Abstract
Puerto Ricans living in the United States mainland present multiple disparities in prevalence of chronic diseases, relative to other racial and ethnic groups. Allostatic load (AL), or the cumulative wear and tear of physiological responses to stressors such as major life events, social and environmental burden, has been proposed as a possible mechanism for the inequalities observed in minority groups, but has not been studied in Puerto Ricans. The aim of this study was to determine the association of AL to six chronic diseases (abdominal obesity, hypertension, diabetes, and self-reported cardiovascular disease (CVD), arthritis and cancer) in Puerto Ricans, and to contrast AL to metabolic syndrome (MetS). Participants of the Boston Puerto Rican Health Study (n=1,116, ages 45–75 years) underwent a home-based interview, where questionnaires were completed and biological samples collected. A summary definition of AL was constructed using clinically-defined cutoffs and medication use for 10 physiological parameters in different body systems. Logistic regression models were run to determine associations between AL score and disease status, controlling for age, sex, smoking, alcohol use, physical activity, total fat intake and energy intake. Parallel models were also run with MetS score replacing AL. We found that increasing categories of AL score were significantly associated with abdominal obesity, hypertension, diabetes and self-reported cardiovascular disease (CVD) and arthritis, but not with self-reported cancer. The strength of associations of AL with all conditions, except diabetes and cancer, was similar to or larger than those of MetS score. In conclusion, Puerto Rican older adults experienced physiological dysregulation that was associated with increased odds of chronic conditions. AL was more strongly associated with most conditions, compared to MetS, suggesting that this cumulative measure may be a better predictor of disease. These results have prospective research implications for Puerto Ricans and other ethnic groups.
Keywords: allostatic load, health disparities, Puerto Ricans, chronic diseases, metabolic syndrome, USA
Introduction
Understanding and reducing racial and ethnic health disparities has been a long standing challenge and a policy priority in the United States (Warnecke, et al., 2008). Puerto Ricans have been reported as having multiple disparities for prevalence of chronic diseases, compared to other racial and ethnic groups (Hajat, Lucas, & Kington, 2000; Zsembik & Fennell, 2005). They exhibit high prevalence of diabetes, heart disease, hypertension and obesity, compared to national data (American Diabetes Association [ADA], 2007; Cleghorn et al., 2004; Denney, Krueger, Rogers, & Boardman, 2004; Oquendo, Lizardi, Greenwald, Weissman, & Mann, 2004). Similar results have been reported for elderly Puerto Ricans living in Massachusetts in contrast to non-Hispanic whites living in the same neighborhoods (Bermudez & Tucker, 2001; Lin, Bermudez, Falcon, & Tucker, 2002; Tucker, Bermudez, & Castaneda, 2000). Four million Puerto Ricans live in the island of Puerto Rico, while another four million live in mainland US, representing the second largest Hispanic group in the US after Mexican-Americans (US Census Bureau, 2004). Responding to the health issues of this growing ethnic group is imperative.
Allostatic load (AL) has been explored as a possible mechanism contributing to health disparities observed in ethnic minority groups (Carlson & Chamberlain, 2005; Tucker, 2005). During the normal and vital process of allostasis, the body adjusts its physiological systems in response to internal and external stressors. However, constant and/or prolonged stressors create a wear and tear of the system. This cumulative burden of physiological responses that exceeds normal operating ranges is referred to as AL (Seeman et al., 2004). The range of values defined as normal versus dysregulated parameters of AL has varied between studies. Nevertheless, suppressed or overactive deviant physiological systems can disturb proper tissues and organ functioning, leading to disease (McEwen, 1998b). Although this model is gaining momentum in studies of racial and ethnic health disparities, no studies have been done on a Puerto Rican cohort (Stewart, 2006).
After a stressor is triggered, the first responders of the allostatic physiological reaction are parameters in the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS) (McEwen & Wingfield, 2003). These neuroendocrine parameters are the primary mediators of AL; they interpret the stressor, elicit a memory of context and initiate a physiological response. Primary mediators then activate secondary physiological parameters, which affect several cellular and system events (McEwen, 1998a; McEwen & Wingfield, 2003; Rosmond, 2005). The response is then repeated when a new stressor, or the anticipation of a stressor based on memory, arises.
Stress itself may be triggered by multiple environmental factors and life events. Puerto Ricans experience some of these factors, namely high poverty levels, language barriers, cultural changes, negative life events, limited social support, violence and criminal activity, and the strain of perceived or real discrimination (Falcon, Todorova, & Tucker, 2009; Moradi & Risco, 2006; Urciuoli, 1996; Zsembik & Fennell, 2005). External stressors alone do not fully explain the pathway from AL to disease; individual biological differences as well as behavioral responses may alter the way a person responds physiologically to perceived stress (McEwen, 1998a). Some behavioral stressors have been reported for Puerto Ricans living in mainland US, including smoking (Fitzgerald et al., 2006; Hajat et al., 2000), alcohol and substance abuse (Lee, Markides, & Ray, 1997; Weingartner, Robison, Fogel, & Gruman, 2002), low levels of physical activity (Fitzgerald et al., 2006), and poor nutritional intake (Gans et al., 2002; Kwan, Bermudez, & Tucker, 2002; Lin, Bermudez, & Tucker, 2003). Thus, individual factors and external stressors likely play a role in the observed high prevalence of chronic conditions in Puerto Ricans. The potential interconnection between stressors, physiological dysregulation and disease is not fully understood; no studies on such associations have been reported for Puerto Ricans.
One criticism of using the AL measure as a predictor of disease is that it reflects many parameters of the metabolic syndrome (MetS), for which association with disease has been already shown (Seeman, McEwen, Rowe, & Singer, 2001). However, AL has been established as an independent predictor of morbidity and mortality, beyond the predictive value of the individual physiological markers (Seeman et al., 2004; Seeman, Singer, Rowe, Horwitz, & McEwen, 1997), and at the same or better strength of association as the secondary mediators, which are traditionally associated with MetS, (Crews, 2007; Karlamangla, Singer, McEwen, Rowe, & Seeman, 2002; Seeman et al., 2001). Thus, although the primary and secondary mediators may not contribute equally to disease (Seeman et al., 2001), the previous studies suggest that the MetS-related parameters alone may not drive the association exclusively. When AL is analyzed as an exposure to disease or death outcomes, the results can add to the level of understanding of the biochemical mechanisms tying the cumulative physiological burden to actual disease state (Seeman et al., 2001). The association of AL with morbidity, as determined in other ethnic groups, may not be applicable to Puerto Ricans, who present multiple environmental and life stress triggers and mediators that may differ from other minority groups in the US. For example, Puerto Ricans have distinct residential segregation patterns (Massey, 1981), and lower income, educational attainment and employment rates than other Hispanic subgroups and non-Hispanic whites (Massey & Bitterman, 1985; National Center for Health Statistics, 2002).
The aim of this study was to determine the association of AL with six chronic conditions in a cohort of Puerto Rican older adults. Secondly, we aimed to assess the strength of the contribution of AL in comparison to MetS. The outcomes (cardiovascular disease (CVD), hypertension, diabetes, obesity, cancer and arthritis) are leading causes of morbidity and mortality in the general US population, as well as in Hispanics (Centers for Disease Control and Prevention [CDC], 2004; CDC, 2007; Ramsey et al., 2008). Their long-term development may reflect lifetime accumulation of physiological dysregulation. We hypothesize that older Puerto Ricans have elevated AL that, in turn, is associated with increased likelihood of chronic diseases. Moreover, we expect AL to have stronger associations with such diseases than MetS, suggesting this cumulative measure may be a better predictor of disease.
