Abstract
Objectives
There is debate whether primary or delayed sternal closure (DSC) is the best strategy following Stage 1 palliation (S1P) for hypoplastic left heart syndrome (HLHS). We describe center variation in DSC following S1P and associated outcomes.
Methods
Society of Thoracic Surgeons Congenital Database participants performing S1P for HLHS from 2000–2007 were included. We examined center variation in DSC, and compared in-hospital mortality, prolonged length of stay (LOS >6wks), and postoperative infection in centers with low (≤25% of cases), middle (26%–74% of cases), and high (≥75% of cases) DSC utilization, adjusting for patient and center factors.
Results
There were 1283 patients (45 centers) included. Median age and weight at surgery were 6d (IQR4-9d) and 3.2 kg (IQR2.8–3.5kg); 59% were male. DSC was used in 74% (range 3–100% of cases/center). In centers with high (n=23) and middle (n=17) vs. low (n=5) DSC utilization, there was a greater proportion of patients with prolonged LOS and infection, and a trend toward increased in-hospital mortality in unadjusted analysis. In multivariable analysis, there was no difference in mortality. Centers with high and middle DSC utilization had prolonged LOS [OR (95%CI): 2.83(1.46–5.47) p=0.002 and 2.23(1.17–4.26) p=0.02] and more infection [2.34(1.20–4.57) p=0.01 and 2.37(1.36–4.16) p=0.003].
Conclusions
Utilization of DSC following S1P varies widely. These observational data suggest more frequent use of DSC is associated with longer LOS and higher postoperative infection rates. Further evaluation of the risks and benefits of DSC in the management of these complex infants is necessary.
Introduction
Delayed sternal closure (DSC) following stage 1 palliation for hypoplastic left heart syndrome (HLHS) is utilized by many centers. Capillary leak syndrome is common after cardiopulmonary bypass and is characterized by increased vascular permeability, generalized edema, impaired pulmonary function, coagulopathy, and cardiac dysfunction (1). Sternal closure immediately after surgery can be associated with cardiac compression, decreased ventricular compliance, and reduced cardiac output, further compromising hemodynamic and respiratory status (2, 3). Thus, the aim of DSC is to minimize post-operative hemodynamic and respiratory instability, and to provide ready access to sites of persistent bleeding. DSC may be utilized routinely due to surgeon or center preference, or selectively, secondary to concern for hemodynamic instability following sternal closure.
However, outcomes associated with DSC are unclear, and a recent survey suggested significant variation in utilization of DSC following stage 1 palliation (4). Single center studies have reported conflicting results regarding the impact of DSC on outcomes including survival to hospital discharge and morbidities such as post-operative infection (5–14). These studies have been limited by small sample size, and in some cases lack of appropriate control groups.
The purpose of this study was to describe center variation in the use of DSC following stage 1 palliation for HLHS, and to evaluate post-operative outcomes associated with DSC utilizing the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database.
Methods
Data Source
As previously described, the STS Congenital Heart Surgery Database collects operative and peri-operative data on all patients undergoing congenital heart surgery at participating centers (15). Data collected include demographics, diagnosis, non-cardiac abnormalities, pre-operative factors, intraoperative details, surgical procedure performed, postoperative complications, and in-hospital mortality. The Duke Clinical Research Institute serves as the data collection and management organization for the STS National Databases. This study was approved by the Duke institutional review board.
Study Population
Infants who underwent stage 1 palliation (Norwood procedure with modified Blalock-Taussig shunt or right ventricle to pulmonary artery conduit) for HLHS between January 2000 and December 2007 were included. Centers with > 15% missing data on mortality, length of stay, or post-operative complications, and those with < 5 eligible cases were excluded. Individual patients (n=7) from remaining centers with missing data on mortality or length of stay were then excluded, leaving a final population of 1283 patients from 45 centers. Missing data for other variables in the final study population was rare (< 0.8% for all).
