Abstract
Context
Patients with early stage but medically inoperable lung cancer patients have a poor rate of primary tumor control (30-40%) and a high rate of mortality (3-year survival 20-35%) with current management.
Objective
To evaluate the toxicity and efficacy of stereotactic body radiation therapy in a high risk population of patients with early stage but medically inoperable lung cancer.
Design, Setting, and Patients
Phase 2 North American multicenter study of patients with biopsy-proven peripheral T1-T2, N0, M0 non-small cell tumors less than 5 cm in diameter and medical conditions precluding surgical treatment. The prescription dose was 18 Gy per fraction times 3 fractions (54 Gy total) delivered in 1½-2 weeks. The study opened May 26, 2004, and closed October 13, 2006; data were analyzed through August 31, 2009.
Main Outcome Measures
The primary endpoint was primary tumor control with overall survival, disease free survival, adverse events, involved lobe, regional, and disseminated recurrence as secondary endpoints.
Results
A total of 59 patients accrued, of which 55 were evaluable (44 T1 and 11 T2 tumors) with a median follow-up of 34.4 months (range, 4.8 to 49.9 months). Only 1 patient had a primary tumor failure; the estimated 3-year primary tumor control rate was 97.6% (95% confidence interval [CI], 84.3%, 99.7%). Three patients had recurrence within the involved lobe; the 3-year primary tumor and involved lobe (local) control rate was 90.6% (95% CI, 76.0%, 96.5%). Two patients experienced regional failure; the local-regional control rate was 87.2% (95%CI, 71.0%, 94.7%). Eleven patients experienced disseminated recurrence; the 3-year rate of disseminated failure was 22.1% (95% CI, 12.3%, 37.8%). The rates of disease-free and overall survival at 3 years were 48.3% (95% CI, 34.4%, 60.8%) and 55.8% (95% CI, 41.6%, 67.9%), respectively. The median overall survival was 48.1 months (95% CI, 29.6% to not reached). Protocol specified treatment-related grade 3 adverse events were reported in 7 patients (12.7%; 95% CI, 9.6%, 15.8%); grade 4 events were reported in 2 patients (3.6%; 95%CI, 2.7%, 4.5%). No grade 5 adverse events were reported.
Conclusion
Patients with inoperable non-small cell lung cancer who received stereotactic body radiation therapy had a survival rate of 55.8% at 3 years, high rates of local tumor control, and moderate treatment-related morbidity.
Introduction
While anatomical resection is the standard treatment for early stage lung cancer, some patients cannot tolerate surgery due to comorbidities such as emphysema and heart disease. These patients are deemed medically inoperable and are generally offered conventional radiotherapy, most commonly given in 20-30 outpatient treatments, or observed without specific cancer therapy. Outcomes are not ideal with either approach since conventional radiotherapy fails to durably control the primary lung tumor in 60-70% of patients1-3, over half of patients ultimately die specifically from progressive lung cancer with observation4-5, and 2-year survival is under 40% with either approach.
Stereotactic Body Radiation Therapy (SBRT) is a non-invasive cancer treatment where numerous small, highly focused, and accurate radiation beams are used to deliver potent doses in 1-5 treatments to tumor targets in extracranial sites6. Consensus publications describing the conduct and technical requirements of SBRT have been published7. Numerous single institution studies have shown that SBRT is an effective and well tolerated treatment for early stage lung cancer in medically inoperable patients8-10. The Radiation Therapy Oncology Group (RTOG) 0236 trial was the first North American multicenter, cooperative group study to test SBRT in treating medically inoperable patients with early stage non-small cell lung cancer. In this report, we describe the 3-year results from RTOG 0236.
