Abstract
Despite G-protein-coupled receptors (GPCRs) being among the most fruitful targets for marketed drugs, intense discovery efforts for several GPCR subtypes have failed to deliver selective drug candidates. Historically, drug discovery programmes for GPCR ligands have been dominated by efforts to develop agonists and antagonists that act at orthosteric sites for endogenous ligands. However, in recent years, there have been tremendous advances in the discovery of novel ligands for GPCRs that act at allosteric sites to regulate receptor function. These compounds provide high selectivity, novel modes of efficacy and may lead to novel therapeutic agents for the treatment of multiple psychiatric and neurological human disorders.
G-protein-coupled receptors (GPCRs) are the largest class of cell-surface receptors and play crucial roles in virtually every organ system (see ref. 1 for review). GPCRs are activated by a diverse range of ligands, including hormones, neurotransmitters, ions, odorants and photons of light, and couple to a wide range of signalling molecules and effector systems. GPCRs have been implicated in a multitude of human disorders and numerous diseases have been linked to mutations and polymorphisms in GPCRs2,3. Thus, it is not surprising that GPCRs are the target of many therapeutic agents that are currently in use. It is estimated that nearly half of all modern drugs regulate GPCR activity in some way.
However, despite the proven success of GPCRs as drug targets, useful ligands do not exist for the majority of GPCRs. GPCRs are encoded by more than 1,000 genes4, yet synthetic ligands for only a small fraction of these are available, and for many receptors intense efforts have failed to yield highly selective ligands that could ultimately be used as drug leads. A number of important issues contribute to the difficulty of discovering small-molecule selective agonists or antagonists that act on the orthosteric site of some GPCRs. For instance, the orthosteric binding sites across members of a single GPCR subfamily for a particular endogenous ligand are often highly conserved, making it difficult to achieve high selectivity for specific GPCR subtypes. Furthermore, ligands at orthosteric sites for some GPCRs, such as peptide or protein receptors, have other physicochemical and pharmacokinetic properties that are incompatible with scaffolds that are useful for small-molecule drug discovery. An alternative approach, which has proven highly successful for ligand-gated ion channels, is the development of selective allosteric modulators of the specific receptor subtypes. These small molecules do not bind to the orthosteric ligand binding site but instead act at an alternatively located binding site (allosteric site), which is distinct from the orthosteric site, to either potentiate or inhibit activation of the receptor by its natural ligand. Benzodiazepines are a classic example of positive allosteric modulators of γ-aminobutyric acid (GABA)A receptors. Benzodiazepines provide an effective and safe approach to the treatment of anxiety and sleep disorders without inducing the potentially lethal effects of direct-acting GABA receptor agonists5. Allosteric modulators of GABAA receptors include compounds with a range of activities, such as positive allosteric modulators (PAMs), which increase the response of the receptor to GABA, negative allosteric modulators (NAMs), which reduce receptor responsiveness, and neutral allosteric ligands, which bind to the allosteric site but have no effects on the responses to the orthosteric ligand.
Although allosteric modulators are well established as research tools and therapeutic agents of ion channels, they have not been a traditional focus of drug discovery efforts for GPCRs. However, in recent years, remarkable progress has been made in the discovery, optimization and clinical development of allosteric modulators of multiple GPCR subtypes. These include PAMs, NAMs and neutral ligands for each of the three major GPCR subfamilies, which offer novel modes of action over orthosteric ligands. These compounds are providing major advances in developing novel drugs, drug leads and research tools for GPCRs, and have potential utility for the treatment of multiple human disorders. Recent efforts have focused on the development of novel strategies for the treatment of psychiatric and neurological disorders, and several potential GPCR drug targets that have been intractable using traditional orthosteric ligand approaches have been identified.
Modes of action and pharmacological properties
Allosteric modulators bind to GPCRs at sites that are topographically distinct from the orthosteric site, leading to a change in receptor conformation. As a consequence, the interactive properties of the GPCR, both with respect to orthosteric ligands and the cellular host environment, can be modified in either a positive or negative direction; in essence, a receptor occupied by an allosteric ligand can be viewed as a ‘novel’ receptor type, with unique behaviour. Allosteric GPCR modulators exhibit one or more of the following pharmacological properties (Fig. 1): affinity modulation — the resulting conformational change can impact the orthosteric binding pocket such that either the association or dissociation rate (or both) of an orthosteric ligand is modified; efficacy modulation — the allosteric effect can change intracellular responses, thus leading to a change in the signalling capacity (or ‘intrinsic efficacy’) of an orthosteric ligand; agonism/ inverse agonism — the allosteric modulator can perturb receptor signalling in either a positive (agonism) or negative (inverse agonism) way, irrespective of the presence or absence of an orthosteric ligand. There are now a number of examples of allosteric GPCR modulators that exhibit one or more of these pharmacological properties6,7. Table 1 provides a list of allosteric modulators of GPCRs that have been identified to date and that will be discussed here (see also refs 6,7).
Figure 1. Modes of action of allosteric modulators.
a | Allosteric ligands bind to a topographically distinct site on a receptor to modulate orthosteric ligand affinity (red) and/or efficacy (blue). Some allosteric ligands can directly perturb signalling in their own right (green). b | Simulations show the effects on the binding (left) or function (right) of an orthosteric agonist mediated by three different allosteric potentiators: the first (red) enhances orthosteric agonist affinity only; the second (blue) enhances orthosteric agonist efficacy only; the third (green) modestly enhances both affinity and efficacy, and also displays allosteric agonism. Note the potential for differences in observed outcome depending on assay format and that the baseline is 50% specific binding.
Table 1.