Methods
Study Population
The sample consisted of 1,449 participants of the Boston Puerto Rican Health Study, which focuses on the interconnections between stress, nutrition, health and aging in Puerto Rican adults (Tucker, 2005). Participants were recruited using US Census track data with at least 25 Puerto Ricans, aged 45–75 years, living in the track. Census blocks with at least 10 Hispanics were selected for door-to-door enumeration and visited 3 to 6 times, on various days and times of the week. One participant per qualified household was randomly invited to participate. Additional recruitment was conducted through to community outreach, including participation in activities with community organizations, referrals from other participants, and advertisement through flyers and local media outlets. Eligible participants had to be able to answer interview questions in either English or Spanish, and be of self-reported Puerto Rican descent, between the ages of 45–75, and living in the Boston, MA area at the time of the study. The age range was selected based on previous studies of elderly Puerto Ricans (≥ 60 years old) living in Massachusetts that showed high prevalence of several chronic diseases, and extended to a younger group to capture more participants before development of conditions. The Institutional Review Board for Human Research at Tufts Medical Center and Northeastern University approved the protocol of the study. Signed consent forms were obtained for all participants. Of eligible participants invited, 83.7% participated. Those who declined did not differ from participants in basic demographics with the exception that they had lived fewer years in the US mainland (29 vs. 33 years). At the time of writing, clean and complete baseline data, obtained during 2004 to 2008, were available for 1,270 participants. A total of 1,116 participants with complete AL data were included in this analysis. The 154 participants without complete AL data had similar baseline characteristics as those with the AL variable, except for significantly lower prevalence of type 2 diabetes and MetS (Appendix 1).
Procedures
All data were obtained during a home-based interview by trained staff, scheduled at the participant’s convenience. Participants were given $50 in cash after data collection. A comprehensive questionnaire was used to obtain demographic and socio-economic information (including age, sex, income, education, years living in the US), self-reported medical diagnoses and medication use, and alcohol use and smoking habits; based on national surveys questionnaires validated against Hispanics and older populations (Block & Subar, 1992; Delgado, Johnson, Roy, & Trevino, 1990; Dreon, John, DiCiccio, & Whittemore, 1993; McDowell et al., 1989; McDowell & Loria, 1989).
A modified 17-item questionnaire was used to assess the degree of acculturation based on the participant’s frequency of use of either Spanish or English language in daily activities (Bermudez, Falcon, & Tucker, 2000; Lin et al., 2003). The questionnaire was adapted from the Bi-dimensional Acculturation Scale for Hispanics (BAS), which focuses on language preference in various settings (Marin & Gamba, 1996). Ranging from zero to 100%, the maximum value represents a fully acculturated participant who speaks fluent English. Perceived stress was obtained from a scaled questionnaire that measured the degree to which situations in one’s life are appraised as stressful (Fillenbaum, Pieper, Cohen, Cornoni-Huntley, & Guralnik, 2000), by asking the participant about feelings and thoughts on specific situations during the month prior to the interview. The questionnaire has been previously tested in other Spanish-speaking groups (Ramirez & Hernandez, 2007; Remor, 2006). The scale ranges from 0 to 75; higher scores represent higher perceived stress.
Physical activity was measured with a modified version of the Paffenbarger questionnaire of the Harvard Alumni Activity Survey (Paffenbarger et al., 1993; Paffenbarger, Wing, Hyde, 1978); it has been effectively tested in an elderly Puerto Rican population (Tucker et al., 2000). A physical activity score was calculated as the sum of hours spent on typical 24-hour activities (heavy, moderate, light, or sedentary activity as well as sleeping) multiplied by weighing factors that parallel the rate of oxygen consumption associated with each category. A physical activity score of <30 is considered indicative of a sedentary lifestyle. Questions about past and current drinking and smoking behaviors were used to determine smoking status (never, past, and current smoker) and alcohol intake (never, past, and current drinker).
Dietary intake was assessed with a food frequency questionnaire (FFQ) designed and validated for this population by including appropriate foods, recipes and portion sizes. This FFQ was shown to capture intakes reported in 24-hour recalls more accurately than the more widely used Block FFQ (Tucker, Bianchi, Maras, & Bermudez, 1998), and has been validated against several plasma nutrients (Bermudez, Ribaya-Mercado, Talegawkar, & Tucker, 2005; Gao, Wilde, Lichtenstein, Bermudez, & Tucker, 2006; Kwan et al., 2002). FFQs were scanned using the OPSCAN program, linked with a portion size entry program, and then with the Minnesota Nutrient Data System (NDS, version 5.0_35) for nutrient analyses. Intakes of macronutrients were expressed as percentages of total energy intake.
Blood pressure was taken by trained interviewers using an electronic sphignomanometer (Dinamap™ Model 8260, Critikon, Tampa, FL), after short rests at three time points, in duplicate, during the home visit. The average of the second and third readings was used as the blood pressure variable. Anthropometric measures, including height, weight, and waist circumference (WC), were taken twice following NHANES III techniques (Chumlea et al., 1998). The average of both readings was used for this analysis.
Blood samples for biochemical analyses of dehydroepiandrosterone sulfate (DHEA-S), glycosylated hemoglobin (HbA1c), C-reactive protein (CRP), total cholesterol (TC), high density lipoprotein (HDL-C), plasma triglycerides and glucose, were drawn by a certified phlebotomist on the day following the home interview. Participants were asked to fast for 12 hours prior to the blood draw; at that point, the phlebotomist asked about fasting status. Only three participants reported they were not fasting. Measures for cortisol, epinephrine and norepinephrine were obtained from 12-hour urine collection samples. Detailed methodology for blood and urine samples is included in Appendix 2.
Independent variable definition
A summary measure of AL was calculated from 10 parameters of biological functioning across a range of regulatory systems, including serum DHEA-S and urinary cortisol (HPA axis); urinary norepinephrine and epinephrine (SNS); systolic blood pressure (SBP) and diastolic blood pressure (DBP) (cardiovascular system); plasma HDL-C and TC (lipid metabolism and long-term atherosclerotic risk); plasma HbA1c (glucose metabolism); and WC (adipose tissue deposition) (Carlson & Chamberlain, 2005; Szanton, Gill, & Allen, 2005). The AL score was constructed by obtaining a sum of the number of parameters for which a participant fell into the highest (or lowest) cutoff point. Additionally, a point was assigned for each class of medication used (hypertension, diabetes, lipid-lowering or testosterone), if the respective AL parameter was within the established cutoff. The 10 parameters were selected because they were previously reported to be associated with disease or mortality (Seeman et al., 1997). However, we adapted them by using clinical cutoff values when available (none were available for neuroendocrine parameters), rather than using cutoff points based on commonly used population-specific quartiles. All analyses were repeated with an 11-parameter AL score that included CRP (as a marker of inflammation).
A variable for MetS was created in the same way that AL was defined: one point was added for any parameter of MetS over the cutoff (plasma glucose, HDL-C, triglycerides, blood pressure and WC, or use of medication), following current guidelines (Grundy et al., 2005). Three MetS categories were defined: having 0 or 1 parameters above or below the cutoff, having 2 parameters, or having 3 or more parameters. Comparison categories for AL were created to parallel those of MetS, so that each level had the same burden of parameters for both predictors: 0 to 2 parameters, 3 to 5 parameters, and 6 or more parameters.
