Abstract
Neisseria meningitidis is a Gram-negative pathogenic bacteria responsible for bacterial meningitis and septicemia. Porins are the most represented outer membrane proteins in the pathogenic Neisseria species, functioning as pores for the exchange of ions, and are characterized by a trimeric β-barrel structure. Neisserial porins have been shown to act as adjuvants in the immune response via activation of B cells and other antigen-presenting cells. Their effect on the immune response is mediated by upregulation of the costimulatory molecule B7-2 (CD86) on the surface of antigen-presenting cells, an effect that is dependent on Toll-like receptor (TLR)2 and MyD88, through a cascade of signal transduction events mediated by direct binding of the porin to the TLR2–TLR1 heterodimer. This article summarizes work carried out investigating the mechanisms of the immune stimulating capacity of the neisserial porins (specifically meningococcal PorB), emphasizing cellular events involved in antigen-presenting cell activation and induction of expression of cell surface molecules involved in the immune response.
Keywords: adjuvant, Neisseria, porin, signal transduction, Toll-like receptor, vaccine
For the past 15 years, our laboratory has been investigating the immune-stimulating capacity of the major outer membrane protein of Neisseria meningitidis, termed PorB. We have characterized this ability, identified the pattern-recognition receptor, Toll-like receptor (TLR)2, as the mediator of this ability, and have been able to use the protein as an immune adjuvant in potential vaccine preparations. This type of activity fulfills the function of Charles Janeway’s description of the “immunologist’s dirty little secret” [1], where immunogens contained contaminants that enhanced their immunogenicity. These ‘contaminants’ were always bacterial components that many investigators have subsequently demonstrated to be TLR ligands, whose adjuvant activity was based on the induction of ‘signal 2’ or enhanced costimulatory activity similar to the mechanism of PorB, which we shall describe in this article.
Neisserial porins & immune adjuvanticity
Approximately 60% of the outer membrane protein content of pathogenic Gram-negative Neisseria consists of porin [2]. In the gonococcus these are termed protein IA (PIA) or IB (PIB), and for the meningococcus PorA (class 1 protein) or PorB (class 2 or 3 protein) [2]. There is significant structural and functional homology amongst Neisserial porins [3–8] and amongst other Gram-negative porins [8,9]. The structure of each porin monomer is a 16-strand β-barrel fold that associates to form the native trimer [8], which acts as a pore allowing the passage of ions and solutes [10,11].
As the major surface-exposed components of the outer membrane of Neisseria consists of porin, it has been investigated as a potential anti-Neisserial vaccine candidate. Studies indicate that the Neisserial porin can induce an immune response in humans and animals in the absence of exogenous adjuvants [12–15]. This led, early on, to investigations into whether the porins could act as immune adjuvants for other poorly immunogenic substances, such as peptides [16,17], as well as alter the immune response to antigens such as capsular polysaccharide (CPS) from a T-cell-independent to a T-cell-dependent response [17–22]. A summary of the properties of the Neisserial porins is presented in Box 1.
To examine the mechanism of the Neisserial porins immune adjuvant activity, in the late 1990s, we began a series of studies to investigate the ability of Neisserial porins, in particular, meningococcal PorB, to stimulate immune cells. Initial studies in our laboratory demonstrated that Neisserial porins can activate murine B cells by upregulating MHC class II and the co-stimulatory ligand B7-2 (CD86), but not B7-1 (CD80) [23]. In addition, porins from Neisseria was shown to induce B-cell proliferation and secretion of immunoglobulin, which were enhanced by coincubation with CD40 ligand [24]. The upregulation of CD86 is essential to the adjuvanticity of porin in vivo, since the administration of anti-CD86 monoclonal antibodies in conjunction with porin conjugated to group C meningococcal CPS greatly diminishes the anti-CPS response in mice [25]. The question then became, how are Neisserial porins activating the immune system, specifically antigen-presenting cells? Our findings revealed that neisserial porins were recognized and induced signaling through the recently discovered pattern-recognition receptors, TLRs, specifically TLR2. We shall briefly review TLRs later in this article to better understand this phenomena.
Box 1. Characteristics of neisserial porins
Major Neisseria outer membrane protein, making up more than 60% of the outer membrane protein contents
Involved in solute diffusion, especially sugars
Gonococci: protein IA or IB; Meningococci: Neisseria meningitidis protein A (class 1) and Neisseria meningitidis protein B (class 2 or 3)
Minimal antigenic variability
Significant homology among themselves and members of the Gram-negative porin superfamily
Investigated as potential vaccines candidates
Immune adjuvant activity and used as vaccine adjuvants in multiple investigative vaccines
Adjuvant activity mediated by induction of CD86 expression on antigen-presenting cells, mediated by Toll-like receptor 2/MyD88
Toll-like receptors
Toll-like receptors are a recently described set of innate immune receptors that recognize structures common to many different pathogens and to some endogenous molecules. Their discovery was based on homology to the Drosophila melanogaster ortholog protein, Toll. Toll is required in the fruit fly for proper embryonal development [26]. Moreover, flies that lack Toll are more susceptible to infection by Aspergillus [27]. This suggested that Toll and related proteins may be important in innate immunity to pathogens. This led to the discovery of the mammalian TLRs [28]. There are currently 12 murine TLRs and 11 human TLRs [29,30]. The first TLR with a described function in mammals was TLR4, the signal-transducing component of the lipopolysac-charide (LPS) binding and signaling complex. For many years investigators knew that LPS can bind to the glycosylphosphatidylinositolanchored surface protein, CD14, but as there was no cytoplasmic portion of the molecule, the method by which LPS would induce cellular stimulation was a mystery. Subsequently, the defect in a specific murine strain that is unresponsive to LPS, C3H/HeJ, was found to be a mutation in the cytoplasmic tail of TLR4, thus, associating this molecule with LPS induction of cellular activation and cytokine release, as seen in endotoxin-mediated events (i.e., shock) [31]. This led to the search for ligands recognized by the other TLRs.
