Abstract
This randomized, double-blind, placebo-controlled study compared the effects of high-dose (100 mg/d) naltrexone versus placebo in a sample of 87 randomized subjects with both cocaine and alcohol dependence. Medication conditions were crossed with two behavioral therapy platforms that examined whether adding contingency management (CM) that targeted cocaine abstinence would enhance naltrexone effects compared to cognitive behavioral therapy (CBT) without CM. Primary outcome measures for cocaine (urine screens) and alcohol use (timeline followback) were collected thrice-weekly during 12 weeks of treatment. Retention in treatment and medication compliance rates were low. Rates of cocaine use and drinks per day did not differ between treatment groups; however naltrexone did reduce frequency of heavy drinking days, as did CBT without CM. Notably, adding CM to CBT did not enhance treatment outcomes. These weak findings suggest that pharmacological and behavioral interventions that have shown efficacy in the treatment of a single drug dependence disorder may not provide the coverage needed when targeting dual drug dependence.
Introduction
Cocaine dependent patients with comorbid alcohol dependence characteristically exhibit a broader and more severe range of problems than individuals with single disorders1–3 resulting in poorer treatment outcomes.2, 4–6 There are no medications of established effectiveness for the treatment of patients concurrently dependent on cocaine and alcohol. The opioid antagonist naltrexone (NTX), at standard doses approved for the treatment of alcohol dependence (50 mg/d), is ineffective in reducing substance use in patients with co-occurring cocaine and alcohol dependence.7, 8
Higher dosages of naltrexone may be more effective in treating cocaine-alcohol dependence. McCaul9 tested NTX at 50 mg/d and 100 mg/d (versus placebo) and reported significant reductions in alcohol consumption in those receiving the higher dose (100 mg/d) during the second month of treatment, but no medication differences at the end of the six month trial. Oslin and colleagues10 conducted an open-label pilot study of naltrexone 150 mg/d in conjunction with psychosocial therapy. At the end of the 12-week trial, 7 of the 8 subjects who completed the study were rated as much or very much improved with significant reductions in percentage of days drinking and daily use of cocaine. More recently, Pettinati and colleagues11 reported reductions in cocaine and alcohol use in men, but not women, treated with 150 mg/d naltrexone. Men receiving the higher naltrexone dose reported less cocaine use, lower drug severity, and greater abstinence from alcohol.
Higher dosages (>50mg/d) of naltrexone sufficient to block the rewarding effects of alcohol, may not completely block the increased reinforcing effects of cocaethylene, the cocaine metabolite formed when cocaine and alcohol are taken together. Thus, NTX's efficacy in the treatment of cocaine-alcohol dependence may depend on the extent to which co-occurring cocaine use is reduced or eliminated. One reasonable method for targeting cocaine abstinence is through behavioral contingency management (CM) procedures. Recently, a number of studies have shown enhanced treatment effects when CM is added to pharmacotherapy for cocaine dependence.12–15 In the case of cocaine-alcohol dependence, CM might reduce or eliminate concurrent cocaine use so that NTX can target alcohol directly rather than the more potent cocaethylene metabolite. At the same time, achievement of cocaine abstinence via CM, might generalize to other behavioral targets, i.e., alcohol, and be further facilitated with NTX support.
The primary aims of this study were to evaluate in a controlled clinical trial the effect of treating cocaine-alcohol dependence with naltrexone (100 mg/d) versus placebo and to determine whether adding CM leads to greater reduction in cocaine use compared to cognitive behavioral therapy (CBT) without CM. We hypothesized that the combination of treatment with naltrexone and CBT plus CM would produce the greatest effects in terms of reduced cocaine and alcohol use.
Method
Subjects
Semi-structured phone screens were conducted on people from the Houston metropolitan area who called in response to media advertisements designed specifically to recruit individuals seeking treatment for both cocaine and alcohol addiction.16 Treatment took place at the outpatient clinic of the Substance Abuse Research Center in Houston, Texas. All participants were at least 18 years old and met DSM-IV17 criteria for current cocaine dependence and current alcohol dependence as assessed by the Structured Clinical Interview for DSM-IV.18 Individuals were excluded if they: (1) met DSM-IV dependence criteria for current non-substance induced Axis I psychotic, depressive, or anxiety disorder; (2) were dependent on drugs other than cannabis or nicotine; (3) had a significant medical contraindication to receiving naltrexone; (4) were pregnant or nursing; (5) were unable to give full informed consent.