Reported allosteric modulators of G-protein-coupled receptors
| receptor | Modulator example(s) |
|---|---|
| GPCR Family A | |
| Adenosine A | PD 81723; LUF 5484 |
| Adenosine A | Amiliorides |
| Adenosine A | VU5455Z; VU8504Z; DU124183 |
| Adrenoceptor α | Amiliorides; benzodiazepines; conopeptide; ρ-TIA |
| Adrenoceptor α, α | Amiliorides |
| Adrenoceptor α | Agmatine |
| Adrenoceptor β | Zinc |
| Cannabinoid CB | Org27569; Org27759; PSNCBAM-1 |
| Chemokine CXCR1 | Reparixin; SCH527123 |
| Chemokine CXCR2 | Reparixin; SCH527123; SB656933 |
| Chemokine CXCR3 | IP-10; I-TAC |
| Chemokine CCR1 | RSVM; ASLW; prichosanthin; plerixafor |
| Chemokine CCR3 | BX-471; CP-481–715; UCB35625 |
| Chemokine CCR5 | Trichosanthin; AK602; AK530; TAKK220; TAK779; SCH 351125; ancriviroc; vicriviroc; aplaviroc; maraviroc |
| Dopamine D | Zinc |
| Dopamine D2 | Amilorides; zinc; L-prolyl-L-leuclylglycin-amide |
| Endothelin ET | Aspirin; sodium salicylate |
| Gonadotropin-releasing hormone receptor |
Furan-1 |
| GH secretagogue | L-692,429; GH-releasing peptide 6 |
| Luteinizing hormone | Org 41841; [3H]Org 43553 |
| mAChR M1 | Brucine; W84; BQCA; TBPB; AC-42; MT3; MT7; 77-LH-28-1; NDMC; tacrine; McN-A-343; staurosporine |
| mAChR M2 | Gallamine |
| mAChR M3 | WIN 62,577 |
| mAChR M4 | Alcuronium; LY2033298; VU10010; VU0152099; VU0152100 |
| Neurokinin NK | Heparin |
| Opioid μ, δ | Cannabidiol |
| Purine P2 | 2,2O-pyridylsatogen tosylate |
| Serotonin 5HT | 5HT moduline |
| Serotonin 5HT, 5HT | Oleamide |
| Serotonin 5HT | Oleamide; PNU-69176E |
| GPCR Family B | |
| CRF1 receptor | Antalarmin; NBI 35965; DMP696; NBI 27914 |
| CGRP receptor | BIBN4096BS |
| Glucagon | Bay27-9955; L-168049 |
| GLP1 receptor | T-0632; NovoNordisk Compounds 1–6 |
| GPCR Family C | |
| Calcium sensing receptor | Fendeline; cinacalcet; NPS 467; NPS 568; L-amino acids; NPS 2143; calhex 231 |
| GABA | CGP7930; CGP13501; GS39783 |
| mGluR1 | (−)-C PC C OEt; Ro 67-7476; Ro 01-6128; BAY36-7620; [3H]R214127; NPS 2390; EM-TBPC; cis-64a; JNJ 16259685 |
| mGluR2 | LY487379; BINA; LY181837; Ro 67-6221 |
| mGluR4 | SIB-1893; MPEP; (−)-PHCCC; VU0155041; VU0080421 |
| mGluR5 | MPEP; MTEP; DFB; DCB; DMeOB; CPPHA; CDPPB: VU-29; ADX-47273 |
| mGluR7 | AMN082 |
CGRP, calcitonin gene related peptide; CRF1, corticotrophin releasing factor 1; GABA, γ-aminobutyric acid; GH, growth hormone; GLP1, glucagon-like peptide 1; mAChR, muscarinic acetylcholine receptor; mGluR, metabotropic glutamate receptor.
The diverse effects on receptor behaviour engendered by allosteric ligands can be described by equations based on various ternary complex mass-action models8–11, that is, reaction schemes involving the formation of a ternary complex between the receptor, orthosteric and allosteric ligands, in addition to binary receptor–orthosteric ligand and receptor–allosteric modulator complexes. within such models, the magnitude and direction of the allos-teric effect that is transmitted from one site to the other, also termed cooperativity, is defined by one or more ‘cooperativity factors’ (Box 1). Although these models are extremely useful for conceptualizing different allosteric modulator effects under various conditions, it is generally difficult to fit such models to real experimental data. As an alternative, ‘operational models’ of allosterism have also been developed; these, combine both mechanistic and empirical parameters to facilitate quantification of experimentally-derived allosteric drug properties in a manner that can facilitate structure–activity studies (Box 2).
Box 1 Mass action receptor models of allosteric interaction.
The simplest allosteric interaction occurs when a modulator has no effect on its own and regulates a single property of the orthosteric ligand, namely its affinity. In this situation, the pharmacological properties of the modulator at its target receptor are characterized by its affinity for the allosteric site, KB and a single cooperativity factor, commonly denoted by the symbol α, which quantifies the magnitude of the allosteric effect exerted between the two sites. Values of α > 1 denote positive cooperativity, or allosteric potentiation, whereas values of α < 1 (but greater than 0) denote negative cooperativity, or allosteric inhibition/antagonism. Thus, α = 10 means that the allosteric ligand can increase the affinity of an orthosteric ligand by a factor of 10, whereas α = 0.1 represents a 10-fold decrease in affinity. Interestingly, some allosteric modulators can posses an α value equal to 1, which means that they do not change the affinity of the orthosteric ligand at equilibrium; this property is referred to as neutral cooperativity. Neutral allosteric ligands can still bind to an allosteric site and, by simple competition, inhibit the actions of other allosteric modulators that bind to that same site. However, there is no a priori reason why the conformational change that is mediated by an allosteric modulator cannot modify the signalling efficacy of the orthosteric ligand, in addition to exerting effects on affinity. If so, then one or more additional cooperativity factors (β, γ, δ, etc.) need to be introduced into the model of allosteric interaction to accommodate this effect on different states of the receptor10,11, leading to an increase in model complexity but also a far greater diversity in the repertoire of pharmacological effects that can be achieved by allosteric receptor modulation.
Box 2 Operational approaches to modelling allosterism.
Equilibrium equations based on operational models of orthosteric drug–receptor interaction108, have proven very useful in their ability to quantify experimentally-derived ligand properties, such as affinity and relative efficacies, from a variety of functional assays in a manner that can be used to guide structure–activity studies. A similar model has recently been developed for allosteric interactions15,19,109, and is illustrated below. KA and KB denote the equilibrium dissociation constants of an orthosteric ligand, A, and allosteric modulator, B, respectively at a receptor (R). The allosteric effect of the modulator on orthosteric ligand affinity, and vice versa, is described by the binding cooperativity factor, α. The letter, S, denotes the stimulus imparted by a ligand-occupied receptor to the cell. The symbol, β, describes an activation cooperativity factor, which quantifies the allosteric effect of the modulator on the signalling efficacy of the orthosteric agonist. In the equilibrium solution to the model, an additional parameter, τ, is introduced as a measure of the direct agonism that each ligand can possess; this parameter is influenced by receptor density, system responsiveness and the intrinsic efficacy of the ligand itself. The remaining parameters are the maximum system response, Em, and the slope, n, of the function that links receptor occupancy to final observed response. Also shown is the application of the model to in-house data of the interaction between the allosteric agonist/modulator, LUF5484, and the orthosteric agonist, R(−)-N6-(2-phenylisopropyl)adenosine (R-PIA), at the human A1adenosine receptor stably expressed in a recombinant Chinese hamster ovary cell line and quantified using an assay of phosphorylation of extracellular signal regulated kinases 1 /2 (pERK1/2). Using this approach, the four properties accounting for the pharmacological actions of the modulator (KB, α, β, τB ) can be derived. Operational models are thus useful in helping to understand the results of allosteric modulator screening programmes.