There is a set of common ligands identified for most of the TLR molecules [29]. The first described was LPS. Interestingly, it was originally mistakenly shown that TLR2 was the signaling molecule responsible for LPS-mediated effects [32]. However, it is now known that this was owing to a lack of purity of the LPS preparations owing to lipoprotein contamination, and these latter molecules were the actual ligands for TLR2 [33,34]. Purified preparations of LPS can not signal through TLR2, but can signal through TLR4 [34]. Other TLR4 ligands include lipid A analogs [35], taxol [36], mycobaterial components [37], Aspergillus hyphae [38], cryptococcal capsule [39], respiratory syncytial virus protein F [40], and endogenous proteins such as heat shock protein [41,42] and fibronectin [43]. dsRNA has been shown to be a ligand for TLR3 [44], bacterial flagellin for TLR5 [45], the antiviral compounds imidazoquinolines for TLR7 and TLR8 [46], and bacterial CpG DNA for TLR9 [47].
TLR2 ligands & specificity
Interestingly, ligand recognition and signaling is a little more complicated when it comes to TLR2. Aderem first suggested that TLR2 mainly recognizes its ligands as a heterodimer [48] with either TLR1 [49] or TLR6 [50]. Signaling does not appear to occur with TLR2 homodimers. This was demonstrated experimentally by Ozinsky et al., when using CD4–TLR2 cytoplasmic domain (TLR–IL-1 Receptor [TIR domain]) fusion proteins. Ozinsky found that TLR2 TIR domain dimerization by anti-CD4 antibody treatment of these constructs did not induce downstream signaling [48]. This is in contrast to the recognition of ligands by other TLRs, where homodimerization is adequate to induce downstream effects, including cell signaling. The implication of the use of TLR2 heterodimers with other TLRs for ligand recognition is significant, as it broadens the family of TLR2-dependent ligands. TLR1/TLR2 heterodimers recognize a variety of bacterial lipopeptides, including the 19-kDa mycobacterial lipoprotein, [51], meningococcal lipoprotein [49], and the synthetic lipoprotein structure, N-palmitoyl-S-[2,3-bis (palmitoyloxy)-(2RS)-propyl]-[R]-cysteinyl-[S]-seryl-[S]-lysyl-[S]-lysyl-[S]-lysyl-[S]-lysine or tripalmitoyl-propyl CysSerLys4 (Pam3CSK4) [50]. TLR6/TLR2 heterodimers recognize mycoplasma lipoproteins [50] and peptidoglycan [52]. One major structural difference between these two groups of molecules (TLR1/2 specific vs TLR2/6 specific) that may account for this differential recognition is that most bacterial lipoproteins and Pam3CYSK4 are triacylated while mycoplasma lipoproteins and peptidoglycan can be diacylated [50,53,54]. This data was confirmed by the use of TLR1, TLR2 or TLR6 knockout (KO) mice [53,55]. Data for the specificity of other ligands is less compelling and may even be contradictory. For example, Flavell et al., using TLR1 or TLR2 knockout mice, have shown that TLR1 and TLR2 are necessary for signaling induced by the Borrelia burgdoferi outer surface lipoprotein (OspA), which is also triacylated [54], while Ariditi et al., using TLR6 dominant negative transfectants, have shown that OspA recognition requires TLR6 [56]. The contrasting data maybe due to the different methods used by these two groups.
Neisserial porins & TLR2-/MyD88-mediated events
As stated previously, our group has demonstrated that the Neisserial porins the and potent immune adjuvants and induce antigen-presenting cell (APC) activation, increase MHC class II and CD86 surface expression and inducing the proliferation of B cells [23], and maturation of dendritic cells (DCs) [57], and activate macrophages. This effect is mediated by TLR2 and myeloid differentiation primary response gene 88 (MyD88) [58]. This immune adjuvant effect is due to the induction of CD86 surface expression [25]. The relationship of TLR-induced activation of APCs and immune adjuvanticity is consistent with Janeway’s original observation that many microbial products are potent vaccine adjuvants and likely work through TLRs [59]. Akira has also described TLRs as adjuvant receptors [60]. Other investigators have identified Gram-negative porins, mainly from Salmonella and Haemophilus, that also activate APCs via a TLR2-related mechanism [61–64]. This is consistent with the finding we have for neisserial porins, and the fact that the porins are indeed, microbial ‘patterns’ recognized by the ‘pattern-recognition receptors’, TLRs.
Prior to our understanding that Neisserial porins, including PorB, transduce their effects via TLR2, we began early investigations into the signal transduction and transcription factor activation ability of PorB, which we shall summarize later. We eventually demonstrated that these signal transduction phenomena and nuclear factor (NF)-κB nuclear translocation by PorB were all mediated by MyD88 and TLR2, as described later and in multiple publications [57,58,65,66]. This was performed mainly using NF-κB-specific reporter gene constructs expressing luciferase transfected into cells, which may also express TLR2.
PorB induction of APC signal transduction & cellular activation
We first investigated the signal transduction events induced by PorB from Neisseria meningitidis in murine B cells, and by the use of inhibitors of these pathways, we began to establish the mechanism by which this bacterial major outer membrane protein induces CD86 upregulation and the proliferation of these cells [67]. PorB was able to induce: protein tyrosine kinase (PTK) activity; the phosphorylation of Erk1 and Erk2; and IκB-α phosphorylation, leading to NF-κB nuclear translocation in B cells in a TLR2-dependent manner. PorB-induced NF-κB nuclear translocation was not dependent on either PTK or Erk1/2 activities. Moreover, B-cell proliferation was dependent on PTK activity and not Erk1/2 activation. These published studies demonstrated that PorB acts through TLR2 as a B-cell mitogen, triggering tyrosine phosphorylation of various cellular proteins that are involved in proliferation and CD86 expression, as well as the phosphorylation of Erk1/2, which is not necessary for CD86 upregulation or for the proliferation of B cells [67]. Further examination of signal transduction events and the role of various MAPKs on PorB induction of CD86 expression in macrophages are presented later.