Of the 281 people who attended the initial intake for study eligibility, 77 men and 10 women met inclusionary criteria and participated in the study. Of those who dropped out or were excluded before randomization (n=194), primary reasons include not showing for return appointments (32.9%), having psychiatric conditions (8.5%), failing to meet cocaine and alcohol dependence criteria (7.9%) having medical conditions (18.9%).
The study protocol was approved by the Committee for the Protection of Human Subjects (CPHS) of the University of Texas Medical School, Houston (Clinicaltrials.gov Identifier: NCT00218569).
Study Design and Procedures
This was a 12-week, randomized, placebo-controlled, double-blind, 2 × 2 trial comparing medication conditions (naltrexone, placebo) with behavioral therapy conditions (CBT only, CBT plus CM). Following a 1-week intake evaluation phase, subjects were assigned to one of four treatment conditions using urn randomization procedures to ensure even distribution of groups with respect to gender, severity of alcohol and cocaine addiction, motivation to change, and family history of alcohol use.19 During the 12-week treatment phase, participants obtained medication and provided urine samples at thrice-weekly (M, W, F) visits.
Medication treatment
Naltrexone (100 mg/d) or matching placebo was administered during clinic visits and given as take-home doses for intervening days. Riboflavin (50 mg/dose) was added to the medication capsules and used as a marker to monitor compliance. Riboflavin analysis was conducted on each urine sample collected. All investigators and staff, except the pharmacist, were blind to medication assignment.
Behavioral therapy
Behavioral therapies consisted of cognitive-behavioral therapy alone, or CBT plus abstinence-based contingency management. All participants received weekly, 1-hour, manual-driven individual CBT therapy sessions that focused on development of coping skills to achieve abstinence from cocaine and alcohol and prevent relapse.20 Coping skills ranged from basic behavioral plans (e.g., avoid or escape the situation) to more elaborate cognitive and interpersonal actions (e.g., changing negative thinking, assertiveness). The therapy manual presented a conceptualization of cocaine and alcohol use as interrelated behaviors, and targeted the functions of both substances when discussing high risk situations and the application of coping skills. CBT was conducted by master's-level or doctoral-level therapists who underwent initial training to establish competence and adherence prior to delivery of the manual driven CBT evaluated previously in21, 22. For ongoing supervision, therapists met weekly with a senior therapist who reviewed manual adherence and clinical case materials.
CM procedures provided monetary rewards for each cocaine-negative urine sample. The reinforcement schedule followed standard recommendations,23 with voucher values starting at $2.50 and increasing by $1.25 for each consecutive cocaine-negative urine sample. A $10 bonus voucher was awarded for providing three consecutive cocaine-negative urine samples. Missed or refused samples were considered positive and reset the voucher value to $2.50. Five consecutive negative urines after submission of a positive urine sample could return the voucher value to its previous level. Subjects received a weekly written and verbal statement indicating their previous week's urine test results. Vouchers earned could be exchanged at any time for gift certificates (e.g., local restaurants, movie theatre) or redeemed as direct cash payments.
Assessments
Psychiatric diagnostic and addiction severity information were collected at intake using the Structured Clinical Interview for DSM-IV18 and the Addiction Severity Index.24 Intake evaluation included a medical history and physical examination, laboratory evaluation of liver and thyroid function, and cardiac functioning (i.e., 12-lead electrocardiogram). Vital signs (including heart rate, blood pressure, and weight) were obtained weekly during treatment. Adverse events were evaluated by the study nurse and physician that included a standardized reporting system when appropriate. Pill taking was observed on clinic visit days and tracked by riboflavin fluorescence testing.25, 26
The primary measure of cocaine use was determined by detection of benzoylecgonine (BE) in urine samples (> 300ng/ml considered positive), obtained thrice-weekly. Onsite analysis (Analytical Neurochemistry Division) was conducted with the Syva EMIT and Varian Thin Layer chromatography Toxi-Lab systems. The Substance Use Report form, a timeline follow back procedure, was used to collect information on daily use of cocaine and alcohol use throughout the study. Treatment outcomes based on cocaine use included the mean proportion of cocaine-positive urines and the Treatment Effectiveness Score (TES). The TES27 assigns one point for each cocaine-negative urine sample. Cocaine-positive and missing samples receive no points. Thus, in this 12-week trial, TES could range from 0 (all samples positive) to 36 (all samples negative for cocaine). Treatment outcomes based on alcohol use included: (1) percent days any drinking; and (2) percent days heavy drinking, defined as ≥ 4 drinks per occasion for women; ≥ 5 drinks per occasion for men.28
Data Analysis
All analyses were performed on the intent-to-treat population using the Statistical Analysis System, Version 9.1329 with statistical significance designated as p < .05. Baseline differences in treatment groups were evaluated using ANOVA and chi-square tests. Kaplan-Meier survival analysis with right censoring was used to test for differences in time to dropout from treatment. Repeated binary outcomes for cocaine and alcohol use were analyzed using mixed models for repeated measures, with each model including the between-subjects factors of medication and therapy, the within-subjects factor of time, with two-way interactions between these variables, as well as the three-way interaction of all three predictors. Intermittent missing data were imputed as drug positive; missing data due to dropout were handled as missing. Each model tested main effect and interaction terms. TES was analyzed using Poisson regression for count variables. For medication compliance, urine samples were coded as positive (> 20% fluorescence) or negative, i.e., noncompliant (≤ 20%) and analyzed to test for between group differences.
