Abstract
Using rat organotypic hippocampal-entorhinal cortical (HEC) slice cultures, we examined whether phospholipase A2 (PLA2) activity is involved in binge alcohol (ethanol)-induced neurodegeneration, and whether docosahexaenoic acid (DHA; 22:6n-3), a fish oil-enriched fatty acid that is anti-inflammatory in brain damage models, is neuroprotective. Assessed with propidium iodide and lactate dehydrogenase (LDH) leakage, neurodamage from ethanol (6 days 100 mM ethanol with four withdrawal periods) was prevented by the PLA2 pan-inhibitor, mepacrine. Also, ethanol-dependent neurodegeneration— particularly in the entorhinal region—was significantly ameliorated by DHA supplementation (25 µM); however, adrenic acid, a 22:4n-6 analog, was ineffective. Consistent with PLA2 activation, [3H] liberation was approximately fivefold greater in [3H]arachidonic acid-preloaded HEC slice cultures during ethanol withdrawal compared to controls, and DHA supplementation suppressed [3H] release to control levels. DHA might antagonize PLA2 activity directly or suppress upstream activators (e.g., oxidative stress); however, other DHA mechanisms could be important in subdueing ethanol-induced PLA2-dependent and independent neuroinflammatory processes.
Keywords: Alcohol, Withdrawal, Hippocampus, Entorhinal cortex, Phospholipase A2, Arachidonic acid, Neuroprotection
Introduction
Chronic binging alcoholics incur brain neurodegeneration [1], but the underlying mechanisms are unresolved. While there are in vitro indications for excitotoxicity [2, 3], in vivo attempts to prove that augmented N-methyl-d-aspartate (NMDA) receptor activation underlies ethanol-induced neurodegeneration have been generally unsuccessful. Binge ethanol-intoxicated adult rats exhibit selective neurodegeneration in the olfactory (entorhinal) cortex, notably layer III pyramidal neurons, and the hippocampus, particularly dentate granule cells [4–6]. Neurodamage in these regions is not significantly diminished by blocking NMDA receptors, voltage-gated calcium channels, or nitric oxide synthase activity [7–9]. However, modest but significant brain edema and electrolyte imbalances occur during the repetitive ethanol intoxication, and diuretics—e.g., furosemide [7] or acetazolamide [10]—in suppressing the edema, exert neuroprotection.
One pathway linked to edema/swelling during trauma or ischemia in brain (esp. astroglia) is phospholipase A2 (PLA2) activation, which leads to disproportionate liberation/mobilization of arachidonic acid (AA, 20:4 n-6) [11]. Elevated AA generates reactive oxygen species (ROS), pro-inflammatory eicosanoids, and other metabolites that promote neuroinflammation and neurodegeneration [12]; then again, in a positive feedback-like scenario, oxidative stress can be upstream of (further) PLA2 activation [13, 14]. Also, AA itself has its direct (pro)apoptotic effects [15–17]. Parenthetically, it is relevant to mention that certain ROS trapping agents protect against binge ethanol-induced neurodegeneration in vivo [8, 18].
In contrast to n-6 AA, long-chain n-3 polyunsaturated fatty acids such as docosahexaenoic acid (DHA, 22:6), enriched in fish oils, are frequently associated with neuroprotection and neuroinflammatory suppression [19]. Of significance, brain concentrations of DHA are reduced by chronic ethanol [20–22]. We examined whether PLA2 inhibition with mepacrine (MEP) or DHA supplementation in our in vitro model of binge ethanol-induced brain damage could reduce neuronal death, assessed principally by propidium iodide (PI) labeling of degenerating neurons [7], and, if so, whether there was an association with AA liberation at early stages of ethanol exposure and withdrawal.
These experiments examining the effects of a PLA2 inhibitor and of DHA utilized long-term cultures of rat organotypic slices of hippocampus and entorhinal cortex, regions mentioned above that are susceptible to binge ethanol-induced neurodamage in rats [4]. Organotypic brain slice cultures have advantages over mixed brain cultures in retaining localized neuronal-glial relationships [23]. Furthermore, unlike slices of hippocampus that are often used in brain slice cultures, hippocampal-entorhinal cortical (HEC) slices in long-term culture have functional perforant pathways [24], which may be important for mechanisms underlying neuronal vulnerability and protection. Our findings with binge ethanol-treated organotypic HEC cultures indicate a role for PLA2 activation and, as in other brain damage models, DHA exerts significant neuroprotection that could involve containment of AA release and associated PLA2-related neuroinflammation, as well as other mechanisms to be investigated.
Materials and Methods
Unless otherwise indicated, supplies were obtained from Fisher (Pittsburgh, PA) and chemicals from Sigma (St. Louis, MO). Rat organotypic HEC slices were prepared from Sprague–Dawley 1-week-old pups (Harlan, Indianapolis, IN) according to Collins et al. [7], as modified from Stoppini et al. [25]. Pups were sacrificed with minimal suffering according to a Loyola Medical Center IACUC-approved protocol. With tissue encompassing the entorhinal/hippocampal complex, transverse slices (350 µm) were taken and placed on 0.4 µm Millicel tissue culture inserts (Millipore, Bedford, MA; 3–4 slices/insert) in 6-well Falcon plates with covers. They were cultured in an atmosphere of 95% O2/5% CO2 at 37°C in MEM media (1 ml/well) containing 25% horse serum (Gibco Invitrogen, Carlsbad, CA) and 6.5 mg/ml glucose; media was changed every 3–4 days. Slices were examined before ethanol or control treatment (below) and those appearing unhealthy as indicated by significant PI labeling or obvious gliosis were discarded.
