Abstract
Toll-like receptors (TLRs) recognize microbial/viral-derived components that trigger innate immune response and conflicting data implicate TLR agonists in cancer, either as protumor or antitumor agents. We previously demonstrated that TLR3 activation mediated by its agonist poly(I:C) induces antitumor signaling, leading to apoptosis of prostate cancer cells LNCaP and PC3 with much more efficiency in the former than in the second more aggressive line. The transcription factor hypoxia-inducible factor 1 (HIF-1) regulates several cellular processes, including apoptosis, in response to hypoxia and to other stimuli also in normoxic conditions. Here we describe a novel protumor machinery triggered by TLR3 activation in PC3 cells consisting of increased expression of the specific I.3 isoform of HIF-1α and nuclear accumulation of HIF-1 complex in normoxia, resulting in reduced apoptosis and in secretion of functional vascular endothelial growth factor (VEGF). Moreover, we report that, in the less aggressive LNCaP cells, TLR3 activation fails to induce nuclear accumulation of HIF-1α. However, the transfection of I.3 isoform of hif-1α in LNCaP cells allows poly(I:C)-induced HIF-1 activation, resulting in apoptosis protection and VEGF secretion. Altogether, our findings demonstrate that differences in the basal level of HIF-1α expression in different prostate cancer cell lines underlie their differential response to TLR3 activation, suggesting a correlation between different stages of malignancy, hypoxic gene expression, and beneficial responsiveness to TLR agonists.
Introduction
Hypoxia-inducible factor 1 (HIF-1) is a basic helix-loop-helix transcription factor that regulates a number of genes required for hypoxic response through binding specific regions of their promoters, named hypoxia-responsive elements [1–3]. HIF-1 is active only as a heterodimer of HIF-1α and HIF-1β subunits. HIF-1β is constitutively expressed in all cell types, whereas HIF-1α levels are tightly controlled. Under normoxia, HIF-1α levels are low because of the proteasomal degradation initiated by oxygen-sensing prolyl hydroxylases. Under hypoxic conditions, HIF-1α is stabilized and freely binds HIF-1β, forming active HIF-1 transcription complex [4]. In humans, three different isoform of hif-1α were recently described: I.1, ubiquitous and responsible for the transcriptional activity of the hypoxic response; I.2, expressed specifically in the testis and plays a dominant-negative function with respect to the I.1 isoform [5]; I.3, has recently been found highly expressed in peripheral blood leukocytes, in the thymus, and in activated T cells [6].
It has been clearly demonstrated that HIF-1 regulates genes relevant to cancer progression reviewed in Dery et al. [7], especially as a predictor of clinical outcome in patients with adenocarcinomas [8]. In particular, HIF-1α has emerged as a potential prognostic biomarker in the proteomic assessment of prostate cancer [9] because clinical observation of high-grade prostate intraepithelial neoplasia lesion (precursor of most prostate adenocarcinoma, PCa) showed increased HIF-1α expression [10], and HIF-1α up-regulation in PCa as well as in prostate cancer bone metastases has been observed [11]. PCa is a prevalent tumor among elderly men, and survival benefit with current PCa therapies is often limited [12]. Indeed, standard pharmacological therapy, consisting of withdrawal of androgens, leads only to transient regression of the disease, and there is no cure for prostate cancer once it becomes refractory to androgen. Although the HIF-1α protein is mainly induced by hypoxic conditions, other stimuli can strongly increase the HIF-1 complex in normoxic conditions and modulate the transcription of hypoxic genes. These stimuli include reactive nitrogen-derived [13] or oxygen-derived radicals [14], cytokines [15,16], growth factors [17], and T-cell receptor stimulation [6,18]. Interestingly, a variety of molecular components derived from bacteria or viruses have also been described to activate HIF-1α in normoxia through specific Toll-like receptors (TLRs) [19–21]. These are a group of transmembrane proteins (11 in humans) that recognize pathogen-associated molecular patterns as well as endogenous damage-associated molecular patterns [22] and elicit pathogen-induced and noninfectious inflammatory responses [23]. TLRs were initially detected only on immune cells [24], but recent studies demonstrate that tumor cells express functional TLRs and that TLR signaling can promote opposite outcomes: tumor growth and immune evasion or apoptosis and cell cycle arrest [25–28]. The TLR3-ligand poly(I:C) mimics the action of double-stranded RNA (dsRNA), the genetic material of many viruses, and TLR3 engagement, directly inhibits cell proliferation, and induces tumor cell death [28,29]. We have previously demonstrated that LNCaP cells, an androgen-dependent human prostate cancer cell line, are sensitive to poly(I:C)-induced apoptosis, whereas PC3 cells, a more aggressive androgen-independent prostate cancer cell line, show a weak sensitivity to the same stimulus [27]. Recently, a number of articles reported the ability of HIF-1 complex to mediate the resistance to several apoptotic stimuli by inducing antiapoptotic genes such as Bcl-xL, survivin, and MCL-1 described to be HIF-1 target genes [30–32]. On the basis of these data, we have hypothesized that the limited response of PC3 cells to poly(I:C) could be due to the induction of a parallel protumoral signal involving HIF-1 complex activation. Here we report evidence showing that poly(I:C) treatment activates TLR3 and enhances the transcription of the I.3 isoform of hif-1α in the prostate cancer cell line PC3 but not in the less aggressive LNCaP cells. We also demonstrate that TLR3 stimulation of PC3 cells induces HIF-1α-dependent vascular endothelial growth factor (VEGF) secretion and resistance to poly(I:C)-induced apoptosis, whereas these responses to poly(I:C) are obtained in LNCaP cells, only forcing overexpression of the hif-1α-I.3 isoform. Taken together, our results suggest that the levels of HIF-1α in prostate cancer cells might play a crucial role in the antitumor potential of TLR3 stimulation.
Materials and Methods
Cell Lines and Reagents
LNCaP and PC3 prostate cancer cells were purchased from ATCC (Manassas, VA) and were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 2 mM l-glutamine, 100 IU/ml penicillin-streptomycin, and 10% fetal calf serum (FCS; Sigma-Aldrich, St Louis, MO). For all the experiments, prostate cancer cell lines were serum-starved for 18 hours and then stimulated with poly(I:C) (InvivoGen, San Diego, CA) in FCS-free medium. Human umbilical vein endothelial cells (HUVECs) were maintained in EGM-2 complete medium (Lonza, Basel, Switzerland). Actinomycin D (Act D), cycloheximide (CHX), and cobalt chloride (CoCl2) were from Sigma. MG-132 was from Calbiochem-Merck (Darmstadt, Germany).