Dependent variables definitions
Abdominal obesity was defined as having WC >102 cm in men or >88 cm in women (National Heart Lung and Blood Institute [NHLBI], 2000). Hypertension was defined as having SBP ≥140 mmHg or DBP ≥ 90 mmHg, or taking hypertension medication (NHLBI, 2004). Fasting plasma glucose concentration ≥ 126 mg/mL or use of anti-diabetes agents was used as the definition of diabetes (ADA, 2008). Self-reported medically diagnosed heart attack, heart disease or stroke comprised the definition of CVD. A heart disease definition that excluded stroke was also evaluated. Diagnoses of arthritis and any type of cancer (excluding skin cancer) were self-reported. All disease variables were dichotomized as having or not having the disease. When evaluating a disease that included one of the parameters of AL in its definition, that parameter, or medication use for the disease, was excluded from the AL sum (e.g., exclusion of WC when assessing abdominal obesity, of HbA1c or diabetes medication for diabetes, and of SBP and DBP or hypertension medication for hypertension).
Statistical Analysis
Pearson’s chi-square statistic was used to determine significant differences in percentages. T-tests were used to compare unadjusted means. The association between each dichotomous health condition with AL and MetS was evaluated using logistic regression models fitted to estimate odds ratios (OR) and 95% confidence intervals (CI), controlling for age, sex, smoking, alcohol intake, physical activity, total dietary fat intake and total energy intake. These covariates were considered potential confounders of the AL response, based on McEwen’s model. To determine how the inclusion of medication use affected the association between AL and health status, we tested additional models with a definition of AL without medication use, and by including the medications in the models as covariates.
Several different models were run to test the consistency of results and to adjust for other potential confounders (income, education, acculturation, years living in the US, perceived stress); none of these altered the associations and were, therefore, not included in final analyses. Standard diagnostic procedures were used to ensure the appropriateness of these models. Statistical analyses were performed using SPSS version 15.0 software. All reported probability tests were two-sided. Tests with P<0.05 were deemed statistically significant.
Results
Few participants fell into the lowest (zero) or highest (7 or more parameters) AL scores; thus, participants with 0 or 1 parameters served as the reference group, and participants with 6 or more parameters were grouped together, resulting in a total of 6 AL categories (0+1, 2, 3, 4, 5, ≥6 parameters). The AL definition used in this study did not include CRP. Analysis repeated with this parameter generated similar results as without it (data not shown), so results are presented with the original 10-parameter score. Results were comparable for men and women, thus they are presented for all participants, to ensure adequate statistical power. Models tested using a definition of AL without consideration of medication use showed similar, albeit weaker, trends as those obtained for the definition including medication. Similarly, including medications in the models as covariates, rather than as part of the definition, attenuated associations but showed the same significant results (data not shown). Thus, presented results use the AL definition that includes medication use.
Puerto Rican women were significantly less acculturated than men, and had lived fewer years in the US mainland (Table 1). They engaged in less physical activity, had greater prevalence of abdominal obesity, MetS and self-reported arthritis, and consumed more carbohydrates than men. Men had significantly lower perceived stress, but higher smoking prevalence and consumption of alcohol, total energy and fat, than women. Other baseline characteristics did not differ significantly by sex.
Table 1.
Baseline characteristics for 1116 participants in the Boston Puerto Rican Health Study
| Men (n=312) | Women (n=804) |
All participants (n=1116) |
|
|---|---|---|---|
| Age (years) | 57.0 (8.0) | 57.5 (7.3) | 57.4 (7.5) |
| Household income ($) | 20,180 (21,130) | 18,130 (38,519) | 18,698 (34,591) |
| Acculturation | 29.1 (22.9) | 22.2 (21.8)* | 24.1 (22.4) |
| Perceived stress score | 21.8 (9.6) | 24.3 (9.3)* | 23.6 (9.4) |
| Years living in the US | 36.0 (11.9) | 33.4 (12.3)* | 34.1 (12.2) |
| Physical activity score | 32.3 (5.6) | 31.0 (3.9)* | 31.4 (4.5) |
| Less than 8th grade | 45.7 | 49.3 | 48.3 |
| Current smoker (%) | 33.9 | 20.9* | 24.5 |
| Current drinker (%) | 49.4 | 35.4* | 39.3 |
| Energy (kcal) | 2386 (859) | 1999 (845)* | 2107 (866) |
| Protein (% from total energy) | 17.1 (3.1) | 17.4 (3.5) | 17.3 (3.4) |
| Total fat (% from total energy) | 32.0 (5.3) | 30.7 (5.2)* | 31.1 (5.2) |
| Carbohydrate (% from total energy) | 50.2 (7.4) | 52.5 (7.5)* | 51.9 (7.5) |
| Abdominal obesitya | 44.6 | 80.2* | 70.3 |
| Hypertensionb | 70.5 | 68.5 | 69.1 |
| Diabetesc | 39.7 | 39.1 | 39.2 |
| Cardiovascular diseased | 21.5 | 20.5 | 20.8 |
| Arthritise | 36.5 | 56.7* | 51.1 |
| Cancere | 4.5 | 7.1 | 6.4 |
| Metabolic Syndromef | 65.4 | 76.9* | 73.7 |
Results presented as mean (standard deviation) or percent
Significantly different by sex at p<0.05
WC >102 cm in men or >88 cm in women
SBP ≥ 140 or diastolic ≥ 90 or taking hypertension medication
Fasting plasma glucose ≥ 126 mg/dl or reported use of insulin or a prescribed oral hypoglycemic medication
Self-reported heart disease, heart attack or stroke
Self-reported
Presence of ≥3 of the following conditions: WC >102 cm in men or >88 cm in women; fasting glucose >100 mg/dL or drug treatment for elevated glucose; blood pressure of >130 or >85 mm Hg (systolic/diastolic) or drug treatment for hypertension; triglycerides >150 mg/dL or drug treatment for elevated triglycerides; HDL-C < 40 mg/dL in men or <50 mg/dL in women or drug treatment for reduced HDL-C.
The mean (standard deviation) for AL score was 3.8 (1.7) for all participants; men had significantly higher mean AL than women (Table 2). Significantly more women than men had higher mean TC, HDL-C, norepinephrine and CRP, whereas more men than women had higher mean SBP, DBP and D-HEAS (Table 2). More women than men had high WC, CRP, and used lipid-lowering medication with normal lipid values. However, women had lower prevalence than men of low HDL-C, and of high SBP, cortisol, norepinephrine or epinephrine. For all participants, the most prevalent parameter was high WC (70.3%), while the least common parameter was high TC (8.9%).
Table 2.