Data Collection
Data on utilization of DSC were collected. Of note, the STS database does not distinguish between “routine” vs. “selective” DSC. Patient demographic information (age, weight, length and gender) and data on any non-cardiac abnormalities were collected, as defined in the database by asplenia, polysplenia, Down syndrome, Turner syndrome, DiGeorge, Williams-Beuren syndrome, Alagille syndrome, 22q11 deletion, Rubella, Marfan syndrome, or any other chromosomal/syndromic abnormality. Data collected regarding pre-operative factors included pre-operative shock, acidosis, arrhythmia, mechanical circulatory support (use of extracorporeal membrane oxygenation or ventricular assist device), mechanical ventilatory support (of note, the database does not distinguish between mechanical ventilatory support required due to respiratory failure vs. that used during transport or in routine pre-operative management), renal failure, sepsis, and neurological deficit. Operative data collected included duration of cardiopulmonary bypass, cross clamp, and circulatory arrest. The data from the era of collection did not specify the use of regional cerebral perfusion. Post-operative data collected included infection, length of stay, and in-hospital mortality. Post-operative infection included sepsis (the current definition of which in the database requires a positive blood culture and excludes line infection), mediastinitis, wound infection, wound dehiscence, and endocarditis. Data on center characteristics were also collected, including annual surgical volume of stage 1 palliation for HLHS, and center region.
Analysis
Data were summarized using frequencies and percentages for categorical variables and median and interquartile range (IQR) for continuous variables. Centers were characterized based on the proportion of cases at each center for which DSC was utilized: low (≤25% of cases), middle (26 – 74% of cases), and high (≥75% of cases). Patient and center characteristics were compared across the DSC groups using chi-square and Kruskal-Wallis tests for categorical and continuous variables, respectively. The relationship between pre-operative factors (any of above) and cardiopulmonary bypass time was evaluated using the Wilcoxon rank-sum test. A generalized estimating equations (GEE) logistic regression analysis was used to evaluate the relationship between center average annual volume of stage 1 palliations performed for HLHS, and DSC utilization.
Outcomes associated with DSC were evaluated utilizing a center-level analysis in an attempt to minimize the impact of patient confounders, and to compare outcomes at centers with “routine” or elective use of DSC vs. those who did not utilize DSC as frequently. In-hospital mortality, prolonged post-operative length of stay (defined as length of stay > 6 weeks, which was the upper quartile of length of stay for the entire cohort: median 22 days, IQR 13–41 days) and post-operative infection (as defined above) were compared across centers with low, middle and high DSC utilization in univariable and multivariable logistic regression, adjusting for patient age, weight, pre-operative factors (as listed above), year of surgery, and center volume. The GEE method was used to account for correlation between outcomes of patients at the same center. Missing data were imputed as “not present” for categorical variables which was the most common value, or the median of non-missing values for continuous variables. Finally, a sensitivity analysis was performed to evaluate the impact of pre-operative factors on outcome. Unadjusted and adjusted odds ratios and 95% confidence intervals are presented. All analyses were conducted using SAS version 8.2 (SAS Institute Inc., Cary, NC).
Results
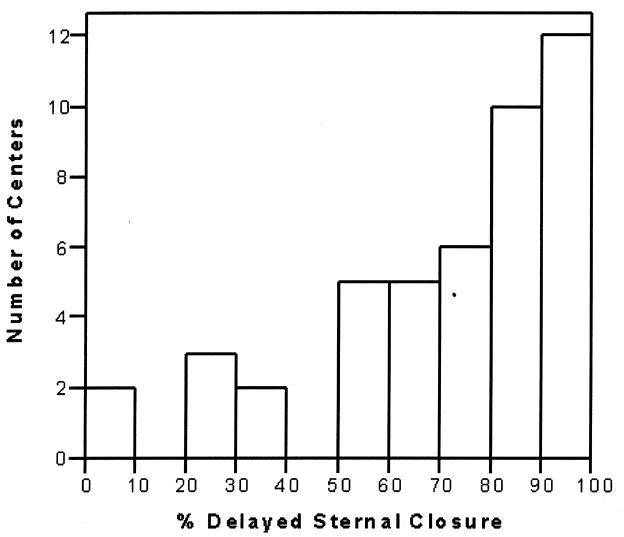
A total of 1283 patients from 45 centers were included. Median age and weight at surgery were 6 days (4–9 days) and 3.2 kg (2.8–3.5 kg), and 59% were male. DSC was utilized in 74% of cases overall (range 3–100% of cases per center; Figure 1). Patient characteristics, pre-operative factors, and operative data in the low, middle, and high DSC groups are displayed in Table 1. Weight at surgery and the presence of any non-cardiac abnormality were similar across groups. The distribution of various pre-operative factors was similar across groups as shown in Table 1, with the exception of acidosis and pre-operative mechanical ventilatory support. Regarding operative data, those with more frequent utilization of DSC had shorter cardiopulmonary bypass times (Table 1). The presence of any of the pre-operative factors listed in Table 1 was associated with shorter cardiopulmonary bypass time [145 min (93–178) vs. 151 min (123–184), p < 0.0001].