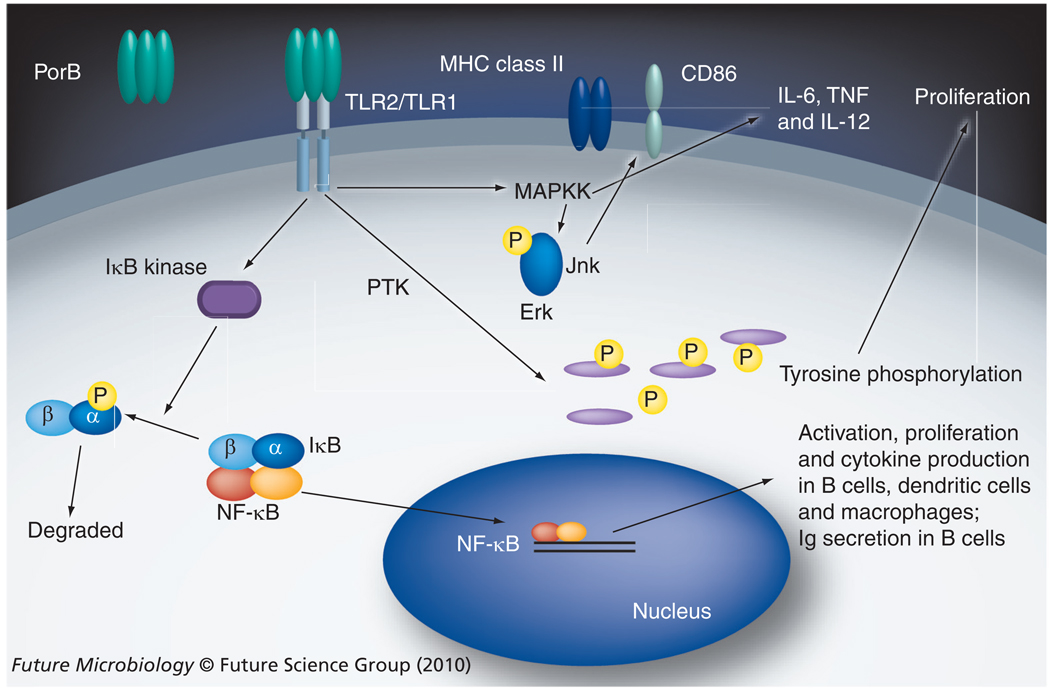
We next examined the ability of PorB to activate other cell types, including the induction of CD86 expression and the production of cytokines, as well as the signal transduction events involved in such phenomena. We next investigated the signal transduction events induced by PorB in murine bone-marrow derived macrophages. By this time, multiple investigators demonstrated that macrophage activation by TLR ligands leads to multiple signal transduction events, including MAPK activation, culminating in surface molecule upregulation and cytokine production, thus creating a bridge between the innate and adaptive immune systems [68–70]. Using specific inhibitors, we examined the involvement of the MAPK pathways in CD86 expression and cytokine production induced by TLR2 and TLR4 agonists. p38 inhibition prevents CD86 upregulation induced by the TLR4 ligand meningococcal lipo-oligosaccharide (LOS), but not by the TLR2 ligands PorB and Pam3CSK4, while JNK inhibition prevented CD86 upregulation induced by PorB and Pam3CSK4, but not by LOS. Erk inhibition had a minimal effect on PorB or LOS induction of CD86, similar to our findings with B cells [67]. The inhibition of any of these MAPKs reduced IL-6 and TNF-α secretion by either PorB and LOS treated macrophages [Unpublished Data]. These unpublished data indicated that TLRs (especially TLR2 and TLR4) use differential signaling pathways to induce CD86 upregulation, but share some common MAPK pathways involved in cytokine production, thus providing a greater understanding of the link between the innate and adaptive immune response. The effect of PorB on antigen presenting cell signaling is shown in Figure 1.
Figure 1. Summary of the known effects of neisserial porin PorB induced by its direct interaction with TLR2/TLR1 heterodimers on antigen-presenting cells.
B cells [67], dendritic cells [57] and macrophages [Unpublished Data] are all activated by PorB via TLR2. PorB induces NF-κB nuclear translocation and IkB degradation, which is not dependent on PTK or MAPK activity (in B cells) [67]. PorB does induce MAPK phosphoryaltion, but only Jnk is required for CD86 upregulation, p38 appears to be more important for TLR4-mediated induction of CD86 surface expression in macrophages. PTKs are involved in B-cell and macrophage proliferation [67], while MAPK and not PTK activity appears to be required for the induction of macrophage proinflammatory cytokine production, including IL-6, IL-12 and TNF.
NF: Nuclear factor; Por: Neisseria meningitidis protein; PTK: Protein tyrosine kinase; TLR: Toll-like receptors.
Due to Neisserial porin’s ability to activate B cells and potentiate immune responses, we hypothesized that porin also employs the potent immune stimulatory function of DCs. We examined the ability of purified N. meningitidis PorB to induce the maturation of murine splenic and bone marrow-derived DC. PorB treatment induced DC maturation, as demonstrated by increased surface expression of CD86 and MHC class I and II molecules. In addition, PorB not only enhanced the allostimulatory activity of DCs, but also augmented the ability of DCs to stimulate T cells in an antigen-specific manner. Importantly, PorB-matured DCs secreted the inflammatory cytokine IL-6, which may have implications for the adjuvant property of porin. The induction of IL-6 by PorB is also significant because IL-6 is one of a number of cytokines produced during infection with N. meningitidis, and may be involved in the inflammatory process observed during infection and disease. We previously demonstrated the requirement of MyD88 and TLR2 for PorB-induced B-cell activation, and had similar findings with regards to DCs. When treated with PorB, DCs from TLR2 or MyD88 KO mice did not have increased expression of CD86, MHC class II nor have an increased induction of IL-6, as seen with DCs from wild-type (WT) mice. This demonstrated that MyD88 and TLR2 were also essential for PorB-induced DC activation [57].