After 16–18 days in culture, slices were treated over a 6-day period with fresh media with or without 100 mM ethanol, a level not unusual in chronic alcoholics [26, 27], according to a “binge” schedule: ethanol-containing media for three consecutive 24 h days, followed by the first overnight withdrawal (ethanol-free media), ethanol 12–14 h daily for the following 2 days, each followed by an overnight withdrawal, and a fourth ethanol exposure for a day and overnight, followed by a ~ 2 h withdrawal, for a total of four withdrawal periods. Although the plates were in Tupperware containers, ethanol concentrations were permitted to diminish during exposure to similate the in vivo situation. With selected cultures MEP (1 µM), DHA (25 µM; Cayman, Ann Arbor, MI) or adrenic acid (ADA, 25 µM; Cayman, Ann Arbor, MI)—an n-6 polyunsaturated fatty acid of the same carbon chain length—was added ~ 3 h prior to the start of ethanol exposure (pre-incubation) and was supplemented throughout ethanol + withdrawal. PI, extensively used to assess specific neurodegeneration in organotypic slices in culture [23, 28], was added to culture media (5 µg/ml) during the last hr of the fourth withdrawal. Measurement of intensity of neuronal PI uptake was accomplished in HEC slices using Image J analysis software and a Nikon DS-5 camera + Nikon TS100 inverted microscope. The percentage of entorhinal cortical (EC) area with PI fluorescence above background was calculated in relation to the total area for each slice; the same threshold level was used for all slices in a given experiment. To verify PI results in the ethanol/MEP experiments, LDH activity—a neurotoxicity measure in slice cultures that correlates with PI labeling [29]—was assayed in media pooled from the final day of ethanol + withdrawal using a Sigma LDC kit and standardized to slice protein, assayed with a bicinchoninic acid method (Pierce, Rockford, IL).
In a modification of an AA release procedure in HEC slices [30], [3H]AA (#0196, ARC, St. Louis, MO; 200 Ci/mmol) was added to experimental and control HEC slice culture media (1 µCi/ml media) 6 h before initiation of ethanol treatment. Measurement of selected HEC tissue extracts from rinsed slices obtained immediately prior to initial ethanol addition indicated that tissue [3H] content, which constituted ~ 70% of the added [3H]AA in agreement with other culture studies [31], did not significantly differ between fatty acid-supplemented and control slices. In media collected 20 min into the first withdrawal, [3H] content representing mainly released AA and AA oxidation products [32] was assessed by scintillation spectroscopy and normalized to protein, determined as above in homogenates of HEC slices. Mean values for PI labeling results and media [3H] were compared with one-way ANOVA and an appropriate post-hoc test as indicated in the figure legends.
Results
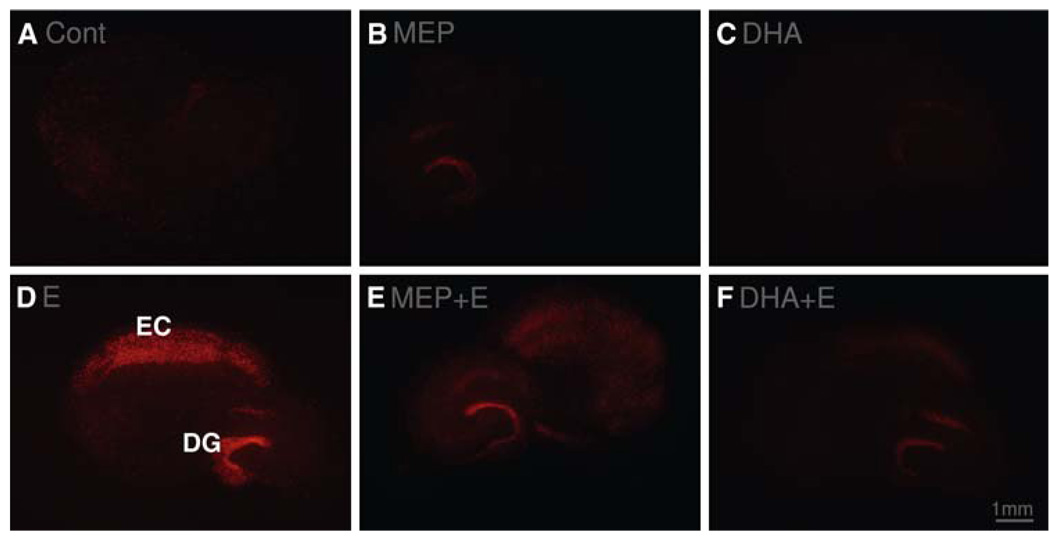
Figure 1 shows representative images of experimental HEC slices in culture, carried through the experimental procedure as described and examined for PI labeling of degenerating neurons. Control HEC slices (Cont) carried through the 6-day protocol including media changes but without ethanol showed minimal PI-labeling. Figure 1B is a control slice exposed to MEP throughout the procedure that incurred no apparent increase over controls in neuronal PI labeling. Likewise, slices supplemented throughout with DHA (top rt) showed no noticeable increases in PI-labeled cells relative to control slices. In Fig. 1D, binge ethanol treatment (Fig. 1E, 100 mM) of HEC slices over 6 days with withdrawals evoked extensive neurodegeneration as indicated by PI labeling of neurons in the EC and the hippocampal dentate gyrus (DG) regions. However, HEC slices exposed to the ethanol + withdrawal protocol and supplemented as described with either PLA2 inhibitor MEP (MEP + E; image in Fig. 1E with slice rotated ~ 90°) or n-3 DHA (Fig. 1F; DHA + E) demonstrated evident reductions in PI-labeled degenerating neurons in both the EC and DG regions of the slices.
Fig. 1.