Figure 1.
Distribution of center utilization of delayed sternal closure
Table 1.
Patient characteristics
| DSC Utilization | |||
|---|---|---|---|
| Low | Middle | High | |
| 5 centers (n=111) | 17 centers (n=406) | 23 centers (n=766) | |
| Patient Characteristics | |||
| Age, days | 8 (5–24) | 6 (4–8) | 6 (4–8) |
| Weight, kg | 3.3 (2.9–3.8) | 3.2 (2.8–3.5) | 3.1 (2.8–3.5) |
| Gender, male | 73 (65.8%) | 240 (59.1%) | 445 (58.1%) |
| Non-Cardiac Abnormality/Syndrome | 19 (17.1%) | 75 (18.5%) | 126 (16.5%) |
| Preoperative Factors | |||
| Shock | 5 (4.5%) | 13 (3.2%) | 32 (4.2%) |
| Acidosis | 8 (7.2%) | 44 (10.8%) | 133 (17.4%) |
| Mechanical circulatory support | 0 (0.0%) | 5 (1.2%) | 7 (0.9%) |
| Mechanical ventilatory support | 24 (21.6%) | 156 (38.4%) | 338 (44.1%) |
| Renal failure | 2 (1.8%) | 8 (2.0%) | 10 (1.3%) |
| Septicemia | 3 (2.7%) | 8 (2.0%) | 12 (1.6%) |
| Neurologic deficit | 1 (0.9%) | 7 (1.7%) | 8 (1.0%) |
| Operative Data | |||
| CPB time, min | 192 (143–266) | 160 (137–193) | 134 (79–169) |
| Cross clamp time, min | 65 (47–95) | 55 (42–71) | 46 (1–62) |
| Circulatory arrest time, min* | 12 (0–41) | 7 (0–34) | 34 (4–48) |
DSC = delayed sternal closure. CPB = Cardiopulmonary bypass.
Utilization of regional cerebral perfusion is not currently captured in the STS Database.
Evaluating center characteristics, there was no association between center average annual volume of stage 1 palliations performed for HLHS and the proportion who received DSC (p=0.1). There was no evidence of variation in the frequency of DSC across geographic regions.
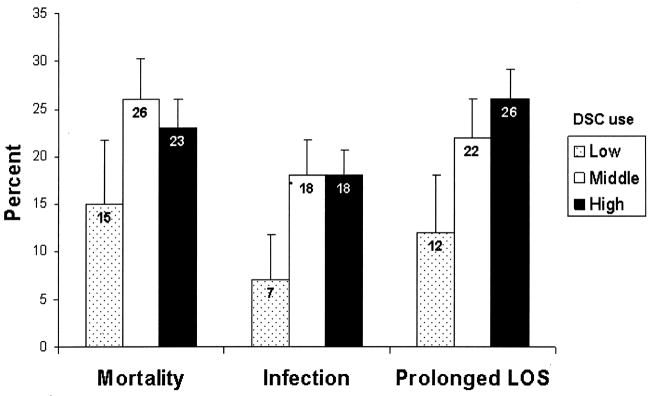
Unadjusted outcomes are displayed in Table 2 and Figure 2. In unadjusted analysis, there was a trend toward greater in-hospital mortality, and a significantly increased proportion with post-operative infection and prolonged length of stay in middle and high DSC groups compared to the low DSC group. Length of stay was 17 days (8–26 days) in the low DSC group, 21 days (12–40 days) in the middle DSC group, and 24 days (14–43 days) in the high DSC group (p < 0.001). Information concerning type of postoperative infection is displayed in Table 3.
Table 2.
