Abstract
Prior work has shown that cannabinoid exposure of zebra finches during sensorimotor stages of vocal development alters song patterns produced in adulthood. We are currently working to identify physiological substrates for this altered song learning. FoxP2 is a transcription factor associated with altered vocal development in both zebra finches and humans. This protein shows a distinct pattern of expression within Area X of striatum that coincides with peak expression of CB1 cannabinoid receptors during sensorimotor learning. Coincident expression in a brain region essential for song learning led us to test for a potential signaling interaction. We have found that cannabinoid agonists acutely increase expression of FoxP2 throughout striatum. When administered during sensorimotor song learning, cannabinoids increase basal levels of striatal FoxP2 expression in adulthood. Thus, song-altering cannabinoid treatments are associated with persistent increases in basal expression of FoxP2 in zebra finch striatum.
Introduction
FoxP2 is a member of the helix-turn-helix family of transcription factors. It is known to play important roles in cellular differentiation during embryonic development of brain, heart and lung (Shu et al., 2001). Several lines of evidence indicate that FoxP2 expression is critical for normal vocal development: (1) the gene encoding FoxP2 is among few known to have undergone positive selection in humans (reviewed by (Kelley and Swanson, 2008); (2) mutation of FoxP2 is associated with a rare disease of altered human vocal development (Lai et al., 2001) and; (3) decreased striatal expression of FoxP2 in zebra finches, an avian vocal learning species, impairs vocal imitation (Haesler et al., 2007).
We have previously found that cannabinoid exposure during periods of vocal learning alters zebra finch vocal development by reducing both song stereotypy and the number of notes incorporated into crystallized song in adulthood (Soderstrom and Johnson, 2003; Soderstrom and Tian, 2004). This, combined with evidence for distinct developmental regulation of CB1 cannabinoid receptor expression during periods of song learning (Soderstrom and Tian, 2006), suggests a role for endogenous cannabinoid signaling in normal vocal developmental processes. CB1 receptors are distinctly expressed within several regions of telencephalon (e.g. lMAN, Area X, auditory Field L2, RA) known to be critical for song learning and production (Soderstrom et al., 2004). The density of this song region receptor expression peaks during sensorimotor learning between 50 and 75 days of age. The timing of this peak coincides with that reported for levels of mRNA encoding FoxP2 within Area X of zebra finch medial striatum (Haesler et al., 2004). This coincidence led us to consider the possibility of a late-postnatal interaction between cannabinoid receptor-mediated signaling and FoxP2 expression. This hypothesis was tested through the experiments described below.
Methods
Except where noted, all materials and reagents were purchased from Sigma or Fisher Scientific. Immunochemicals were purchased from Vector laboratories (Burlingame, CA) and Santa Cruz Biotechnology (Santa Cruz, CA). We have employed the recently revised system of nomenclature in descriptions of zebra finch neuroanatomy (Reiner, 2004). Equithesin was prepared from reagents (40 % propylene glycol, 10 % ETOH, 5 % chloral hydrate, 1 % pentobarbital).
Animals
Adult male zebra finches bred in our aviary and sexed at ~ 25 days via PCR (Soderstrom et al., 2007) were used in these experiments. Prior to the start of experiments, birds were housed with an adult male song tutor in flight aviaries and provided free access to mixed seeds (SunSeed VitaFinch), grit, water, and cuttlebone. Each flight aviary contained several perches. The light–dark cycle was controlled at L:D 14:10 h and ambient temperature was maintained at 78° F.
Animals were cared for and experiments conducted according to protocols approved by East Carolina University’s Animal Care and Use Committee.