Compounds that possess an allosteric mode of action can display a number of theoretical advantages over orthosteric ligands as potential therapeutic agents. For example, allosteric modulators that do not display any agonism are quiescent in the absence of endogenous orthosteric activity and only exert their effect in the presence of a released orthosteric agonist. Thus, such allosteric modulators have the potential to maintain activity dependence and both temporal and spatial aspects of endogenous physiological signalling. A second potential advantage of allosteric ligands is the prospect of greater receptor selectivity due to either higher sequence divergence in allosteric sites across receptor subtypes relative to the conserved orthosteric domain12, or due to selective cooperativity at a given subtype to the exclusion of others13. Alternatively, selectivity might be engendered by combining both or thosteric and a llosteric pharmacophores within the same molecule to yield a novel class of ‘bitopic’ GPCR ligand14. A third advantage is that allosteric modulators with limited positive or negative cooperativity would impose a ‘ceiling’ on the magnitude of their allosteric effect7. This property allows for a high degree of titratability of the pharmacological effect, which means that large doses of allosteric modulators can be administered with a lower propensity towards target-based toxicity than orthosteric agonists or antagonists. Moreover, limited cooperativity modulators introduce a new level of pharmacological responsiveness, whereby they can allow for a subtle re-setting of endogenous agonist activity. For allosteric antagonists, this would manifest phenomenologically as a partial antagonism, where the response is reduced to a new level, but not completely abrogated. These potential advantages of allosteric modulators apply regardless of the particular therapeutic area and are of equal importance for pharmacological modulation of receptors located within the CNs and peripherally.
In recent years, there has been a growing appreciation of a much broader spectrum of GPCR behaviour than just G-protein and second messenger activation, and of the fact that agonist binding need not trigger all these events in a sequential fashion15. Thus, depending on the functional endpoint being measured (for example, activation, accessory-protein coupling, dimerization, phosphorylation, internalization or desensitization), the same drug acting at the same receptor and in the same cellular background might display an entire spectrum of efficacies ranging from full to partial to inverse agonism. The ability of different ligands to induce unique GPCR conformational states that activate a discrete subset of the possible cellular behaviours that are linked to that GPCR has been termed ‘stimulus-trafficking’, ‘biased agonism’ or ‘functional selectivity’16. Given that allosteric modulators themselves cause a conformational change in GPCR structure, it is possible that some will also engender functional selectivity in the actions of orthosteric ligands that co-bind to the receptor. This will be dependent on the type and degree of allosteric modulation of the signalling pathway being measured. For example, 1-(4-ethoxyphenyl)-5-methoxy-2-methylindole-3-carboxylic acid (Fig. 2a; compound 1), an allosteric modulator of the chemoattractant receptor-homologous molecule on T helper 2 cells (CRTH2), is inactive against prostaglandin D2-induced G-protein-linked pathways but is a potent antagonist of G-protein-independent, β-arrestin coupling to the same receptor17. In another recent study, performed on native metabotropic gluta-mate receptor 5 (mGluR5) expressed in cultured rat cortical astrocytes, the allosteric modulator, CPPHA (Fig. 2a; compound 2), had different effects on calcium mobilization and eRK1/2 phosphorylation, even though both pathways were linked to the endogenous mGluR5 receptor18 (Fig. 2b). The ability to modulate selected signalling pathways that are associated with a given GPCR introduces an additional opportunity for fine-tuning intracellular signalling with allosteric modulators19.
Figure 2. Allosteric modulators of gPcr-associated signalling pathways.
a | Chemical structures of allosteric modulators known to differentially modulate signalling pathways that are associated with a given G-protein-coupled receptor in a common cellular background. b | Differential effects of the allosteric modulator, N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA), on dihydroxyphenylglycol (DHPG)-mediated calcium signalling (left), or extracellular signal regulated kinase (ERK) 1/2 phosphorylation (right) through the endogenous mGluR5 receptor in rat cortical astrocytes. Data from ref. 17. M, motor; pERK1/2, phosphorylated ERK1/2; tERK1/2, total ERK1/2.
The fact that allosteric mechanisms are governed not only by the affinity (KB, Boxes 1,2) of the allosteric modulator, but also by one or more cooperativity factors also presents significant practical implications and challenges for drug discovery. For instance, the potency of an allosteric modulator in a given assay will be determined by both its affinity and its cooperativity with the orthosteric ligand. Thus, there are at least two ligand properties that will need to be optimized simultaneously, in contrast to standard orthosteric antagonist programmes in which drug potency is determined primarily by ligand affinity. In addition, the cooperativity between an allosteric modulator and the orthosteric site can change depending on the orthosteric ligand that is used to probe receptor function — sometimes referred to as ‘probe dependence’15,20 — and means that the choice of orthosteric ligand can be crucial to the success of modulator screening campaigns. As a general rule, the endogenous orthosteric agonist for a given target GPCR should be used as the probe in screening assays to increase the likelihood that this will translate to the endogenous (physiological) situation. If the nature of the assay (or target) precludes the use of the endogenous agonist when screening for novel allosteric modulators, caution needs to be exercised in the interpretation of data derived from screens based on surrogate orthosteric agonists due to the potential for probe-dependent pharmacology. Furthermore, if cooperativity is limited, it might be difficult to achieve an appreciable signal-to-noise window to detect the effects of weak modulators12, even though such compounds could represent promising drug leads. Another important issue relates to the potential for species differences across allosteric sites. Although there are limited examples of putative endogenous allosteric modulators for some GPCRs (see ref. 7), allosteric sites need not have evolved to accommodate an endogenous ligand and are thus more likely to show sequence divergence between subtypes, and indeed between species. This becomes an important consideration in drug discovery when attempting to progress initial hits and leads from human GPCR-based in vitro screens to in vivo animal models of a given disease. A lack of in vivo animal efficacy might be due to differences in the allosteric binding sites between the human and animal target GPCRs. Finally, the fact that the same allosteric modulator can in some cases show differential effects on orthosteric ligand affinity versus signalling efficacy means that different results may be obtained depending on the type of assay that is used. A similar consideration applies in situations in which the modulator engenders functional selectivity in the actions of the orthosteric agonist (Fig. 2b). The real challenge in this case relates to our current inability to conclusively link a given functional endpoint to the desired therapeutic outcome. Thus, when possible, screening should not rely on a single functional assay as pathway-dependent allosteric modulation will not be detected. In the past, such findings may have led to the abandonment of a drug discovery campaign, but assay-dependent pharmacology may actually be a hallmark of an allosteric receptor-based effect.
Allosteric GPCR modulation in CNS disorders
Two allosteric modulators of GPCRs have now entered the market (Fig. 3), generating further excitement in the field about the prospect of this new mode of GPCR regulation. The first such drug was cinacalcet (sensipar/Mimpara; Amgen. Fig. 3; compound 3), a PAM of the calcium sensing receptor (CasR)21, that increases sensitivity to circulating calcium. CasR is involved in the regulation of calcium homeostasis and renal calcium resorption, as well as in the maintenance of intracellular inositol triphosphate levels. For disorders in which a CasR-related deficiency occurs, such as hyperparathyroidism, cinacalcet can play an integral role in therapy. More recently, maraviroc (Celsentri/selzentry; Pfeizer. Fig. 3; compound 4), a NAM of the chemokine receptor CCR5, was launched for the treatment of HIv infections. Maraviroc binds to CCR5, stabilizing a receptor conformation that has lower affinity for the HIv virus, thereby blocking CCR5-dependent entry of HIv-1 into cells22. These exciting advances provide clear proof of concept for the clinical utility of allosteric modulators of GPCRs. It will be important to further advance our understanding of this new approach to regulate GPCR function and develop molecules with appropriate properties for the treatment of a broader range of human disorders. The remainder of this Review will focus on the application of this approach to the understanding and treatment of CNs disorders as this remains a therapeutic area with one of the highest attrition rates in drug discovery23.