Definition and prevalence of allostatic load and its parameters in the Boston Puerto Rican Health Study
| Parameter | Mean ± SD or % | Cutoff | % | ||||
|---|---|---|---|---|---|---|---|
| Men | Women | All participants | Men | Women | All participants |
||
| Allostatic load | 4.0 (1.8) | 3.7 (1.6)* | 3.8 (1.7) | 0+1 parameter | 7.7 | 9.2 | 8.8 |
| 2 | 11.5 | 15.0 | 14.1 | ||||
| 3 | 19.6 | 18.7 | 18.9 | ||||
| 4 | 23.1 | 23.9 | 23.7 | ||||
| 5 | 17.9 | 19.7 | 19.2 | ||||
| ≥ 6 parameters | 20.2 | 13.6 | 15.4 | ||||
| WC (cm) | 101.9 (14.6) | 101.1 (15.2) | 101.3 (15.0) | Men, > 102 Women, > 88a |
44.6 | 80.2* | 70.3 |
| TC (mg/dl) | 173.3 (42.5) | 188.4 (40.8)* | 184.2 (41.9) | ≥240b | 7.1 | 9.6 | 8.9 |
| HDL-C (mg/dl) | 40.0 (12.3) | 47.1 (12.3)* | 45.1 (12.7) | < 40b | 55.1 | 26.6* | 34.6 |
| SBP (mmHg) | 137.7 (18.4) | 134.7 (19.2)* | 135.5 (19.0) | > 140c | 42.9 | 36.3* | 38.2 |
| DBP (mmHg) | 82.7 (11.2) | 80.3 (10.3)* | 81.0 (10.5) | > 90c | 21.5 | 17.4 | 18.5 |
| HbA1c (%) | 7.0 (1.9) | 7.0 (1.7) | 7.0 (1.8) | > 7.0d | 33.3 | 32.6 | 32.8 |
| D-HEAS (ng/mL) | 1153.4 (868.0) | 670.9 (489.2)* | 805.8 (655.3) | Men, ≤589.5 Women, ≤368.5e |
27.2 | 28.5 | 28.1 |
| Cortisol (µg/g creatinine) |
31.3 (19.5) | 29.7 (26.0) | 30.1 (24.3) | Men, ≥41.5 Women, ≥49.5f |
23.1 | 11.6* | 14.8 |
| Norepinephrine (µg/g creatinine) |
35.4 (28.2) | 40.1 (26.1)* | 38.8 (26.8) | Men, ≥30.5 Women, ≥46.9f |
45.5 | 28.1* | 33.0 |
| Epinephrine (µg/g creatinine) |
4.0 (3.6) | 3.7 (3.3) | 3.8 (3.4) | Men, ≥2.8 Women, ≥3.6f |
54.5 | 40.0* | 44.1 |
| CRP (mg/L) | 4.9 (9.8) | 6.4 (7.6)* | 6.0 (8.3) | > 3g | 42.9 | 60.5* | 55.6 |
| Total medication use for diabetes |
32.4 | 32.7 | 32.6 | Medication use for diabetes and HbA1c≤7.0 |
7.4 | 7.2 | 7.3 |
| Total medication use for hypertension |
53.5 | 55.5 | 54.9 | Medication use for hypertension and SBP≤140 and DBP ≤90 |
24.0 | 28.7 | 27.4 |
| Total use of lipid- lowering medication |
38.1 | 41.7 | 40.7 | Use of lipid-lowering medication and HDL-C≥40 and TC<240 |
16.0 | 26.0* | 23.2 |
Results presented as mean (standard deviation) or percent
Significantly different by sex at p<0.05
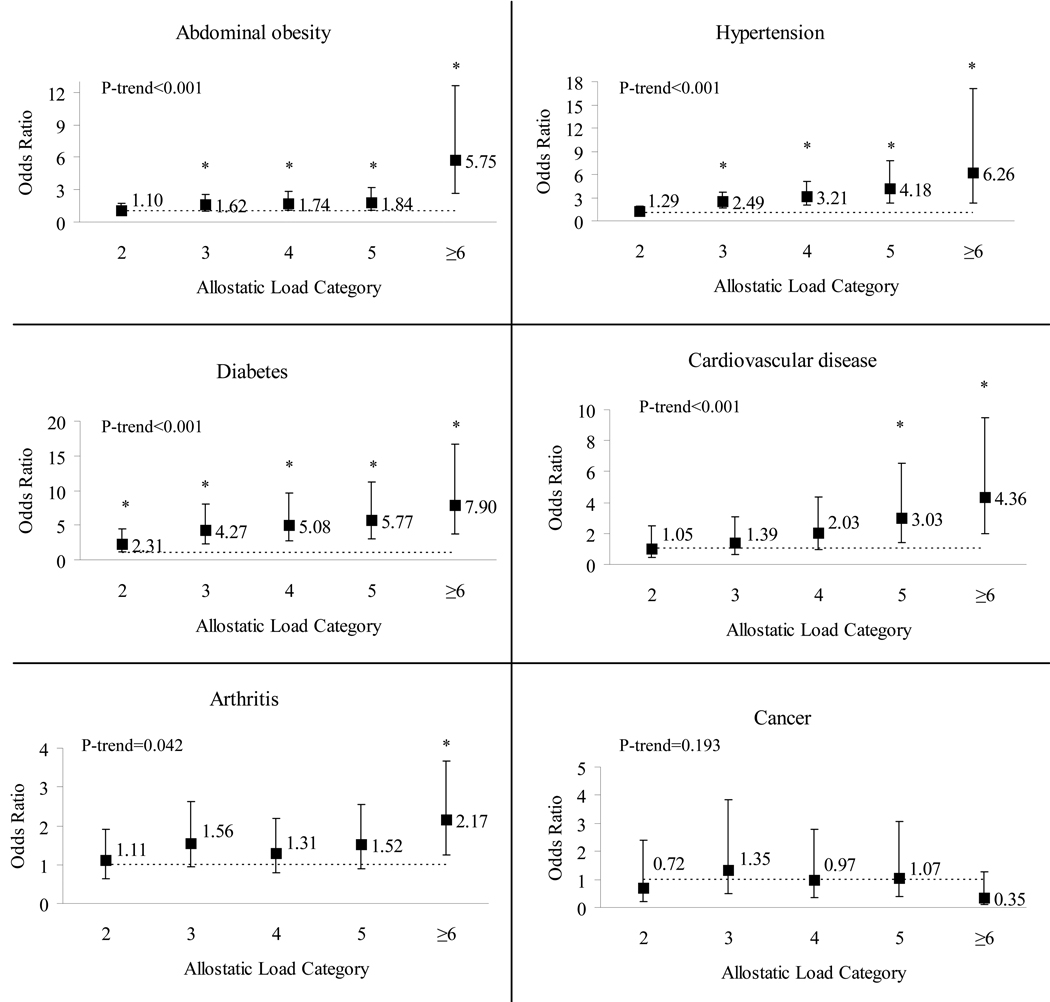
Logistic regression models, controlling for age, sex, smoking, alcohol intake, physical activity, total dietary fat intake and total energy intake show that the four highest categories of AL (excluding WC) were significantly associated with abdominal obesity (OR (95%CI) = 1.62 (1.03–2.55), 1.74 (1.08–2.80), 1.84 (1.06–3.20) and 5.75 (2.22–12.61) for categories 3, 4, 5 and ≥6, respectively) compared to participants with 0 or 1 AL parameters (Figure 1). Participants with 3, 4, 5 and ≥6 AL parameters (excluding blood pressure) were significantly more likely to have hypertension than those in the reference category (2.49 (1.66–3.75), 3.21 (2.03–5.06), 4.18 (2.25–7.79) and 6.26 (2.28–17.17), respectively). Participants in all categories of AL (excluding HbA1c) had higher odds of having diabetes than those in the reference category (2.31 (1.18–4.53), 4.27 (2.26–8.08), 5.08 (2.68–9.63), 5.77 (2.96–11.23), and 7.90 (2.96–11.23) for those with 2, 3, 4, 5, and ≥6 parameters, respectively). Participants in the two highest categories of AL (5 and ≥6) were significantly more likely to report CVD than those with 0 or 1 AL parameters (3.03 (1.41–6.52) and 4.36 (2.00–9.47), respectively). Results obtained with a definition of heart conditions excluding stroke did not differ from those including it. Additionally, to remove any potential direct effect of hypertension on progression of CVD (Rosmond, 2005), analysis of the association of CVD was run with an AL definition that did not include SBP, DBP or hypertension medication, but the results did not differ from those with inclusion of those parameters (data not shown). The highest category of AL (≥6) was significantly associated with self-reported arthritis (2.17 (1.25–3.67)) compared to participants with 0 or 1 AL parameters. The trend for all conditions, except self-reported history of cancer, was significant.