Binge ethanol-dependent PI labeling of degenerating neurons in the EC and DG in representative organotypic HEC slices in culture and suppression by MEP or by DHA. (A) Control slice; (B) slice supplemented with DHA (25 µM); (C) slice supplemented with MEP (1 µM); (D) binge ethanol (100 mM)-treated slice; (E) binge ethanol-treated and MEP-supplemented slice; (F) binge ethanol-treated and DHA-supplemented slice
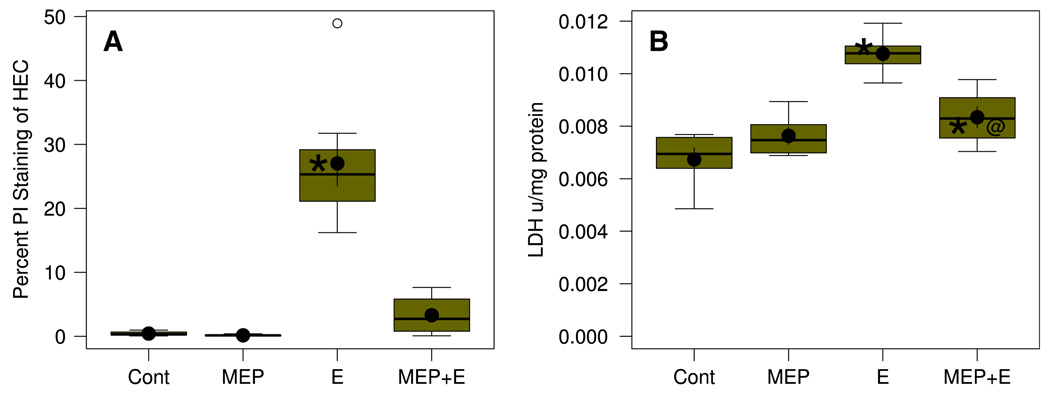
In Fig. 2 is shown quantitation of neurotoxicity in HEC slice experiments with 100 mM binge ethanol in the absence and presence of MEP. In Fig. 2A, quantitation of PI-labeled EC neurons represented in Fig. 1 demonstrated that binge ethanol treatment caused extensive neurodegeneration. MEP alone caused no increase in neurodegeneration, but its addition effectively prevented most of the binge ethanol-induced neurodamage (MEP + E). Further confirmation of cytotoxicity due to binge ethanol and significant reduction by MEP co-treatment is in Fig. 2B, which shows the effects of these treatments on LDH leakage in HEC slice culture. In accord with earlier HEC slice culture LDH results [7], binge ethanol treatment caused significantly increased media LDH activity (E) relative to controls (Cont), and this leakage representing degenerating cells was significantly—but not completely—suppressed by MEP co-treatment (MEP + E).
Fig. 2.
Neurodegeneration in HEC slices in culture is significantly increased over control (Cont) by binge ethanol (E) treatment and inhibited by supplementation with MEP. Box in boxplot illustrates the extent of the middle two quartiles and defines the interquartile range (IQR), along with the median (horizontal line). Filled circle inside box shows the mean with error bars showing SEM. Whiskers extending from box depict maximum range of values within 1.5 IQR, while small open circles denote “outlier” values. N = 6–9 wells/grp. (A) Neurodegeneration expressed as percent PI labeling. *Group mean was significantly greater (P < .01) than the mean of the Control (Cont) group by Holm-Bonferroni t-tests. Overall one-way ANOVA was significant (F3.20 = 65.34, P = 2.03 × 10−14). (B) Neurodegeneration expressed as media LDH activity/mg slice protein. *Group mean significantly greater (P < .05) than the mean of the Control (Cont) group by Holm-Bonferroni t-test. @Group mean significantly less (P <0.05) than the mean for the Ethanol (E) group by Holm-Bonferroni t-test. Overall one-way ANOVA was significant (F3,25 = 21.04, P = 2.13 × 10−16)
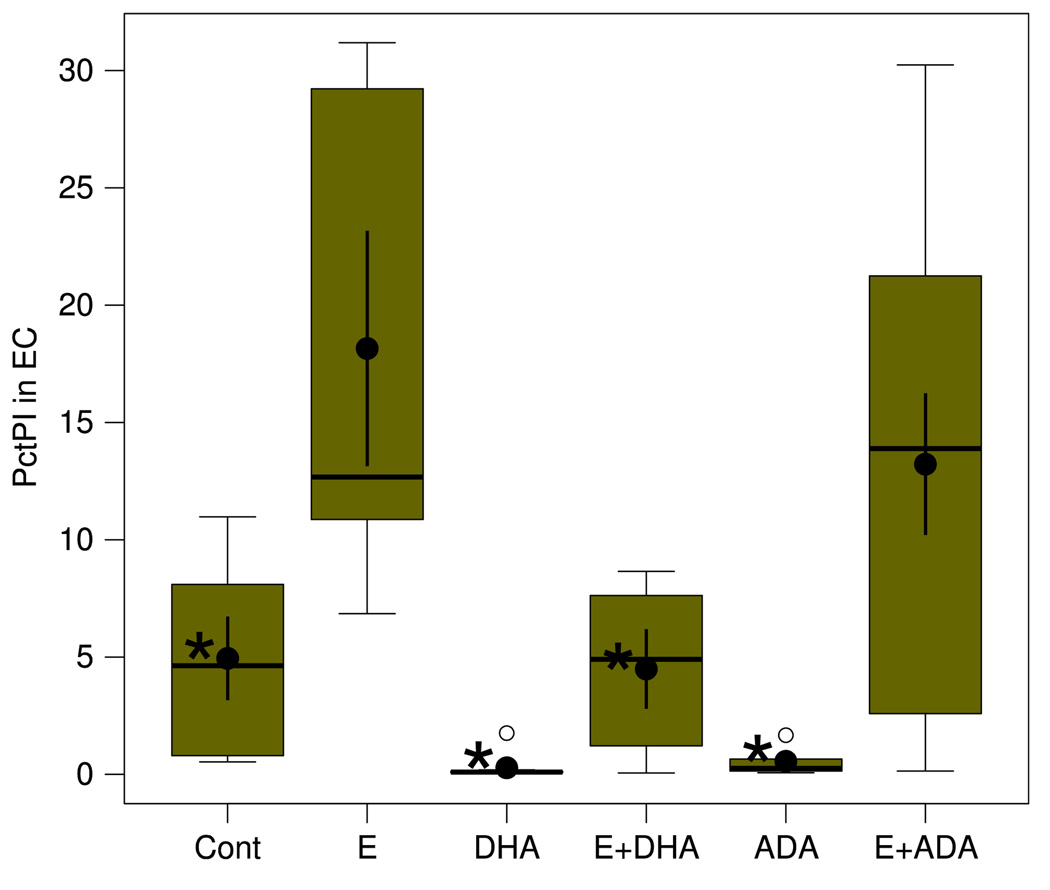
Figure 3 displays the effects on binge ethanol-induced neurodegeneration (PI labeling of EC neurons) of supplementation with two polyunsaturated fatty acids, DHA and ADA, a 22:4 analog that at times has been used as a n-6 polyunsaturated fatty acid comparison for DHA. In Fig. 3, pre-incubation/supplementation with DHA (25 µM), while having no effects on control slice PI labeling, completely blocked (E + DHA) the EC neurodegeneration caused by binge ethanol treatment (E) of HEC slices. Comparatively, Fig. 3 shows that supplementation with 25 µM n-6 ADA had no significant effect (E + ADA) on EC neurodegeneration in the HEC slice cultures. DG cell PI labeling in the ethanol-treated slices, while clearly suppressed by MEP or DHA (Fig. 1), was not quantitated.