Figure 3. structures of the two marketed gPcr allosteric modulators.
Cinacalet (compound 3) is a positive allosteric modulator of the calcium sensing receptor and is used to treat hyperparathyroidism. Maraviroc (compound 4) is a negative allosteric modulator of chemokine receptor 5 that inhibits HIV entry into cells and is used to treat HIV infections.
Negative allosteric modulators of mGluR5 for the treatment of anxiety disorders
One of the most well studied GPCRs with regards to allosteric modulators is the mGluR5 subtype of metabotropic glutamate receptor. mGluRs are members of the class C GPCRs, which possess a large extracellular domain that includes the orthosteric gluta-mate binding site and a seven transmembrane (7TM) spanning domain with a topology that is similar to that of the 7TM region of class A and class B GPCRs. Many allosteric modulators for this receptor have been discovered and they span the entire range of potential activities outlined above (PAMs, NAMs and neutral allosteric ligands). In addition, radioligands exist for one allosteric site on mGluR5; these have been key to understanding the pharmacological properties of allosteric modulators that are directed to this specific binding site.
A major aim of efforts to discover mGluR5 antagonists has been for the treatment of anxiety disorders. The mGluR5 receptor is localized at postsynaptic sites in brain regions involved in anxiety disorders25, where it increases excitability by modulating various ion channels and potentiates currents through ionotropic n-methyl-d-aspartate (NMDA) receptors26. Based on these actions, it was postulated that an antagonist of mGluR5 might dampen the activity in glutamatergic circuits, which are thought to underlie anxiety disorders. However, until highly selective pharmacological tools that distinguish between mGluR1 and mGluR5 became available (Table 2), this hypothesis could not be tested. The first compound that was identified as a negative allosteric modulator of the mGluR family was CPCCoet27, a structurally novel mGluR1 inhibitor. varney et al.28 published the first description of negative allosteric modulators of mGluR5, sIB-1757 and sIB-1893, which exhibited selectivity and micromolar potency for mGluR5. subsequent structural analogues, including MPeP29 and MTeP30, have increased potency, selectivity and brain penetrance. since the discovery of these prototypical compounds, a large number of highly selective mGluR5 NAMs have been identified and optimized31. Also, radioligands and positron emission tomography (PeT) ligands for the MPeP allosteric site now exist that might be useful for dose-finding studies in clinical development32. The focus on allosteric modulators was key to the discovery of selective inhibitors of mGluR5, leading to selectivity that had not previously been achieved with orthosteric ligands for any of the mGluR subtypes.
Table 2.
Potential indications for allosteric modulators of mGluRs
| compound structure | compound name (reference) |
mglur subtype |
study data |
|---|---|---|---|
| Pain | |||
 |
CPCCOEt (27) |
1 NAM | 1st allosteric modulator of an mGluR |
| Anxiety, fragile X syndrome, GERD, chronic pain, depression, migraine | |||
 |
SIB-1757 (28) |
5 NAM | One of the first allosteric modulators of mGluR 5 |
 |
SIB-1893 (28) |
5 NAM | One of the first allosteric modulators of mGluR 5 |
 |
MPEP (29) |
5 NAM | Anxiolytic activity in animal models Established mGluR theory of fragile X syndrome |
 |
MTEP (30) | 5 NAM | Anxiolytic activity in animal models |
 |
Fenobam (34) |
5 NAM | Anxiolytic activity in animal models Efficacious in clinical trials in humans for anxiety and GERD |
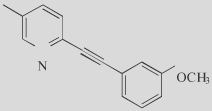 |
M-5MPEP (41) |
5 Partial antagonist |
One of the first partial mGluR5 antagonists |
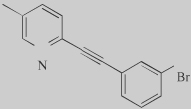 |
Br-5MPEPy (41) |
5 Partial antagonist |
One of the first partial mGluR5 antagonists |
| Schizophrenia, cognition, extinction | |||
 |
DFB (48) | 5 PAM | Acts at MPEP site |
 |
CDPPB (51) | 5 PAM | Acts at MPEP site Antipsychotic-like activity in animal models |
 |
ADX47273 (56) | 5 PAM | Acts at MPEP site Antipsychotic-like and cognition-enhancing activity in animal models |
| Anxiety disorders, schizophrenia | |||
 |
LY354740 (58) | 2/3 agonist |
Anxiolytic and antipsychotic activity in animal models |
 |
LY341495 | 2/3 antagonist |
Anxiolytic and an tipsychotic activity in animal models |
 |
LY487379 (66) | 2 PAM | Anxiolytic and antipsychotic activity in animal models |
 |
BINA (70) | 2 PAM | Anxiolytic and an tipsychotic activity in animal models |
| Parkinson’s disease, movement disorders | |||
 |
(−)-PHCCC (86, 88) | 4 PAM | Robust activity in rodent models of PD |
 |
VU0155041 (87) | 4 PAM/ Allosteric agonist |
Robust activity in rodent models of PD |
GERD, gastroesophageal reflux disease; mGluR, metabotropic glutamate receptor; NAM, negative allosteric modulator; PAM, positive allosteric modulator; PD, Parkinson’s disease.
The discovery of MPeP and related compounds allowed extensive investigation of the potential utility of mGluR5 NAMs for the treatment of anxiety disorders. Multiple studies have established robust efficacy of mGluR5 NAMs in several animal models of anxiolytic activity33. Clinical validation for efficacy of mGluR5 NAMs came from the finding that a novel anxiolytic, fenobam (Neuropharm Group Plc), is a selective and potent allosteric antagonist of mGluR5 (ref. 34). Fenobam is active in preclinical models of anxiety35 and has demonstrated clinical efficacy36, providing strong support for the potential utility of mGluR5 allosteric antagonists as anxiolytic agents. In addition a number of studies suggest that mGluR5 NAMs have potential utility in other CNs disorders, including fragile X syndrome, chronic pain, addictive disorders, depression, gastroesophogeal reflux, migraine and neurodegenerative disorders37,38. It is hoped that ongoing clinical development programmes will lead to clearer tests of efficacy for these indications.
A potential concern with mGluR5 NAMs is that they might induce target-related adverse effects that limit their use, including possible impairments of cognition or psychotomimetic effects39,40. Interestingly, Rodriguez et al.41 recently reported the discovery of mGluR5 allos-teric partial antagonists. Partial allosteric antagonists fully occupy the MPeP binding site on the mGluR5 receptor but, due to limited negative cooperativity, only partially block agonist responses resulting in partial mGluR5 inhibition. M-5MPeP and Br-5MPePy represent the first partial allosteric antagonists of mGluR5 (Table 2), induci ng a maximal mGluR5 inhibition of approximately 50% at concentrations that fully occupy the receptor. This might represent a novel approach to reducing mGluR5-mediated responses while maintaining some level of activity of the receptor, and could be advantageous in terms of the adverse-effect profile.