Unadjusted and adjusted odds ratios for outcomes associated with center utilization of delayed sternal closure.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| In-hospital Mortality | ||||
| DSC use: | ||||
| Low | Reference | |||
| Middle | 1.90 (0.93–3.89) | 0.08 | 1.28 (0.80–2.07) | 0.31 |
| High | 1.63 (0.85–3.11) | 0.14 | 1.08 (0.68–1.71) | 0.74 |
| Postoperative Infection | ||||
| DSC use: | ||||
| Low | Reference | |||
| Middle | 2.82 (1.53–5.21) | 0.001 | 2.37 (1.36–4.16) | 0.003 |
| High | 2.75 (1.28–5.92) | 0.009 | 2.34 (1.20–4.57) | 0.01 |
| Prolonged LOS | ||||
| DSC use: | ||||
| Low | Reference | |||
| Middle | 2.09 (1.07–4.05) | 0.03 | 2.23 (1.17–4.26) | 0.02 |
| High | 2.59 (1.37–4.91) | 0.004 | 2.83 (1.46–5.47) | 0.002 |
LOS = length of stay
Figure 2.
Unadjusted outcomes associated with center utilization of delayed sternal closure. Comparing the middle and high DSC group vs. low DSC group respectively, p=0.08 and p=0.14 for mortality, p=0.001 and p=0.009 for infection, and p=0.03 and p=0.004 for prolonged LOS. Lines extending from the bars indicate 95% confidence intervals. DSC = delayed sternal closure. LOS = length of stay
Table 3.
Type of post-operative infection
| DSC Utilization | ||||
|---|---|---|---|---|
| Low | Middle | High | ||
| 5 centers (n=111) |
17 centers (n=406) |
23 centers (n=766) |
p-value |
|
| Wound dehiscence | 0 (0%) | 10 (2.5%) | 7 (0.9%) | 0.04 |
| Wound infection | 3 (2.7%) | 16 (3.9%) | 32 (4.2%) | 0.76 |
| Postoperative sepsis | 5 (4.5%) | 55 (13.6%) | 95 (12.4%) | 0.03 |
| Mediastinitis | 1 (0.9%) | 2 (0.5%) | 14 (1.8%) | 0.15 |
| Postoperative endocarditis | 0 (0%) | 1 (0.3%) | 3 (0.4%) | 0.76 |
In multivariable analysis, there was no significant difference in in-hospital mortality between groups, but post-operative infection and prolonged length of stay were significantly greater in the middle and high DSC groups compared to the low DSC group (Table 2).
To evaluate the impact of pre-operative factors on outcome, pre-operative factors were removed from the models and the analysis repeated, with similar results (vs. Table 2): in-hospital mortality [middle DSC group OR (95% CI) 1.35 (0.83–2.22) p=0.23 and high DSC group 1.21 (0.77–1.90) p=0.40], infection [middle DSC group 2.49 (1.40–4.40) p=0.002 and high DSC group 2.56 (1.30–5.01) p=0.006], and prolonged length of stay [middle DSC group 2.30 (1.17–4.52) p=0.02 and high DSC group 2.99 (1.51–5.92 p=0.002].
Discussion
There is debate regarding optimal timing of sternal closure following stage 1 palliation for infants with HLHS. While it is hypothesized that DSC may promote greater hemodynamic and respiratory stability in the post-operative period, outcomes associated with this approach are unclear. Evaluating 1283 infants with HLHS we found that utilization of DSC following stage1 palliation varied widely among 45 US centers. More frequent use of DSC was associated with prolonged length of stay and higher rates of post-operative infection. DSC was not significantly associated with in-hospital mortality.
Survival following staged palliation for patients with HLHS has improved significantly during the past 2 decades (16–19). However, evidence to guide optimal care in this patient population is still evolving. A recent survey of centers caring for infants with HLHS suggested that peri-operative care varied widely by center, including differences in models of care delivery, operative techniques, medications utilized, feeding regimens, and type of monitoring (4). Wide variations in practice may reflect the lack of evidence to define best practices.