PorB directly binds to TLR2
We have gathered enough evidence that PorB mediates its cell-stimulating ability via TLR2 and MyD88, but up until this point, no TLR2 ligand had been shown to actually bind to TLR2. We investigated the interaction of PorB with TLR2 and described the direct binding of a bacterial protein to TLR2 for the first time. Using flurochrome-labeled PorB, we demonstrated its binding to TLR2 when purified TLR2 was bound to ELISA plates. We then demonstrated that PorB could bind to TLR2, in both a cell free system in vitro when it is over-expressed, and also on the surface of human embryonic kidney (HEK) 293 cells. We also demonstrated that TLR2-mediated binding of PorB is directly related to cellular activation. In addition, using HEK 293 cells expressing the chimeric TLR2/TLR1 and TLR2/TLR6 complexes, we report the selectivity of PorB binding to the TLR2/TLR1 heterodimer, which is required for initiating signaling in transfected HEK 293 and murine B cells. Together, these data provide new evidence that TLR2 recognizes PorB through direct binding, and that PorB-induced cell activation is mediated by a TLR2/TLR1 complex [65,71].
To further characterize PorB interactions with TLR2/TLR1, we then performed a set of inhibition studies to determine whether binding of one TLR2 ligand affects the binding of another. We had initially performed binding competition studies using unlabeled PorB and had demonstrated that it can inhibit the binding of flurochrome-labeled PorB to HEK cells expressing TLR2. We then expanded our studies to other TLR2 ligands, such as Pam3CSK4, (TLR2/TLR1) and Pam2CSK4 (TLR2/TLR6), and demonstrated that PorB binding to TLR2 is inhibited by excessive amounts of Pam3CSK4 [65]. We also demonstrated the same phenomena using native murine B cells [Wetzler LM, Unpublished Data]. We have further examined whether Pam2CSK4, the TLR2/6 ligand, can inhibit PorB binding to TLR2 by incubating labeled PorB alone or in the presence of 100 µg/ml of Pam2CSK4 with 293-TLR2 cells. We demonstrated a nonsignificant reduction in fluorescence-associated PorB to the cell surface, indicative of a lack of competition between these two ligands. We hypothesize that there could be differences in the specific binding site on TLR2 for TLR2/1 (PorB and Pam3CSK4) and TLR2/6 ligands (Pam2CSK4). However, a specific role of the different coreceptors cannot be excluded. The mechanism of TLR1 recruitment by Pam3CSK4 has been recently elucidated but little is known about the mechanism by which TLR6 associates into a functional heterodimer with TLR2 [72]. Although we have shown that purified PorB is free of LOS contamination [73], we have also examined whether Neisserial LOS–a TLR4 ligand – might affect PorB binding to TLR2, and whether LOS fails to prevent PorB binding to TLR2.
As a control for expression of functional TLR2 on these cells and for ligand activity, HEK cells were cotransfected with TLR2 and a luciferase-NF-κB reporter construct as described previously [58,74] luciferase was measured activity in response to various TLR2 ligands, including PorB, Pam3CSK4 and Pam2CSK4. In addition, we used TNF-α (50 ng/ml) as a non-TLR ligand positive control. The cells expressing TLR2 responded similarly to Pam3CSK4, Pam2CSK4 and PorB. HEK cells transfected with a control plasmid, HEK pcDNA, failed to respond to any TLR2 ligand but responded to TNF-α. Furthermore, the cells failed to respond to the TLR4 ligand, LPS [Unpublished Data].
PorB requires TLR2/TLR1 heterodimers for optimal function
We have published data demonstrating that PorB induced cell activation is mediated by TLR2 and MyD88 [58]. As stated previously, more recent work has now demonstrated that this activation via TLR2 occurs in association with TLR1 [65]. In this work, we demonstrated that PorB bound to HEK transfects cells expressing TLR2/TLR1 and induces cell activation, as determined by IL-8 secretion and NF-κB-mediated luciferase activity. We have also determined that murine B cells from WT mice respond to PorB by upregulating the costimulatory surface molecules CD86, CD40 and MHC class II [58]. We have subsequently examined the role of TLR2, TLR1 or TLR6 in PorB induction of B cell cytokine production. We first determined the level of IL-6 production in response to PorB in B cells from WT mice as compared with B cells from TLR2 KO mice. We have demonstrated that the IL-6 failed to be induced by PorB in the absence of TLR2, although the coreceptors TLR1 and TLR6 were present on both types of B cells. Similar results were obtained when induction of B cell TNF-α production was measured [Wetzler LM, Unpublished Data].
We then examined the role of TLR1 in the PorB induction of IL-6 in B cells. B cells from TLR6 KO mice responded to PorB and to Pam3CSK4, both being TLR2/TLR1 ligands and hence not affected by the lack of TLR6. Furthermore, these cells responded to LOS by producing high amounts of IL-6. B cells from TLR1 KO mice produced a much lower amount of IL-6 in response to either PorB or Pam3CSK4, indicating that the deletion of TLR1 affects cell activation mediated by these ligands. TLR4 KO B cells produced IL-6 in response to all ligands, except for Neisserial LOS, a known TLR4 ligand. All the cells responded greatly to heat-killed N. meningitidis, as these organisms can stimulate B cells via either TLR2 or TLR4. Interestingly, we found that PorB was still able to induce a small amount of IL-6 production even in the absence of TLR1, as opposed to Pam3CSK4, which could not induce any IL-6 production in these B cells. This indicates that the induction of cytokine production by PorB may not be dependent on the presence of TLR1, but does appear to require the presence of TLR2. Whether a TLR2 homodimer or a TLR2 heterodimer with another TLR is needed for PorB induction of IL-6 is unclear and deserves further study, as does investigation of this phenomena in other cell types.