Figure 1.
Odds ratio (OR) and 95% confidence interval (CI) for chronic conditions by allostatic load in the Boston Puerto Rican Health Study. Reference category includes sums of zero and one parameters. Exclusions from allostatic load sum were: WC for abdominal obesity; SBP, DBP or hypertension medication for hypertension, HbA1c or diabetes medication for diabetes. Logistic regression models were adjusted for sex, age, smoking, alcohol intake, physical activity, total dietary fat intake and total energy intake.
*Significant at p<0.05
Logistic regression models were run, adjusted for the same covariates, to compare the association of AL versus MetS with chronic conditions. AL showed stronger OR, and more significant associations, than MetS, for all conditions except diabetes, and self-reported cancer, where neither predictor showed significant association (Table 3). Exploratory stepwise logistic regression analyses were run for each health condition with all AL parameters as independent variables. Results showed that the primary mediators D-HEAS, urinary cortisol and norepinephrine were each significantly and independently associated with all of the health conditions tested, except abdominal obesity and cancer (data not shown).
Table 3.
Odds ratio (OR) and 95% confidence interval (CI) for chronic disease by number of parameters of metabolic syndrome or allostatic load for participants of the Boston Puerto Rican Health Study
| Chronic disease | Metabolic Syndrome | Allostatic Load | ||||
|---|---|---|---|---|---|---|
| Category | OR (95% CI) | P-value | Category | OR (95% CI) | P-value | |
| Abdominal obesitya | 0, 1 | 1.00 | 0–2 | 1.00 | ||
| 2 | 1.56 (1.02–2.38) | 0.039 | 3–5 | 1.66 (1.21–2.28) | 0.002 | |
| ≥3 | 3.52 (2.39–5.17) | <0.001 | ≥6 | 5.53 (2.66–11.63) | <0.001 | |
| Trend | <0.001 | Trend | <0.001 | |||
| Hypertensionb | 0, 1 | 1.00 | 0–2 | 1.00 | ||
| 2 | 1.77 (1.20–2.60) | 0.004 | 3–5 | 2.52 (1.90–3.35) | <0.001 | |
| ≥3 | 4.98 (3.43–7.21) | <0.001 | ≥6 | 5.24 (1.96–14.02) | 0.001 | |
| Trend | <0.001 | Trend | <0.001 | |||
| Diabetesc | 0, 1 | 1.00 | 0–2 | 1.00 | ||
| 2 | 1.89 (1.12–3.19) | 0.018 | 3–5 | 2.72 (1.96–3.77) | <0.001 | |
| ≥3 | 5.22 (3.24–8.40) | <0.001 | ≥6 | 4.19 (2.44–7.20) | <0.001 | |
| Trend | <0.001 | Trend | <0.001 | |||
| Cardiovascular diseased | 0, 1 | 1.00 | 0–2 | 1.00 | ||
| 2 | 1.05 (0.48–2.31) | 0.904 | 3–5 | 2.02 (1.27–3.22) | 0.003 | |
| ≥3 | 2.39 (1.23–4.63) | 0.010 | ≥6 | 4.15 (2.42–7.12) | <0.001 | |
| Trend | <0.001 | Trend | <0.001 | |||
| Arthritise | 0, 1 | 1.00 | 0–2 | 1.00 | ||
| 2 | 1.68 (0.99–2.85) | 0.053 | 3–5 | 1.38 (1.01–1.90) | 0.047 | |
| ≥3 | 1.73 (1.09–2.73) | 0.020 | ≥6 | 2.07 (1.33–3.21) | 0.001 | |
| Trend | 0.064 | Trend | 0.005 | |||
| Cancere | 0, 1 | 1.00 | 0–2 | 1.00 | ||
| 2 | 0.52 (0.16–1.69) | 0.220 | 3–5 | 1.32 (0.68–2.58) | 0.412 | |
| ≥3 | 0.91 (0.37–2.25) | 0.846 | ≥6 | 0.43 (0.14–1.29) | 0.130 | |
| Trend | 0.430 | Trend | 0.056 | |||
WC >102 cm in men or >88 cm in women; excluded from AL and MetS sum
SBP ≥ 140 or DBP ≥ 90 or taking hypertension medication; excluded from AL and MetS sum
Fasting plasma glucose ≥ 126 mg/dl or use of insulin or prescribed oral hypoglycemic medication; medication and HbA1c excluded from AL sum, and medication and glucose excluded from MetS sum
Self-reported heart disease, heart attack or stroke
Self-reported
Logistic regression models adjusted for sex, age, smoking, alcohol intake, physical activity, total dietary fat intake and total energy intake.
Discussion
Our results show that AL was associated with several chronic diseases in a cohort of Puerto Rican older adults. Increasing categories of AL were significantly associated with abdominal obesity, hypertension, diabetes and self-reported CVD and arthritis, but not with self-reported cancer. Moreover, AL showed stronger associations with CVD, arthritis and hypertension, and abdominal obesity, than parallel categories of MetS. Only diabetes showed stronger OR when MetS was the predictor; neither predictor was significantly associated with self-reported cancer.
The mean (SD) for AL score in our cohort was 3.8 (1.7) for all participants. It is difficult to compare overall measures of AL across studies due to variation in definitions. Yet, considering AL as a number of extreme parameters, our results are comparable to what has been reported in the MacArthur Studies of Successful Aging (3.9 (2.1)), a study on factors affecting physical and cognitive functioning (Seeman et al., 2004). However, the MacArthur cohort consists of high-functioning, but older, participants than our group (70–79 versus 45–75 years), suggesting that this population of Puerto Ricans experience high levels of AL at younger ages. The men in our study experienced higher dysregulation than women. Cross-culturally, women have been found to have higher AL scores than men (Stewart, 2006). It is possible that the specific set of parameters and clinical definitions that we selected drove this observation, as some of the cutoff points used here differ for men and women, in contrast to the combined population-specific cutoffs used by other studies. The basis for higher disrupted clinically-relevant parameters in Puerto Rican men should be further studied.
Increasing categories of AL (excluding respective definitive parameters) were significantly associated with higher odds of abdominal obesity, hypertension, diabetes, and self-reported CVD and arthritis, in agreement with previous reports (Crews, 2007; Seeman et al., 2001). These trends suggest that Puerto Ricans should aim to maintain the number of overloaded allostatic parameters to less than two, as the odds for disease were greatly reduced under that value. Notably, we found that AL was associated with both metabolic and non-metabolic conditions (e.g., arthritis), suggesting that disease status in this population may relate to overall dysregulation across various body systems and is not limited to metabolic responses. Interestingly, including CRP in the AL definition did not alter the observations obtained with the original 10-parameter score, although it has been previously associated with cancer, arthritis and obesity (Emery, Gabay, Kraan, & Gomez-Reino, 2007; Florez et al., 2006; Heikkila et al., 2008; Otterness, 1994).