This prior survey also suggested variation in utilization of DSC following stage 1 palliation, which was confirmed in our study (4). We did not find any correlation between the use of DSC and center characteristics. Regarding patient characteristics, we found that centers who utilized DSC more frequently tended to report a higher prevalence of factors such as acidosis and pre-operative mechanical ventilatory support. This may reflect differences in patient pre-operative status, or differences in coding of these variables. Of note, the database does not distinguish between mechanical ventilation required due to respiratory failure vs. that used in the routine pre-operative management of these infants. However, one may hypothesize that due to the presence of these factors, these patients were “sicker” going into surgery, leading to longer cardiopulmonary bypass times, greater post-operative edema, and more frequent use of DSC. Previous studies have suggested that longer cardiopulmonary bypass times are associated with greater capillary leak and post-operative inflammation, and greater utilization of DSC (20, 21, 22). However, we found that patients with pre-operative “risk” factors actually had shorter cardiopulmonary bypass times. Centers with more frequent utilization of DSC also had shorter cardiopulmonary bypass times. In addition, in a sensitivity analysis to evaluate the impact of pre-operative factors on outcome, we found similar results whether or not these factors were included in our models. Thus, our data suggest that the presence of these pre-operative factors or longer duration of cardiopulmonary bypass do not lead to more frequent utilization of DSC, and that these pre-operative factors do not appear to impact the relationship between DSC and outcome. Other factors not captured in the STS Database such as hemodynamic status at the conclusion of surgery, experience of ICU staff in managing infants who have undergone primary sternal closure, and availability of 24-hour in-house surgical support, may impact center or surgeon preference for primary vs. delayed sternal closure. In addition, other pre-operative variables including variations in anatomic substrate such as the presence of aortic atresia or the presence of a restrictive atrial septum may also impact outcome (9, 23).
Results of prior studies evaluating outcomes associated with DSC are conflicting. Studies of DSC in infants and children undergoing congenital heart surgery for a variety of defects have reported increased mortality associated with DSC (5, 6). However in these studies, DSC was only performed in patients who were hemodynamically unstable. Therefore, higher mortality in this group may be expected. In studies focusing on patients undergoing stage 1 palliation, some have found DSC to be associated with increased mortality, while others have not found a significant relationship (7–9). In our analysis, we did not find a significant relationship between in-hospital mortality and DSC. Despite potential alterations in hemodynamic status associated with primary sternal closure, and hypothesized benefits of DSC, a survival benefit was not evident in our analysis (2, 3).
In contrast, we did find that utilization of DSC was associated with increased post-operative morbidity, including infection and prolonged length of stay. Prior studies regarding the relationship between DSC and post-operative infection have had mixed results. Several have reported no increase in mediastinitis or blood stream infections associated with the use of DSC (10, 11). However, others have shown an increase in gram-negative mediastinitis and surgical site infections (12–14). Our data show the higher rate of infection is primarily related to blood stream infections. Previous studies evaluating the relationship of DSC with length of stay are limited. A prior single center study in the arterial switch population reported no difference in survival, post-operative length of stay, or post-operative infection associated with DSC (24). This study was limited by small sample size (n=52). It is likely that the increased frequency of postoperative infection found in our study is related to the prolonged length of stay. Postoperative infection has been previously shown to be associated with prolonged length of stay and increased mortality (13). Due to the limitations of the database, we were not able to evaluate the impact of DSC on duration of mechanical ventilation, however it is likely that utilization of DSC is associated with longer duration of intubation, which may also impact length of stay. Alternatively, it is possible that length of stay was impacted by other factors not related to DSC such as post-operative feeding difficulties, the management of which may vary by center. Such variation would not lead to a spurious association between DSC and length of stay unless these factors differed systematically across the different categories of delayed sternal closure. In addition, to reduce the potential for confounding by center-level factors, our risk model explicitly adjusted for hospital level variables, including center volume. Finally, our analytic strategy accounts for unexplained between-hospital variation by treating observations within a hospital as clustered (correlated) observations. Peri-operative morbidities such as prolonged length of stay may have an impact on longer term outcomes as well, as prior studies have shown prolonged hospital stay to be an independent predictor of future neurodevelopmental status in HLHS patients (25).
Limitations
This study is subject to the limitations associated with all observational investigations including selection bias and the potential impact of confounders. We performed a center-level analysis in an attempt to minimize the impact of patient confounders, and adjusted for patient and center factors in addition to accounting for within-center clustering of outcomes in our models. We also performed a sensitivity analysis to further evaluate the potential impact of patient pre-operative factors on outcome.