Positive allosteric modulators of mGluR5 for the treatment of schizophrenia and disorders of cognitive function
A large number of cellular and behavioural studies suggest that activators of mGluR5 could provide novel therapeutic agents for the treatment of both psychosis and cognitive disruption in patients suffering from schizophrenia39,40,42–47. This has led to efforts aimed at the discovery of the first mGluR5-selective PAMs, which include three series, represented by DFB, CPPHA and CDPPB48–52 (Fig. 2; Table 2). These mGluR5 PAMs can induce up to 10-fold leftward shifts in the glutamate concentration response curves and have potencies in the low nM to µM range. Interestingly, these compounds increase glutamate potency without increasing glutamate affinity at the orthosteric site and act by interacting with at least two allosteric sites. DFB and CDPPB bind to the same site in the 7TM domain of the receptor as the allosteric antagonist MPeP, and extensive studies revealed that PAM activity of members of the CDPPB series, such as vu29, are mediated by activity at this site53. By contrast, CPPHA does not interact with the MPeP site but acts at another site in the 7TM domain54.
Medicinal chemistry efforts with these mGluR5 PAMs illustrate the difficulties that are often encountered when designing ligands for allosteric sites. within the DFB series, slight structural changes afforded a large spectrum of pharmacological responses (from positive to negative allosteric modulation and neutral cooperativity), which presented insurmountable hurdles for rational ligand design48. CPPHA possessed a ‘flat’ structure–activity relationship (sAR) — out of 995 analogues, only 45 had any activity52. efforts around CDPPB were more fruitful, but did not provide major advances relative to CDPPB itself55. Despite these challenges, these compounds enabled validation of the hypothesis that mGluR5 PAMs potentiate mGluR5-mediated electrophysiological responses in brain circuits41,49,53 and have robust anti-psychotic-like effects in animal models50,51. More recently, Addex pharmaceuticals reported a distinct mGluR5 PAM chemotype, represented by ADX47273, which also afforded in vivo efficacy in preclinical behavioural models in which known antipsychotics provide similar positive results56. Moreover, these mGluR5 PAMs improved cognitive function in animals in which object recognition was impaired56, and also reversed deficits in set-shift task performance induced by NMDA receptor antagonists, demonstrating behavioural flexibility57. These exciting findings provide direct support for the hypothesis that mGluR5 PAMs have potential utility as novel antipsychotic and cognition-enhancing agents.
mGluR2 PAMs for the treatment of schizophrenia and anxiety disorders
A large number of preclinical and clinical studies have provided evidence that agonists of group II mGluRs represent a novel approach to treating anxiety disorders and schizophrenia. selective group II mGluR agonists, such as ly354740 and related compounds, have demonstrated robust efficacy in a broad range of animal models used to predict efficacy in the treatment of anxiolytic disorders58,59 and schizophrenia60,61. This has led to clinical studies that revealed efficacy of group II mGluR agonists in the treatment of both types of disease58,59,62,63. In each of these trials, no major adverse events were reported and there was no evidence of liabilities associated with current anxiolytic and antipsychotic medications.
Despite the tremendous advances in the development of group II mGluR agonists, it is not yet clear whether orthosteric agonists of these receptors will reach the market for broad clinical use. To date, mGluR2/3 agonists include only one major chemical scaffold and it is unlikely that significant deviation from these structures will be possible. In addition, these orthosteric agonists activate both mGluR2 and mGluR3, and preclinical studies with mGluR2 and mGluR3 knockout mice suggest that mGluR2 is likely to be responsible for clinical efficacy64. Finally, chronic administration of group II mGluR agonists induces robust tolerance in at least one rodent model that has been used to predict antipsychotic efficacy65. However, it is possible that positive allosteric modulators of mGluR2 and/or mGluR3 could provide an alternative approach that might offer greater selectivity, along with other potential advantages, over orthosteric agonists.
Multiple novel compounds have now been identified that act as allosteric potentiators of mGluR2. Most of these molecules are structurally related to two prototypical mGluR2 PAMs, termed ly487379 (refS 65,66) and BINA67–71. These compounds are highly selective for mGluR2 relative to mGluR3 or any other mGluR subtype. In most systems, these compounds have no agonist activity on mGluR2 but induce a leftward shift of the concentration response curve to glutamate. Mutation analysis has identified three amino acids in the 7TM domain that are critical for the actions of mGluR2 PAMs68,72.
The mGluR2 PAMs have robust effects in potentiating the ability of group II mGluR agonists to reduce transmission at several glutamatergic synapses66,68,71,73,74. Interestingly, psychomimetic agents increase transmission at glutamatergic synapses in the prefrontal cortex and this has been postulated to play an important role in the pathophysiology of schizophrenia75,76. These effects are blocked by group II mGluR agonists75–77 and by the mGluR2 PAMs74. Furthermore, the efficacy of mGluR2 PAMs in multiple animal models that are predictive of antipsychotic activity is close to that observed with the mGluR2/3 orthosteric agonists65,69,74,78,79. mGluR2 PAMs also have similar effects to mGluR2/3 agonists in animal models that predict anxiolytic activity65,66, raising the exciting possibility that selective mGluR2 PAMs might provide a novel treatment for schizophrenia and anxiety disorders that may be devoid of the adverse effects that are associated with currently available drugs.
mGluR4 PAMs as a novel approach for the treatment of Parkinson’s disease
Another mGluR subtype, mGluR4, has emerged as a new target for the treatment of Parkinson’s disease (PD)80. PD is a common neurodegenerative disorder that is characterized by disabling motor impairments such as tremor, rigidity and bradykinesia. The primary pathological change that gives rise to the symptoms of PD is the loss of dopaminergic neurons in the substantia nigra, which project to the striatum to regulate activity through the basal ganglia. The most effective pharmacological agents for the treatment of PD include l-DoPA, the immediate precursor of dopamine, and other drugs that replace dopaminergic modulation of basal ganglia function. unfortunately, dopamine replacement therapy fails in most patients due to loss of efficacy and the occurrence of severe adverse effects as the disease progresses81.
Dopamine acts in the striatum to reduce activity of striatal neurons projecting to neurons in the external globus pallidus82. loss of dopaminergic neurons in patients with PD leads to a pathological increase in activity at these synapses and dopamine replacement therapies are aimed at restoring striatal dopamine function. Interestingly, activation of mGluR4 reduces transmission at the striato–pallidal synapse80,83,84 and orthosteric agonists of mGluR4 have robust efficacy in several rodent models of PD84,85. Thus, selective activators of mGluR4 could provide an exciting new approach to the treatment of PD and may lack the adverse effects and limitations in efficacy that are associated with l-DoPA.
Recently, (−)-PHCCC was identified as a selective mGluR4 PAM86–88 (Table 2). PHCCC has no mGluR4 agonist activity but increases the potency of glutamate at mGluR4. Consistent with its effects in recombinant systems, PHCCC potentiates mGluR4-mediated effects at several synapses86,89,90 including the striato–pallidal synapse, but has no effect at synapses in which mGluR4 does not regulate synaptic transmission86,91. Furthermore, PHCCC has a robust anti-parkinsonian action in rodent models86 and reduces loss of dopamine neurons in a rodent model of dopaminergic cell death92. These data provide strong validation of mGluR4 as a target for the development of novel therapeutic agents with the potential for the treatment of PD.
