How can we decrease the body’s energy efficiency? The answer to this could be used to fight the exploding obesity crisis. Our ability to accumulate and retain energy reserves once provided a survival advantage. However, these ingrained energy-conservation pathways are now driving unprecedented weight gain in modern societies where calorie-dense food pervades. Burning off excess fuel (analogous to heating a house in winter with the windows open) may be an effective therapeutic avenue to reduce obesity when diet and exercise are not enough. In this week’s Science Express, Vegiopoulos et al. demonstrate that the fatty acid prostaglandin encourages adipocytes (fat cells) to do exactly this—waste energy through increased heat production (1).
In contrast to the familiar white adipocytes that specialize in lipid storage, brown adipocytes burn fat to produce heat (thermogenesis). Infants have prominent depots of brown fat that regulate body temperature. Al-though these “newborn” depots regress during childhood, appreciable amounts of brown fat persist in adults and correlate with adult leanness (2–5). Similarly, brown fat counteracts obesity and metabolic disease in rodents (6) Therefore, enhancing brown fat thermo-genesis is an attractive target for anti-obesity therapy. Vegiopoulos et al. show that white fat can be induced to form intermediate “beige” adipocytes with many of the properties of brown adipocytes, but have a completely independent developmental origin (7) (see the figure).
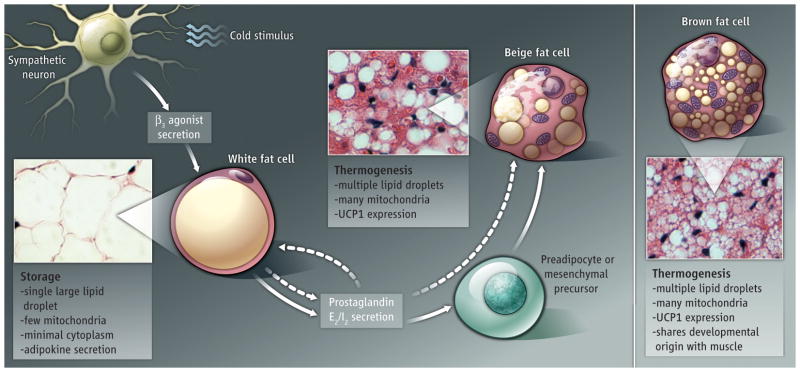
Figure 1.
White to beige. Fat cells can be classified as white storage cells, brown thermogenic cells, or intermediate beige cells. Prostaglandins produced by white fat cells (in response to β3-adrenergic agonists) or from the circulation stimulate the formation of thermogenic beige cells in white fat. The origin of beige cells is unclear.
The thermogenic activity of fat tissue is controlled by the sympathetic nervous system. Cold exposure in rodents and humans causes sympathetic nerve fibers to release norepinephrine in fat tissues (6). Norepinephrine then activates β-adrenergic receptors on brown fat cells to stimulate lipolysis and heat production. In white fat depots, prolonged exposure to cold or β3-adrenergic agonists causes tissue transdifferentiation from white to beige (6). Unfortunately, β3-adrenergic agonists have not reached successful clinical application for weight loss because of technical, efficacy and safety issues (8).
Vegiopoulos et al. have now discovered an alternative--prostaglandins drive beige fat development in white fat. This signaling pathway may lead to new therapies for obesity. Specifically, the authors found that white fat cells in mice increase expression of the enzyme cyclooxygenase-2 (COX-2) in response to cold or β3-adrenergic agonists, which consequently produces prostaglandins. Prostaglandins then act locally, triggering brown fat–specific gene expression in white fat cells. In mice engineered to express COX-2 in the skin, a systemic increase of two prostaglandins (PGE2 and PGI2) caused white fat pads to become beige. By contrast, brown fat depots were unresponsive to increased prostaglandin synthesis. Whether housed in cool or warm conditions, the transgenic animals ate more than their wild-type counterparts and remained lean, even when fed a high-fat diet. The lean phenotype was due to elevated energy expenditure and increased body temperature, similar to findings in rats given PGE2 (9). Although many factors suppress beige fat development, prostaglandin is thefirst positive acting signal that is both necessary and sufficient to promote thermogenesis selectively in the white fat compartment.