Treatments
Drug treatments were given by IM injection of 50 μl into the pectoralis muscle. Drug dilutions for injection were made from 10 mM stocks (in DMSO) to produce a final vehicle of 1:1:18 DMSO:Alkamuls (Rhodia, Cranberry, NJ):PBS (pH = 7.4). The cannabinoid agonist WIN55212-2 (WIN) was purchased from Sigma. The CB1 receptor-selective antagonist SR141716A (SR) was a gift from Sanofi Recherche. The evening prior to experiments, animals were moved to isolation within recording chambers where potential singing could be monitored. Because zebra finches are inactive and don’t sing in the dark, treatments were given in the morning, immediately prior to the beginning of light cycles to avoid potential song- and activity-related FoxP2 expression. Preliminary experiments suggested that peak FoxP2 expression occurs 90 min following WIN treatment, and therefore this period was adopted for all studies. For antagonist experiments, SR was given ten minutes prior to the agonist WIN, which was given immediately preceding the beginning of light phases, and 90 min prior to perfusion for immunohistochemistry. Due to the potential for singing-related alterations in FoxP2 expression, our plan was to remove birds that sang during to 90 post-lights-on period from the study. This plan proved unnecessary as none of the subjects sang prior to perfusion (possibly due to handling and the novel recording room environment).
For developmental experiments, once-daily injections of vehicle (VEH- groups Fig 4) or 1 mg/kg WIN (WIN- groups Fig 4) were given from 50 to 75 days of age (during the sensorimotor period of zebra finch vocal learning). WIN treatment during this period is well-documented to alter both song stereotypy and incorporation of notes into mature song (see (Soderstrom and Johnson, 2003; Soderstrom and Tian, 2004). Following completion of treatments, animals were allowed to mature to at least 110 days of age in visual isolation. Upon maturation, groups of animals were either given no further treatment (as a handling control, see VEH and WIN groups Fig 4), given a single acute vehicle injection (VEH-VEH and WIN-VEH groups Fig 4), or given a single acute injection of 3 mg/kg WIN in order to stimulate FoxP2 expression (VEH-WIN and WIN-WIN groups Fig 4). This dosage of WIN has also been found to acutely decrease both singing and locomotor activity in these animals (Soderstrom and Johnson, 2001). For antagonist experiments, similar treatment regimens were employed, with the exception that 3 mg/kg of SR was injected 10 min prior to each of the 25 daily VEH or WIN injections given during development (VEH+SR- and WIN+SR- groups, Fig 6). Note that treatment groups are designated by the repeated, developmental treatment indicated first, and separated by a hyphen from the indication of a second final acute treatment given in adulthood (to induce potential rapid FoxP2 responses). Treatment groups and designations are summarized for clarity in Table 1.
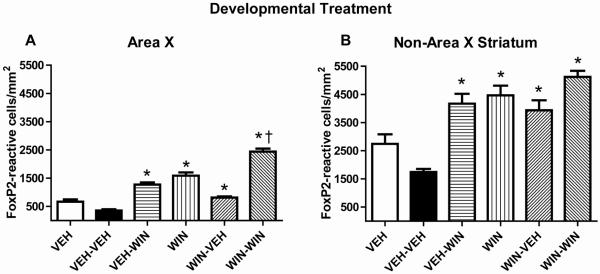
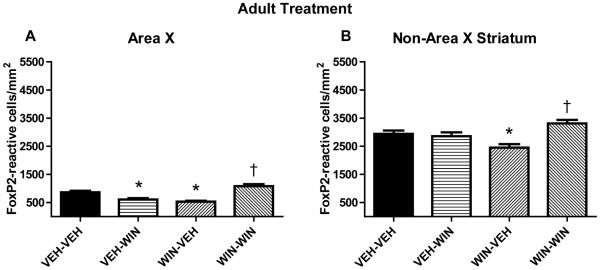
Fig. 4.
Developmental cannabinoid exposure persistently increases basal densities of FoxP2-reactive nuclei within both Area X and other regions of striatum. Initial daily treatments over 25 days are indicated by first designations (VEH-, WIN-). Later, single acute treatments to stimulate FoxP2 expression in adulthood are indicated second (-VEH, -WIN, see Table 1). (A) Effects of treatments within Area X and; (B) in non-Area X-containing striatum. Treatments were delivered during sensorimotor song learning (from 50-75 days) and measured in adulthood (> 110 days). Basal levels of FoxP2 expression are elevated following repeated WIN exposure during development (compare VEH-VEH to WIN-VEH). Acute responsiveness persists (e.g. VEH-WIN and WIN-WIN). Asterisks indicate differences from VEH-VEH treatment groups (p < 0.05, one-way ANOVA followed by Tukey post-tests). Dagger indicates a difference from WIN-VEH group (p < 0.05, one-way ANOVA followed by Tukey post-test).