Patients and Methods
Patient Eligibility
Patients had to be ≥18 years old with Zubrod performance status 0 (fully active, unrestricted), 1 (restricted activities but able to work), or 2 (cares for self but unable to work). Cytologic or histologic proof of non-small cell cancer was required for entry. Eligible patients could have American Joint Committee on Cancer (AJCC) stages T1, T2 (≤5 cm), or T3 (≤5 cm peripheral tumors only), N0, M0 cancer based on both mandatory computed tomography (CT) and positron emission tomography (PET) screening. While a subset of T3 patients were eligible, ultimately none were enrolled making the study results not necessarily pertinent to the T3 subset. The treated tumor was required to be >2 cm in all directions from the “proximal bronchial tree” defined as the distal 2 cm of trachea, carina, and named major lobar bronchi up to their first bifurcation. Patients were ineligible if they had a synchronous malignancy within 2 years of entry. Patients with history of prior radiotherapy to the thorax, active systemic, pulmonary, or pericardial infection, and pregnant or lactating women were ineligible. Those with plans to receive conventional radiotherapy, chemotherapy, biological therapy, vaccine therapy, or surgery as treatment (except at disease progression) were ineligible. Operable patients were ineligible. Data regarding race and ethnicity were collected from the registering site as per reporting requirements of the study sponsor (the United States National Cancer Institute); however, this information was not used in patient selection. All patients were required to sign informed consent prior to study registration.
Prior to enrollment, patients were required to be evaluated by an experienced thoracic surgeon or pulmonologist to determine “operability.” Standard indicators defining a patient to be “medically inoperable” included baseline Forced Expiratory Volume in 1 second (FEV-1) <40% predicted, predicted post-operative FEV-1 <30% predicted, carbon monoxide diffusing capacity <40% predicted, baseline hypoxemia or hypercapnia, severe pulmonary hypertension, diabetes mellitus with end organ damage, severe cerebral, cardiovascular or peripheral vascular disease, or severe chronic heart disease.
Radiotherapy Specifications
The gross tumor volume was outlined on pulmonary CT windows, excluding soft tissue densities with standard uptake values (SUV) on PET less than 2 (likely to be atelectasis). No additional margin was added for possible microscopic extension. An institution appropriate error margin beyond this gross tumor volume (defined as the planning target volume) which included both set-up error and error related to motion, was limited to no more than 5 mm in the axial dimension and 10 mm in the craniocaudal dimension.
Patients received 60 Gy in 3 fractions of 20 Gy per fraction, which was prescribed to the edge of the planning target volume. Each fraction was separated by at least 40 hours (at most 8 days). The entire 3 fraction regimen was required to be completed within 14 days. Only 4 to 10 MV photon beams were allowed. For planning, no tissue density heterogeneity correction was allowed. Later analysis using proper accounting of density heterogeneity showed RTOG 0236 over-predicted the actual planning target volume dose such that the delivered dose was actually closer to 54 Gy in 3 fractions of 18 Gy11.
Image guidance capable of confirming the position of the target with each treatment was required. Tumor motion related to respiration was required to be quantified using fluoroscopy or 4-dimensional (4-D) CT scans. If the motion confirmed with free breathing was greater than the maximum planning target volume expansions allowed by the protocol, a method of motion control such as abdominal compression, gating, breath holding was required.
Adequate target coverage was achieved when 95% of the planning target volume was covered by 60 Gy and when 99% of the planning target volume received at least 54 Gy. High dose conformality was controlled such that the volume of tissue outside of the planning target volume receiving a dose greater than 63 Gy must be less than 15% of the planning target volume and the target conformality index (ratio of the volume receiving 60 Gy to the planning target volume) was ≤1.2. Moderate dose conformality and gradient quality were controlled by the parameters listed in Table 1. The treatment plans also had to meet a number of contoured organ dose constraints (Table 2).
Table 1.