The relative roles of PGE2 and PGI2 in beige fat development remain to be determined. Genetic deletion of the PGI2 receptor reduces the expression of brown fat-specific genes in subcutaneous but not in visceral fat. Interestingly, the “browning” effect of β3-adrenergic agonists is most prominent in subcutaneous fat. Thus, differential activity of the PGI2 receptor may un-derlie fat depot–selective metabolic effects of β3-adrenergic agonists.
Prostaglandins have a broad array of functions in numerous tissues, with PGE2 acting through four different receptors (EP1-4), and PGI2 acting through the prostacyclin (IP) receptor (10). The complexities of prostaglandin-receptor signaling make therapeutic side effects a concern. For example, prostaglandin powerfully induces the inflammatory response through various combinations of ligand-receptor interactions (11). However, Vegiopoulos et al. did not observe increased systemic inflammation in the transgenic mice that overexpress COX-2. Regardless, unrave-ling the molecular mechanism of prostaglandin action in white fat may reveal more selective methods to promote beige fat development and avoid undesirable and unpredictable side effects. Conceivably, systemic delivery of a highly specific prostag-landin or a synthetic analog could treat obesity
Prostaglandins appear to act directly on fat cells, because treatment of isolated white fat precursors with PGI2 stimulated the expression of brown fat-related genes. PGI2 treatment also amplified this effect in response to norepinephrine. Thus, prostaglandins likely potentiate the response of fat cells to sympathetic nerve activity in tissues. Consistent with this, inhibition of COX-2 suppressed the emergence of beige fat in white fat depots of cold-exposed or β3-adrenergic agonist–treated mice. The intracellular signaling pathways stimulated by norepinephrine involve adenylate cyclase and production of adenosine 3′,5′-monophosphate (cyclic AMP). Prostaglandin receptors also converge on adenylate cyclase (12) and thus may promote the production of beige fat by further activating a common signaling cascade.
The question of where beige cellscome from remains unanswered. The study by Vegiopoulos et al. suggests that progenitor cells are pushed to develop into beige fat by prostaglandins. Alternatively (or additionally), white fat cells could directly convert into beige cells. Regardless of how they arise, the development of beige adipocytes in white fat correlates extremely well with protection against obesity and associated disease. Nonsurgical therapies for obesity are desperately needed. Healthy diet and exercise are accessible and affordable treatments, with broad health benefits beyond weight reduction. However, behavioral modifications are often insufficient. Stimulating white to beige fat conversion through prostaglandin signaling can hopefully be translated into an effective standalone or adjunct therapy to raise energy expenditure and promote weight loss.
References and Notes
- 1.Vegiopoulos A, et al. Science. 2010 May 6; doi: 10.1126/science.1186034. [DOI] [Google Scholar]
- 2.van Marken Lichtenbelt WD, et al. N Engl J Med. 2009;360:1500. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 3.Virtanen KA, et al. N Engl J Med. 2009;360:1518. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, et al. N Engl J Med. 2009;360:1509. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M, et al. Diabetes. 2009;58:1526. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seale P, et al. Genes Dev. 2009;23:788. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seale P, et al. Nature. 2008;454:961. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arch JR. Eur J Pharmacol. 2002;440:99. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 9.Long NC, et al. J Physiol. 1991;444:363. doi: 10.1113/jphysiol.1991.sp018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimoto Y, Narumiya S. J Biol Chem. 2007;282:11613. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 11.Tilley SL, et al. J Clin Invest. 2001;108:15. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimer R, et al. Prostaglandins. 1992;44:485. doi: 10.1016/0090-6980(92)90142-g. [DOI] [PubMed] [Google Scholar]
- 13.Our work on this topic is supported by NIH grant DK081605 (to P.S.) and by the Institute for Diabetes and Metabolism at the University of Pennsylvania School of Medicine.