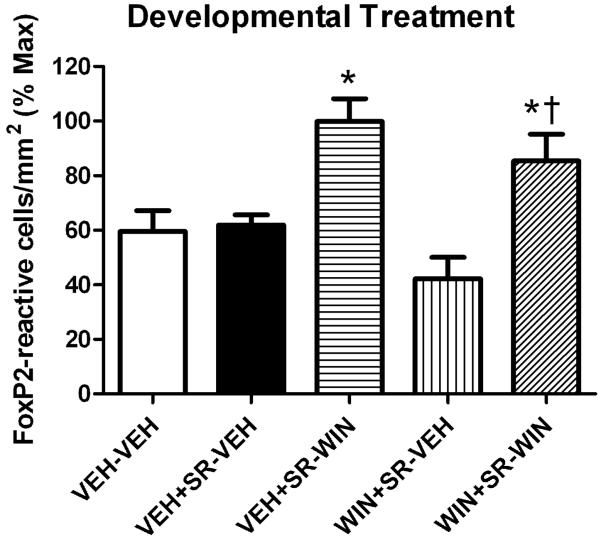
Fig 6.
Developmental WIN treatments administered after the CB1-selective antagonist SR do not persistently alter basal densities of FoxP2-labeled nuclei or alter acute WIN responsiveness in adulthood. Asterisk indicates difference from VEH+SR-VEH, dagger indicates difference from VEH+SR-VEH (p < 0.05, one-way ANOVA, Tukey post-test).
Table 1. Summary of Treatment Groups.
| #Animals | Developmental Tx | Acute Tx | |
|---|---|---|---|
| Designation | (#Young/#Adult)a | (QD for 25 days) | 1X in adulthood |
| VEH | 6/0 | Vehicle | None |
| WIN | 6/0 | 1 mg/kg WIN | None |
| VEH-VEH | 10/4 | Vehicle | Vehicle |
| VEH-WIN | 6/4 | Vehicle | 3 mg/kg WIN |
| WIN-VEH | 6/4 | 1 mg/kg WIN | Vehicle |
| WIN-WIN | 6/4 | 1 mg/kg WIN | 3 mg/kg WIN |
| VEH+SR-VEH | 4/0 | Vehicle + 3 mg/kg SR | Vehicle |
| VEH+SR-WIN | 4/0 | Vehicle + 3 mg/kg SR | 3 mg/kg WIN |
| WIN+SR-VEH | 4/0 | 1 mg/kg WIN + 3 mg/kg SR | Vehicle |
| WIN+SR-WIN | 4/0 | 1 mg/kg WIN + 3 mg/kg SR | 3 mg/kg WIN |
# young birds given treatment/#adult birds given treatments
Western Blotting
Each adult male zebra finch was overdosed with anesthetic and the brain removed to 20 ml ice-cold RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 % Triton X-100, 1% Na-deoxycholate, 0.1% SDS, 1 mM EDTA, pH = 7.4). Tissue was homogenized with a Polytron. The homogenate was frozen overnight at −80 C, thawed on ice, and centrifuged at 16,000 x g for 30 min at 4 C. The supernatant was collected and protein concentration determined by the Bradford method. Fifty μg of soluble protein was separated by SDS-PAGE (using a 10 % gel, and BioRad pre-stained low-range markers), and blotted to a nylon membrane using standard protocols. The resulting blot was blocked for 2 hr at 25 C in a 1 X casein solution (diluted from 10X stock, Vector # SP-5020). After blocking, the membrane was incubated overnight with a 1:2000 dilution of the anti-FoxP2 antibody in 1 X casein blocking solution. Following primary antibody incubation, the blot was washed 3 X with TBS-T (Tris-buffered saline [pH = 7.4] with 0.05 % Tween-20) and placed in a secondary antibody solution containing biotinylated anti-goat antibody (1:2000) for 1 hr (the secondary antibody was obtained as part of the Vector ABC kit, cat # PK-4005). The blot was washed again and developed using diaminobenzidine (Vector cat # SK-4100). Estimated mass of stained bands were interpolated from migrations of standards and generation of a curve fit using a second-order polynomial equation using GraphPad Prism software.