In vivo demonstration of PorB adjuvant activity & the role of TLR2
We have determined that the ability of PorB to induce immune stimulation is dependent on its interaction with TLR2 and TLR1, as well as the subsequent TLR-dependent activation of immune cells and epithelial cells expressing such receptors. We have published evidence of the actual adjuvant activity of PorB demonstrating that it can enhance the humoral immune response against bacterial CPS [25], bacterial oligosaccharides derived from LPS [75], and protein test antigens such as ovalbumin (OVA) [71]. Moreover, we have shown that the PorB immune enhancement activity is dependent on an increased CD86 expression of APCs [23,25], which we subsequently demonstrated to require TLR2 and MyD88 [58].
Based on this evidence, we have hypothesized that TLR2 plays a role in the adjuvant activity of PorB. To test our hypothesis, we compared the adjuvant effect of PorB on the induction of immune responses to a prototype antigen, OVA, in the presence or absence of TLR2. We immunized wild-type mice and TLR2 KO mice with a mixture of PorB and OVA, three times at 2-week intervals, for a total of three immunizations. Sera were collected prior to each immunization and 4 weeks after the final immunization. Control mice were immunized with OVA alone. OVA was obtained from commercial chicken egg whites by freeze-drying followed by lyophilization and resuspension of the total proteins in sterile phosphosphate-buffered saline, as previously described [57].
Mice immunized with OVA (without additional adjuvants) responded by producing anti-OVA IgM, and the level in wild-type C57Bl/6 mice was similar to those levels obtained in OVA immunized TLR2 KO mice, indicative of a TLR2-independent response to this antigen. When we examined the response of TLR2 KO mice to immunization with OVA plus PorB as an adjuvant, we detected little or no variation of the specific anti-OVA IgM in this group, indicating that IgM production was likely due to the baseline production of IgM to OVA, which did not require an adjuvant.
However, when we then determined the levels of specific anti-OVA IgG in WT mice and TLR2 KO mice, we found that when PorB was used as an adjuvant, the anti-OVA IgG concentration in the immune mice sera was ten-times greater than the anti-OVA IgG in mice immunized with OVA alone. In support of our hypothesis of a role for TLR2 in the adjuvant activity of Neisserial PorB, sera from TLR2 KO mice immunized with PorB/OVA had similar low levels of anti-OVA IgG as compared with mice immunized with OVA alone. This is the first direct evidence that the adjuvant effect of Neisserial PorB is dependent on TLR2 expression. Anti-OVA IgG subtypes were not determined due to the paucity of sera obtained, however further studies are planned to examine these parameters as the ratio of IgG subtypes can be altered by the adjuvant used.
We have continued to use PorB as a vaccine adjuvant in the induction of protection against actual infectious agents. We have examined the role of PorB as an immune adjuvant using a T-cell-independent antigen, namely Francisella tularensis LPS, and the ability of PorB to enhance protection against murine infection with Francisella. BALB/c mice were immunized with LPS isolated from the F. tularensis subsp. holarctica live vaccine strain (LVS), with or without PorB, and the antibody levels induced during the vaccination regimen and the level of protection against intranasal challenge with LVS were determined. Antigen administered alone induced a specific F. tularensis LPS IgM response that was not maintained long-term and which conferred protection to only 25% of the mice when challenged 4 weeks after the final immunization. By contrast, F. tularensis LPS administered in combination with Neisserial PorB induced consistent levels of specific IgM throughout the immunization, and increased the proportion of surviving mice to 70% [75]. Further unpublished observations demonstrated a similar effect when the vaccine was administered intranasally, including enhanced induction of both anti-LPS IgM and IgG, and improved protection of LVS pulmonary infection as compared with mice immunized with LPS alone [Chiavolini D, Wetzler, LM, Unpublished Data]. This is an extremely important observation as it demonstrates the viability of the use of PorB as an adjuvant when administered intranasally, which may be more pertinent route of delivery for protection from mucosal or pulmonary infections. Others have also demonstrated the use of Neisserial porins as potent immune adjuvants when given intranasally [76–80], thus confirming the viability of this route of immunization and PorB adjuvant activity.
Conclusion
As described in this review, Neisserial porins, as characterized by PorB, bind to TLR2/TLR1. Through this interaction, they have a set of pleiotropic effects on TLR2/TLR1-expressing cells, inducing cell activation, enhancing MHC class II and CD86 surface expression, and inducing significant production of proinflammatory cytokines. We have gathered experimental evidence that this induction of CD86 is involved in the adjuvant activity of porins. It is likely that cytokine induction and cell proliferation, including the induction of increased B-cell immunoglobulin secretion, are also involved in the porins’ immune enhancing activity. Moreover, we have begun to dissect the use of intracellular signal transduction pathways that are involved in the porins’ ability to induce CD86, cell proliferation and cytokine production, mainly demonstrating the importance of the MAPK Jnk for TLR2-mediated events. Our increased knowledge of the innate immune function of PorB as summarized in this article, will enhance our ability to use this protein as a vaccine adjuvant, and will add to our understanding of innate immune responses to Gram-negative bacteria that contain these porins.