In addition to PHCCC, two mGluR5 NAMs, sIB-1893 and MPeP, possess mGluR4 PAM activity93, although these compounds have low potency and efficacy at mGluR4 (Table 2). More interestingly, Niswender et al.87 identified multiple novel mGluR4 PAMs in a high throughput screening campaign. one of these compounds, termed vu0155041 is structurally distinct from PHCCC and provides significant improvements in terms of aqueous solubility, potency and selectivity for mGluR4 (Table 2). In contrast to PHCCC, however, vu0155041 has allosteric agonist activity at mGluR4. vu0155041 also has robust activity in rodent models of PD, providing further support for the hypothesis that selective mGluR4 PAMs may represent novel anti-parkinsonian agents.
Allosteric modulators of muscarinic acetylcholine receptors for the treatment of Alzheimer’s disease and schizophrenia
Muscarinic acetylcholine receptors (mAChRs) are a family of five receptors (classed M1–M5) that are involved in a wide variety of biological processes and disease states, including pain, Alzheimer’s disease, schizophrenia, diabetes and obesity94. Numerous non-selective mAChR agonists have advanced into clinical development and show efficacy in improving cognition and ameliorating psychotic symptoms in patients suffering from Alzheimer’s disease or schizophrenia61,95–97. Despite the tremendous promise of ligands that selectively target specific mAChR subtypes, the discovery of highly selective ligands has been hampered by the high sequence homology shared between their orthosteric binding domains. The therapeutic activity of mAChR agonists for the treatment of Alzheimer’s disease and schizophrenia is thought to be mediated by activation of the M1 or M4 subtypes. However, activation of peripheral M2 and M3 leads to adverse effects that have resulted in the discontinuation of development of these compounds98.
A range of allosteric ligands have been reported for M1 mAChRs. AC-42 and 77-lH-28-1 (ref. 99) are allosteric M1 agonists that activate the receptor in the absence of an orthosteric agonist and are highly selective for M1 versus M2–M5 mAChRs100 (Table 3). N-desmethlyclozapine is another M1 agonist that potentiates NMDA receptor activity and exhibits a mode of interaction with the M1 receptor that differs from that of classic orthosteric ligands101. A third compound, brucine, is an M1 PAM that selectively increases the affinity of M1 for ACh and thereby acts as an allosteric potentiator102. These early compounds lack the M1 potency and the clean ancillary pharmacology that is required to probe the effects of selective M1 mAChR activation in vitro or in vivo, but provide strong evidence that targeting allosteric sites can confer M1 subtype selectivity.
Table 3.
Potential indications for allosteric modulators of muscarinic receptors
| compound structure | compound name (reference) |
mAchr subtype | study data |
|---|---|---|---|
| Schizophrenia, cognition, Alzheimer’s disease (palliative and disease-modifying), movement disorders | |||
 |
AC-42 (100) | 1 Ectopic agonist | First ectopic or allosteric agonist for M1 |
 |
N-desmethylclozapine (101) |
1 Allosteric agonist | Allosteric agonist Ancillary pharmacology prohibits use as a tool |
 |
Brucine (102) | 1 PAM | Increases the affinity of M1 for ACh, but also a weak M1 PAM |
 |
TBPB (103) | 1 allosteric agonist | Antipsychotic activity in animal models Shift APP processing Lowers Aβ |
 |
77-LH-28-1 (99) | 1 Ectopic agonist | Initiates gamma frequency oscillations |
 |
Thiochrome (13) | 4 PAM | First M4 PAM Weak, low affinity |
 |
LY2033928 (104) | 4 PAM | Antipsychotic-like and activity in animal models (CAR and PPI) |
 |
VU0152099 (106) | 4 PAM | Centrally active Antipsychotic-like and activity in animal models Amphetamine-induced hyperlocomotion |
 |
VU0152100 (106) | 4 PAM | Centrally active Antipsychotic-like and activity in animal models Amphetamine-induced hyperlocomotion |
Aβ, beta-amyloid ; Ach, acetylcholine; APP, amyloid precursor protein; CAR, conditioned avoidance response; mAchR, muscarinic acetylcholine receptor; PAM, positive allosteric modulator; PPI, prepulse inhibition.
Recently, jones et al.103 reported the characterization of a novel highly selective agonist for the M1 mAChR without agonist activity on any of the other mAChR subtypes, termed TBPB. TBPB activates M1 mAChRs through an allosteric site rather than the orthosteric site, which is likely to be crucial for this unprecedented selectivity. TBPB potentiates M1-induced regulation of NMDA receptor currents in hippocampal pyramidal cells, but does not alter responses that are thought to be mediated by M2 and M4 mAChRs. Furthermore, TBPB was efficacious in multiple rodent models that are predictive of antipsychotic-like activity at doses that did not produce catalepsy or peripheral adverse effects, which are associated with other mAChR agonists. Finally, TBPB had effects on processing of the amyloid precursor protein towards the non-amyloidogenic pathway and decreased Aβ production in vitro. Taken together, these data suggest that selective activation of M1 may provide a novel approach for the treatment of symptoms associated with schizophrenia and Alzheimer’s disease.
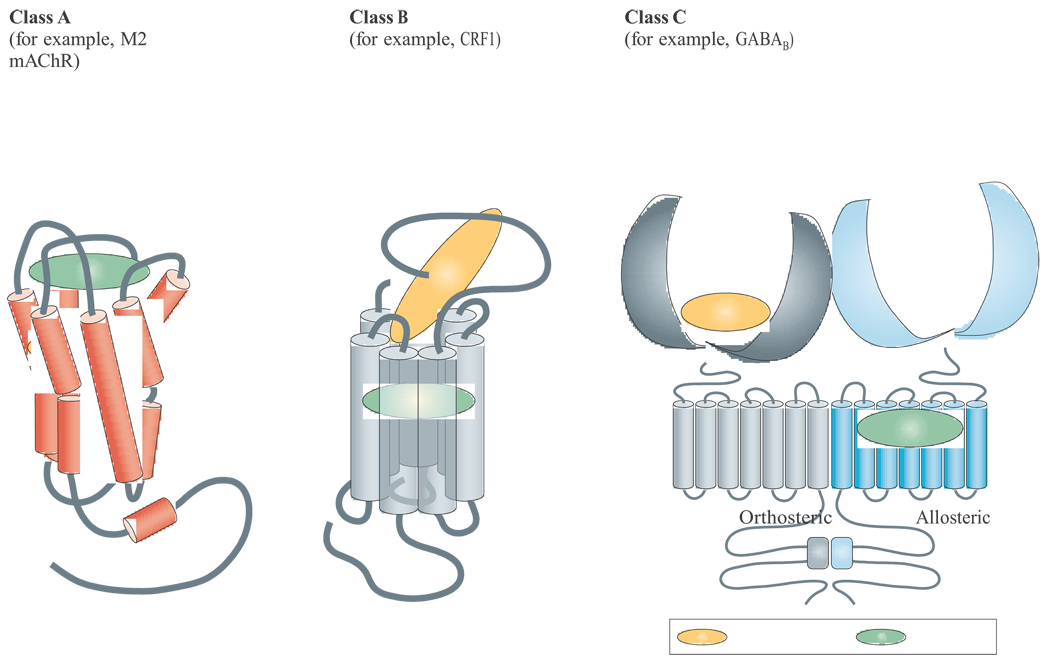
Box 3 Structural biology of GPCR orthosteric and allosteric sites.