Anti-FoxP2 immunohistochemistry
Ninety minutes following treatments, birds were killed by Equithesin overdose and transcardially perfused with phosphate-buffered saline (PBS, pH = 7.4) followed by phosphate-buffered 4 % paraformaldehyde, pH = 7.0. After brains were removed and immersed overnight in buffered 4 % paraformaldehyde, they were blocked down the midline and left hemispheres were sectioned parasaggitally (lateral to medial) on a vibrating microtome. Immunohistochemistry was performed using a standard protocol reported in (Whitney et al., 2000) except that anti-FoxP2 primary antibody was employed. For immunohistochemistry experiments, 30 micron sections of zebra finch brain were reacted with a 1:3000 dilution of polyclonal anti-FoxP2 antibody raised in goat (Santa Cruz Biotechnology, cat# sc-21069). Tissue sections were rinsed in 0.1 % H2O2 for 30 min, blocked with 5 % rabbit serum for 30 min, and incubated overnight in blocking solution containing anti-FoxP2 antibody (1:5000). After antibody exposure, sections were rinsed in PBS (pH = 7.4), incubated in blocking solution containing biotinylated anti-goat antiserum (1:500) for 1 hour, rinsed with PBS again, and then submerged in avidin-biotin-peroxidase complex solution (purchased as a kit from Vector Laboratories, cat # PK-4005) for 1 hour. Antibody labeling was visualized with DAB solution (Vector cat # SK-4100). Control sections that were not incubated in primary antibody were not immunoreactive. To eliminate possible variance associated with reaction conditions, tissue from equal numbers of animals from each treatment group within an experiment were processed simultaneously.
Staining was examined in various brain regions at 40 X using an Olympus BX51 microscope under brightfield conditions. Multiple images were captured using a Spot Insight QE digital camera and Image-Pro Plus software (MediaCybernetics, Silver Spring, MD) under identical, calibrated exposure conditions. These images were background-corrected, converted to grey scale and assembled into montages to create complete images of striatum (see Fig 1). The borders of striata and Area X were traced manually. Two dimensional counts of labeled nuclei (Mizutani et al., 2007) from montage images, and areas enclosed within traced areas were determined without knowledge of treatment condition from five separate sections per animal using Image-Pro Plus software. Threshold mean grey scale values used to distinguish labeled vs unlabeled nuclei were set for each antibody reaction (due to variance in staining intensities across reactions). Because each treatment group was represented in each antibody reaction, variance associated with different threshold values were spread evenly across replicates. Counts were made independently by two investigators and pooled for analysis. Mean densities (within region counts of stained nuclei/area of the region) were compared across treatment group using one-way ANOVA as described below.
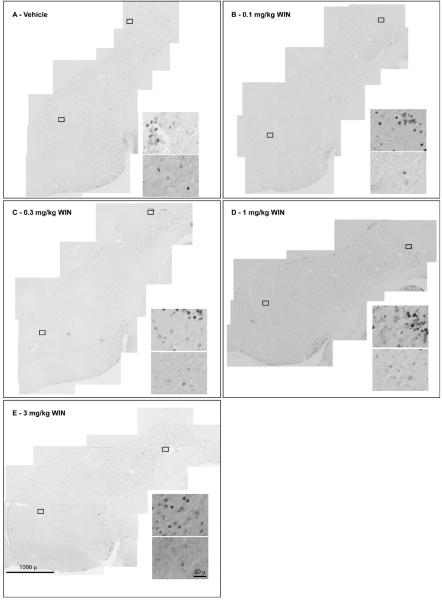
Fig. 1.
Montages of 100X micrographs of parasagittal sections of adult male zebra finch telencephalon stained with anti-FoxP2 antibody. Sections shown were reacted together and represent tissue from animals treated with: (A) Vehicle, (B) 0.1 mg/kg, (C) 0.3 mg/kg, (D) 1 mg/kg and, (E) 3 mg/kg WIN. Diffuse labeled nuclei appear throughout striatum. Distinctly higher densities are observed within caudal regions of lateral striatum. Along caudal and caudal-dorsal borders of lateral striatum, characteristic punctate aggregations of labeled nuclei occur. Insets are 1000X DIC images from within striatum as indicated by boxes. Upper high-power panels were taken within regions of lateral striatum, lower panels within regions of Area X of striatum.