Comparison of parallel AL and MetS categories showed that associations of AL with abdominal obesity, CVD, hypertension and arthritis were stronger than for MetS. Only the OR for diabetes were stronger when using MetS. Reports from the MacArthur Studies show that AL predicted morbidity and mortality similarly to MetS, and they attributed the largest contribution to the secondary mediators (Karlamangla et al., 2002; Seeman et al., 2001). Still, it is possible that the non-MetS primary parameters play a role on the association of AL with disease. Our exploratory analysis with individual parameters of AL showed that several primary mediators contributed to the final models in association with several conditions, supporting our observations. For diabetes, the stronger association for MetS may be due to the inclusion of triglycerides as a parameter; higher levels of this lipid are associated with risk of diabetes. The AL definition does not include triglycerides, but rather cholesterol, which is not as strongly linked to diabetes. Nonetheless, our results support previously reported observations, further strengthening the premise that AL may be a better predictor of disease than MetS alone.
The prevalence of the chronic diseases studied in this Puerto Rican community was high, relative to most other populations. This is a major concern, as these chronic conditions impact the societal and economic burdens for this group, exacerbating existing disparities. Our results suggest a likely contribution of AL to such disparities; thus, studies and interventions aimed at understanding and reducing AL may be helpful in curbing prevalence of chronic disease in Puerto Ricans and other similar high risk populations. Self-reported cancer prevalence in our sample was unexpectedly low, as previous reports show high numbers of co-morbidities in cancer patients (Extermann, 2007) and relatively high cancer rates in mainland Puerto Ricans (Ho et al., 2009; Pinheiro et al., 2009). Bias in study participation or in self report cannot be ruled out.
Our study had some limitations. First, the Census track method of identifying Puerto Ricans limited the selection to areas with high concentration of Hispanics. Ethnic density has been associated with common social and environmental stressors (Pickett & Wilkinson, 2008), as well as a shared physical environment that may not promote healthy lifestyles. Although we cannot ascertain the effect of density in our cohort, it is possible that this might have influenced the observed high prevalence of some conditions. Nonetheless, we adjusted for some lifestyle behaviors that may be shared by those living in ethnically-dense areas. Selection bias could influence observed associations, as those declining participation in the study had lived in the US longer and were somewhat more acculturated than those participating. However, neither acculturation nor years of living in the US were found to be significant covariates in our models. Expanding the recruitment efforts with community approaches may have improved our ability to capture a more inclusive sample of this population. There were no significant differences between those with complete AL data and those without, except for lower prevalence of diabetes and MetS in the latter group. This may be due to lower acceptance of the blood draw by individuals with diabetes, as they might not want to fast. As the prevalence of these conditions was high in our sample, we do not expect that this would be a major source of bias. Based on our recruitment strategies, high participation rates, and few differences between participants and non-participants, we believe that our sample is reasonably representative of Puerto Rican adults living in urban communities in mainland US.
Another limitation is that we are examining cross-sectional data, based on the assumption that this population has been under stress for a long time in order to develop the cumulative dysregulation. This limits our ability to define causal direction. A loop mechanism could be operating, where disease status affects other factors that increase stress and propagates AL (Crews, 2007; Szanton et al., 2005). This cycle perpetuates and the disease state may be accelerated. Longitudinal data will be very valuable in validating our results and establishing the predictive value of AL on disease. Finally, recent evidence suggests that the neuroendocrine markers used here may reflect a transient state rather than long-term response to stress (Gersten, 2008). Circadian changes in some neuroendocrine parameters (Hansen, Garde, Skovgaard, & Christensen, 2001) and blood pressure (Cicconetti, Donadio, Pazzaglia, D'Ambrosio, & Marigliano, 2007) have also been reported; this may bias the estimation of the associations, as those whose interviews were taken in the evening may have different characteristics that those interviewed during the day. Other primary mediators, such as salivary alpha-amylase, or both high and low cutoffs for cortisol, as well as repeated measures for fluctuating parameters, may better reflect long-term effects (Loucks, Justerb, & Pruessner, 2008), and should be examined in future studies.
Our study had several strengths. First, we used a definition of AL that has not been explored previously. Other groups have used different cutoff points (sample-based quartiles or z-scores) and methods (weighted summation and partitioning) within their population to define AL (Logan & Barksdale, 2008). Although a previous study used clinical cutoffs, it did not include the same set of parameters and guidelines used here (Seeman et al., 2008). Our summation of clinical cutoffs included medication use in the classification, as is done to define MetS (Grundy et al., 2005), considering parameters that had been stabilized by drug treatment and that cannot be captured with cross-sectional measures. This definition resulted in stronger associations than when excluding medication use or adjusting for them in the models, suggesting that medication use by these participants reflected their overloaded parameters. The use of clinical cutoffs in future studies could allow for more accurate inferences and comparisons to other populations.
We carefully adjusted our models for behavioral cofactors, including physical activity and dietary intake, which are not explored often, but play a role in the AL model. Increased total fat and energy intake have been associated with stressful eating (Oliver, Wardle, & Gibson, 2000; Rutters, Nieuwenhuizen, Lemmens, Born, & Westerterp-Plantenga, 2008), and may also contribute to chronic health conditions. Nonetheless, it is possible that other factors not studied here (outlook in life, social support, self esteem, social position, resilience, genomic and non-genomic (not altering gene expression) effects, or other dietary factors) could also influence AL (McEwen, 2008; Rosmond, 2005). Further research on the contributing factors to AL in this population should be pursued.
Our study has several implications. First, our results, while obtained as cross-sectional data, add to the body of evidence that AL is associated to some chronic diseases beyond the use of traditional MetS markers. This could lead to the potential inclusion of additional parameters as risk assessment markers for such conditions in future studies and guidelines. Studies on minority groups are increasingly employing the AL model as a possible mechanism to explain health disparities. Thus, if confirmed in longitudinal studies and diverse populations, AL may be adopted as a measure of cumulative physiological dysregulation and a predictor of disease in the same way that MetS has been studied as a marker of metabolic dysregulation in the past. Studies focusing on health disparities, on the aging process, on psychosocial aspects of health, and on stress, could benefit from using this measure. Our results also support the importance of development of interventions for reducing AL and thus, prevalence of chronic conditions in Puerto Ricans. Targeted interventions at the individual and community level could include smoking cessation and stress-reduction programs, low-fat dietary advice, and activities that increase physical activity (McEwen, 2008).
In conclusion, Puerto Rican older adults experience physiological dysregulation that is associated with increased odds of abdominal obesity, hypertension, diabetes and self-reported CVD and arthritis, but not with self-reported cancer. AL showed stronger associations with most conditions, than parallel categories of MetS, suggesting that AL may be a better marker of disease beyond traditional metabolic parameters. These results have prospective policy and research implications for Puerto Ricans and other ethnic groups.