Western Blot Analysis and Nuclear Extracts Preparation
Cell lysates were prepared in cell lysis buffer (Cell Signaling, Danvers, MA). Equal amounts of proteins (40 µg) were subjected to SDS-polyacrylamide gel electrophoresis and were transferred onto a nitrocellulose membrane saturated with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20. Membranes were incubated with primary antibody and subsequently with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Membranes were washed with Tris-buffered saline with 0.1% Tween 20 and developed using the chemiluminescence system (Amersham Bioscience, Piscataway, NJ). Antibody anti-HIF-1α was from BD Biosciences (San Diego, CA), cleaved caspase-3 (Asp175) was from Cell Signaling, and anti-β-actin was from Sigma. Secondary antibodies were horseradish peroxidase-conjugated goat antimouse or antirabbit (Bio-Rad, Hercules, CA).
To perform Western blot analysis of nuclear extracts, cells were washed in ice-cold PBS, scraped, collected in buffer A (10 mM Tris, pH 7.8, 1.5 Mm MgCl2, and 10mM KCl), and kept on ice for 10 minutes before Dounce homogenization. Nuclei were then pelleted at 1000g for 7 minutes at 4°C, resuspended in 150 µl of ice-cold lysis buffer, incubated for 30 minutes on ice in the presence of proteases inhibitor cocktail (Sigma), and then stored at -80°C. Protein concentration was determined by the micro bicinchoninic acid method (Pierce, Rockford, IL).
Transfection Assay
One day after plating (1 x 105 cells/ml), cells were transiently transfected with 1 µg of a plasmid encoding I.3 isoform of hif-1α [6] or relative control plasmid (pcDNA3) or with 1 µg of a plasmid containing an interfering sequence targeting hif-1α (int.hif-1α) or the relative control (int. scramble) previously described [33]. Cells were transfected for 5 hours with Lipofectamine LTX/plus (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Stimulation with poly(I:C) was performed 18 hours later.
To obtain stably transfected PC3 cells with nonfunctional TLR3, cells were transfected with 1 µg of TLR3 dominant-negative plasmid (TLR3-DN; pZERO-hTLR3; InvivoGen) or with 1 µg of plasmid containing only the resistance to puromycin (pPURO), using Lipofectamine LTX/plus as described previously. Forty-eight hours later, the cells were trypsinized and seeded at different dilutions onto 100-mm plates in complete DMEM supplemented with 2 µg/ml puromycin (InvivoGen). After incubating at 37°C for 2 to 3 weeks, individual colonies were isolated using sterile cloning rings, trypsinized, and plated onto 12-well plates. The clones obtained were expanded and maintained in DMEM supplemented with 2 µg/ml puromycin.
Total DNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocol, and to confirm the presence of TLR3-DN plasmid in the clones selected, semiquantitative polymerase chain reaction (PCR) was performed using 1 µg of total DNA, specific primers (10 µM) (forward, 5′-GAACGTTCTTTTTCGCAACG-3′; reverse, 5′-CTCATTGTGCTGGAGGTTCA-3′), and 1.5 U of Taq DNA polymerase (Invitrogen). Data were normalized for the expression of β-actin.
RNA Extraction and Real-time Quantitative Reverse Transcription-PCR Analyses
Total RNA was extracted using TRIzol reagent (Invitrogen) and used 3 µg for the reverse transcription reaction by using a SuperScript First-Strand Synthesis System Kit (Invitrogen). One microgram of total complementary DNA was used for quantitative reverse transcription (qRT)-PCR analysis performed in triplicate for each sample. cDNA was mixed with 0.5 µM of both forward and reverse primers and with 20 µl of MasterMix (SYBR Green JumpStart; Sigma). Reactions were performed by a 7500 Fast Real-time PCR System (Applied Biosystems, Carlsbad, CA). Primer sequences were as follows: generic hif-1α, forward 5′TGGCCTTGTGAAAAAGGGT3′ and reverse 5′ TTGATGGGTGAGGAATGGGT3′; and vegf, forward 5′CCTTGCTGCTCTACCTCCAC3′ and reverse 5′TGGTGATGTTGGACTCCTCA3′. Amounts of the specific isoform of hif-1α were estimated by the comparative Ct method using ribosomal protein L32 messenger RNA (mRNA; forward 5′CATCTCCTTCTCGGCATCA3′ and reverse 5′AACCCTGTTGTCAATGCCTC3′) and I.3 isoform mRNA (forward 5′TGGTGGTTACTCAGCACTTTTA-GA3′ and reverse 5′CTCCGACATTGGGAGCTCAT3′).
ELISA
The level of VEGF was evaluated by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol and was normalized to the number of adherent cells counted at the time of collection.
In Vitro Morphogenesis
A total of 5 x 104 HUVECs were seeded in 48-well microtiter plates containing polymerized Matrigel and incubated with conditioned medium (CM) obtained from either untreated or poly(I:C)-treated cells or in the presence of specific human VEGF neutralizing antibody (1 µg/ml; R&D Systems). After 6 hours, cells were fixed with 4% formaldehyde in PBS for 30 minutes at room temperature, washed, and stored in PBS at 4°C. The number of cell intersections in six random microscopic fields was counted in triplicate wells at 10x original magnification, using the inverted phase-contrast microscope Leitz Fluovert (Wetzlar, Germany).
Apoptosis Assay
For propidium iodide (PI) staining, cells were detached with trypsin, washed with cold PBS plus 5% FCS, and then fixed in 70% ethanol for 24 hours. After washing with PBS, cells were incubated with 1 µg/ml PI for 3 hours at 25°C before FACS analysis by a Coulter Epics XL flow cytometer (Beckman Coulter, Fullerton, CA). Cells were considered apoptotic when their DNA content was <2N (sub-G1 cell population). Results were analyzed with Win MDI software (Scripps Research Institute, La Jolla, CA) and were presented as a percentage of specific apoptosis determined using the following formula: [(% apoptotic cells in experimental sample - % apoptotic cells in control sample) / (100 - % apoptotic cells in control sample) x 100].
Statistical Analysis
Statistical differences were determined either by Student's t test for paired samples or by one-way analysis of variance followed by Student's t test with the Bonferroni correction. P ≤ .05 was considered significant. Densitometric analysis was performed using AIDA software.