Statistical Analyses
Relationships between drug treatments and anti-FoxP2-reactive cell densities were determined through one-way ANOVA by treatment. Following ANOVA determination that mean values of cell densities differed across treatment (p ≤ 0.05), Tukey post-tests were done.
Results
Western blotting confirmed that the anti-FoxP2 antibody labeled an apparently single primary band of approximately 80.1 kDa (Fig 2). The mass of this labeled protein is consistent with that reported previously for full-length zebra finch FoxP2 protein expressed in vitro (Miller et al., 2008). The anti-FoxP2 antibody that we have used is directed against the 16 N-terminal amino acids of Human FoxP2 (NCBI protein database accession # 015409). This epitope is identical to that of three isoforms of zebra finch FoxP2 represented with sequence information in GenBank (accession # AAR28756.1, AAS55874.1 and AAS55875.1). The predicted mass of these zebra finch FoxP2 isoforms range from 76.9 to 79.5 kDa, which is in reasonable agreement with the migration of the primary band labeled in Fig. 2. Furthermore, the distinct staining of cell nuclei in immunhistochemistry experiments is consistent with the cellular distribution expected for the transcription factor, FoxP2 (e.g. Fig 1 and (Mizutani et al., 2007).
Fig 2.
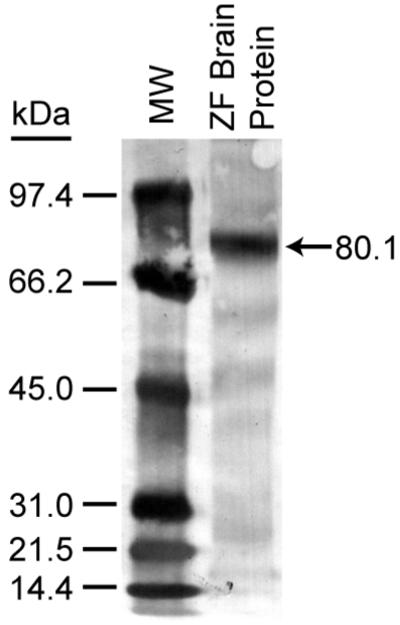
Western blot analysis of anti-FoxP2 staining of proteins isolated from the brain of an adult male zebra finch and separated by 10 % PAGE (40 μg protein). The arrow indicates labeling of a primary band of 80.1 kDa, consistent with the mass reported for full-length zebra finch FoxP2 protein (Miller et al., 2008).
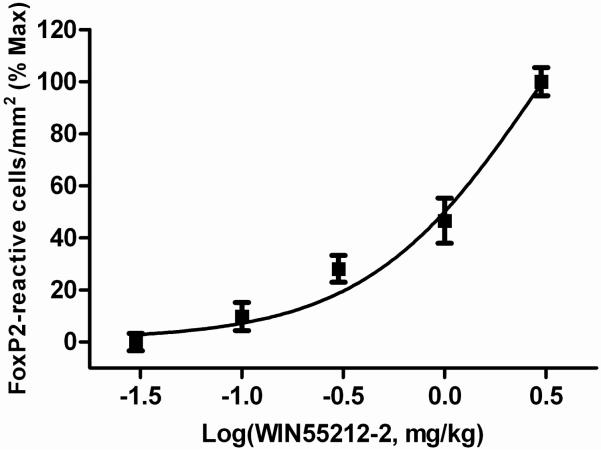
Initial experiments indicated significant increases in striatal anti-FoxP2 immunoreactive nuclei 90 min following the 3 mg/kg injections of the cannabinoid agonist, WIN55212-2 (WIN, data not shown). In addition to this rapid, cannabinoid-induced response, there was an interesting pattern of diffuse labeled nuclei throughout striatum (notably including the song region, Area X), with distinct, nuclear concentrations within lateral striatum at the border with nidopallium (Fig 1). Larger experiments were done to assess the dose-dependence of cannabinoid-stimulated FoxP2 expression. Results of these dose-response experiments are summarized in Fig 3. As indicated by Fig 3, densities of anti-FoxP2-reactive nuclei increased as a function of WIN dosage. Although densities of FoxP2-expressing nuclei outside of striatum were not measured, we did note general patterns of expression in other brain regions known important to vocal behavior. Within the vocal-motor regions of caudal telencephalon, notably distinct expression was observed within HVC and a distinct lack of expression was observed within RA. Staining within lMAN and L2 was not distinct, and was similar to the diffuse labeling of nuclei throughout telencephalon (with the exception of RA). Distinct staining was observed within the thalamic regions DLM and ovoidalis, but not within nucleus rotundus. Very dense staining of thalamic cell nuclei surrounding DLM, ovoidalis and nucleus rotundus was noted. Within cerebellum, distinct labeling of some, but not all, Golgi cell nuclei was observed.