Results
Sample Characteristics
The demographic and substance use characteristics of participants at randomization are presented in Table 1. The total sample of 87 subjects had a mean age of 34.41 years (SD = 4.55) and a mean education level of 12.14 years (SD = 1.74). Most were African American (71.3%), male (87.3%), and unemployed (63.2%). Reported use of cocaine and alcohol in the 30 days prior to treatment was 16.56 (SD = 8.45) and 20.88 days (SD = 8.22), respectively. Cocaine use was primarily in the form of smoked crack cocaine (81.2%), with 16.5% of subjects reporting intranasal administration and 2.4% reporting IV injection of cocaine. The majority of subjects (52.9%) reported previous drug abuse treatment and 47.1% reported previous treatment for alcohol.
Table 1.
Pretreatment characteristics.
| Placebo CBT |
Placebo CBT+CM |
Naltrexone CBT |
Naltrexone CBT+CM |
|
|---|---|---|---|---|
| Demographics (N) | N=27 | N=14 | N==20 | N=25 |
| Age in years (M, SD) | 33.00 (1.29) | 41.97 (7.40) | 32.82 (1.16) | 32.95 (1.28) |
| Male (%, N) | 96.3% (N=26) | 92.9% (N=13) | 90.0% (N=18) | 76% (N=19) |
| Caucasian (%, N) | 14.8% (N=4) | 7.1% (N=1) | 15% (N=3) | 16% (N=4) |
| Employed (%, N) | 37.0% (N=10) | 28.5% (N=4) | 40.1% (N=8) | 36.0% (N=9) |
| Years education (M, SD) | 12.33 (1.96) | 11.57 (1.51) | 12.47 (1.54) | 12.00 (1.76) |
| Days using substance/month | ||||
| Cocaine (M, SD) | 16.85 (8.29) | 17.43 (8.74) | 16.74 (8.77) | 15.64 (8.66) |
| Alcohol (M, SD) | 22.00 (8.54) | 22.43 (7.09) | 19.53 (8.24) | 19.84 (8.61) |
| Years using substance/lifetime | ||||
| Cocaine (M, SD) | 14.33 (6.16) | 12.29 (7.25) | 10.63 (5.51) | 14.88 (5.75) |
| Alcohol (M, SD) | 21.17 (6.45) | 22.93 (7.87) | 21.11 (8.65) | 22.40 (6.06) |
Note: CBT = Cognitive behavioral therapy. CM = Contingency management.
Retention and Adherence
Of the 87 participants randomized (intent-to-treat sample), 34 (40.5%) completed at least 6 weeks of treatment and 21 (24%) completed all 12 weeks of treatment. Kaplan-Meier analysis failed to show differences in dropout across the four groups X2(3) = 1.52, p = 0.68, with median survival times by group as follows: 31.5 days for naltrexone with CBT; 30.0 days for naltrexone with CBT+CM; 34.0 days for placebo with CBT; and 36.5 days for placebo with CBT+CM. Medication adherence based on urinary riboflavin results showed that compliance rates, ranging from 50% to 80%, were comparable for all four treatment groups, X2(3) = 3.40, p = 0.33.