| Planning target volume, PTV (cc) |
Ratio of 50% Prescription Isodose Volume to the PTV, R50% |
Maximum Dose 2 cm from PTV in any Direction, D2cm (Gy) |
||
|---|---|---|---|---|
| Deviation | Deviation | |||
| none | minor* | none | minor | |
| 1.8 | <3.9 | 3.9-4.1 | <28.1 | 28.1-30.1 |
| 3.8 | <3.9 | 3.9-4.1 | <28.1 | 28.1-30.1 |
| 7.4 | <3.9 | 3.9-4.1 | <28.1 | 28.1-30.1 |
| 13.2 | <3.9 | 3.9-4.1 | <28.1 | 28.1-30.1 |
| 21.9 | <3.8 | 3.8-4.0 | <30.4 | 30.4-32.4 |
| 33.8 | <3.7 | 3.7-3.9 | <32.7 | 32.7-34.7 |
| 49.6 | <3.6 | 3.6-3.8 | <35.1 | 35.1-37.1 |
| 69.9 | <3.5 | 3.5-3.7 | <37.4 | 37.4-41.7 |
| 95.1 | <3.3 | 3.3-3.5 | <39.7 | 39.7-41.7 |
| 125.8 | <3.1 | 3.1-3.3 | <42.0 | 42.0-44.0 |
| 162.6 | <2.9 | 2.9-3.1 | <44.3 | 44.3-46.3 |
For values of PTV dimension or volume not specified, linear interpolation between table entries was required.
Protocol deviations greater than listed here as ‘minor’ were classified as ‘major’ for protocol compliance.
Table 2.
Organ tolerance dose limits for Radiation Therapy Oncology Group 0236a
| Organ | Volume | Total Dose |
|---|---|---|
| Spinal Cord | Any point | 18 Gy maximum |
| Esophagus | Any point | 27 Gy maximum |
| Ipsilateral Brachial Plexus | Any point | 24 Gy maximum |
| Heart | Any point | 30 Gy maximum |
| Trachea and Ipsilateral Bronchus | Any point | 30 Gy maximum |
| Whole Lung (Right & Left) | <10% of volume | 20 Gyb |
Exceeding organ limits by more than 2.5% constituted a ‘minor’ protocol violation. Exceeding these organ limits by more than 5% constituted a ‘major’ protocol violation.
Also known as V-20 or volume of total lung getting 20 Gy or more.
Institutional Review and Accreditation
Prior to enrollment of any patients, sites were required to have both local Institutional Review Board/Ethics approval and pass central credentialing standards for protocol participation defined by the Advanced Technology Consortium. This credentialing included irradiation of a standard chest phantom (supplied by the Radiologic Physics Center, Houston, TX). In addition, all sites were required to obtain central approval of their methods of immobilization, motion assessment and control, and target verification. Finally, upon enrollment of the first patient onto the protocol from each center, a central review of the contouring and dosimetry by the PI was facilitated by the Image Guided Therapy QA Center at Washington University (St. Louis, MO) to assure that protocol objectives were met.
Follow-up and Endpoints
Patients were seen every three months during years 1 and 2 post treatment and then every 6 months until 4 years post treatment. Imaging was required at each visit for response and toxicity assessment using CT scans. Follow-up PET scans were required if progressive soft tissue abnormalities where noted on CT. Pulmonary function tests (FEV-1, DLCO, and arterial blood gases) were to be performed every 3 months for year 1 posttreatment and every 6 months for year 2 post treatment. Tumor measurements at each follow-up were carried out using the Response Evaluation Criteria in Solid Tumors (RECIST)12 where a complete response (CR) is total tumor disappearance and partial response (PR) is decrease in the longest tumor diameter by 30% or more.
The primary endpoint of the study was two-year actuarial primary tumor control. Primary tumor control was defined as the absence of primary tumor failure. Primary tumor failure was defined based on meeting both of two criteria: 1. Local enlargement defined as at least a 20% increase in the longest diameter of the gross tumor volume per CT, and 2. Evidence of tumor viability. Tumor viability could be affirmed by either demonstrating PET imaging with uptake of a similar intensity as the pretreatment staging PET, or by repeat biopsy confirming carcinoma. Primary tumor failure included marginal failures occurring within 1 cm of the planning target volume (1.5-2.0 cm from the gross tumor volume). Failure beyond the primary tumor but within the involved lobe was collected separately from disseminated failure within uninvolved lobes. Local failure is the combination of primary tumor and involved lobe failure with local control being the absence of local failure.
Secondary endpoints included assessments of treatment-related toxicity, disease free survival and overall survival. Disease-free survival included separate assessments of local-regional failure (within the primary site, involved lobe, hilum, and mediastinum) and disseminated recurrence (failure beyond the local and regional sites).