Despite having a common structural architecture, G-protein-coupled receptors (GPCRs) display multiple orthosteric binding modes due to the substantial diversity of endogenous ligands that they recognize110. For example, many small-molecule class A GPCR orthosteric agonists use a cavity that is formed by the transmembrane (TM) helical bundle, as illustrated schematically for the M2 muscarinic acetylcholine receptor (mAchR), whereas most class A and B peptide agonist-recognizing GPCRs, including the corticotrophin releasing factor 1 (CRF1) receptor, use regions in the amino terminus and/or extracellular loops as the main points of orthosteric contact. Class C GPCRs, such as the metabotropic glutamate receptors (mGluRs), the calcium sensing receptor and the γ-aminobutyric acid (GABA)B receptor, are dimers that use the amino terminus exclusively to bind orthosteric ligands. Because of this diversity, the orthosteric domain on one type of GPCR might represent a potential allosteric modulator domain on another. Mutagenesis experiments have provided evidence to support this idea. For instance, extensive studies on the muscarinic receptors have found at least one allosteric site that uses epitopes in the extracellular loops and the top of the TM domains7,111–120. By contrast, studies on peptide GPCRs, such as those for chemokines or CRF1, have localized allosteric sites to TM regions away from the extracellular domains that are used by the orthosteric peptides121–127. Finally, studies on the class C GPCRs have revealed the most striking separation between orthosteric and allosteric sites, with the entire TM region of these GPCRs presenting potentially more than one allosteric pocket for small-molecule modulators68,128–135. Moreover, it is possible for an allosteric ligand to bind in one monomer of a dimer to modulate the binding and/or function of the orthosteric ligand in the other monomer136. The propensity of many GPCRs to form dimers or higher order oligomers also allows orthosteric sites to allosterically modulate each other via cooperative binding7 and to induce a striking functional rescue of ACh efficacy at a mutant M4 receptor with impaired orthosteric-site signalling107. Collectively, the studies with M1 and M4 allosteric modulators demonstrate the potential of targeting allosteric sites for developing highly selective activators of individual mAChR subtypes and suggest that both M1 and M4 may provide viable targets for the development of novel antipsychotic agents.
Major progress has also been made in the discovery of selective allosteric modulators of M4 mAChRs. The thiamine metabolite, thiochrome (Table 3), was one of the first truly selective M4 PAMs to be reported13, but it had a very low affinity for the receptor. More recently, two structurally related molecules, ly2033298 (ref.104) and vu10010 (ref. 105) were reported as highly selective M4 PAMs (Table 3). These compounds do not bind to the orthosteric ACh binding site and display variable (ly2033298) or no (vu10010) agonism in the absence of ACh. However, they both induce robust (approximately 50-fold) shifts in the ACh concentration response curve by increasing both the affinity and efficacy of ACh at the M4 mAChR. vu10010 has activity in hippocampal neurons, where it selectively increases M4-mediated depression of transmission at excitatory synapses. Analogues of vu10010 were recently optimized to develop two centrally active compounds, vu0152099 and vu0152100 (ref. 106), both of which achieve significant levels in the brain after systemic administration and reverse amphetamine-induced hyperlocomotion in rats. ly2033298 has also been shown to possess in vivo efficacy in rodent paradigms that are predictive of antipsychotic drug efficacy, such as reductions in conditioned avoidance response and apomorphine-induced suppression of prepulse-inhibition104, and to induce a striking functional rescue of ACh efficacy at a mutant M4 receptor with impaired orthostericsite signalling107. Collectively, the studies with M1 and M4 allosteric modulators demonstrate the potential of targeting allosteric sites for developing highly selective activators of individual mAChR subtypes and suggest that both M1 and M4 may provide viable targets for the development of novel antipsychotic agents.
Summary and conclusions
Over the past decade major advances have been made in establishing allosteric modulators of GPCRs as a novel approach to regulating this important class of drug targets. These include PAMs, NAMs, neutral allosteric modulators and allosteric agonists, and highlight the tremendous potential for fine tuning cellular signalling processes in either a positive (for example, allosteric potentiator) or negative (for example, partial allosteric antagonist) direction. Potent and selective GPCR modulators have now been developed for each of the three major subfamilies of GPCRs. These have provided novel tools and drug leads for multiple receptors for which efforts aimed at discovery of orthosteric ligands had been unsuccessful. Multiple allosteric modulators of GPCRs have been developed for use in animal studies and have robust effects in animal models that predict efficacy in schizophrenia, anxiety disorders, Parkinson’s disease and other neurological and psychiatric disorders. This recent preclinical work, coupled with the launch of cinacalcet and maraviroc as the first marketed GPCR allosteric modulators, provide strong validation of the potential clinical utility of both GPCR PAMs and NAMs. The discovery and development of GPCR subtype-selective allosteric modulators is a relatively recent endeavour. The studies reported to date provide proof of concept that could fuel the discovery of highly selective ligands for other GPCRs that have been previously intractable, such as D1 dopamine receptors, other mAChR subtypes, receptors for various sphingolipids and other lipid mediators, and a range of peptide receptors. The major advances in discovery and characterization of novel GPCR modulators are providing fundamental new insights into the mechanisms of action, range of activities and keys to chemical optimization of these compounds as therapeutic agents. These advances are providing crucial information that will guide drug discovery efforts aimed at developing the next generation of therapeutic agents that target GPCR allosteric sites for the treatment of a range of CNs disorders as well as multiple peripheral indications.
Glossary
- Orthosteric site
The binding site for the endogenous ligand on a receptor.
- Allosteric modulator
A ligand that binds to an allosteric site and modulates the binding and/or signalling of an orthosteric ligand.
- Allosteric site
A binding site on a receptor that is topographically distinct from the orthosteric site.
- Cooperativity
The binding of two or more molecules of the same ligand to a receptor complex to initiate a response. Also used in a less strict sense to describe the allosteric interaction between more than one molecule of any chemical type on a receptor complex.
- Stimulus-trafficking
The ability of a ligand to preferentially stabilize specific GPCr conformations, each associated with its own repertoire of stimuli and signalling behaviours, to the exclusion of other possible receptor states.
- Allosteric agonist
A ligand that binds to an allosteric site and causes receptor activation (allosteric agonist) or reduces constitutive receptor activity (allosteric inverse agonist) in the absence of an orthosteric ligand.
Footnotes
Competing interests statement
The authors declare competing financial interests: see web version for details.