Fig. 3.
Neurodegeneration in the EC is significantly increased compared to control (Cont) by binge ethanol (E) treatment and inhibited by supplementation with DHA but not ADA. Neurodegeneration expressed as percent PI labeling. N = 6–9 wells/grp. See Fig. 2 legend for explanation of box plots. *Groups whose means were significantly less (P <. 05) than the mean of the ethanol (E) group by Holm-Bonferroni t-test. Overall one-way ANOVA was significant (F5,36 = 6.77, P = .00015)
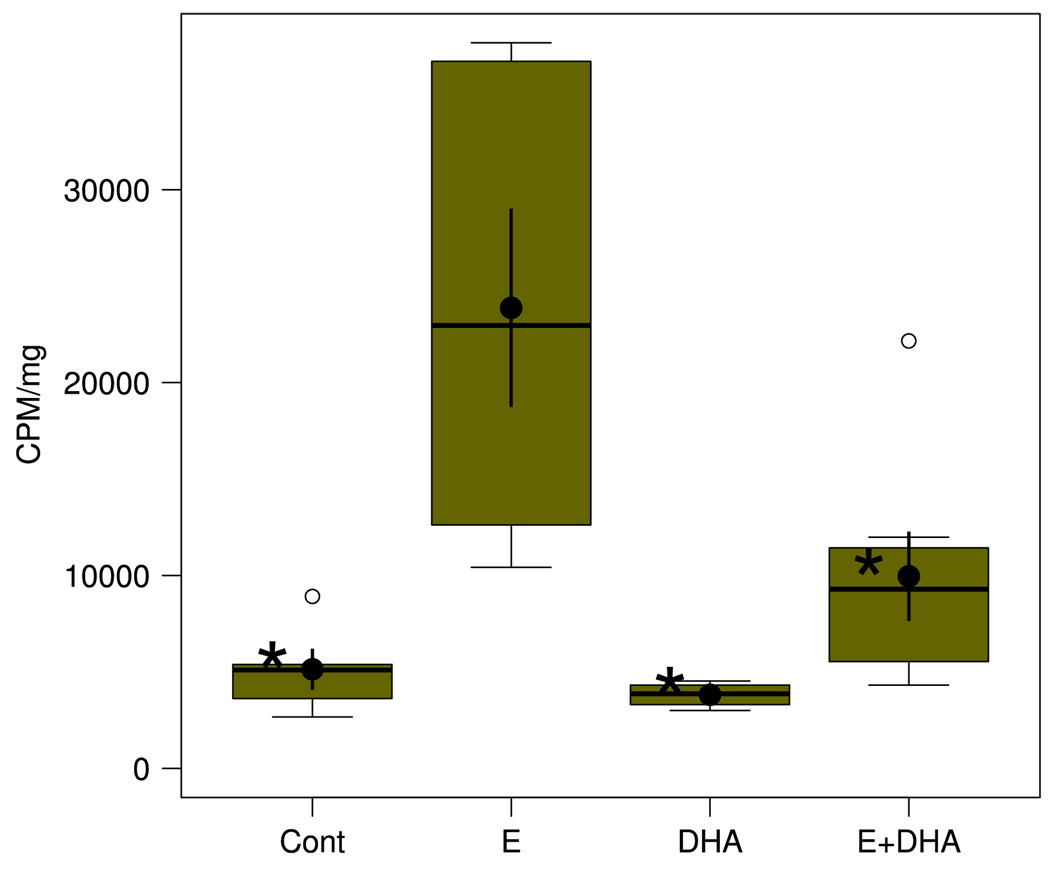
The box plots in Fig. 4 demonstrate the effect of the neurotoxic binge ethanol exposure on tritium release from [3H]AA-pre-incorporated HEC slices during the first of the withdrawal periods, and the effect on release of neuroprotective DHA pre-incubation. Assayed in media taken 20 min into the first withdrawal period, the [3H] results showed, when compared to release from control (Cont) cultures, robust (approximately fivefold) release of [3H] associated with ethanol withdrawal (E) that indicates significant activation of PLA2 activity due to binge ethanol. Subsequent withdrawal periods also showed increased [3H] release compared to controls, but to lesser extents (not shown). Pre-incubation/supplementation with DHA as in Fig. 3 did not alter basal (control) [3H] release, but it completely abrogated the increase in [3H] release due to binge ethanol (E + DHA), signifying that DHA supplementation effectively suppresses binge ethanol-dependent activation of PLA2-dependent mechanisms.
Fig. 4.