The National Cancer Institute’s Common Toxicity Criteria (CTC) Version 3.0 was used for grading of adverse events13. Certain adverse events attributable to study therapy were specified prospectively within the protocol for use in evaluating the secondary endpoint of treatment related toxicity including grade 3 measures of lung injury, esophageal injury, heart injury, and nerve damage as well as any grade 4-5 toxicity felt related to treatment. However, all adverse events reported by participating centers were collected and assessed.
Statistical Design
The study design aimed to improve the two-year primary tumor control rate from 60% to 80%. Since conventional radiation provides poor primary tumor control (<40%), a phase II study comparing SBRT with conventional radiation would result in a sample size too small for meaningful assessment. Instead, a rate of 60% was chosen as the lowest acceptable primary tumor control rate after taking into consideration the >80% primary tumor control rate seen in the Indiana University study9. Assuming an exponential distribution of time to primary tumor progression, the hazard rate for a primary tumor control rate of 80% was projected to be 0.0093 per month, and the hazard rate for a 60% primary tumor control rate was projected to be 0.02128. Eighteen failures were required for a one-sided Type 1 error rate of 0.05 with 80% statistical power to detect a difference in primary tumor control rates of at least 20%, resulting in a required sample size of 49 and two years of follow-up. After adjusting for a potential ineligibility rate of 5% (based on pre-treatment characteristics), the final sample size was 52 patients.
The secondary endpoint of treatment-related toxicity used the null hypothesis that the toxicity rate is less than or equal to 25% with an overall Type I error rate of no more than 0.1014. The null hypothesis would be rejected if there were 17 or more patients with unacceptable toxicity, defined as any grade 3 or 4 AE related to the symptoms shown in Table 3 or any grade 4 or 5 AE attributed to SBRT, of the first 49 evaluable patients who met all eligibility criteria and received at least some portion of protocol treatment. Three interim analyses of toxicity were planned after 25%, 50%, and 75% of the total number of evaluable patients were accrued.
Table 3.
Pretreatment Characteristics of Patients Enrolled in Radiation Therapy Oncology Group 0236
| Characteristic | Patients No. (%) (N=55)a |
|
|---|---|---|
| Age, median (range) | 72 (48-89) | |
| Median | ||
| Range | ||
| Number of Patients | ||
| Racial Category | ||
| Asian | 2 (4) | |
| Black or African American |
2 (4) | |
| White | 51 (93) | |
| Gender | ||
| Male | 21 (38) | |
| Female | 34 (62) | |
| Zubrod Performance Statusb | ||
| 0 | 12 (22) | |
| 1 | 35 (64) | |
| 2 | 8 (15) | |
| Stage | ||
| IA | 44 (80) | |
| IB | 11 (20) | |
| Histology | ||
| Squamous cell carcinoma |
17 (31) | |
| Adenocarcinoma | 19 (35) | |
| Large cell undifferentiated |
3 (5) | |
| Non-small cell carcinoma not otherwise specified |
16 (29) | |
Unless otherwise specified
Defined as fully active, unrestricted for those with a score of 0, restricted activities but able to work for those with a score of 1, and cares for self but unable to work for those with a score of 2.
The hazard rate of primary tumor control at two years was estimated using life table estimates with a time span of two years. Patients who died within 2 years without primary tumor failure were censored at the time of death. Primary tumor control time was measured from the start of treatment to date of failure or date of censoring. A one-sided Z-test was used to test the significance between the logarithm of the estimated hazard rate (λEST) and the hypothesized hazard rate (λhyp =0.0093) with a variance equal to the reciprocal of the number of cases with a primary tumor progression observed within 2 years. Thus, the null hypothesis would be rejected at a significance level of 0.05 when the test statistic Z had a value less than −1.645.
Tumor control rates as well as the secondary endpoints of DFS and OS were estimated using the Kaplan-Meier product limit method15. A failure for DFS was defined as the first of the following: local failure, marginal failure, regional failure, disseminated recurrence, or death; patients who were alive without failure were censored at the date of last follow-up. A failure for OS was death due to any cause; patients still alive were censored at the date of last follow-up. All endpoints were measured from the date of study registration to allow for the risk of toxicity and failure prior to or during SBRT. An unplanned exploratory analysis was done examining outcomes within T1 patients and within T2 patients; no statistical comparisons were done between these two groups. All analyses were performed using Statistical Analysis Software (SAS®) version 9.2.