Fig 3.
Acute administration of the cannabinoid agonist, WIN55212-2 dose-dependently stimulates FoxP2 expression within adult male zebra finch striatum. Animals were perfused 90 min after treatment, and brains removed and processed for anti-FoxP2 immunohistochemistry. Densities of anti-FoxP2 reactive nuclei were determined and transformed to % of maximum stimulation (produced by 3 mg/kg WIN).
Developmental experiments were done to assess whether song-altering cannabinoid treatments result in changes in basal and cannabinoid-stimulated striatal FoxP2 expression (Fig 4). Following once-daily injections of 1 mg/kg WIN from 50 -75 days of age, and maturation to early adulthood, basal levels of striatal FoxP2 expression were significantly elevated (VEH vs WIN, Fig 4 A and B). Despite an apparent trend toward inhibition, control basal levels and WIN-stimulated basal levels were not significantly altered by the handling stress of receiving acute injections at adulthood (VEH vs VEH-VEH, and WIN vs WIN-VEH). Interestingly, WIN treatment in animals not developmentally exposed to cannabinoids acutely increased FoxP2 expression to levels indistinguishable from developmental WIN-altered basal levels (compare VEH-WIN to WIN-VEH, Fig 4 A and B). Animals with elevated basal FoxP2 expression levels following developmental WIN exposure were capable of additional, acute responses following WIN treatment in adulthood (compare WIN-VEH to WIN-WIN, Fig 4 A and B).
To determine whether FoxP2 expression sensitivity to WIN is restricted to developmental exposure, experiments employing the same 25 day treatment regimen were conducted in adults. Because statistically significant handling effects on FoxP2 expression were not observed in earlier experiments, in an effort to reduce animal impact, these controls were not included in adult experiments. Results of experiments in adults are summarized in Fig 5, and demonstrate that, in contrast to treatment effects during sensorimotor song learning, repeated cannabinoid dosing over 25 days in adults resulted in a persistent, modest decrease in basal FoxP2 expression levels (compare VEH-VEH to WIN-VEH, Fig. 5 A and B).
Fig. 5.
Repeated cannabinoid treatment during adulthood decreases basal densities of FoxP2-reactive nuclei within both Area X and other regions of striatum. Initial daily treatments over 25 days are indicated by first designations (VEH-, WIN-). Later, single acute treatments to stimulate FoxP2 expression are indicated second (-VEH, -WIN, see Table 1). Basal levels of FoxP2 expression are decreased following repeated WIN exposure in adulthood in both (A) Area X and (B) other regions of striatum (compare VEH-VEH to WIN-VEH). Acute responsiveness persists only after repeated cannabinoid treatments (e.g. WIN-VEH and WIN-WIN). Asterisks indicate differences from VEH-VEH treatment groups (p < 0.05, one-way ANOVA followed by Tukey post-tests). Dagger indicates a difference from WIN-VEH group (p < 0.05, one-way ANOVA followed by Tukey post-test).