Future perspective
We have shown that PorB has a significant immune-stimulating capacity and can act as an vaccine adjuvant. The mechanism of its activity is likely mediated by TLR2 and the innate immune system. PorB has been used in experimental vaccines, but its adjuvant activity needs to be compared with other known vaccine adjuvants (i.e., alum) and investigative adjuvants, whether they are TLR ligands or not (including CpG DNA, monophosporyl lipid A, QS-21 and saponins). Moreover, we have evidence that PorB stimulates immune cells in a more robust manner compared with other TLR2 ligands, and its adjuvant capacity needs to be compared with other TLR2 ligands that are potential adjuvants, like Pam3CSK. The adjuvant capacity of other Gram-negative porins also needs to be examined as this may allow Gramnegative organism-specific vaccine adjuvants to be developed. Finally, beyond the adjuvant activity of PorB, the role of PorB and its recognition by TLR2 in innate immunity towards Neisserial infections, along with the induction of Neisserial-specific inflammation, needs to be further characterized. Similar studies can also be performed for other Gram-negative organisms containing similar molecules as part of the Gram-negative porin superfamily.
Executive summary
Neisserial porins & immune adjuvanticity
Neisserial porins (especially meningococcal PorB) are immunogenic, with a need for additional adjuvants.
Meningococcal PorB can activate B cells, dendritic cells and macrophages, increasing expression of MHC class II and the T-cell costimulatory molecule CD86.
An increase in CD86 on antigen-presenting cells induced by PorB is likely related to its immune adjuvant activity.
PorB induces B-cell proliferation and immunoglobulin secretion.
Toll-like receptors
Toll-like receptors (TLRs) are pattern-recognition receptors essential to innate and adaptive immunity.
There are 12 murine TLRs and 11 human TLRs.
TLR4 is the endotoxin receptor (with MD2).
dsRNA has been shown to be a ligand for TLR3, bacterial flagellin for TLR5, the antiviral compounds imidazoquinolines for TLR7 and TLR8, and bacterial CpG DNA for TLR9.
TLR2 ligands & specificity
TLR2 forms heterodimers with TLR1 or TLR6 to recognize their ligands.
TLR2 ligands include di- and tri-acylated lipoproteins, peptidoglycans and bacterial porins.
Neisserial porins & TLR2/MyD88-mediated events
PorB induction of antigen-presenting cell signal transduction and cellular activation.
PorB induces phosphotyrosein kinase activity, ERK activity and NF-κB nuclear translocation.
PorB induces B-cell proliferation. This is dependent on phosphotyrosein kinase activity but not Erk1/2 activation.
PorB directly binds to TLR2
PorB binds to purified TLR2 or TLR2/1 dimers, but not TLR2/6 dimers.
The TLR2/1 ligand Pam3CSK4 inhibits PorB binding to TLR2, but the TLR2/6 ligand Pam2CSK4 does not inhibit binding.
PorB requires TLR2/TLR1 heterodimers for optimal function
B cells from TLR1 knockout mice had a significantly diminished response to PorB (as measured by IL-6 production) as compared with B cells from wild-type mice.
Response to PorB was similar for B cells from TLR6 knockout mice and B cells from wild-type mice (as measured by IL-6 production).
The presence of TLR1 enhanced PorB binding to TLR2-transfected HEK cells, while TLR6 cotransfection had no effect.
The presence of TLR1 enhanced the PorB induction of IL-8 by TLR2-transfected HEK cells, while TLR6 cotransfection had no effect.
In vivo demonstration of PorB adjuvant activity & the role of TLR2
PorB enhancement of anti-ovalbumin IgG is diminished in TLR2 knockout mice but anti-ovalbumin IgM induction was not affected.
PorB enhances anti-Francisella tularensis supsp. holarctica lipopolysaccharide IgG and IgM production, and enhances immunity in mice towards Francisella turlarenisis subsp. holarctica infection.
Acknowledgements
The author would like to thank all the present and past members of his laboratory, and his previous mentors. Without any of you, this work could never have been performed.
This work was supported by NIH A1040944 years 1–14.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Janeway CA., Jr Immunogenecity: signal 1,2,3…and 0. Immunol. Today. 1989;10(9):283–287. doi: 10.1016/0167-5699(89)90081-9. [DOI] [PubMed] [Google Scholar]
- 2.Lytton EJ, Blake MS. Isolation and partial characterization of the reduction-modifiable protein of Neisseria gonorrhoeae. J. Exp. Med. 1986;164(5):1749–1759. doi: 10.1084/jem.164.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward MJ, Lambden PR, Heckels JE. Sequence analysis and relationships between meningococcal class 3 serotype proteins and other porins from pathogenic and non-pathogenic Neisseria species. FEMS Microbiology Letters. 1992;94:283–290. doi: 10.1016/0378-1097(92)90644-4. [DOI] [PubMed] [Google Scholar]
- 4.Butcher S, Sarvas M, Runeberg-Nyman K. Class 3 porin of Neisseria meningitidis: cloning and structure of the gene. Gene. 1991;105(1):125–128. doi: 10.1016/0378-1119(91)90523-e. [DOI] [PubMed] [Google Scholar]
- 5.Carbonetti NH, Sparling PF. Molecular cloning and chracterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc. Natl Acad. Sci. USA. 1987;84(24):9084–9088. doi: 10.1073/pnas.84.24.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gotschlich EC, Seiff ME, Blake MS, Koomey M. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc. Natl Acad. Sa. USA. 1987;84:8135–8139. doi: 10.1073/pnas.84.22.8135. ▪ First paper describing the cloning of neisserial porin.
- 7.Murakami K, Gotschlich EC, Seiff ME. Cloning and characterization of the structural gene for the class 2 protein of Neisseria meningitides. Infect. Immun. 1989;57:2318–2323. doi: 10.1128/iai.57.8.2318-2323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derrick JP, Urwin R, Suker J, Feavers IM, Maiden MC. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect. Immun. 1999;67:2406–2413. doi: 10.1128/iai.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeanteur D, Lakey JH, Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Molec. Microbiol. 1991;5:2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. ▪ First description of the bacterial porin superfamily and the relationship in structure and function of Gram-negative porins.