This study is also subject to the limitations of data collected. We were unable to evaluate elective vs. emergent use of delayed sternal closure as this is not defined in the database. However, utilizing a center-level analysis did allow us to compare outcomes in those with “routine” or elective (high) use of DSC vs. those who did not utilize DSC as frequently. We were also unable to evaluate the impact of duration of time the chest is open following surgery on outcomes as this information is not uniformly captured in the database currently. The database is also limited in that institutions may differ in their coding of different variables. More uniform definitions of variables were added to the database in 2005, however these may still be interpreted differently by different institutions. In addition we were not able to evaluate the impact of factors such as variations in anatomic substrate, hemodynamic status at the conclusion of surgery, peri-operative antibiotic or corticosteroid use, and center model of post-operative care on the relationship between DSC and outcome, as these variables are not collected in the database. Finally, although this represents the largest study to date evaluating DSC in this population, our sample size may have limited our power to detect certain differences between groups.
Conclusions
This multicenter study is the largest to date evaluating utilization of DSC following stage 1 palliation for patients with HLHS. We found that utilization of DSC varies widely, although the majority of centers use DSC in more than half of cases. This observational study suggests that more frequent use of DSC is associated with no difference in survival, and greater post-operative morbidity, including prolonged length of stay, and higher rates of post-operative infection, primarily related to sepsis. Further evaluation of the risks and benefits of DSC in the management of these complex infants is warranted, particularly in cases involving elective use of DSC. Given the wide variation in practice of DSC by institution and potential variations in other aspects of care for these infants, a trial performed in this area may need to utilize stratification of randomization by institution and/or the implementation of standardized management protocols across institutions.
Acknowledgments
Dr. Pasquali receives grant support (KL2 RR024127-02) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and from the American Heart Association (AHA) Mid-Atlantic Affiliate Clinical Research Program. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIH, or AHA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seghaye MC, Grabitz RG, Duchateau J, Busse S, Dabritz S, Koch D. Inflammatory reaction and capillary leak syndrome related to cardiopulmonary bypass in neonates undergoing cardiac operations. J Thorac Cardiovasc Surg. 1996;112:687–97. doi: 10.1016/s0022-5223(96)70053-3. [DOI] [PubMed] [Google Scholar]
- 2.McElhinney DB, Reddy VM, Parry AJ, Johnson L, Fineman JR, Hanley FL. Management and outcomes of delayed sternal closure after cardiac surgery in neonates and infants. Crit Care Med. 2000;28:1180–4. doi: 10.1097/00003246-200004000-00044. [DOI] [PubMed] [Google Scholar]
- 3.Kay PH, Brass T, Lincoln C. The pathophysiology of atypical tamponade in infants undergoing cardiac surgery. Eur J Cardiothorac Surg. 1989;3:255–61. doi: 10.1016/1010-7940(89)90075-4. [DOI] [PubMed] [Google Scholar]
- 4.Wernovsky G, Ghanayem N, Ohye RG, Bacha EA, Jacobs JP, Gaynor JW, et al. Hypoplastic left heart syndrome: consensus and controversies in 2007. Cardiol Young. 2007;17:S75–86. doi: 10.1017/S1047951107001187. [DOI] [PubMed] [Google Scholar]
- 5.Riphagen S, McDougall M, Tibby SM, Alphonso N, Anderson D, Austin C, et al. “Early” delayed sternal closure following pediatric cardiac surgery. Ann Thorac Surg. 2005;80:678–84. doi: 10.1016/j.athoracsur.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Alexi-Meskishvili V, Weng Y, Uhlemann F, Lange PE, Hetzer R. Prolonged open sternotomy after pediatric open heart operation: experience with 113 patients. Ann Thorac Surg. 1995;59:379–83. doi: 10.1016/0003-4975(94)00840-4. [DOI] [PubMed] [Google Scholar]