In order to assess the CB1 receptor dependence of developmental WIN-altered FoxP2 expression, the CB1-selective antagonist, SR141716A (SR) was employed. Results of this experiment are summarized in Fig. 6 and demonstrate that repeated injections of 1 mg/kg WIN, when given 10 min following 3 mg/kg of the SR antagonist, did not result in altered basal levels of FoxP2 expression (compare VEH-VEH and WIN+SR-VEH, Fig. 6). Developmental administration of the SR antagonist alone or following WIN did not alter later acute responsiveness to WIN in adulthood (compare VEH+SR-VEH to VEH+SR-WIN, and WIN+SR-VEH to WIN+SR-WIN, Fig. 6).
Discussion
Because cannabinoid treatments are established to acutely alter zebra finch CNS expression of other transcription factors (e.g. ZENK, (Whitney et al., 2003) and c-fos (Soderstrom and Tian, 2008), it was an important first step to determine if FoxP2 expression was subject to similar rapid alterations. Indeed, FoxP2 expression was rapidly and dose-dependently stimulated following acute injections of the cannabinoid agonist WIN to naïve adult animals (Fig 3). The pattern of striatal distribution of cannabinoid stimulated FoxP2 expression was not expected. Although increased FoxP2-expressing nuclei were observed throughout striatum (notably including Area X), the most distinct expression was apparent in regions of lateral striatum, including notably within cell clusters at the border with nidopallium (Fig. 1). The identity and functional significance of these distinct clusters have (to our knowledge) not been established. We expected to find more-distinct cannabinoid-related differences in FoxP2 expression within Area X of medial striatum, a region known to be essential for song learning (Bottjer et al., 1984; Sohrabji et al., 1990; Scharff and Nottebohm, 1991). This expectation was based on: (1) Elevated expression of mRNA encoding FoxP2 within Area X at 50 days during sensorimotor learning (Haesler et al., 2004) and; (2) the fact that CB1 cannabinoid receptors are distinctly and densely expressed within Area X (Soderstrom and Tian, 2006). Therefore conspicuous expression within lateral striatum (a region implicated in processing of visual information, (Krutzfeldt and Wild, 2004) was unexpected. Lack of distinct responsiveness within CB1-expressing Area X suggests that acute cannabinoid modulation of FoxP2 expression in striatum may not be due to effects downstream of striatal CB1 receptor populations, and depend on input from other brain regions. Distinct CB1 receptor expression within zebra finch thalamus (nucleus rotundus, DLM, ovoidalis) and midbrain (MLD) suggest possible brain regions involved (Soderstrom and Tian, 2006).
In adult birds given the same treatment regimen used in developmental experiments (25 daily treatments followed by at least 35 days prior to acute injections) acute WIN stimulation of FoxP2 expression was blunted (there was a reduction in Area X [Fig 5A] and no difference between VEH-VEH and VEH-WIN within other regions of striatum [Fig. 5B]). This result contrasts with that found following repeated treatment of developing animals (note the difference between VEH-VEH and VEH-WIN, Fig. 4 A and B). This reduced sensitivity to acute WIN following prior repeated treatment of adults suggests: (1) that initial novel experience of handling and injection (such as that associated with the dose-response study [Fig 2]) may potentiate WIN-induced FoxP2 expression and; (2) a persistent tolerance to this potentiation follows repeated handling and injection of drug in adulthood, but not during sensorimotor learning from days 50 to 75. Interestingly, adults that received repeated WIN injections maintained later acute responsiveness throughout striatum (compare WIN-VEH to WIN-WIN in Fig 5 A and B). From this it appears that cannabinoid exposure prevented the handling effect observed in the VEH- group adults. Well-established acute inhibition of memory consolidation by cannabinoid agonists suggests a potential mechanism for lack of tolerance in the WIN- group animals (Wise et al., 2009).
Changes in basal FoxP2 expression levels produced by WIN during sensorimotor learning, but not in adulthood, demonstrates a distinct developmental period of cannabinoid sensitivity. Our prior work has documented similar distinct periods of cannabinoid sensitivity to influence features of vocal behavior that persist through adulthood (Soderstrom and Tian, 2004). These periods of sensitivity may be due to decreases in CB1 expression that occur over normal development (Soderstrom and Tian, 2006), or to yet uncharacterized differences between developing and mature brain. Persistent changes in basal levels of transcription factor expression are well-documented as key physiological changes responsible for neuronal differentiation underlying CNS development (reviewed by (Corbin et al., 2008). This, combined with the established importance of FoxP2 in vocal development (Enard et al., 2002; Haesler et al., 2004; MacDermot et al., 2005), suggests that the mechanism of cannabinoid-altered song learning may involve increased basal levels of FoxP2 expression.