Results
Between May 2004 and October 2006, 59 patients were enrolled to the study of which 55 were evaluable; four patients of the 59 enrolled were deemed not evaluable because they did not meet eligibility requirements (3 patients) or did not receive SBRT (1 patient). Pretreatment characteristics for evaluable patients are shown in Table 3.
Median follow-up for all patients (n=55) was 34.4 months (range 4.8-49.9 months). Among patients still living (n=29), median follow-up was 38.7 months (range 30.2 to 49.9 months). One patient did not return for follow-up evaluations after treatment was completed. It was found through the Social Security Death Index that the patient passed away 7 months after completion of treatment. This patient was censored for all endpoints except overall survival for which the patient was a failure. All other patients were able to be evaluated for response at least once. If at any given follow-up a patient was not evaluated for disease status, it was assumed that the disease status reported from the previous follow-up was still applicable. Reasons for a patient not being evaluated were not collected.
Digital data submitted to the ITC was reviewed for contouring and dosimetry compliance. There were no deviations recorded in contouring primary tumor targets. Tumor coverage was scored acceptable for all but 1 patient enrolled (98% tumor coverage compliance). Normal tissue dose constraints were appropriately respected in 40 patients (73% normal tissue dose constraint compliance). There were no reports of using non-protocol therapy in combination with SBRT.
Twenty-eight patients (51% (95% confidence interval, CI: 42%, 60%)) had a CR occurring a median of 6.5 months (range 1.6 to 42.6 months) from completion of SBRT. PR was recorded in 21 patients; the rate of complete plus partial response after therapy was 89% (CI: 81%, 97%).
Only one patient (T2, N0, M0 at diagnosis) experienced a documented recurrence or progression at the primary site. There were no reported marginal recurrences. The 3-year primary tumor control rate was 97.6% (95% CI, 84.3%, 99.7%), with a hazard ratio of 0.001. The corresponding Z statistic was −2.226 which is less than −1.645 with a p-value of 0.013 consistent with a hypothesis that the primary tumor control rate would be at least 80%. Three patients had recurrence within the involved lobe; the 3-year primary tumor and involved lobe (local) control rate was 90.6% (95% CI, 76.0, 96.5).
Regional failures were reported in 2 patients, one occurring at 33.0 and the other at 36.1 months post protocol therapy. Combining local and regional failures, the three year local-regional control rate was 87.2% (95% CI, 71.0%, 94.7%). Disseminated recurrence as some component of recurrence was reported in 11 patients. Collectively, 14 patients had recurrence of cancer. Their patterns of failure included: 1 primary alone, 1 involved lobe alone, 2 involved lobe and disseminated, 1 hilum alone, 1 mediastinum and disseminated, and 8 disseminated alone. The 3-year rate of disseminated failure was 22.1% (95% CI, 12.3%, 37.8%) with 8 such failures occurring prior to 24 months. The 3-year rate of disseminated recurrence for T1 patients was 14.7% (95% CI, 6.2%, 32.7%); however, this rate was 47.0% (95% CI, 22.7%, 79.1%) for patients with T2 tumors. The 3-year rates of disseminated recurrence were 5.9% (95% CI, 0.9%, 35.0%) for squamous histology and 30.7% (95%CI, 16.8%, 51.8%) for non-squamous histology.
Twenty-six patients died during the period of observation after treatment. Ten patients (18% of entire study population (95% CI, 8%, 13%)) died of lung cancer. Two patients died of non-protocol related medical interventions and 5 of co-morbid problems, specifically stroke, myocardial infarction in two patients, aggravation of emphysema, and 2nd malignancy. Nine patients died of unknown causes.