Cocaine Use Outcomes
The probability of having a cocaine-positive urine sample did not change over time as a function of medication, therapy, or their interaction. Evidence for a quadratic time effect emerged, F (1, 359) = 4.46, p ≤.04, showing that across treatments, any potential decrease in the probability of cocaine-positive urines was gradually reversed. No differences were found for urine-based TES as a function of medication, therapy, or their interaction. Mean (SD) TES scores were 6.55 (9.39) for naltrexone with CBT, 4.81 (9.90) for naltrexone with CBT+CM, 3.63 (4.70) for placebo with CBT and 7.93 (12.30) for placebo with CBT+CM.
Drinking Outcomes
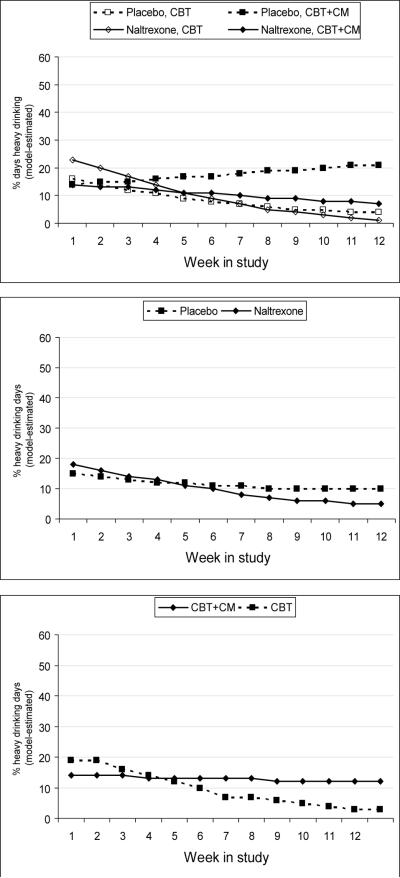
The probability of drinking days (any drinking) showed an effect for time, F (1, 365) = 5.27, p ≤.02, such that for each successive week in treatment the odds of drinking decreased by a factor of 0.94 (95% C.I. 0.89–0.99). No medication or therapy group differences emerged. Mean percent drinking days were 40% for naltrexone with CBT, 33% for naltrexone with CBT+CM, 23% for placebo with CBT, and 33%for placebo with CBT+CM. As shown in Figure 1, analysis of the probability of a heavy drinking day revealed a reliable effect of time, F (1, 57) = 12.51, p ≤.01; time by therapy, F (1, 308) = 4.71, p ≤.01; and time by medication, F (1, 308) = 13.02, p ≤.01. For participants in the CBT group, the odds of heavy drinking decreased by a factor of 0.81 over time in treatment (95% C.I. 0.74–0.88), whereas for participants in the CBT+CM group, the odds of heavy drinking remained stable over time (O.R. = 0.99, 95% C.I. 0.92–1.06). Likewise, for participants receiving naltrexone, the odds of a heavy drinking day decreased over time by a factor of 0.83 (95% C.I. 0.78–0.88). For participants receiving placebo, the odds of heavy drinking did not change over time (O.R. = 0.96, 95% C.I. 0.87–1.07).
Figure 1.
Model-estimated percentages of heavy drinking days each week across the four medication by therapy treatment groups [top panel]. The Medication x Time interaction [middle panel] and the Therapy x Time interaction [bottom panel] were significant. Note. CBT = Cognitive behavioral therapy. CM = Contingency management.
Adverse events
The most frequent adverse events reported by naltrexone-treated subjects were nausea (17%), feeling down (23%) and headache (10%). Comparable rates were reported in the placebo group; nausea (15%), feeling down (27%), and headache (20%). None of these events differed across groups. No serious adverse events emerged, nor did any subject reportedly withdraw from the study due to medication-related problems.
Discussion
High-dose naltrexone (100 mg/d) was expected to improve cocaine use and drinking outcomes, particularly when combined with behavioral therapy that included contingency management procedures rewarding cocaine abstinence, but findings failed to support this hypothesis. The type of medication and therapy did not affect cocaine use, which remained high throughout treatment. Naltrexone did show benefit in reducing the frequency of heavy drinking over time, as did cognitive behavioral therapy alone. Overall, rates of retention and medication compliance indicated suboptimal adherence with the protocol which needs to be considered in interpreting these results.