Results
HIF-1α Is Upregulated by Poly(I:C) in PC3 Cells
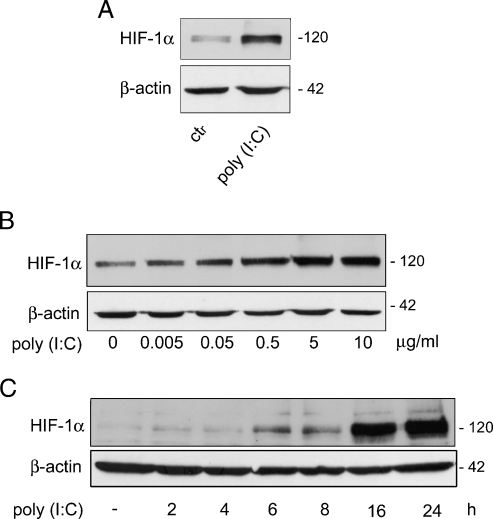
Because it has been reported that the TLR4 and 7/8 agonists induce hypoxic genes in macrophages in normoxic condition [19,20], we examined the possibility that TLR3 stimulation could modulate HIF-1α protein in PC3 cells. Cells were treated with the TLR3 agonist poly(I:C) and levels of HIF-1α determined by Western blot. As shown in Figure 1A, poly(I:C) induces a marked up-regulation of HIF-1α levels in total cell lysates. Because activated HIF-1α rapidly moves into the nucleus, cells were treated with increasing doses of poly(I:C) for 6 hours, and HIF-1α expression was evaluated in nuclear extracts. As shown in Figure 1B, nuclear accumulation of HIF-1α was strongly induced by poly(I:C) in a dose-dependent manner. Time course analysis showed that an increase of HIF-1α was first observed in the nuclear extracts after 6 hours of exposure to 5 µg/ml poly(I:C), and maximal induction was obtained after 16 hours (Figure 1C). These results demonstrate that, in PC3 cells, stimulation with poly(I:C) induces accumulation of HIF-1α and its translocation to the nucleus.
Figure 1.
Poly(I:C) treatment induces time- and dose-dependent HIF-1α nuclear accumulation in PC3 cells Western blot analysis. (A) Cells were treated with poly(I:C) (25 µg/ml) for 16 hours, and whole-cell extracts were analyzed for HIF-1α. (B) Cells were treated with the indicated doses of poly(I:C) for 6 hours, and nuclear extracts were analyzed for HIF-1α. (C) Nuclear extracts from cells treated with 5 µg/ml poly(I:C) for the indicated times. β-Actin was used as control for equalamounts (30 µg/lane) of protein loaded. Data shown are typical of three separate experiments with similar results.
HIF-1α Accumulation on Poly(I:C) Treatment Is Mostly Accounted for by the Induction of a Specific Isoform of HIF-1α mRNA
It is well known that protein stabilization is the predominant mechanism by which HIF-1α protein is increased during hypoxia, whereas the regulation of HIF-1 under normoxic conditions is less clear [7]. As several articles reported that increased transcription of the hif-1α gene accounts for HIF-1α protein induction under normoxic conditions [34], we hypothesized that this mechanism could be implicated in the induction of HIF-1α by poly(I:C) in PC3 cells and investigated whether hif-1α mRNA was directly upregulated in a time course qRT-PCR experiment. A statistically significant increase in the levels of hif-1α mRNA was induced within 4 hours of poly(I:C) stimulation, and a further boost was observed after 6 hours (Figure 2A). To confirm that the increase of hif-1α mRNA is important for elevated protein levels, PC3 cells were treated with poly(I:C) for 6 hours, then transcriptional inhibitor Act D or protein synthesis inhibitor CHX were added and incubated for 15 minutes. Figure 2B (upper panel) shows that both Act D and CHX completely abolished poly(I:C)-dependent HIF-1α protein induction, whereas in cells treated with CoCl2, a chemical hypoxia-mimic that induces stabilization of HIF-1α protein, CHX and Act D did not inhibit HIF-1α protein accumulation, as expected. Indeed, this observation is consistent with previous studies showing that CoCl2 as well as hypoxia had no effect on HIF-1α protein synthesis but blocked its degradation. On the contrary, our results indicated that the induction of HIF-1α protein by poly(I:C) needed de novo synthesis of both hif-1α mRNA and protein in PC3 cells. Next, we analyzed whether poly(I:C) enhances HIF-1α protein stability, and to this end, we treated PC3 cells with the proteasome inhibitor MG-132 to block the degradation of HIF-1α. As shown in Figure 2B (lower panel), in the absence of poly(I:C), MG-132 induced an increase in the level of HIF-1α. When the MG-132-treated cells were also exposed to poly(I:C), we observed a substantial increase of HIF-1α protein level compared with MG-132 alone, whereas the addition of CHX reverted the increase to the level of MG-132 alone, confirming a crucial role of de novo synthesis of HIF-1α protein in poly(I:C)-induced HIF-1α accumulation. In contrast, exposure of PC3 cells to CoCl2 combined with MG-132 resulted in a greater increment of HIF-1α compared with CoCl2 alone, an increase which was not significantly inhibited by CHX, as expected (Figure 2B, lower panel). In accordance with this result, we did not observe, after poly(I:C) treatment, reactive oxygen species accumulation, which has been implicated in TLR-mediated HIF-1α stabilization because of the down-regulation of prolyl hydroxylase [35] (data not shown).
Figure 2.
Poly(I:C) stimulation specifically induces the I.3 isoform of HIF-1α in PC3 cells. (A) Cells untreated or treated with 5 µg/ml poly(I:C) for the indicated times were analyzed for hif-1α mRNA levels by qRT-PCR. Each point represents the mean of triplicate samples from three independent experiments with SD as error bars, *P ≤ .05. (B) Cells were treated with 5 µg/ml poly(I:C) for 5 hours 45 minutes and then with CHX (2 µg/ml) or Act D (2 µg/ml) for 15 minutes (upper panel). Cells were treated with 5 µg/ml poly(I:C) for 20 hours 30 minutes with or without 5 µg/ml MG-132 for the last 3 hours in the presence or absence of CHX (2 µg/ml) for 30 minutes (lower panel). Treatment with 100 µM CoCl2 for 3 hours was used as hypoxic stimulus. HIF-1α protein was detected by Western blot on whole-cell lysates. β-Actin was used as loading control. (C) qRT-PCR with specific primers was used to evaluate the different hif-1α isoforms induced after poly(I:C) stimulation. Data shown in panel B are typical of three separate experiments with similar results. The histogramin panel C represents the mean of triplicate samples from three independent experiments with SD as error bars, *P ≤ .05, **P = .01.