Disease-free survival and overall survival at 3 years were 48.3% (95% CI, 34.4%, 60.8%) and 55.8% (95% CI, 41.6%, 67.9%), respectively (Figure). Median disease-free survival and overall survival for all patients were 34.4 months (95% CI, 25.0 months to not reached) and 48.1 months (95% CI, 29.6 months to not reached), respectively. For T1 patients, median disease-free survival was 36.1 months (95% CI, 25.0 months to not reached) and median overall survival was not reached, which was similar to T2 patients whose disease-free survival and overall survival were 30.8 months (95% CI, 4.9 months to not reached) and 33.7 months (95% CI, 11.1 months to not reached), respectively.
Figure.
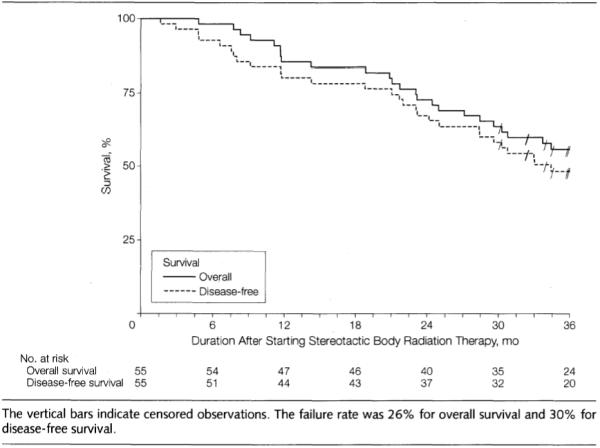
Patient Course After Initiation of Stereotactic Body Radiation Therapy
Seven patients (12.7%; 95% CI, 9.6%, 15.8%) and 2 patients (3.6%; 95% CI, 2.7, 4.5) were reported to experience protocol specified treatment-related grade 3 and 4 adverse events, respectively. No grade 5 treatment-related adverse events were reported. An additional 6 patients (10.9%; 95% CI, 8.2%, 13.6%) were reported to have adverse events attributable to SBRT that were not classified prospectively as protocol specified. Three of these non-protocol specified serious adverse events were related to complications of the skin or ribs. All grades of reported SBRT-related adverse events appear in Table 4. Protocol specified adverse events only appear in Table 5. Because only 9 of the first 49 evaluable patients experienced a protocol-specified adverse event, the null hypothesis that the true adverse event rate is 25% or less cannot be rejected.
Table 4.
Adverse Events Related to Stereotactic Body Radiation Therapya
| All Patients (n=55) |
First 49 Evaluable Patients (n=49) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||||
| Category | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
|
| ||||||||||
| Blood/Bone Marrow | 3 | 1 | 2 | 0 | 0 | 3 | 1 | 2 | 0 | 0 |
| Cardiovascular (general) | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Coagulation | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Constitutional symptoms | 11 | 8 | 1 | 0 | 0 | 11 | 7 | 1 | 0 | 0 |
| Dermatology/Skin | 3 | 2 | 2 | 0 | 0 | 3 | 1 | 2 | 0 | 0 |
| Gastrointestinal | 4 | 1 | 1 | 0 | 0 | 4 | 1 | 1 | 0 | 0 |
| Hemorrhage/Bleeding | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Infection | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Lymphatics | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Metabolic/Laboratory | 2 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 0 |
| Musculoskeletal/Soft Tissue | 3 | 5 | 3 | 0 | 0 | 3 | 3 | 3 | 0 | 0 |
| Neurology | 3 | 2 | 1 | 0 | 0 | 3 | 2 | 1 | 0 | 0 |
| Pain | 5 | 9 | 0 | 0 | 0 | 5 | 6 | 0 | 0 | 0 |
| Pulmonary/Upper Respiratory |
11 | 13 | 8 | 1 | 0 | 11 | 11 | 8 | 1 | 0 |
| Renal/Genitourinary | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
|
| ||||||||||
| Worst non-hematologic | 13 (24%) |
17 (31%) |
13 (24%) |
2 (4%) |
0 (0%) |
13 (27%) |
14 (29%) |
13 (27%) |
2 (4%) |
0 (0%) |
|
| ||||||||||
| Worst overall | 13 (24%) |
17 (31%) |
13 (24%) |
2 (4%) |
0 (0%) |
13 (27%) |
14 (29%) |
13 (27%) |
2 (4%) |
0 (0%) |
Includes adverse events where relationship to treatment was missing
Table 5.