- 10.Douglas JT, Lee MD, Nikaido H. Protein I of Neisseria gonorrhoeae outer membrane is a porin. FEMS Microbiol. Lett. 1981;12:305–309. [Google Scholar]
- 11.Heasley FA, Danielsson D, Normark S. Genetics and Immunobiology of neisseria gonorrhoeae. Umea, Sweden: University of Umea; 1980. Reconstitution and characterization of the N. gonorrhoeae outer membrane permeability barrier; pp. 12–15. [Google Scholar]
- 12.Wetzler LM, Blake MS, Barry K, Gotschlich EC. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes and blebs isolated from rmp deletion mutants. J. Infect. Dis. 1992;166:551–555. doi: 10.1093/infdis/166.3.551. [DOI] [PubMed] [Google Scholar]
- 13.Zollinger WD, Woodrow GC, Levine MM. New Generation Vaccines. NY, USA: Marcel Dekker, Inc.; 1990. New and improved vaccines against meningococcal disease; pp. 325–348. [Google Scholar]
- 14.Bjune G, Hoiby EA, Gronnesby JK, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 15.Poolman JT. Meningococcal vaccines. J. Med. Microbiol. 1988;26:170–172. [PubMed] [Google Scholar]
- 16.Lowell GH, Smith LF, Seid RC, Zollinger WD. Peptides bound to proteosomes via hydrophobic feet become highly immunogenic without adjuvant. J. Exp. Med. 1988;167:658–663. doi: 10.1084/jem.167.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowell GH, Ballou WR, Smith LF, Wirtz RA, Zollinger WD, Hockmeyer WT. Proteosome–lipopeptide vaccines: enhancement of immunogenicity for malaria CS peptides. Science. 1988;6(240):800–802. doi: 10.1126/science.2452484. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly JJ, Deck RR, Liu MA. Immunogenicity of a Haemophilus influenzae polysaccharide–Neisseria meningitidis outer membrane protein vaccine. J. Immunol. 1990;145:3071–3079. [PubMed] [Google Scholar]
- 19. Lowell GH, Woodrow GC, Levine MM. New Generation Vaccines. NY, USA: Marcel Dekker, Inc.; 1990. Proteosomes, hydrophobic anchors, iscoms, and liposomes for improved presentation of peptides and protein vaccines; pp. 141–160. ▪ Review of early studies demonstrating the adjuvant activity of neisserial porin.
- 20.Wetzler LM. Immunopotentiating ability of neisserial major outer membrane proteins. Use as an adjuvant for poorly immunogenic substances and potential use in vaccines. Ann. NY Acad. Sci. 1994;730:367–370. doi: 10.1111/j.1749-6632.1994.tb44295.x. [DOI] [PubMed] [Google Scholar]
- 21.Livingston PO, Calves MJ, Helling F, Zollinger WD, Blake MS, Lowell GH. GD3/proteosome vaccines induce consistent IgM antibodies against the ganglioside GD3. Vaccine. 1993;11:1199–1204. doi: 10.1016/0264-410x(93)90043-w. [DOI] [PubMed] [Google Scholar]
- 22.Fusco PC, Michon F, Laude-Sharp M, et al. Preclinical studies on a recombinant group B meningococcal porin as a carrier for a novel Haemophilus influenzae type B conjugate vaccine. Vaccine. 1998;16:1842–1849. doi: 10.1016/s0264-410x(98)00174-1. [DOI] [PubMed] [Google Scholar]
- 23. Wetzler LM, Ho Y, Reiser H. Neisserial porins induce B lymphocytes to express costimulatory B7–2 molecules and to proliferate. J. Exp. Med. 1996;183:1151–1159. doi: 10.1084/jem.183.3.1151. ▪ First description of studies demonstrating Neisseria meningitidis protein (PorB) stimulation of antigen-presenting cells and an induction of the enhanced expression of the T-cell costimulatory molecule CD86, which is essential for the adjuvant activity of PorB.
- 24.Snapper CM, Rosas FR, Kehry MR, Mond JJ, Wetzler LM. Neisserial porins may provide critical second signals to polysaccharide-activated murine B cells for induction of immunoglobulin secretion. Infect. Immun. 1997;65:3203–3208. doi: 10.1128/iai.65.8.3203-3208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mackinnon FG, Ho Y, Blake MS, et al. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J. Infect. Dis. 1999;180:755–761. doi: 10.1086/314966. ▪ First demonstrated the essential role of CD86 in the adjuvant activity of PorB.
- 26.Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 27.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 28.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc. Natl Acad. Sci. USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uematsu S, Akira S. Toll-Like receptors (TLRs) and their ligands. Handb. Exp. Pharmacol. 2008;183:1–20. doi: 10.1007/978-3-540-72167-3_1. [DOI] [PubMed] [Google Scholar]
- 30.Roach JC, Glusman G, Rowen L, et al. The evolution of vertebrate Toll-like receptors. Proc. Natl Acad. Sci USA. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. ▪ First paper to describe the role of TLR4 in lipopolysaccharide innate immune recognition.