Because we recently described a novel mRNA isoform I.3 of HIF-1α that is upregulated in activated human T lymphocytes, we investigated the levels of different hif-1α isoforms in PC3 cells and their regulation through TLR3. Because the primers used for the previously mentioned qRT-PCR amplify both I.1 and I.3 hif-1α isoforms, we used isoform-specific primers to study which isoform of hif-1α was induced by poly(I:C). As shown in Figure 2C, I.1 isoform of hif-1α gene, canonically activated by hypoxia, is not affected by poly(I:C) stimulation, whereas I.3 isoform mRNA is upregulated after 6 hours of treatment at the same degree observed in qRT-PCR for total hif-1α mRNA shown in Figure 2A.
Poly(I:C)-Induced HIF-1α Accumulation Is TLR3-Dependent
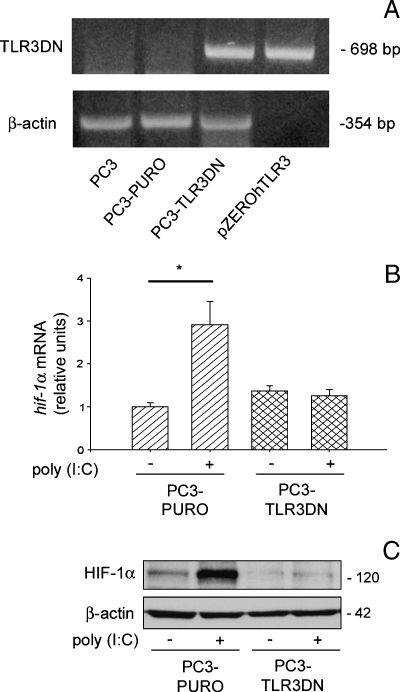
Different molecules besides TLR3 are involved in dsRNA recognition, because poly(I:C) is able to activate also different pathways mediated by cytosolic sensors [36,37]. To determine whether the observed HIF-1α accumulation was directly dependent on TLR3 stimulation, we produced a PC3 cell line (PC3-TLR3-DN) stably transfected with a dominant-negative form of TLR3 (pZERO-hTLR3) and another (PC3-PURO) with a plasmid containing solely the resistance to puromycin. DNA analysis with specific primers showed the presence of the pZERO-hTLR3 plasmid only in the PC3-TLR3-DN cells (Figure 3A). Hence, both transfected clones were treated with poly(I:C), and the increase in hif-1α mRNA and nuclear protein accumulation were evaluated by qRT-PCR and Western blot, respectively. As shown in Figure 3, B and C, the expression of TLR3-DN completely inhibits poly(I:C)-induced HIF-1α transcription and nuclear protein accumulation, whereas HIF-1α up-regulation was confirmed in poly(I:C)-treated PC3-PURO control cells. These data demonstrate the key role of TLR3 in mediating the induction of HIF-1α triggered by poly(I:C).
Figure 3.
Poly(I:C)-induced HIF-1α accumulation is TLR3-dependent. (A) PC3 cells stably transfected with a plasmid containing the dominant-negative form of TLR3 (PC3-TLR3-DN) or with a plasmid containing only the resistance to puromycin (PC3-PURO) were subjected to total DNA extraction, and the presence of the TLR3-DN plasmid was assessed using PCR with specific primers. Nontransfected PC3 cells and the plasmid used for the transfection (pZERO-hTLR3) were used as negative and positive controls, respectively. β-Actin was used as a control for equal amounts of DNA loaded. (B and C) Both stably transfected cell lines were treated for 6 hours with 5 µg/ml poly(I:C), and the increase of hif-1α mRNA and nuclear protein accumulation were evaluated by qRT-PCR (B) and Western blot analysis (C). β-Actin was used to normalize both qRT-PCR and Western blot. Data shown in panels A and C are typical of three separate experiments with similar results. The histogramin panel B represent the mean of triplicate samples from three independent experiments with SD as error bars, *P = .05.
Poly(I:C) Induces HIF-1α-Mediated VEGF Transcription and Secretion
To test whether the enhanced HIF-1α protein level induced by poly(I:C) is paralleled by an increase in its transcriptional activity, PC3 cells were stimulated with poly(I:C) for different lengths of time, and transcription of veg f, a known HIF-1 target gene, was analyzed by qRT-PCR. VEGF mRNA levels were significantly enhanced in PC3 cells after 16 and 24 hours of poly(I:C) treatment (Figure 4A). Using ELISA, we also evaluated the amount of VEGF secreted by poly(I:C)-treated PC3 cells, in CM collected at various time points during poly(I:C) stimulation. The kinetics of VEGF accumulation is subsequent to the induction of vegf mRNA, with a significant increase of VEGF protein in PC3-CM already detectable after 24 hours of stimulation and strong accumulation after 48 hours (Figure 4B). To validate the angiogenic potential of VEGF secreted under poly(I:C) stimulation, the tubulogenic activity of CM was tested on the endothelial cell line HUVEC. As shown in Figure 4, C and D, poly(I:C)-treated PC3 cell CM was found to stimulate the formation of more capillary-like structures than the CM from control PC3 cells. Moreover, anti-VEGF antibody significantly reverted this proangiogenic function, confirming that VEGF is the main mediator of poly(I:C)-induced in vitro angiogenesis (Figure 4D). No direct effect of poly(I:C) on tubulogenesis was observed (Figure 4D). To clearly demonstrate the involvement of HIF-1α in the stimulation of VEGF secretion, PC3 cells were transiently transfected with a vector encoding an RNA interference targeting hif-1α (int. HIF-1α) or with a vector encoding a scramble RNA interference sequence (int.scramble). At first, we verified that poly(I:C)-induced HIF-1α protein was significantly reduced in cells with int.HIF-1α (Figure 4E). Transfected cells were treated with poly(I:C) for 24 hours, and veg f expression was analyzed by qRT-PCR. The RNA interfering against hif-1α, but not scramble sequence, significantly inhibited poly(I:C)-induced veg f transcription (Figure 4F), demonstrating that poly(I:C) can induce VEGF production by increasing HIF-1α protein expression and the formation of a transcriptionally active HIF-1 complex.
Figure 4.