In summary, CB1 cannabinoid receptor activation acutely and rapidly increases striatal FoxP2 expression. Acute increases in FoxP2 expression were observed throughout striatum as well as within the song region, Area X. Exposure to vocal development-altering cannabinoid treatments during sensorimotor development, but not in adulthood, persistently increased basal expression levels of FoxP2 in zebra finch striatum. Thus, persistent changes in basal striatal FoxP2 expression are associated with cannabinoid-altered vocal development.
Acknowledgments
Financial Support: National Institute on Drug Abuse R01DA020109
Literature Cited
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Juliano SL, Poluch S, Stancik E, Haydar TF. Regulation of neural progenitor cell development in the nervous system. J Neurochem. 2008;106:2272–2287. doi: 10.1111/j.1471-4159.2008.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Paabo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JL, Swanson WJ. Positive selection in the human genome: from genome scans to biological significance. Annu Rev Genomics Hum Genet. 2008;9:143–160. doi: 10.1146/annurev.genom.9.081307.164411. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt NO, Wild JM. Definition and connections of the entopallium in the zebra finch (Taeniopygia guttata) J Comp Neurol. 2004;468:452–465. doi: 10.1002/cne.10972. [DOI] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE. Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet. 2005;76:1074–1080. doi: 10.1086/430841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, Spiteri E, Condro MC, Dosumu-Johnson RT, Geschwind DH, White SA. Birdsong decreases protein levels of FoxP2, a molecule required for human speech. J Neurophysiol. 2008;100:2015–2025. doi: 10.1152/jn.90415.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A, Matsuzaki A, Momoi MY, Fujita E, Tanabe Y, Momoi T. Intracellular distribution of a speech/language disorder associated FOXP2 mutant. Biochem Biophys Res Commun. 2007;353:869–874. doi: 10.1016/j.bbrc.2006.12.130. [DOI] [PubMed] [Google Scholar]
- Reiner AP, Bruce L, Butler AB, Csillag A, Kunzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. D.J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Yang H, Zhang L, Lu MM, Morrisey EE. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem. 2001;276:27488–27497. doi: 10.1074/jbc.M100636200. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. The Zebra Finch CB1 Cannabinoid Receptor: Pharmacology and In Vivo and In Vitro Effects of Activation. J Pharmacol Exp Ther. 2001;297:189–197. [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. Cannabinoid exposure alters learning of zebra finch vocal patterns. Brain Res Dev Brain Res. 2003;142:215–217. doi: 10.1016/s0165-3806(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Qin W, Leggett MH. A minimally invasive procedure for sexing young zebra finches. J Neurosci Methods. 2007 doi: 10.1016/j.jneumeth.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Distinct periods of cannabinoid sensitivity during zebra finch vocal development. Developmental Brain Research. 2004;153:225–232. doi: 10.1016/j.devbrainres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Developmental pattern of CB1 cannabinoid receptor immunoreactivity in brain regions important to zebra finch (Taeniopygia guttata) song learning and control. J Comp Neurol. 2006;496:739–758. doi: 10.1002/cne.20963. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. CB(1) cannabinoid receptor activation dose dependently modulates neuronal activity within caudal but not rostral song control regions of adult zebra finch telencephalon. Psychopharmacology (Berl) 2008;199:265–273. doi: 10.1007/s00213-008-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q, Valenti M, Di Marzo V. Endocannabinoids link feeding state and auditory perception-related gene expression. J Neurosci. 2004;24:10013–10021. doi: 10.1523/JNEUROSCI.3298-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. Post-transcriptional regulation of zenk expression associated with zebra finch vocal development. Brain Res Mol Brain Res. 2000;80:279–290. doi: 10.1016/s0169-328x(00)00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. CB1 cannabinoid receptor activation inhibits a neural correlate of song recognition in an auditory/perceptual region of the zebra finch telencephalon. J Neurobiol. 2003;56:266–274. doi: 10.1002/neu.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]