Supplementary Material
Acknowledgments
We would like to thank LoriLyn Price and Jason Nelson for their assistance with the dataset compilation. This study was supported by the National Institutes of Health-National Institute on Aging, Grant Number P01-AG023394 and P01-AG023394-S1, and by the United States Department of Agriculture, Agricultural Research Service agreement number 58-1950-7-707.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Josiemer Mattei, Tufts University.
Serkalem Demissie, Boston University.
Luis M Falcon, Northeastern University.
Jose M Ordovas, Tufts University.
Katherine L. Tucker, Email: kl.tucker@neu.edu, Northeastern University.
References
- American Diabetes Association. Total prevalence of diabetes & pre-diabetes. 2007 http://www.diabetes.org/diabetes-statistics/prevalence.jsp.
- American Diabetes Association. Standards of medical care in diabetes--2008. Diabetes Care. 2008;(31 Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Bermudez OI, Falcon LM, Tucker KL. Intake and food sources of macronutrients among older Hispanic adults: association with ethnicity, acculturation, and length of residence in the United States. Journal of the American Dietetic Association. 2000;100(6):665–673. doi: 10.1016/s0002-8223(00)00195-4. [DOI] [PubMed] [Google Scholar]
- Bermudez OI, Ribaya-Mercado JD, Talegawkar SA, Tucker KL. Hispanic and non-Hispanic white elders from Massachusetts have different patterns of carotenoid intake and plasma concentrations. Journal of Nutrition. 2005;135(6):1496–1502. doi: 10.1093/jn/135.6.1496. [DOI] [PubMed] [Google Scholar]
- Bermudez OI, Tucker KL. Total and central obesity among elderly Hispanics and the association with Type 2 diabetes. Obesity Research. 2001;9(8):443–451. doi: 10.1038/oby.2001.58. [DOI] [PubMed] [Google Scholar]
- Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. Journal of the American Dietetic Association. 1992;92(8):969–977. [PubMed] [Google Scholar]
- Carlson ED, Chamberlain RM. Allostatic load and health disparities: a theoretical orientation. Research in Nursing & Health. 2005;28(4):306–315. doi: 10.1002/nur.20084. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Health disparities experienced by Hispanics--United States. Morbidity and Mortality Weekly Report. 2004;53(40):935–937. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Deaths: Leading causes for 2004. National Vital Statistics Reports. 2007 http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_05.pdf.
- Chumlea WC, Guo SS, Wholihan K, Cockram D, Kuczmarski RJ, Johnson CL. Stature prediction equations for elderly non-Hispanic white, non-Hispanic black, and Mexican-American persons developed from NHANES III data. Journal of the American Dietetic Association. 1998;98(2):137–142. doi: 10.1016/S0002-8223(98)00036-4. [DOI] [PubMed] [Google Scholar]
- Cicconetti P, Donadio C, Pazzaglia MC, D'Ambrosio F, Marigliano V. [Circadian rhythm of blood pressure: non-dipping pattern and cardiovascular risk] Recenti Progressi in Medicina. 2007;98(7–8):401–406. [PubMed] [Google Scholar]
- Cleghorn GD, Nguyen M, Roberts B, Duran G, Tellez T, Alecon M. Practice-based interventions to improve health care for Latinos with diabetes. Ethnicity & Disease. 2004;14(3 Suppl 1):S117–S121. [PubMed] [Google Scholar]
- Crews DE. Composite estimates of physiological stress, age, and diabetes in American Samoans. American Journal of Physical Anthropology. 2007;133(3):1028–1034. doi: 10.1002/ajpa.20612. [DOI] [PubMed] [Google Scholar]
- Delgado JL, Johnson CL, Roy I, Trevino FM. Hispanic Health and Nutrition Examination Survey: methodological considerations. American Journal of Public Health. 1990;80 Suppl:6–10. doi: 10.2105/ajph.80.suppl.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney JT, Krueger PM, Rogers RG, Boardman JD. Race/ethnic and sex differentials in body mass among US adults. Ethnicity & Disease. 2004;14(3):389–398. [PubMed] [Google Scholar]
- Dreon DM, John EM, DiCiccio Y, Whittemore AS. Use of NHANES data to assign nutrient densities to food groups in a multiethnic diet history questionnaire. Nutrition and Cancer. 1993;20(3):223–230. doi: 10.1080/01635589309514290. [DOI] [PubMed] [Google Scholar]
- Emery P, Gabay C, Kraan M, Gomez-Reino J. Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatology International. 2007;27(9):793–806. doi: 10.1007/s00296-007-0357-y. [DOI] [PubMed] [Google Scholar]
- Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14(1):13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- Falcon LM, Todorova I, Tucker K. Social support, life events, and psychological distress among the Puerto Rican population in the Boston area of the United States. Aging & Mental Health. 2009;13(6):863–873. doi: 10.1080/13607860903046552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum GG, Pieper CF, Cohen HJ, Cornoni-Huntley JC, Guralnik JM. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(2):M84–M89. doi: 10.1093/gerona/55.2.m84. [DOI] [PubMed] [Google Scholar]
- Fitzgerald N, Himmelgreen D, Damio G, Segura-Perez S, Peng YK, Perez-Escamilla R. Acculturation, socioeconomic status, obesity and lifestyle factors among low-income Puerto Rican women in Connecticut, U.S., 1998–1999. Revista Panamericana de Salud Publica. 2006;19(5):306–313. doi: 10.1590/s1020-49892006000500003. [DOI] [PubMed] [Google Scholar]
- Florez H, Castillo-Florez S, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Lee D, et al. C-reactive protein is elevated in obese patients with the metabolic syndrome. Diabetes Research and Clinical Practice. 2006;71(1):92–100. doi: 10.1016/j.diabres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Gans KM, Burkholder GJ, Upegui DI, Risica PM, Lasater TM, Fortunet R. Comparison of baseline fat-related eating behaviors of Puerto Rican, Dominican, Colombian, and Guatemalan participants who joined a cholesterol education project. Journal of Nutrition Education and Behavior. 2002;34(4):202–210. doi: 10.1016/s1499-4046(06)60094-8. [DOI] [PubMed] [Google Scholar]
- Gao X, Wilde PE, Lichtenstein AH, Bermudez OI, Tucker KL. The maximal amount of dietary alpha-tocopherol intake in U.S. adults (NHANES 2001–2002) Journal of Nutrition. 2006;136(4):1021–1026. doi: 10.1093/jn/136.4.1021. [DOI] [PubMed] [Google Scholar]
- Gersten O. Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in Taiwan. Social Science & Medicine. 2008;66(3):507–519. doi: 10.1016/j.socscimed.2007.09.004. discussion 520–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Weinstein M, Cornman J, Singer B, Seeman T, Chang MC. Sex differentials in biological risk factors for chronic disease: estimates from population-based surveys. Journal of Women's Health. 2004;13(4):393–403. doi: 10.1089/154099904323087088. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Hajat A, Lucas JB, Kington R. Health outcomes among Hispanic subgroups: data from the National Health Interview Survey, 1992–95. Advanced Data. 2000;(310):1–14. [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Skovgaard LT, Christensen JM. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clinica Chimica Acta. 2001;309(1):25–35. doi: 10.1016/s0009-8981(01)00493-4. [DOI] [PubMed] [Google Scholar]
- Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes & Control. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- Ho GY, Figueroa-Valles NR, De La Torre-Feliciano T, Tucker KL, Tortolero-Luna G, Rivera WT, et al. Cancer disparities between mainland and island Puerto Ricans. Revista Panamericana de Salud Publica. 2009;25(5):394–400. doi: 10.1590/s1020-49892009000500003. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. Journal of Clinical Epidemiology. 2002;55(7):696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- Kwan LL, Bermudez OI, Tucker KL. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. Journal of Nutrition. 2002;132(7):2059–2064. doi: 10.1093/jn/132.7.2059. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Markides KS, Ray LA. Epidemiology of self-reported past heavy drinking in Hispanic adults. Ethnicity & Health. 1997;2(1–2):77–88. doi: 10.1080/13557858.1997.9961817. [DOI] [PubMed] [Google Scholar]