Protocol-Specified Adverse Events Related to Stereotactic Body Radiation Therapya
| All Patients (n=55) |
First 49 Evaluable Patients (n=49) |
|||||
|---|---|---|---|---|---|---|
| Adverse Event | 3 | 4 | 5 | 3 | 4 | 5 |
|
| ||||||
| Forced expiratory volume | 2 | 0 | 0 | 2 | 0 | 0 |
| Hypocalcemia | 0 | 1 | 0 | 0 | 1 | 0 |
| Hypoxia | 2 | 0 | 0 | 2 | 0 | 0 |
| Pneumonitis NOS | 2 | 0 | 0 | 2 | 0 | 0 |
| Pulmonary function test NOS decreased | 3 | 1 | 0 | 3 | 1 | 0 |
|
| ||||||
| Maximum protocol-specific adverse event | 7 (13%) |
2 (4%) |
0 (0%) |
7 (14%) |
2 (4%) |
0 (0%) |
Includes adverse events where relationship to treatment is missing
Comment
The main finding in this prospective study was the high rate of primary tumor control (97.6% at 3 years). Primary tumor control is an essential requirement for the cure of lung cancer. Treatments applied for curative intent must be judged at least partly on their ability to control gross disease. Stereotactic body radiation therapy as delivered in RTOG 0236 provided more than double the rate of primary tumor control than reports describing conventional radiotherapy1, 3, 16-17. Admittedly, patients deemed medically inoperable have other competing causes of death besides lung cancer4. Series reporting results from conventional radiotherapy for similar patient groups report 2-3-year OS in the 20-35% range2-3, 18, considerably lower than the 55.8% rate at 3 years in this report.
In contrast to the trial from Indiana University where the dose levels used in this trial were first piloted9, there were no reported SBRT-related patient deaths in RTOG 0236. Perhaps, this is because patients with centrally located tumors were not eligible for RTOG 0236. In contrast, as described in the update by Fakiris19, the Indiana University experience included 31% of patients with central tumors. They had 5 treatment related deaths out of the cohort of 70 patient and 3 year overall survival of 42.7%. While we attempted to obtain complete follow-up on all patients, our study was flawed in the fact that few patients had autopsy at time of death and 9 patients died of unknown causes.
The most disappointing finding in this trial was the rate of disseminated recurrence (22.1% at 3 years). Because the primary tumor, involved lobe, and regional failure rates were all low and metastases appeared fairly soon after SBRT, it might be assumed that many of these patients harbored occult tumor at diagnosis that went undetected by initial staging (i.e., CT and PET). Given that the regional failure rate in the hilum and mediastinum was quite low (only 2 patients), adding pre-SBRT hilar or mediastinal pathological staging, as is commonly done in operable patients, would not likely have altered the rate of disseminated recurrence. Instead, these results would imply that either better whole body staging to identify patients with occult metastatic disease or effective adjuvant therapies to eradicate such disease is necessary to improve the outcomes.
Because RTOG 0236 was the first North American cooperative group trial using SBRT, considerable effort was expended in developing the infrastructure to ensure a consistent and high quality treatment across all enrolling centers. The products of these interactions were the compliance criteria, the accreditation and credentialing criteria, the quality assurance assessment criteria and mechanisms, and the data collection and monitoring program all specific to SBRT20. This infrastructure greatly facilitated the high compliance observed for this protocol and will eventually allow evaluation of relationships between dosimetry, compliance, and adverse events with longer follow-up (more events) from completed trials.
RTOG 0236 was limited largely by the patient population’s ability to undergo invasive procedures. Even the initial biopsy required to confirm malignancy for eligibility was potentially threatening to this frail population. Importantly, the trial did not require and seldom used invasive pathological staging and histological confirmation of recurrence. Rather, non-invasive tests like CT and PET were used both of which are associated with problems with accuracy. Control was consistently evaluated by diagnostic CT, but PET was only used if CT showed progressive changes. Collectively, these staging and evaluation methods make this experience difficult to compare to results after surgical management of healthier patients where both invasive staging and histological confirmation of recurrence is more common.