Four studies, in addition to this one, have evaluated high dose naltrexone for treatment of cocaine-alcohol dependence. Collectively, the findings have been less than robust. Positive effects, when reported, have been short-lived9 or in combination with disulfiram.30 Pettinati11 found reduced cocaine and alcohol use in men treated with 150 mg/d naltrexone, but not in women. The modest benefit associated with dosages greater than 100 mg/d may be offset by tolerability issues, particularly in women. Medication compliance, found to be poor in this study and others,30, 31 is another clinical concern affecting naltrexone's therapeutic potential. Newer sustained release formulations of naltrexone have been shown to enhance compliance and efficacy in the treatment of alcoholism,32 but remain to be evaluated for treatment of co-occurring cocaine-alcohol dependence.
There are several possible reasons why adding contingency management procedures to the behavioral therapy platform did not enhance medication effects. First, we did not deliver CM within the context of a comprehensive community reinforcement approach, as recommended.23, 33 Second, we used the standard reinforcement schedule, starting at $2.50, which may have been of insufficient magnitude to compensate for the powerful reinforcing effects of cocaethylene. Third, by targeting cocaine abstinence only, and not alcohol use, continued drinking may have undermined efforts to resist using cocaine. Alternatively, those who were rewarded for cocaine abstinence may have engaged in compensatory use of alcohol, thus biasing the results. Finally, only targeting one substance in a dually dependent population may have altered the reported use or actual use of the both drugs. Given that so few subjects in this study actually made successful contact with contingent rewards (N=22, 54%), the true effects of this combined behavioral treatment cannot be determined. Thus, it remains possible that subjects who actually experienced CM reinforcement for sustained abstinence from cocaine may have been more likely to benefit from naltrexone. Nevertheless, the finding that CBT alone decreased heavy drinking greater than CBT with CM may indicate that CM is detrimental to the treatment of alcohol dependence in dual-dependent patients.
In this study naltrexone was effective in reducing frequency of heavy drinking days, consistent with the premise that the medication works by inhibiting reinforcement after initial drinking.34 These relapse-reducing properties have resulted in study designs that include prior detoxification from alcohol before starting naltrexone treatment. In the Oslin et al. study10 subjects were required to successfully complete detoxification from alcohol and to have a urine toxicology screen negative for benzoylecgonine prior to starting naltrexone (150 mg/d) treatment. Pettinati et al.11 also required short-term abstinence from alcohol and cocaine in order to evaluate naltrexone as a relapse-prevention medication. Thus, it is possible that ongoing cocaine and alcohol use in the present sample may have negatively impacted the effectiveness naltrexone pharmacotherapy.
Major limitations of this study are the small size of the treatment groups combined with overall low retention in treatment. Low power may have precluded detection of less than large effects. Our finding that fewer than half of the participants completed 6 and 12 weeks of treatment is consistent with retention rates reported in other cocaine-alcohol treatment studies,2, 10, 35 which tend to be considerably lower than retention in single drug treatment studies. Strategies for retaining dual-substance dependent patients in treatment are needed. In this study, neither the pharmacological or behavioral interventions appeared to provide the reinforcement needed to enhance retention in this difficult-to-treat population. Contingency management procedures targeting clinic visit attendance directly have been shown to enhance retention36 and should be considered in this context.
The present findings fail to support the use of naltrexone 100mg/d as a general treatment strategy for cocaine-alcohol dependence. On a broader level, this study adds to a growing literature showing that the positive effects of pharmacological and behavioral interventions used in treatment of single diagnosis cocaine or alcohol dependence, do not generalize directly when used to treat co-occurring drug dependence.
Acknowledgements
This study was funded by grant R01-DA-015801 from the National Institute on Drug Abuse (Dr. Schmitz).
Footnotes
Preliminary results were presented at the annual meeting of the College on Problems of Drug Dependence, San Juan, Puerto Rico, June 2008.
