Lenardo MJ. Qualitative and quantitative contributions of the T cell receptor zeta chain to mature T cell apoptosis. J Exp Med. 1996;183:2109–2117. doi: 10.1084/jem.183.5.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.She J, Matsui K, Terhorst C, Ju ST. Activation-induced apoptosis of mature T cells is dependent upon the level of surface TCR but not on the presence of the CD3 zeta ITAM. Int Immunol. 1998;10:1733–1740. doi: 10.1093/intimm/10.11.1733. [DOI] [PubMed] [Google Scholar]
- 95.Boehme SA, Zheng L, Lenardo MJ. Analysis of the CD4 coreceptor and activation-induced costimulatory molecules in antigen-mediated mature T lymphocyte death. J Immunol. 1995;155:1703–1712. [PubMed] [Google Scholar]
- 96.Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan's disease). Lancet. 1975;1:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 97.Seemayer TA, et al. X-linked lymphoproliferative disease: twenty-five years after the discovery. Pediatr Res. 1995;38:471–478. doi: 10.1203/00006450-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 99.Sayos J, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 100.Veillette A, Dong Z, Perez-Quintero LA, Zhong MC, Cruz-Munoz ME. Importance and mechanism of ‘switch’ function of SAP family adapters. Immunol Rev. 2009;232:229–239. doi: 10.1111/j.1600-065X.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- 101.Bottino C, et al. NTB-A [correction of GNTB-A], a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. J Exp Med. 2001;194:235–246. doi: 10.1084/jem.194.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 103.Cannons JL, et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 105.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bassiri H, Janice Yeo WC, Rothman J, Koretzky GA, Nichols KE. X-linked lymphoproliferative disease (XLP): a model of impaired anti-viral, anti-tumor and humoral immune responses. Immunol Res. 2008;42:145–159. doi: 10.1007/s12026-008-8048-7. [DOI] [PubMed] [Google Scholar]
- 107.Crotty S, McCausland MM, Aubert RD, Wherry EJ, Ahmed R. Hypogammaglobulinemia and exacerbated CD8 T-cell-mediated immunopathology in SAP-deficient mice with chronic LCMV infection mimics human XLP disease. Blood. 2006;108:3085–3093. doi: 10.1182/blood-2006-04-018929. [DOI] [PubMed] [Google Scholar]
- 108.Czar MJ, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yin L, et al. Mice deficient in the X-linked lymphoproliferative disease gene sap exhibit increased susceptibility to murine gammaherpesvirus-68 and hypo-gammaglobulinemia. J Med Virol. 2003;71:446–455. doi: 10.1002/jmv.10504. [DOI] [PubMed] [Google Scholar]
- 110.Chen G, Tai AK, Lin M, Chang F, Terhorst C, Huber BT. Increased proliferation of CD8+ T cells in SAP-deficient mice is associated with impaired activation-induced cell death. Eur J Immunol. 2007;37:663–674. doi: 10.1002/eji.200636417. [DOI] [PubMed] [Google Scholar]
- 111.Nagy N, Klein E. Deficiency of the proapoptotic SAP function in X-linked lymphoproliferative disease aggravates Epstein-Barr virus (EBV) induced mononucleosis and promotes lymphoma development. Immunol Lett. 2010;130:13–18. doi: 10.1016/j.imlet.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Nagy N, Matskova L, Kis LL, Hellman U, Klein G, Klein E. The proapoptotic function of SAP provides a clue to the clinical picture of X-linked lymphoproliferative disease. Proc Natl Acad Sci USA. 2009;106:11966–11971. doi: 10.1073/pnas.0905691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Critchfield JM, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 114.Mitchison NA. Induction of Immunological Paralysis in Two Zones of Dosage. Proc R Soc Lond B Biol Sci. 1964;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- 115.Fehr T, et al. Rapid deletional peripheral CD8 T cell tolerance induced by allogeneic bone marrow: role of donor class II MHC and B cells. J Immunol. 2008;181:4371–4380. doi: 10.4049/jimmunol.181.6.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parish IA, et al. The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood. 2009;113:4575–4585. doi: 10.1182/blood-2008-10-185223. [DOI] [PMC free article] [PubMed] [Google Scholar]