- Lin H, Bermudez OI, Falcon LM, Tucker KL. Hypertension among Hispanic elders of a Caribbean origin in Massachusetts. Ethnicity & Disease. 2002;12(4):499–507. [PubMed] [Google Scholar]
- Lin H, Bermudez OI, Tucker KL. Dietary patterns of Hispanic elders are associated with acculturation and obesity. Journal of Nutrition. 2003;133(11):3651–3657. doi: 10.1093/jn/133.11.3651. [DOI] [PubMed] [Google Scholar]
- Logan JG, Barksdale DJ. Allostasis and allostatic load: expanding the discourse on stress and cardiovascular disease. Journal of Clinical Nursing. 2008;17(7B):201–208. doi: 10.1111/j.1365-2702.2008.02347.x. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Justerb RP, Pruessner JC. Neuroendocrine biomarkers, allostatic load, and the challenge of measurement: A commentary on Gersten. Social Science & Medicine. 2008;66(3):525–530. [Google Scholar]
- Marin G, Gamba RJ. A New Measurement of Acculturation for Hispanics: The Bidimensional Acculturation Scale for Hispanics (BAS) Hispanic Journal of Behavioral Science. 1996;18(3):297–316. [Google Scholar]
- Massey DS. Hispanic residential segregation: A comparison of Mexican, Cubans, and Puerto Ricans. Sociology and Social Research. 1981;65:311–322. [Google Scholar]
- Massey DS, Bitterman B. Explaining the paradox of Puerto Rican segregation. Social Forces. 1985;64(2):306–331. [Google Scholar]
- McDowell M, Briefiel RR, Warren RA, Buzzard IM, Feskanich D, Gardner SN. The dietary data collection system - An automated interview and coding system for NHANES III; Fourteenth National Nutrient Databank Conference; Iowa City, IA; 1989. [Google Scholar]
- McDowell M, Loria CM. Cultural considerations in analyzing dietary data from the Hispanic Health and Nutrition Examination Survey. National Center for Health Statistics; National Nutrition Database Conference; 1989. pp. 43–46. [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998a;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Science. 1998b;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Moradi B, Risco C. Perceived Discrimination Experiences and Mental Health of Latina/o American Persons. Journal of Counseling Psychology. 2006;53(4):411–421. [Google Scholar]
- National Center for Health Statistics at the Centers for Disease Control and Prevention: National Health Interview Survey. National Hispanic Health Leadership Summit; A demographic and health snapshot of the U.S. Hispanic/Latino Population 2002. 2002 http://www.cdc.gov/NCHS/data/hpdata2010/chcsummit.pdf.
- National Heart Lung and Blood Institute, National Institutes of Health. The practical guide: Identification, evaluation, and treatment of overweight and obesity in adults. 2000 http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf.
- National Heart Lung and Blood Institute, National Institutes of Health. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- National Heart Lung and Blood Institute, National Institutes of Health. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. 2004 http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. [PubMed]
- Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosomatic Medicine. 2000;62(6):853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Lizardi D, Greenwald S, Weissman MM, Mann JJ. Rates of lifetime suicide attempt and rates of lifetime major depression in different ethnic groups in the United States. Acta Psychiatrica Scandinavica. 2004;110(6):446–451. doi: 10.1111/j.1600-0447.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- Otterness IG. The value of C-reactive protein measurement in rheumatoid arthritis. Seminars in Arthritis and Rheumatism. 1994;24(2):91–104. doi: 10.1016/s0049-0172(05)80003-4. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. New England Journal of Medicine. 1993;328(8):538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. American Journal of Epidemiology. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Wilkinson RG. People like us: ethnic group density effects on health. Ethnicty & Health. 2008;13(4):321–334. doi: 10.1080/13557850701882928. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiology, Biomarkers & Prevention. 2009;18(8):2162–2169. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- Ramirez MT, Hernandez RL. Factor structure of the Perceived Stress Scale (PSS) in a sample from Mexico. The Spanish J Psychology. 2007;10(1):199–206. doi: 10.1017/s1138741600006466. [DOI] [PubMed] [Google Scholar]
- Ramsey F, Ussery-Hall A, Garcia D, McDonald G, Easton A, Kambon M, et al. Prevalence of selected risk behaviors and chronic diseases--Behavioral Risk Factor Surveillance System (BRFSS), 39 steps communities, United States, 2005. MMWR: Surveillance Summaries. 2008;57(11):1–20. [PubMed] [Google Scholar]
- Remor E. Psychometric properties of a European Spanish version of the Perceived Stress Scale (PSS) The Spanish Journal of Psychology. 2006;9(1):86–93. doi: 10.1017/s1138741600006004. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute Stress-related Changes in Eating in the Absence of Hunger. Obesity. 2009;17(1):72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine. 2004;58(10):1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Procedures of the National Academy of Sciences. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Merkin SS, Crimmins E, Koretz B, Charette S, Karlamangla A. Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988–1994) Social Science & Medicine. 2008;66(1):72–87. doi: 10.1016/j.socscimed.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- Stewart JA. The detrimental effects of allostasis: allostatic load as a measure of cumulative stress. Journal of Physiological Anthropology. 2006;25(1):133–145. doi: 10.2114/jpa2.25.133. [DOI] [PubMed] [Google Scholar]
- Szanton SL, Gill JM, Allen JK. Allostatic load: a mechanism of socioeconomic health disparities? Biological Research for Nursing. 2005;7(1):7–15. doi: 10.1177/1099800405278216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. Journal of Clinical Endocrinology and Metabolism. 2001;86(9):4171–4177. doi: 10.1210/jcem.86.9.7838. [DOI] [PubMed] [Google Scholar]
- Tucker KL. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. Journal of Medical Investigation. 2005;52 Suppl:252–258. doi: 10.2152/jmi.52.252. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. American Journal of Public Health. 2000;90(8):1288–1293. doi: 10.2105/ajph.90.8.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. American Journal of Epidemiology. 1998;148(5):507–518. doi: 10.1093/oxfordjournals.aje.a009676. [DOI] [PubMed] [Google Scholar]
- Urciuoli B. Exposing prejudice: Puerto Rican experiences of language, race, and class. Boulder, Colorado: Westview Press; 1996. [Google Scholar]
- Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. American Journal of Public Health. 2008;98(9):1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartner K, Robison J, Fogel D, Gruman C. Depression and substance use in a middle aged and older Puerto Rican population. Journal of Cross-Cultural Gerontology. 2002;17(2):173–193. doi: 10.1023/a:1015861002809. [DOI] [PubMed] [Google Scholar]
- Zsembik BA, Fennell D. Ethnic variation in health and the determinants of health among Latinos. Social Science & Medicine. 2005;61(1):53–63. doi: 10.1016/j.socscimed.2004.11.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.