Mobilization of [3H] from incorporated [3H]AA in HEC slices in culture is significantly increased by ethanol + withdrawal treatment and normalized by supplementation with DHA. Results expressed as cpm/mg slice protein. N = 6–9 wells/grp. See Fig. 2 legend for explanation of box plot. *Groups whose means were significantly less (P < .05) than the mean of the ethanol (E) group by Holm-Bonferroni t-tests. Overall one-way ANOVA was significant (F3,18 = 7.91, P = .0014)
Discussion
The findings show that the neurodegeneration provoked in organotypic HEC slices by subchronic binge ethanol exposure involves augmented PLA2 activity as evidenced by the extensive neuroprotection from a general PLA2 inhibitor (MEP), and the [3H] release experiments further suggest that substantially elevated mobilization of n-6 AA, a well-documented neuroinflammatory accomplice, occurs early in the binge ethanol protocol. This is the first experimental data to our knowledge that directly implicates PLA2 activity with “binge alcoholic brain damage,” and current studies with selective inhibitors are underway to determine the specific PLA2 forms involved [33]. We also find that supplementation with n-3 DHA—but not n-6 ADA, a 22-carbon elongation product of AA—affords essentially complete neuroprotection in concert with blockade of the induced AA mobilization. These results are consistent with binge or episodic ethanol-induced brain damage involving to an appreciable extent neuroinflammatory PLA2 activation, excess AA mobilization and oxidative stress that are conceivably downstream of neuroglial edema/electrolyte dysregulation [7, 34]. Brain (esp. cellular) swelling is known to increase PLA2 activity [11, 35, 36], and in positive feedback-like fashion, excessively released AA can potentially instigate more brain edema [37, 38] as well as increase oxidative stress (ROS)—which can trigger further PLA2 activation [13].
On the other hand, when supplemented or potentiated, n-3 polyunsaturated fatty acids—in particular, DHA—frequently have neuroprotective, anti-inflammatory, and survival effects [19, 39]. Much of the anti-inflammatory evidence for DHA is in vivo; however, the molecule has been linked to neuroprotection in various brain and other culture models as well [40–43]. Pertinent to our case is a study with rat organotypic hippocampal slice cultures in which DHA pre-incubation protected against ischemia-induced brain damage [44]. Also, the n-3 fatty acid had positive effects on survival, neurite outgrowth and neuronal differentiation in brain cultures [45, 46], and suppressed epileptiform activity in acute hippocampal slices [47]. As mentioned, ADA, the 22-carbon n-6 fatty acid elongation product of AA that is enriched in human brain plasmalogens [48], was not effective in suppressing ethanol-induced neurodamage. Unlike the potent neuroprotective effect of DHA, which is incorporated to a great extent into neuronal membrane [49], ADA’s lack of neuroprotection might in part relate to its high incorporation in myelin [50, 51] and consequently its relatively slow turnover. We acknowledge that a more appropriate (although not essential at this point) comparison for DHA is docosapentaenoic acid (22:5 n-6), a fatty acid shown to be reciprocally related to DHA in neuronal membranes [52] and which we plan to use in future studies.
Several possibilities might explain DHA’s inhibition of induced liberation of AA in DHA-supplemented brain slices during neurotoxic binge ethanol exposure. There are indications, albeit limited, that supplemented DHA can inhibit PLA2 [53]; and conversely, reduced tissue DHA levels (dietary n-3 deprivation) have been associated with elevations in rat brain cortical secretory and cytosolic PLA2 isoforms [54]. Since supplemented DHA is incorporated and released by PLA2 isoforms along with AA, as demonstrated in pathological situations [13, 55], it is also possible, that suppressed AA release in DHA-supplemented slices during binge ethanol treatment reflects competition with incorporated DHA; however, since AA may be released by a different PLA2 isoform than DHA, as reported with ATP-stimulated glia [56], such competition might not be relevant. Parenthetically, it is possible that inhibition by MEP actually increases endogenous neuroprotective DHA. A further mechanism is that through its potential antioxidative effects [57], DHA could counter oxidative stress that in some studies is upstream (in addition to downstream) of PLA2 activation [13, 14]. Potentially related to this possibility is that DHA neuroprotection from glutamate toxicity in brain cultures or rat brain in vivo is associated with increased glutathione, antioxidant enzymes, and stabilization of intracellular calcium [40, 58], anti-oxidative actions that could affect PLA2 activity in our DHA-supplemented ethanol model. An additional AA-related mechanism for DHA is its reported inhibition of cyclooxygenase-mediated AA metabolism [59], which would lessen formation of neurodeleterious eicosanoids, as well as derived ROS.
However, mechanisms for DHA neuroprotection against ethanol-induced neurodamage other than or in addition to abrogation of excessive AA liberation and/or metabolism could well be important and, if so, it would suggest alternative neurotoxic mechanisms for binge ethanol as well. DHA incorporation specifically into neuronal membrane phosphatidylserine (PS) has recently been demonstrated [60], and research linking this phenomenon to neuronal survival reviewed and summarized [61]. In brief, prolonged ethanol exposure can deplete neuronal PS [62] as well as DHA within membrane PS [21]. Through its ability to increase PS in inner leaflets of neuronal membranes, added DHA can facilitate translocation of PKB/Akt, a key anti-inflammatory kinase which antagonizes apoptotic signaling [52]. An equally intriguing possibility is that free or liberated DHA can be oxygenated (apparently in glia) by lipoxygenase-like enzymes to neuroprotectin-1 and resolvin molecules, which have marked—possibly receptor-mediated—neuroprotective properties [63–65]. These possible neuroprotective mechanisms for DHA need to be evaluated in future studies of the n-3 fatty acid’s propitious actions on binge ethanol-induced neurodegeneration. Regardless of these latter possibilities, the significantly increased [3H] mobilization from incorporated [3H]AA during binge ethanol treatment (further analyses will ascertain the AA content of the released [3H]) and its suppression by neuroprotective doses of DHA remain consistent with a model in which PLA2, AA and potentially derived ROS have roles in binge ethanol-dependent neurodegeneration.