Poly(I:C) increases the production of active VEGF through HIF-1α. (A and B) vegf mRNA and protein levels of PC3 cells were evaluated after 5 µg/ml poly(I:C) stimulation for the indicated time points using qRT-PCR (A) and ELISA (B), respectively. (C and D) Formation of capillary-like structures. Representative phase-contrast microphotographs of capillary-like structures (C) and quantitative evaluation of their morphogenesis in HUVEC cultures (D). HUVECs were exposed to supernatants of PC3 cells untreated (ctr) or treated with poly(I:C) for 48 hours in the presence or absence of VEGF neutralizing antibodies (anti-VEGF Ab). HUVEC exposed to serum-free medium (DMEM) with or without poly(I:C) for 48 hours were used as negative controls. (E) Cells were transiently transfected with a vector encoding an RNA interfering hif-1α (int. hif-1α) or with a vector encoding a scramble RNA sequence (int. scramble), then treated with 5 µg/ml poly(I:C) for 6 hours and analyzed for total HIF-1α protein level using Western blot. β-Actin was used as a control for equal amounts of protein loaded. (F) Transfected cells were treated with poly(I:C) for 24 hours, and vegf mRNA expression was evaluated by qRT-PCR. The histograms in A, B, D, and F represent the mean of triplicate samples from three independent experiments with SD as error bars, *P ≤. 05, **P ≤. 01. Data shown in panel E are typical of three separate experiments with similar results.
Poly(I:C) Induces VEGF Secretion in LNCaP Cells Overexpressing I.3 hif-1α Isoform
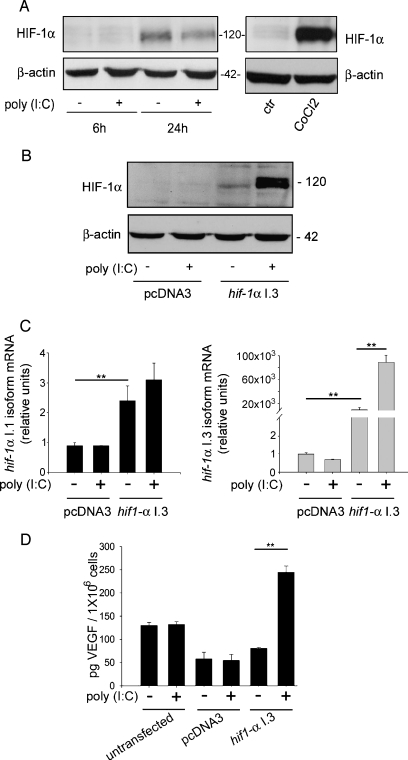
It has been published that LNCaP cells have the lowest HIF-1α levels among human and rat PCa cell lines, suggesting that HIF-1α expression may increase in cancer progression [38]. Therefore, we studied whether TLR3 stimulation can induce HIF-1α nuclear accumulation also in LNCaP cells and found that poly(I:C) stimulation failed to induce nuclear accumulation of HIF-1α (Figure 5A). Total lysates of LNCaP cells treated with CoCl2 were used as positive control of HIF-1α accumulation. The poly(I:C)-independent weak accumulation of HIF-1α at 24 hours is an effect of serum starvation, as previously described [39]. To study the effects of I.3 hif-1α overexpression in LNCaP cells treated or not with poly(I:C), we transfected this cell line with a plasmid containing I.3 isoform of hif-1a (hif-1a I.3) and treated the cells with poly(I:C). Overexpression of hif-1a I.3 isoform in LNCaP cells induced a slight increase of HIF-1α protein, strongly raised on poly(I:C) treatment (Figure 5B). The mechanism of poly(I:C)-induced HIF-1α accumulation in transfected LNCaP cells was further investigated by qRT-PCR and revealed that the transfection with hif-1a I.3 induces a 2000-fold accumulation of this mRNA compared with untransfected LNCaP cells and that poly(I:C) stimulation induces a further five-fold accumulation above the basal levels of the transfected cells (Figure 5C, right panel). The left panel of Figure 5C shows that I.3 isoform transfection causes I.1 hif-1a mRNA to increase by approximately three-fold over the basal levels and that poly(I:C) fails to induce additional accumulation. These data confirm the induction of the specific I.3 isoform of hif-1α mRNA by poly(I:C), suggesting an important role of the basal levels of this isoform in this process. To understand whether poly(I:C)-induced HIF-1α protein was functional in hif-1α I.3-transfected LNCaP cells, we checked for VEGF accumulation in LNCaP CM by ELISA. Figure 5D shows that poly(I:C) fails to induce VEGF accumulation in LNCaP cells transfected with pcDNA3 control plasmid, in accordance with the lack of HIF-1α accumulation shown in Figure 5A. In the CM of the cells transfected with the I.3 plasmid, the accumulation of VEGF is only weak with respect to the medium of the cells transfected with the control plasmid, but a very strong accumulation of VEGF is detectable in the medium of the cells transfected with hif-1α I.3 and then treated with poly(I:C) (Figure 5D).
Figure 5.
Overexpression of I.3 hif-1α in LNCaP cells determines poly(I:C)-induced VEGF production. (A) LNCaP cells were treated for the indicated times with 25 µg/ml poly(I:C), and Western blot analysis of HIF-1α was performed on nuclear extracts. Cells treated with 100 µM CoCl2 for 3 hours were used as positive control for HIF-1α accumulation. β-Actin was used as a loading control. (B, C, and D) LNCaP cells were transiently transfected with a plasmid containing the I.3 isoform of HIF-1α (hif-1α I.3) or with a control plasmid (pcDNA3). Cells were then treated with 5 µg/ml poly(I:C) for 6 hours, and HIF-1α protein levels were analyzed using Western blot (B). Analysis of the I.1 and I.3 isoform of hif-1α mRNA was performed by using qRT-PCR (C). ELISA was used to analyze VEGF concentration in the CM of untransfected or transfected cells with the indicated plasmids and then treated or left untreated with poly(I:C) (D), the histogram represents the mean ± SEM of three independent experiments. **P ≤ .01. Data shown in panels A and B are typical experiments repeated three times with similar results. Histograms in panels C and D represent the mean of triplicate samples from three independent experiments with SD as error bars, *P ≤ .05, **P ≤ .01.