The RTOG 0236 trial demonstrated that technologically intensive treatments like SBRT can be carried out in a cooperative group so long as proper infrastructure and support are put in place. The RTOG will be building on RTOG 0236 to complete a trial to determine a safe and effective dose for central lung tumors (RTOG 0813), complete a trial to refine the dose of SBRT for peripheral tumors (RTOG 0915), and design a trial to address the rather high rate of disseminated failure observed after treatment.
Acknowledgments
Supported by grant numbers (RTOG U10 CA21661, CCOP U10 CA37422, Stat U10 CA32115) from the National Cancer Institute and the ATC grant (U24 CA 81647)
References
- 1.Armstrong JG, Minsky BD. Radiation therapy for medically inoperable stage I and II non-small cell lung cancer. Cancer Treat Rev. 1989 Dec;16(4):247–255. doi: 10.1016/0305-7372(89)90044-3. [DOI] [PubMed] [Google Scholar]
- 2.Dosoretz DE, Katin MJ, Blitzer PH, et al. Medically Inoperable Lung Carcinoma: The Role of Radiation Therapy. Semin Radiat Oncol. 1996 Apr;6(2):98–104. doi: 10.1053/SRAO00600098. [DOI] [PubMed] [Google Scholar]
- 3.Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush C. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1993 Oct 20;27(3):517–523. doi: 10.1016/0360-3016(93)90374-5. [DOI] [PubMed] [Google Scholar]
- 4.McGarry RC, Song G, des Rosiers P, Timmerman R. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest. 2002 Apr;121(4):1155–1158. doi: 10.1378/chest.121.4.1155. [DOI] [PubMed] [Google Scholar]
- 5.Raz DJ, Zell JA, Ou SH, Gandara DR, Anton-Culver H, Jablons DM. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest. 2007 Jul;132(1):193–199. doi: 10.1378/chest.06-3096. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007 Mar 10;25(8):947–952. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 7.Potters L, Steinberg M, Rose C, et al. American Society for Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2004 Nov 15;60(4):1026–1032. doi: 10.1016/j.ijrobp.2004.07.701. [DOI] [PubMed] [Google Scholar]
- 8.Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005 Dec 1;63(5):1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006 Oct 20;24(30):4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 10.Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008 Mar 1;70(3):685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009 Mar 15;73(4):1235–1242. doi: 10.1016/j.ijrobp.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000 Feb 2;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003 Jul;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 14.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982 Mar;38(1):143–151. [PubMed] [Google Scholar]
- 15.Kaplan E, Meier P. Nonparametric Estimation from Incomplete Observations. Amer Stat Assoc J. 1958 June; [Google Scholar]
- 16.Dosoretz DE, Galmarini D, Rubenstein JH, et al. Local control in medically inoperable lung cancer: an analysis of its importance in outcome and factors determining the probability of tumor eradication. Int J Radiat Oncol Biol Phys. 1993 Oct 20;27(3):507–516. doi: 10.1016/0360-3016(93)90373-4. [DOI] [PubMed] [Google Scholar]
- 17.Dosoretz DE, Katin MJ, Blitzer PH, et al. Radiation therapy in the management of medically inoperable carcinoma of the lung: results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys. 1992;24(1):3–9. doi: 10.1016/0360-3016(92)91013-d. [DOI] [PubMed] [Google Scholar]
- 18.Haffty BG, Goldberg NB, Gerstley J, Fischer DB, Peschel RE. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1988 Jul;15(1):69–73. doi: 10.1016/0360-3016(88)90348-3. [DOI] [PubMed] [Google Scholar]
- 19.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009 Nov 1;75(3):677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Timmerman R, Galvin J, Michalski J, et al. Accreditation and quality assurance for Radiation Therapy Oncology Group: Multicenter clinical trials using Stereotactic Body Radiation Therapy in lung cancer. Acta Oncol. 2006;45(7):779–786. doi: 10.1080/02841860600902213. [DOI] [PubMed] [Google Scholar]