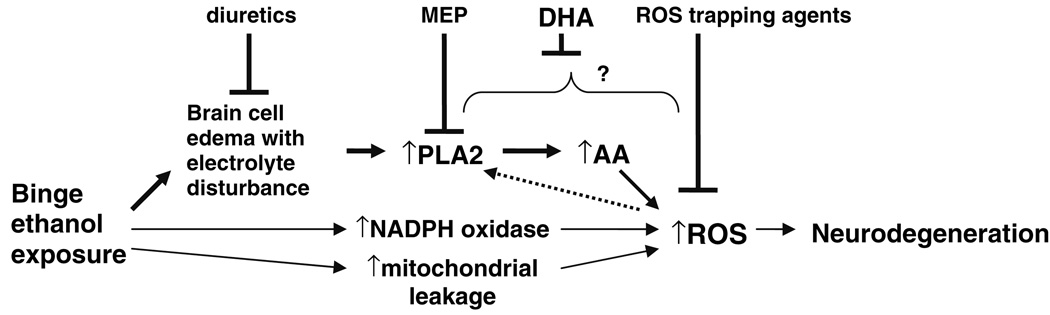
The PLA2-based mechanistic scheme in Fig. 5 depicts how diuretic treatment, inhibition by MEP, DHA acting possibly at several steps, and ROS trapping agents might exert neuroprotection. Also added is the aforementioned possibility of upstream activation (dashed arrow) of PLA2 isoforms by ROS [13], enabling a “neurotoxic cycle.” For completeness, Fig. 5 includes as well the possibility that ROS might arise during binge ethanol exposure via increased membrane NADPH oxidase activity, and/or from brain mitochondrial damage and ROS leakage. The first of these latter two possibilities is indicated, for example, by evidence that preconditioning-like neuroprotection against ischemia/reperfusion that is exerted by an acute ethanol dose in gerbils involves NADPH oxidase-derived ROS [66]. Additionally, mitochondrial dysfunction and derived ROS due to toxic ethanol treatment, mostly in liver but also in brain cultures, have been reported [67, 68]. However, whether these two latter pathways are linked to ethanol-induced brain edema and cell swelling is an open question.
Fig. 5.
Proposed mechanistic scheme for binge ethanol-induced neurodegeneration. Upper pathway depicts focus of this report: brain edema, PLA2 activation, AA mobilization, and ROS generation, and inhibition (neuroprotection) by diuretics, MEP, DHA, and ROS trapping agents at various potential steps. The roles of edema and ROS in the model are predicated on the basis of in vivo results with binge ethanol rat models [7, 8, 18]. Two lower pathways depict possible ROS generation during ethanol exposure from increased NADPH oxidase activity and mitochondrial leakage/damage. See text for further discussion
High ethanol intake or exposure is linked to reductions in brain DHA levels [20, 22]. There are several possible reasons, and an important one is that ethanol can potentiate DHA oxidation [69], contributing to its brain depletion. Added to this are epidemiological studies showing that binge-drinking individuals tend to have lower n-3 dietary intakes than nondrinkers [70]. Given DHA’s essential role in brain cognition [71, 72], its potential deficit in alcohol abuse could be a factor underlying increased vulnerability of the brain to synaptic and neuronal dysfunction. In association with others [71], we concur that dietary DHA or fish oil supplementation might afford a significant element of neuroprotection as well as functional cognitive benefits in active alcoholics.
Acknowledgments
Supported by the Loyola University Medical Center (LUMC) Alcohol Research Program (NIH T32 AA13527) and an LUMC Potts award to M.A.C.
Abbreviations
- AA
Arachidonic acid
- ADA
Adrenic acid
- DHA
Docosahexaenoic acid
- HEC
Hippocampal-entorhinal cortical
- MEP
Mepacrine
- PKB
Protein kinase B
- PLA2
Phospholipase A2
- PS
Phosphatidylserine
- ROS
Reactive oxygen species
References
- 1.Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10:559–561. doi: 10.1016/0741-8329(93)90083-z. doi:10.1016/0741-8329(93)90083-Z. [DOI] [PubMed] [Google Scholar]
- 2.Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, et al. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. doi:10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, 2nd, Gibson DA, Holley RC, et al. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. Neuroscience. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. doi:10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. doi:10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 5.Switzer RC, Majchrowicz E, Weight F. Ethanol-induced argyrophilia in entorhinal cortex of rat. Anat Rec. 1982;202:186A. [Google Scholar]
- 6.Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. doi:10.1111/j.1530-0277.2000.tb01973.x. [PubMed] [Google Scholar]
- 7.Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB J. 1998;12:221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- 8.Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314:780–788. doi: 10.1124/jpet.105.085779. doi:10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6, 7-dinitro-quinoxaline-2, 3-dione, and MK-801. Alcohol Clin Exp Res. 1998;22:217–224. [PubMed] [Google Scholar]
- 10.Brown JC, III, Belmadani A, Kumar S, Neafsey EJ, Collins MA. Neuroinflammatory-like mechanisms in alcohol-induced brain damage. J Neurochem. 2005;94:90. [Google Scholar]
- 11.Lambert IH, Pedersen SF, Poulsen KA, Lambert IH, Pedersen SF, Poulsen KA. Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol (Oxf) 2006;187:75–85. doi: 10.1111/j.1748-1716.2006.01557.x. doi: 10.1111/j.1748-1716.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- 12.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. doi:10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J Neurochem. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. doi:10.1111/j.1471-4159.2007.05003.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinez J, Moreno JJ. Role of Ca2+-independent phospholipase A2 on arachidonic acid release induced by reactive oxygen species. Arch Biochem Biophys. 2001;392:257–262. doi: 10.1006/abbi.2001.2439. doi: 10.1006/abbi.2001.2439. [DOI] [PubMed] [Google Scholar]
- 15.Caro AA, Cederbaum AI. Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic Biol Med. 2006;40:364–375. doi: 10.1016/j.freeradbiomed.2005.10.044. doi:10.1016/j.freeradbiomed.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Maia RC, Culver CA, Laster SM, Maia RC, Culver CA, Laster SM. Evidence against calcium as a mediator of mitochondrial dysfunction during apoptosis induced by arachidonic acid and other free fatty acids. J Immunol. 2006;177:6398–6404. doi: 10.4049/jimmunol.177.9.6398. [DOI] [PubMed] [Google Scholar]