- 7.Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, et al. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2008;136:94–9. doi: 10.1016/j.jtcvs.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002;22(1):82–9. doi: 10.1016/s1010-7940(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 9.Forbess JM, Cook N, Roth SJ, Serraf A, Mayer JE, Jonas RA. Ten-year institutional experience with palliative surgery for hypoplastic left heart syndrome. Risk factors related to stage 1 mortality. Circulation. 1995;92:II262–6. doi: 10.1161/01.cir.92.9.262. [DOI] [PubMed] [Google Scholar]
- 10.Al-Sehly AA, Robinson JL, Lee BE, Taylor G, Ross DB, Robertson M, et al. Pediatric poststernotomy mediastinitis. Ann Thorac Surg. 2005;80:2314–20. doi: 10.1016/j.athoracsur.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Shah SS, Kagen J, Lautenbach E, Bilker WB, Matro J, Dominguez TE, et al. Bloodstream infections after median sternotomy at a children’s hospital. J Thorac Cardiovasc Surg. 2007;133:435–40. doi: 10.1016/j.jtcvs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Long CB, Shah SS, Lautenbach E, Coffin SE, Tabbutt S, Gaynor JW, et al. Postoperative mediastinitis in children: epidemiology, microbiology and risk factors for gram-negative pathogens. Pediatr Infect Dis J. 2005;24:315–9. doi: 10.1097/01.inf.0000157205.31624.ed. [DOI] [PubMed] [Google Scholar]
- 13.Holzmann-Pazgal G, Hopkins-Broyles D, Recktenwald A, Hohrein M, Kieffer P, Huddleston C, et al. Case-control study of pediatric cardiothoracic surgical site infections. Infect Control Hosp Epidemiol. 2008;29:76–9. doi: 10.1086/524323. [DOI] [PubMed] [Google Scholar]
- 14.Levy I, Ovadia B, Erez E, Rinat S, Ashkenazi S, Birk E, et al. Nosocomial infections after cardiac surgery in infants and children: incidence and risk factors. J Hosp Infect. 2003;53:111–6. doi: 10.1053/jhin.2002.1359. [DOI] [PubMed] [Google Scholar]
- 15.Curzon CL, Milford-Beland S, Li JS, O’Brien SM, Jacobs JP, Jacobs ML, et al. Cardiac surgery in infants with low birth weight is associated with increased mortality: Analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg. 2008;135:546–51. doi: 10.1016/j.jtcvs.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 16.Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106(12 Suppl 1):I82–9. [PubMed] [Google Scholar]
- 17.Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ., III Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000;102:III136–41. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 18.Tabbutt S, Dominguez TE, Ravishankar C, Marino BS, Gruber PJ, Wernovsky G, et al. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock Taussig shunt. Ann Thorac Surg. 2005;80:1582–90. doi: 10.1016/j.athoracsur.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Azakie T, Merklinger SL, McCrindle BW, Van Arsdell GS, Lee KJ, Benson LN, et al. Evolving strategies and improving outcomes of the modified Norwood procedure: a 10-year single-institution experience. Ann Thorac Surg. 2001;72:1349–53. doi: 10.1016/s0003-4975(01)02795-3. [DOI] [PubMed] [Google Scholar]
- 20.Maehara T, Novak I, Wyse RK, Elliot MJ. Perioperative monitoring of total body water by bio-electrical impedance in children undergoing open heart surgery. Eur J Cardiothorac Surg. 1991;5:258–64. doi: 10.1016/1010-7940(91)90174-i. [DOI] [PubMed] [Google Scholar]
- 21.Stiller B, Sonntag J, Dähnert I, Alexi-Meskishvili V, Hetzer R, Fischer T, et al. Capillary leak syndrome in children who undergo cardiopulmonary bypass: clinical outcome in comparison with complement activation and C1 inhibitor. Intensive Care Med. 2001;27:193–200. doi: 10.1007/s001340000704. [DOI] [PubMed] [Google Scholar]
- 22.Samir K, Riberi A, Ghez O, Ali M, Metras D, Kreitmann B. Delayed sternal closure: a life-saving measure in neonatal open heart surgery; could it be predictable? Eur J Cardiothorac Surg. 2002;21:787–93. doi: 10.1016/s1010-7940(02)00100-8. [DOI] [PubMed] [Google Scholar]
- 23.Glatz JA, Tabbutt S, Gaynor JW, Rome JJ, Montenegro L, Spray TL, et al. Hypoplastic left heart syndrome with atrial level restriction in the era of prenatal diagnosis. Ann Thorac Surg. 2007;84(5):1633–8. doi: 10.1016/j.athoracsur.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 24.Owens WA, Vitale N, Hasan A, Hamilton JRL. A policy of elective delayed sternal closure does not improve the outcome after arterial switch. Ann Thorac Surg. 2001;71:1553–5. doi: 10.1016/s0003-4975(01)02395-5. [DOI] [PubMed] [Google Scholar]
- 25.Mahle WT, Visconti KJ, Freier C, Kanne SM, Hamilton WG, Sharkey AM, et al. Relationship of surgical approach to neurodevelopmental outcomes in hypoplastic left heart syndrome. Pediatrics. 2006;117:e90–e97. doi: 10.1542/peds.2005-0575. [DOI] [PubMed] [Google Scholar]