- 32.Kirschning CJ, Wesche H, Merrill AT, Rothe M. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 34.Heine H, Kirschning CJ, Lien E, Monks BG, Rothe M, Golenbock DT. Cutting edge: cells that carry a null allele for Toll-like receptor 2 are capable of responding to endotoxin. J. Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 35.Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perera PY, Mayadas TN, Takeuchi O, et al. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 2001;166:574–581. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 37.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 38.Mambula SS, Sau K, Henneke P, Golenbock DT, Levitz SM. Toll-like receptor (TLR) signaling in response to Aspergillus fumigatus. J. Biol. Chem. 2002;277:39320–39326. doi: 10.1074/jbc.M201683200. [DOI] [PubMed] [Google Scholar]
- 39.Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 2001;166:4620–4626. doi: 10.4049/jimmunol.166.7.4620. [DOI] [PubMed] [Google Scholar]
- 40.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 41.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 42.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/ interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 43.Okamura Y, Watari M, Jerud ES, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 44.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll- like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 46.Jurk M, Heil F, Vollmer J, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 47.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl Acad. Sci. USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl Acad. Sci. USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyllie DH, Kiss-Toth E, Visintin A, et al. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J. Immunol. 2000;165:7125–7132. doi: 10.4049/jimmunol.165.12.7125. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi O, Kawai T, Muhlradt PF, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi O, Sato S, Horiuchi T, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 52.Imler JL, Hoffmann JA. Toll receptors in innate immunity. Trends Cell Biol. 2001;11:304–311. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 53.Akira S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 54.Alexopoulou L, Thomas V, Schnare M, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 55.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 56.Bulut Y, Faure E, Thomas L, Equils O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 2001;167:987–994. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- 57.Singleton TE, Massari P, Wetzler LM. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J. Immunol. 2005;174:3545–3550. doi: 10.4049/jimmunol.174.6.3545. [DOI] [PubMed] [Google Scholar]
- 58. Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. ▪ Original description of the importance of TLR2 recognition of PorB for its ability to stimulate antigen-presenting cells and increase CD86 expression.
- 59.Medzhitov R, Janeway CA., Jr Innate immune induction of the adaptive immune response. Cold Spring Harb. Symp. Quant. Biol. 1999;64:429–435. doi: 10.1101/sqb.1999.64.429. [DOI] [PubMed] [Google Scholar]
- 60.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 61.Galdiero M, Finamore E, Rossano F, et al. Haemophilus influenzae porin induces Toll-like receptor 2-mediated cytokine production in human monocytes and mouse macrophages. Infect. Immun. 2004;72:1204–1209. doi: 10.1128/IAI.72.2.1204-1209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ray A, Chatterjee NS, Bhattacharya SK, Biswas T. Porin of Shigella dysenteriae enhances mRNA levels for Toll-like receptor 2 and MyD88, up-regulates CD80 of murine macrophage, and induces the release of interleukin-12. FEMS Immunol. Med. Microbiol. 2003;39:213–219. doi: 10.1016/S0928-8244(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 63.Ray A, Karmakar P, Biswas T. Up-regulation of CD80-CD86 and IgA on mouse peritoneal B-1 cells by porin of Shigella dysenteriae is Toll-like receptors 2 and 6 dependent. Mol. Immunol. 2004;41:1167–1175. doi: 10.1016/j.molimm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Ray A, Biswas T. Porin of Shigella dysenteriae enhances Toll-like receptors 2 and 6 of mouse peritoneal B-2 cells and induces the expression of immunoglobulin M, immunoglobulin G2a and immunoglobulin A. Immunology. 2005;114:94–100. doi: 10.1111/j.1365-2567.2004.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Massari P, Visintin A, Gunawardana J, et al. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 2006;176:2373–2380. doi: 10.4049/jimmunol.176.4.2373. ▪ First-time description of a TLR2 ligand directly binding to TLR2.
- 66.Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 2003;11:87–93. doi: 10.1016/s0966-842x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 67.MacLeod H, Bhasin N, Wetzler LM. Role of protein tyrosine kinase and Erk1/2 activities in the Toll-like receptor 2-induced cellular activation of murine B cells by neisserial porin. Clin. Vaccine Immunol. 2008;15:630–637. doi: 10.1128/CVI.00435-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones BW, Heldwein KA, Means TK, Saukkonen JJ, Fenton MJ. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann. Rheum. Dis. 2001;60(Suppl. 3):6–12. doi: 10.1136/ard.60.90003.iii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirschfeld M, Weis JJ, Toshchakov V, et al. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 71.Liu X, Wetzler LM, Massari P. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine. 2008;26:786–796. doi: 10.1016/j.vaccine.2007.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brodsky I, Medzhitov R. Two modes of ligand recognition by TLRs. Cell. 2007;130:979–981. doi: 10.1016/j.cell.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Massari P, King CA, Macleod H, Wetzler LM. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein Expr. Purif. 2005;44:136–146. doi: 10.1016/j.pep.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 74.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274(16):10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 75.Chiavolini D, Weir S, Murphy JR, Wetzler LM. Neisseria meningitidis PorB, a Toll-like receptor 2 ligand, improves the protective capacity of Francisella tularensis LPS against murine experimental tularemia. Clin. Vaccine Immunol. 2008;15:1322–1329. doi: 10.1128/CVI.00125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Langley JM, Halperin SA, McNeil S, et al. Safety and immunogenicity of a proteosome -trivalent inactivated influenza vaccine, given nasally to healthy adults. Vaccine. 2006;24:1601–1608. doi: 10.1016/j.vaccine.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 77.Chabot S, Brewer A, Lowell G, et al. A novel intranasal protollin-based measles vaccine induces mucosal and systemic neutralizing antibody responses and cell-mediated immunity in mice. Vaccine. 2005;23:1374–1383. doi: 10.1016/j.vaccine.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 78.Hall MA, Stroop SD, Hu MC, et al. Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections. Infect. Immun. 2004;72:2507–2512. doi: 10.1128/IAI.72.5.2507-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oftung F, Naess LM, Wetzler LM, et al. Antigen-specific T-cell responses in humans after intranasal immunization with a meningococcal serogroup B outer membrane vesicle vaccine. Infect. Immun. 1999;67:921–927. doi: 10.1128/iai.67.2.921-927.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haneberg B, Dalseg R, Wedege E, et al. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect. Immun. 1998;66:1334–1341. doi: 10.1128/iai.66.4.1334-1341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]