- 17.Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem. 2008;104:1177–1189. doi: 10.1111/j.1471-4159.2007.05022.x. doi:10.1111/j.1471-4159.2007.05022.x. [DOI] [PubMed] [Google Scholar]
- 18.Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, et al. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 19.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. doi:10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 20.Pawlosky RJ, Salem N., Jr Ethanol exposure causes a decrease in docosahexaenoic acid and an increase in docosapentaenoic acid in feline brains and retinas. Am J Clin Nutr. 1995;61:1284–1289. doi: 10.1093/ajcn/61.6.1284. [DOI] [PubMed] [Google Scholar]
- 21.Gustavsson L. Brain lipid changes after ethanol exposure. Ups J Med Sci. 1990 Suppl 48:245–266. [PubMed] [Google Scholar]
- 22.Pawlosky RJ, Bacher J, Salem N., Jr Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: nutritional consequences of a low n-3 fatty acid diet. Alcohol Clin Exp Res. 2001;25:1758–1765. doi: 10.1111/j.1530-0277.2001.tb02187.x. [PubMed] [Google Scholar]
- 23.Noraberg J, Kristensen BW, Zimmer J. Markers for neuronal degeneration in organotypic slice cultures. Brain Res Brain Res Protoc. 1999;3:278–290. doi: 10.1016/s1385-299x(98)00050-6. doi:10.1016/S1385-299X(98)00050-6. [DOI] [PubMed] [Google Scholar]
- 24.Diekmann S, Nitsch R, Ohm TG. The organotypic entorhinal-hippocampal complex slice culture of adolescent rats. A model to study transcellular changes in a circuit particularly vulnerable in neurodegenerative disorders. J Neural Transm. 1994 Suppl 44:61–71. doi: 10.1007/978-3-7091-9350-1_5. [DOI] [PubMed] [Google Scholar]
- 25.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. doi:10.1016/0165-0270(91)90128-M. [DOI] [PubMed] [Google Scholar]
- 26.Jones AW. The drunkest drinking driver in Sweden: blood alcohol concentration 0.545% w/v. J Stud Alcohol. 1999;60:400–406. doi: 10.15288/jsa.1999.60.400. [DOI] [PubMed] [Google Scholar]
- 27.Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52:448–453. doi: 10.15288/jsa.1991.52.448. [DOI] [PubMed] [Google Scholar]
- 28.Mulholland PJ, Self RL, Harris BR, Little HJ, Littleton JM, Prendergast MA. Corticosterone increases damage and cytosolic calcium accumulation associated with ethanol withdrawal in rat hippocampal slice cultures. Alcohol Clin Exp Res. 2005;29:871–881. doi: 10.1097/01.alc.0000163509.27577.da. doi:10.1097/01.ALC.0000163509.27577.DA. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer J, Kristensen BW, Jakobsen B, Noraberg J. Excitatory amino acid neurotoxicity and modulation of glutamate receptor expression in organotypic brain slice cultures. Amino Acids. 2000;19:7–21. doi: 10.1007/s007260070029. doi:10.1007/s007260070029. [DOI] [PubMed] [Google Scholar]
- 30.Belmadani A, Zou JY, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca2+-mediated neurotoxicity. Neuroscience. 2001;104:769–781. doi: 10.1016/s0306-4522(01)00139-7. doi:10.1016/S0306-4522(01)00139-7. [DOI] [PubMed] [Google Scholar]
- 31.Ushijima H, Nishio O, Klocking R, Perovic S, Muller WE. Exposure to gp120 of HIV-1 induces an increased release of arachidonic acid in rat primary neuronal cell culture followed by NMDA receptor-mediated neurotoxicity. Eur J Neurosci. 1995;7:1353–1359. doi: 10.1111/j.1460-9568.1995.tb01126.x. doi:10.1111/j.1460-9568.1995.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 32.Dreyer EB, Lipton SA. The coat protein gp120 of HIV-1 inhibits astrocyte uptake of excitatory amino acids via macrophage arachidonic acid. Eur J Neurosci. 1995;7:2502–2507. doi: 10.1111/j.1460-9568.1995.tb01048.x. doi: 10.1111/j.1460-9568.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown J, III, Achille N, Neafsey EJ, Collins MA. Identification of phospholipase A2 isoforms which contribute to binge alcohol-mediated neurodegeneration. Alcohol. 2006;39:112. doi: 10.1016/j.alcohol.2006.09.010. [Google Scholar]
- 34.Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res. 2004;28:350–364. doi: 10.1097/01.alc.0000113416.65546.01. doi:10.1097/01.ALC.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- 35.Basavappa S, Pedersen SF, Jorgensen NK, Ellory JC, Hoffmann EK. Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. J Neurophysiol. 1998;79:1441–1449. doi: 10.1152/jn.1998.79.3.1441. [DOI] [PubMed] [Google Scholar]
- 36.Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys J. 1995;68:1888–1894. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staub F, Winkler A, Peters J, Kempski O, Baethmann A. Mechanisms of glial swelling by arachidonic acid. Acta Neurochir Suppl (Wien) 1994;60:20–23. doi: 10.1007/978-3-7091-9334-1_5. [DOI] [PubMed] [Google Scholar]
- 38.Staub F, Winkler A, Peters J, Kempski O, Kachel V, Baethmann A. Swelling, acidosis, and irreversible damage of glial cells from exposure to arachidonic acid in vitro. J Cereb Blood Flow Metab. 1994;14:1030–1039. doi: 10.1038/jcbfm.1994.135. [DOI] [PubMed] [Google Scholar]
- 39.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. doi:10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Zhao X, Mao ZY, Wang XM, Liu ZL. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14:2457–2461. doi: 10.1097/00001756-200312190-00033. doi:10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 41.Kishida E, Tajiri M, Masuzawa Y, Kishida E, Tajiri M, Masuzawa Y. Docosahexaenoic acid enrichment can reduce L929 cell necrosis induced by tumor necrosis factor. Biochim Biophys Acta. 2006;1761:454–462. doi: 10.1016/j.bbalip.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Tada M, Takahata K, Tomizawa K, Matsui H. Inhibitory effect of polyunsaturated fatty acids on apoptosis induced by etoposide, okadaic acid and AraC in Neuro2a cells. Acta Med Okayama. 2007;61:147–152. doi: 10.18926/AMO/32903. [DOI] [PubMed] [Google Scholar]