HIF-1α Levels Regulate Poly(I:C)-Induced Apoptosis in PC3 and LNCaP Cells
So far, we have demonstrated that, unlike PC3 cells, LNCaP cells fail to undergo HIF-1α increase on TLR3 stimulation. Given that previous articles reported an antiapoptotic effect for HIF-1α [40] and given the low sensitivity of PC3 cells to poly(I:C)-induced apoptosis [27], we hypothesized a role of HIF-1α in their resistance to this apoptotic stimulus. To test this hypothesis, PC3 cells were transfected with int.HIF-1α or with int.scramble, treated with poly(I:C), and apoptosis induction was analyzed. PI staining revealed that PC3 cells transfected with int.HIF-1α were more susceptible to poly(I:C)-induced apoptosis than the cells transfected with int.scramble (Figure 6A). Moreover, Western blot analysis of the activated form of caspase-3 showed that induction of this apoptotic effector is stronger in cells transfected with int.hif-1α than that transfected with int.scramble, confirming the importance of HIF-1α in regulation of apoptotic susceptibility.
Figure 6.
Up-regulation of HIF-1α inhibits poly(I:C)-induced apoptosis in LNCaP and PC3 cells. PC3 cells transfected with scramble or hif-1α interfering plasmids, treated or not with 5 µg/ml poly(I:C) for 24 hours, were analyzed for the apoptotic rate using PI staining (A) and caspase-3 activation (B). LNCaP cells overexpressing I.3 isoform of hif-1α were treated with 5 µg/ml poly(I:C) for 24 hours, and PI staining (C) and caspase-3 activation (D) were performed to evaluate the apoptotic rate. Histograms in panels A and C represent the mean of triplicate samples from three independent experiments with SD as error bars, **P ≤ .01. Data shown in panels B and D are typical experiments repeated three times with similar results.
To confirm the antiapoptotic function of the poly(I:C)-induced HIF-1α protein observed in PC3 cells, we transfected LNCaP cells with the plasmid containing I.3 hif-1α. Transfected LNCaP cells were treated with poly(I:C), and their apoptotic rate was evaluated by flow cytometry. Figure 6C shows that the sub-G1 population represents 12% of I.3 hif-1α-transfected cells after poly(I:C) treatment, whereas this population significantly increases (up to approximately 25%) in cells transfected with the control plasmid and treated with poly(I:C). We also analyzed caspase-3 activation in transfected LNCaP cells treated or not with poly(I:C) and observed that activation of caspase-3 was lower in the cells transfected with I.3 hif-1α than in the cells transfected with the control plasmid (Figure 6D).
Discussion
Nonhypoxic stimuli such as lipopolysaccharide (LPS), interleukin-1β (IL-1β), insulin growth factor (IGF), and tumor necrosis factor α (TNFα), usually associated with inflammatory conditions, have been described to induce HIF-1α protein accumulation and transcription of HIF-1 target genes in several cellular models [7]. Stimulation of TLRs by microbial components may represent the signal promoting a proinflammatory environment that enhances tumor growth and chemoresistance. Here we report that stimulation of TLR3 with poly(I:C) can increase HIF-1α mRNA and HIF-1α protein nuclear translocation in the highly aggressive prostate cancer cell line PC3. A recent article reported that transfected poly(I:C) induces HIF-1α in a glioblastoma cell line, suggesting a role of this transcription factor in response to viral infection [41]. Here we extend to prostate cancer cells the involvement of HIF-1α in poly(I:C) response and, for the first time, demonstrate a key role of TLR3 in poly(I:C)-induced HIF-1α activation, using a TLR3 dominant-negative stably transfected PC3 cell line. In fact, whereas transfected poly(I:C) mimics intracellular dsRNA generated during viral replication, extracellular administration of poly(I:C) involves TLR3 activation and mimics viral infection through the pinocytosis of dsRNA released by lysed, infected cells. TLR agonists are under clinical trial for the treatment of various carcinoma and even a GMP-grade synthetic analog of poly(I:C) has been proposed as an adjuvant for cancer immunotherapy [42], but this strategy is currently discussed because several articles report opposite protumor or antitumor response after TLR stimulation [43,44]. We recently described that poly(I:C) induces a strong TLR3-mediated apoptotic response in LNCaP cells, whereas PC3 cells seem weakly sensitive to this stimulus [27]. The lower sensitivity of PC3 cells to poly(I:C)-induced apoptosis compared with LNCaP cells led us to hypothesize that, in the more aggressive PC3 cell line, poly(I:C) stimulation may activate genes inducing an antiapoptotic response. Besides the described antiapoptotic action of androgens [45], the resistance to apoptosis is linked to HIF-1α function in prostate cancer [39,46]. Using an RNA interference approach, we observed a significant increase of apoptosis in HIF-1α knockdown PC3 cells, demonstrating a direct role of HIF-1α in the protection of PC3 cells against poly(I:C)-induced apoptosis.
Beside HIF-1α-mediated antiapoptotic effect, we observed also a consistent increase in poly(I:C)-induced VEGF production. VEGF is one of the principal HIF-1 target genes considered the main factor responsible for the induction of neovascularization in hypoxic tumor sites, favoring cancer progression [47]. The ability of poly(I:C) to induce TLR3-dependent VEGF secretion has been recently reported in a mesothelioma cell line [48], but the mechanism of induction was not described. In the present article, using a plasmid containing an interference sequence for HIF-1α, we demonstrate that poly(I:C)-induced HIF-1α activation is responsible for VEGF production in PC3 cells, revealing a new protumor face of TLR3 signaling in prostate cancer.
HIF-1α stabilization is the key event in the induction of HIF-1 complex in hypoxic conditions, but its activation mechanism in normoxic condition is still not clear, probably depending on the nature of the stimulus and on the cell types. There is accumulating evidence that the main mechanism implicated in the nonhypoxic induction of HIF-1α is an increase in HIF-1α protein translation and that the rate of degradation of HIF-1α remains high [7]. As for the mechanisms underlying TLR3-mediated HIF-1α up-regulation, here we demonstrated by pharmacological inhibitors that the HIF-1α increase in PC3 cells requires new mRNA transcription and de novo protein synthesis, showing for the first time that poly(I:C) enhances HIF-1α expression through a translation-dependent mechanism rather than inhibition of protein degradation. Regarding the different mRNA isoforms of HIF-1α, it has been initially shown that, in mice, HIF-1α is encoded by two alternative isoforms, which are expressed from different promoters and uses two distinct exons: I.1 or I.2 [18,49]. The I.1 isoform, which is analogous to the I.3 human isoform described in the present manuscript, was expressed in tissue-specific manner and was strongly induced by activation, whereas the I.2 isoform (analog of the human I.1 isoform) demonstrated a ubiquitous pattern of expression and was present in all analyzed tissues. Studies of the murine and human HIF-1α promoters [49,50] revealed that the ubiquitous isoform is expressed continuously from the housekeeping-type CpG island-rich promoter. Nevertheless, murine I.1 isoform and corresponding human I.3 isoform were shown to be dramatically upregulated by activating stimuli in T lymphocytes [6,18], possibly because of the presence of a number of activation response binding sites for transcriptional factors in the promoter [49]. Thus, it was expected that the HIF-1α isoform upregulated after TLR3 activation was the I.3 but not the ubiquitous I.1 in human cells. Here we investigated for the first time the levels of different hif-α isoforms in tumor cells and report that activation of HIF-1 complex after poly(I:C) stimulation in PC3 cells is mainly dependent on the transcription of the HIF-1α I.3 isoform.