References
- 1.Heil SH, Badger GJ, Higgins ST. Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. J Stud Alcohol. 2001;62:14–22. doi: 10.15288/jsa.2001.62.14. [DOI] [PubMed] [Google Scholar]
- 2.Carroll KM, Rounsaville BJ, Gordon LT, et al. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54:199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- 3.Weiss RD, Mirin SM, Griffin ML, Michael JL. Psychopathology in cocaine abusers. Changing trends. J Nerv Ment Dis. 1988;176:719–725. doi: 10.1097/00005053-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz JM, Bordnick PS, Kearney ML, Fuller SM, Breckenridge JK. Treatment outcome of cocaine-alcohol dependent patients. Drug Alcohol Depend. 1997;47:55–61. doi: 10.1016/s0376-8716(97)00069-0. [DOI] [PubMed] [Google Scholar]
- 5.Brady KT, Sonne S, Randall CL, Adinoff B, Malcolm R. Features of cocaine dependence with concurrent alcohol abuse. Drug Alcohol Depend. 1995;39:69–71. doi: 10.1016/0376-8716(95)01128-l. [DOI] [PubMed] [Google Scholar]
- 6.Brower KJ, Blow FC, Hill EM, Mudd SA. Treatment outcome of alcoholics with and without cocaine disorders. Alcohol Clin Exp Res. 1994;18:734–739. doi: 10.1111/j.1530-0277.1994.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 7.Hersh D, Van Kirk, JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology (Berl) 1998;139:44–52. doi: 10.1007/s002130050688. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Grabowski J. Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. Am J Addict. 2004;13:333–341. doi: 10.1080/10550490490480982. [DOI] [PubMed] [Google Scholar]
- 9.McCaul ME. Efficacy of natrexone for alcoholics with and without comorbid opiate or cocaine dependence. Alcoholism: Clinical and Experimental Research. 1996;20:216A–218A. doi: 10.1111/j.1530-0277.1996.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 10.Oslin DW, Pettinati HM, Volpicelli JR, Wolf AL, Kampman KM, O'Brien CP. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16:163–167. doi: 10.1016/s0740-5472(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 11.Pettinati HM, Kampman KM, Lynch KG, et al. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abuse Treat. 2008;34:378–390. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosten TR, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 13.Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz JM, Rhoades HM, Elk R, Creson D, Hussein I, Grabowski J. Medication take-home doses and contingency management. Exp Clin Psychopharmacol. 1998;6:162–168. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayre SL, Evans M, Hokanson PS, et al. “Who gets in?” Recruitment and screening processes of outpatient substance abuse trials. Addict Behav. 2004;29:389–398. doi: 10.1016/j.addbeh.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID -I / P, Version 2.0) Biometric Research Department; NY: 1995. [Google Scholar]
- 19.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 20.Marlatt GA. Relapse prevention: theoretical rationale and overview of the model. In: Marlatt GA, Gordon JR, editors. Relapse Prevention. Guilford Press; New York: 1985. pp. 3–70. [Google Scholar]
- 21.Schmitz JM, Oswald LM, Jacks SD, Rustin T, Rhoades HM, Grabowski J. Relapse prevention treatment for cocaine dependence: group vs. individual format. Addict Behav. 1997;22:405–418. doi: 10.1016/s0306-4603(96)00047-0. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 23.Budney AJ, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. Therapy Manuals for Drug Addiction: USDHHS NIH. 1998 Pub No 98-4309. [Google Scholar]
- 24.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 25.Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 26.Mooney ME, Sayre SL, Green C, Rhoades H, Schmitz JM. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addictive Disorders & Their Treatment. 2004;3:165–173. [Google Scholar]
- 27.Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Res Monogr. 1997;175:208–220. [PubMed] [Google Scholar]
- 28.Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: 1-year results from three studies with problem drinkers. Am J Public Health. 1995;85:823–828. doi: 10.2105/ajph.85.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The SAS System for Windows (Version 9.13) Version 8.1. SAS Institute Inc.; Cary, NC: 2006. computer program. [Google Scholar]
- 30.Pettinati HM, Kampman KM, Lynch KG, et al. A double blind, placebo-controlled trial that combines disulfiram and naltrexone for treating co-occurring cocaine and alcohol dependence. Addict Behav. 2008;33:651–667. doi: 10.1016/j.addbeh.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oslin DW, Lynch KG, Pettinati HM, et al. A placebo-controlled randomized clinical trial of naltrexone in the context of different levels of psychosocial intervention. Alcohol Clin Exp Res. 2008;32:1299–1308. doi: 10.1111/j.1530-0277.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garbutt JC, Kranzler HR, O'Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 33.Smith JE, Meyers RJ, Miller WR. The community reinforcement approach to the treatment of substance use disorders. Am J Addict. 2001;10(Suppl):51–59. doi: 10.1080/10550490150504137. [DOI] [PubMed] [Google Scholar]
- 34.Volpicelli JR, Watson NT, King AC, Sherman CE, O'Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- 35.Grassi MC, Cioce AM, Giudici FD, Antonilli L, Nencini P. Short-term efficacy of disulfiram or naltrexone in reducing positive urinalysis for both cocaine and cocaethylene in cocaine abusers: A pilot study. Pharmacol Res. 2007;55:117–121. doi: 10.1016/j.phrs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]