- 43.Florent S, Malaplate-Armand C, Youssef I, Kriem B, Koziel V, Escanye MC, et al. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J Neurochem. 2006;96:385–395. doi: 10.1111/j.1471-4159.2005.03541.x. doi:10.1111/j.1471-4159.2005.03541.x. [DOI] [PubMed] [Google Scholar]
- 44.Strokin M, Chechneva O, Reymann KG, Reiser G, Strokin M, Chechneva O, et al. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose deprivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phospholipids by calcium independent phospholipase A2. Neuroscience. 2006;140:547–553. doi: 10.1016/j.neuroscience.2006.02.026. doi:10.1016/j.neuroscience.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Cao D, Xue R, Xu J, Liu Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem. 2005;16:538–546. doi: 10.1016/j.jnutbio.2005.02.002. doi:10.1016/j.jnutbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. doi:10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 47.Young C, Gean PW, Chiou LC, Shen YZ. Docosahexaenoic acid inhibits synaptic transmission and epileptiform activity in the rat hippocampus. Synapse. 2000;37:90–94. doi: 10.1002/1098-2396(200008)37:2<90::AID-SYN2>3.0.CO;2-Z. doi:10.1002/1098-2396(200008)37:2<90::AID-SYN2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 48.Martinez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J Neurochem. 1998;71:2528–2533. doi: 10.1046/j.1471-4159.1998.71062528.x. [DOI] [PubMed] [Google Scholar]
- 49.Salem N, Jr, Kim H-Y, Yergey JA. Docosahexaenoic acid in membrane function and metabolism. In: Simopoulos A, Martin R, Kifer R, editors. The health effects of polyunsaturated fatty acids in seafoods. New York: Academic Press; pp. 263–317. [Google Scholar]
- 50.Wilson R, Bell MV. Molecular species composition of glycerophospholipids from white matter of human brain. Lipids. 1993;28:13–17. doi: 10.1007/BF02536353. doi:10.1007/BF02536353. [DOI] [PubMed] [Google Scholar]
- 51.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24:69–176. doi: 10.1016/0163-7827(85)90011-6. doi:10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 52.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. doi:10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin RE. Docosahexaenoic acid decreases phospholipase A2 activity in the neurites/nerve growth cones of PC12 cells. J Neurosci Res. 1998;54:805–813. doi: 10.1002/(SICI)1097-4547(19981215)54:6<805::AID-JNR8>3.0.CO;2-4. doi:10.1002/(SICI)1097-4547(19981215)54:6<805::AID-JNR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. doi:10.1038/sj.mp. 4001887. [DOI] [PubMed] [Google Scholar]
- 55.Bazan NG., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970;218:1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- 56.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. doi:10.1038/sj.bjp. 0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yavin E, Brand A, Green P. Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr Neurosci. 2002;5:149–157. doi: 10.1080/10284150290003159. doi:10.1080/10284150290003159. [DOI] [PubMed] [Google Scholar]
- 58.Hossain MS, Hashimoto M, Gamoh S, Masumura S. Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem. 1999;72:1133–1138. doi: 10.1046/j.1471-4159.1999.0721133.x. doi:10.1046/j.1471-4159.1999.0721133.x. [DOI] [PubMed] [Google Scholar]
- 59.Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, Bourcier T, et al. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci USA. 2006;103:15184–15189. doi: 10.1073/pnas.0510086103. doi:10.1073/pnas.0510086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo M, Stockert L, Akbar M, Kim HY. Neuronal specific increase of phosphatidylserine by docosahexaenoic acid. J Mol Neurosci. 2007;33:67–73. doi: 10.1007/s12031-007-0046-z. doi:10.1007/s12031-007-0046-z. [DOI] [PubMed] [Google Scholar]
- 61.Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. doi:10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 62.Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. doi:10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- 63.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. doi:10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 64.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. doi:10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Sun AY, Simonyi A, Kalogeris TJ, Miller DK, Sun GY, et al. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidasederived ROS. Free Radic Biol Med. 2007;43:1048–1060. doi: 10.1016/j.freeradbiomed.2007.06.018. doi:10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bailey SM. A review of the role of reactive oxygen and nitrogen species in alcohol-induced mitochondrial dysfunction. Free Radic Res. 2003;37:585–596. doi: 10.1080/1071576031000091711. doi:10.1080/1071576031000091711. [DOI] [PubMed] [Google Scholar]
- 68.Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–588. doi: 10.1002/jnr.10767. doi:10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- 69.Milne GL, Morrow JD, Picklo MJ., Sr Elevated oxidation of docosahexaenoic acid, 22:6 (n-3), in brain regions of rats undergoing ethanol withdrawal. Neurosci Lett. 2006;405:172–174. doi: 10.1016/j.neulet.2006.06.058. doi:10.1016/j.neulet.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 70.Kim SY, Breslow RA, Ahn J, Salem N., Jr Alcohol consumption and fatty acid intakes in the 2001–2002 National Health and Nutrition Examination Survey. Alcohol Clin Exp Res. 2007;31:1407–1414. doi: 10.1111/j.1530-0277.2007.00442.x. doi:10.1111/j.1530-0277.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- 71.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. doi:10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 72.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159–164. doi: 10.1016/j.neures.2006.06.010. doi:10.1016/j.neures.2006.06.010. [DOI] [PubMed] [Google Scholar]