A previous article reported amplification of the HIF-1α gene in PC3 cells resulting in a higher basal level of the HIF-1α protein present in the nucleus of these cells with respect to other prostate cancer cell lines such as LNCaP [38]. Moreover, the apoptotic signaling mechanism triggered by poly(I:C) in LNCaP cells is mediated by PKC-α [27] without increasing HIF-1α, whereas PKC-α seems to play a role in HIF-1α accumulation in PC3 cells (data not shown), indicating complex transduction pathways evoked by poly(I:C) in prostate cancer cell lines, which deserves further investigation. In view of the different features of these two cell lines, a common mechanism of HIF-1α activation after poly(I:C) stimulation is highly unlikely. Accordingly, our data show that poly(I:C) induced up-regulation of endogenous HIF-1α in PC3 cells but not in LNCaP cells. Surprisingly, transfection of the I.3 HIF-1α isoform, although enhancing approximately 2000-fold HIF-1α basal mRNA, only slightly increased HIF-1α protein, failing to induce VEGF secretion. Interestingly, in LNCaP cells overexpressing exogenous HIF-1α I.3, stimulation with poly(I:C) strongly increases HIF-1α protein, suggesting that TLR3 activation is capable of counteracting the mechanisms that can basally control HIF-1α protein accumulation. The possibility that the effects of this forced overexpression may partially depend on an artificial responsiveness of the exogenous promoter cannot be ruled out. Noteworthy, poly(I:C) stimulation of LNCaP cells overexpressing HIF-1α leads to a strong induction of HIF-1α and VEGF proteins paralleled by the capability to resist to poly(I:C)-induced apoptosis. These results also demonstrate that, in LNCaP, a high basal expression of I.3 HIF-1α is essential for poly(I:C)-induced HIF-1α accumulation. In view of our data, the deregulation of HIF-1α basal levels seems to be crucial in controlling the mechanisms involved in cancer progression.
HIF-1 has become an important therapeutic target in solid tumors, and efficient drugs capable of decreasing HIF-1 are currently under development [51,52]. Moreover, activation of hypoxic genes in prostate, breast, and ovarian cancers as predictor of adverse clinical outcome has been reported, suggesting that HIF-1 complex represents a key transcription factor in endocrine tumors [9]. In particular, HIF-1a/β and HIF-2α, together with abnormal estrogen receptor β and endothelial nitric oxide synthase, have been recently described to drive prostate cancer toward a more aggressive phenotype [53].
In conclusion, our results are the first evidence showing a correlation between HIF-1α I.3 isoform expression and ability of TLR3 to regulate apoptosis and induce VEGF production in human tumor cell lines. Future studies with gene-targeted knockdown of the HIF-1α I.3 isoform may provide conclusive evidence for its role in TLR3-mediated effects in prostate cancer, contributing to the refinement of diagnostic tools and possibly the development of targeted therapeutic strategies.
Acknowledgment
The authors thank Fioretta Palombi for helpful discussion and critical reading of the manuscript.
Abbreviations
- HIF
hypoxia-inducible factor
- HUVEC
human umbilical vein endothelial cell
- TLR
Toll-like receptor
- VEGF
vascular endothelial growth factor
Footnotes
This work was supported by grants from MIUR PRIN 2007 to E.Z. and from Agenzia Spaziale Italiana no. I/065/08/0-227/(09) to A.F. This work was partially supported by National Institutes of Health grant R21 AI068816-01A1. The authors declare no conflict of interest.
References
- 1.Pugh CW, Tan CC, Jones RW, Ratcliffe PJ. Functional analysis of an oxygen-regulated transcriptional enhancer lying 3′ to the mouse erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:10553–10557. doi: 10.1073/pnas.88.23.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 4.Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009;122:1055–1057. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- 5.Depping R, Hagele S, Wagner KF, Wiesner RJ, Camenisch G, Wenger RH, Katschinski DM. A dominant-negative isoform of hypoxia-inducible factor-1α specifically expressed in human testis. Biol Reprod. 2004;71:331–339. doi: 10.1095/biolreprod.104.027797. [DOI] [PubMed] [Google Scholar]
- 6.Lukashev D, Sitkovsky M. Preferential expression of the novel alternative isoform I.3 of hypoxia-inducible factor 1a in activated human T lymphocytes. Hum Immunol. 2008;69:421–425. doi: 10.1016/j.humimm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann AC, Mori R, Vallbohmer D, Brabender J, Klein E, Drebber U, Baldus SE, Cooc J, Azuma M, Metzger R, et al. High expression of HIF1α is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 2008;10:674–679. doi: 10.1593/neo.08292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimbro KS, Simons JW. Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer. 2006;13:739–749. doi: 10.1677/erc.1.00728. [DOI] [PubMed] [Google Scholar]
- 10.Zhong H, Semenza GL, Simons JW, De Marzo AM. Up-regulation of hypoxia-inducible factor 1α is an early event in prostate carcinogenesis. Cancer Detect Prev. 2004;28:88–93. doi: 10.1016/j.cdp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxiainducible factor 1a in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 12.Gomella LG, Johannes J, Trabulsi EJ. Current prostate cancer treatments: effect on quality of life. Urology. 2009;73:S28–S35. doi: 10.1016/j.urology.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Brune B, Zhou J. The role of nitric oxide (NO) in stability regulation of hypoxia inducible factor-1α (HIF-1α) Curr Med Chem. 2003;10:845–855. doi: 10.2174/0929867033457746. [DOI] [PubMed] [Google Scholar]
- 14.Gorlach A, Kietzmann T. Superoxide and derived reactive oxygen species in the regulation of hypoxia-inducible factors. ethods Enzymol. 2007;435:421–446. doi: 10.1016/S0076-6879(07)35022-2. [DOI] [PubMed] [Google Scholar]
- 15.Gerber SA, Pober JS. IFN-α induces transcription of hypoxia-inducible factor-1α to inhibit proliferation of human endothelial cells. J Immunol. 2008;181:1052–1062. doi: 10.4049/jimmunol.181.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor κB activation. Biochem J. 2003;370:1011–1017. doi: 10.1042/BJ20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukashev D, Caldwell C, Ohta A, Chen P, Sitkovsky M. Differential regulation of two alternatively spliced isoforms of hypoxia-inducible factor-1α in activated T lymphocytes. J Biol Chem. 2001;276:48754–48763. doi: 10.1074/jbc.M104782200. [DOI] [PubMed] [Google Scholar]
- 19.Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1α. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 20.Nicholas SA, Sumbayev VV. The involvement of hypoxia-inducible factor 1 α in Toll-like receptor 7/8-mediated inflammatory response. Cell Res. 2009;19:973–983. doi: 10.1038/cr.2009.44. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan M, Luo W, Csoka B, Hasko G, Lukashev D, Sitkovsky MV, Leibovich SJ. Differential regulation of HIF-1α isoforms in murine macrophages by TLR4 and adenosine A(2A) receptor agonists. J LeukocBiol. 2009;86:681–689. doi: 10.1189/jlb.0109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 25.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 26.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 27.Paone A, Starace D, Galli R, Padula F, De CP, Filippini A, Ziparo E, Riccioli A. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-α-dependent mechanism. Carcinogenesis. 2008;29:1334–1342. doi: 10.1093/carcin/bgn149. [DOI] [PubMed] [Google Scholar]
- 28.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 29.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res. 2007;13:4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 30.Chen N, Chen X, Huang R, Zeng H, Gong J, Meng W, Lu Y, Zhao F, Wang L, Zhou Q. BCL-xL is a target gene regulated by hypoxia-inducible factor-1α. J Biol Chem. 2009;284:10004–10012. doi: 10.1074/jbc.M805997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1α signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903–25914. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piret JP, Minet E, Cosse JP, Ninane N, Debacq C, Raes M, Michiels C. Hypoxia-inducible factor-1-dependent overexpression of myeloid cell factor-1 protects hypoxic cells against tert-butyl hydroperoxide-induced apoptosis. J Biol Chem. 2005;280:9336–9344. doi: 10.1074/jbc.M411858200. [DOI] [PubMed] [Google Scholar]
- 33.Bonello S, Zahringer C, Belaiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1α promoter via a functional NFκB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 34.Gorlach A. Regulation of HIF-1α at the transcriptional level. Curr Pharm Des. 2009;15:3844–3852. doi: 10.2174/138161209789649420. [DOI] [PubMed] [Google Scholar]
- 35.Nicholas SA, Sumbayev VV. The role of redox-dependent mechanisms in the downregulation of ligand-induced Toll-like receptors 7, 8 and 4-mediated HIF-1 α prolyl hydroxylation. Immunol Cell Biol. 2010;88:180–186. doi: 10.1038/icb.2009.76. [DOI] [PubMed] [Google Scholar]
- 36.Clemens MJ, Hershey JW, Hovanessian AC, Jacobs BC, Katze MG, Kaufman RJ, Lengyel P, Samuel CE, Sen GC, Williams BR. PKR: proposed nomenclature for the RNA-dependent protein kinase induced by interferon. J Interferon Res. 1993;13:241. doi: 10.1089/jir.1993.13.241. [DOI] [PubMed] [Google Scholar]
- 37.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 38.Zhong H, Agani F, Baccala AA, Laughner E, Rioseco-Camacho N, Isaacs WB, Simons JW, Semenza GL. Increased expression of hypoxia inducible factor-1α in rat and human prostate cancer. Cancer Res. 1998;58:5280–5284. [PubMed] [Google Scholar]
- 39.Thomas R, Kim MH. HIF-1α: a key survival factor for serum-deprived prostate cancer cells. Prostate. 2008;68:1405–1415. doi: 10.1002/pros.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong SW, Yoo JW, Kang HS, Kim S, Lee DK. HIF-1α-dependent gene expression program during the nucleic acid-triggered antiviral innate immune responses. Mol Cells. 2009;27:243–250. doi: 10.1007/s10059-009-0030-2. [DOI] [PubMed] [Google Scholar]
- 42.Jasani B, Navabi H, Adams M. Ampligen: a potential toll-like 3 receptor adjuvant for immunotherapy of cancer. Vaccine. 2009;27:3401–3404. doi: 10.1016/j.vaccine.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27:168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 44.Wolska A, Lech-Maranda E, Robak T. Toll-like receptors and their role in carcinogenesis and anti-tumor treatment. Cell Mol Biol Lett. 2009;14:248–272. doi: 10.2478/s11658-008-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenzo PI, Saatcioglu F. Inhibition of apoptosis in prostate cancer cells by androgens is mediated through downregulation of c-Jun N-terminal kinase activation. Neoplasia. 2008;10:418–428. doi: 10.1593/neo.07985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J, Schmid T, Schnitzer S, Brune B. Tumor hypoxia and cancer progression. Cancer Lett. 2006;237:10–21. doi: 10.1016/j.canlet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 47.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 48.Wornle M, Sauter M, Kastenmuller K, Ribeiro A, Roeder M, Mussack T, Ladurner R, Sitter T. Role of viral induced vascular endothelial growth factor (VEGF) production in pleural effusion and malignant mesothelioma. Cell Biol Int. 2009;33:180–186. doi: 10.1016/j.cellbi.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Wenger RH, Rolfs A, Spielmann P, Zimmermann DR, Gassmann M. Mouse hypoxia-inducible factor-1α is encoded by two different mRNA isoforms: expression from a tissue-specific and a housekeeping-type promoter. Blood. 1998;91:3471–3480. [PubMed] [Google Scholar]
- 50.Iyer NV, Leung SW, Semenza GL. The human hypoxia-inducible factor 1α gene: HIF1A structure and evolutionary conservation. Genomics. 1998;52:159–165. doi: 10.1006/geno.1998.5416. [DOI] [PubMed] [Google Scholar]
- 51.Melillo G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev. 2007;26:341–352. doi: 10.1007/s10555-007-9059-x. [DOI] [PubMed] [Google Scholar]
- 52.Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–2786. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, Colussi C, Lirangi V, Illi B, D'Eletto M, et al. Endothelial NOS, estrogen receptor β, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest. 2009;119:1093–1108. doi: 10.1172/JCI35079. [DOI] [PMC free article] [PubMed] [Google Scholar]