DATABASES
UniProtKB: http://ca.expasy.org/sprot
CASR | CCR5 | CRTH2 | M1 MACHR | M4 MACHR | MGLUR1 | MGLUR2 | MGLUR3 | MGLUR4 | MGLUR5
FURTHER INFORMATION
Jeffrey Conn’s homepage: http://www.connlab.com
References
- 1.George SR, O’Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nature Rev. Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel AM. Mutations in G proteins and G protein-coupled receptors in endocrine disease. J. Clin. Endocrinol. Metab. 1996;81:2434–2442. doi: 10.1210/jcem.81.7.8675557. [DOI] [PubMed] [Google Scholar]
- 3.Rana BK, Shiina T, Insel PA. Genetic variations and polymorphisms of G protein-coupled receptors: functional and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2001;41:593–624. doi: 10.1146/annurev.pharmtox.41.1.593. [DOI] [PubMed] [Google Scholar]
- 4.Howard AD, et al. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol. Sci. 2001;22:132–140. doi: 10.1016/s0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- 5.Mohler HF, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Langmead CJ, Christopoulos A. Allosteric agonists of 7TM receptors: expanding the pharmacological toolbox. Trends Pharmacol. Sci. 2006;27:475–481. doi: 10.1016/j.tips.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 7.May LT, Leach K, Sexton PM, Christopoulos A.Allosteric modulation of G protein-coupled receptors Annu. Rev. Pharmacol. Toxicol 2007471–51. Comprehensive overview of principles, mechanisms and examples of GPCR allosterism [DOI] [PubMed] [Google Scholar]
- 8.Lazareno S, Birdsall NJ. Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholineat muscarinic receptors. Mol. Pharmacol. 1995;48:362–378. [PubMed] [Google Scholar]
- 9.Ehlert FJ. Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol. Pharmacol. 1988;33:187–194. [PubMed] [Google Scholar]
- 10.Hall DA.Modeling the functional effects of allosteric modulators at pharmacological receptors: an extension of the two-state model of receptor activation Mol. Pharmacol 2000581412–1423. Together with reference 9, important theoretical papers describing the application and extension of the ternary complex model to allosteric interactions [DOI] [PubMed] [Google Scholar]
- 11.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 12.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nature Rev. Drug Discov. 2002;1:198–210. [Google Scholar]
- 13.Lazareno S, Dolezal V, Popham A, Birdsall NJ. Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol. Pharmacol. 2004;65:257–266. doi: 10.1124/mol.65.1.257. [DOI] [PubMed] [Google Scholar]
- 14.Valant C, et al. A novel mechanism of G protein-coupled receptor functional selectivity: Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/ allosteric ligand J. Biol. Chem 2008. Discovery and validation of a bitopic orthosteric/ allosteric mode of action of a functionally selective GPCR ligand [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nature Rev. Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- 16.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 17.Mathiesen JM, et al. Identification of indole derivatives exclusively interfering with a G protein-independent signaling pathway of the prostaglandin D2 receptor CRTH2 Mol. Pharmacol 200568393–402. One of the earliest and most striking examples of allosteric modulator-mediated stimulus trafficking [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J. Pharmacol. Exp. Ther. 2005;315:1212–1219. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
- 19.Leach K, Sexton PM, Christopoulos A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 2007;28:382–389. doi: 10.1016/j.tips.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 20.May LT, Christopoulos A. Allosteric modulators of G-protein-coupled receptors. Curr. Opin. Pharmacol. 2003;3:551–556. doi: 10.1016/s1471-4892(03)00107-3. [DOI] [PubMed] [Google Scholar]
- 21.Harrington PE, Fotsch C. Calcium sensing receptor activators: calcimimetics. Curr. Med. Chem. 2007;14:3027–3034. doi: 10.2174/092986707782794096. [DOI] [PubMed] [Google Scholar]
- 22.Dorr P, et al. Maraviroc (UK-427, 857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 24.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 25.Romano C, Sesma MA, Mcdonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 26.Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J. Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- 27.Annoura H, Fukunaga A, Uesugi M, Tatsouka T, Horikawa Y. A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino) cyclopropchromen-1acarboxylates. Bioorg Med. Chem. Lett. 1996;6:763–766. [Google Scholar]
- 28.Varney MA, et al. SIB-1757 and SIB-1893: selective, noncompetitive antagonists of metabotropic glutamate receptor type 5 J. Pharmacol. Exp. Ther 1999290170–181. Together with reference 27, seminal papers reporting the discovery of early, novel GPCR negative allosteric modulators (NAMS) for metabotropic glutamate receptors [PubMed] [Google Scholar]
- 29.Gasparini F, et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 30.Cosford ND, et al. 3-[(2-Methyl-1, 3-thiazol-4-yl) ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- 31.Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu M. Recent developments of the PET imaging agents for metabotropic glutamate receptor subtype 5. Curr. Top. Med. Chem. 2007;7:1800–1805. doi: 10.2174/156802607782507394. [DOI] [PubMed] [Google Scholar]
- 33.Swanson CJ, et al. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Rev. Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 34.Porter RH, et al. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther. 2005;315:711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- 35.Patel JB, Martin C, Malick JB. Differential antagonism of the anticonflict effects of typical and atypical anxiolytics. Eur. J. Pharmacol. 1982;86:295–298. doi: 10.1016/0014-2999(82)90331-4. [DOI] [PubMed] [Google Scholar]
- 36.Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T. Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J. Clin. Psychopharmacol. 1982;2:129–133. [PubMed] [Google Scholar]
- 37.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Slassi A, et al. Recent advances in non-competitive mGlu5 receptor antagonists and their potential therapeutic applications. Curr. Top. Med. Chem. 2005;5:897–911. doi: 10.2174/1568026054750236. [DOI] [PubMed] [Google Scholar]
- 39.Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J. Pharmacol. Exp. Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- 40.Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl.) 2004;175:310–318. doi: 10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez A, et al. A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators Mol. Pharmacol 2005681793–1802. This study reports the initial discovery of a GPCR NAM that has partial antagonist activity as well as being a novel, neutral allosteric-site ligand [DOI] [PubMed] [Google Scholar]
- 42.Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor. Curr. Drug Target CNS Neurol. Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- 43.Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl.) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- 44.Alagarsamy S, Sorensen SD, Conn PJ. Coordinate regulation of metabotropic glutamate receptors. Curr. Opin. Neurobiol. 2001;11:357–362. doi: 10.1016/s0959-4388(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 45.Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J. Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindsley CW, et al. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr. Top. Med. Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- 47.Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nature Neurosci. 1999a;2:234–240. doi: 10.1038/6338. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien JA, et al. A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5 Mol. Pharmacol 200364731–740. Initial discovery of mGluR5-positive allosteric modulators (PAMs) and demonstration that compounds in a single structural class can have PAM, NAM and neutral allosteric site activity [DOI] [PubMed] [Google Scholar]
- 49.O’Brien JA, et al. A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J. Pharmacol. Exp. Ther. 2004;309:568–577. doi: 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- 50.Lindsley CW, et al. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1, 3-diphe nyl-1H-pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J. Med. Chem. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- 51.Kinney GG, et al. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J. Pharmacol. Exp. Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Z, et al. Challenges in the development of mGluR5 positive allosteric modulators: the discovery of CPPHA. Bioorg Med. Chem. Lett. 2007;17:1386–1391. doi: 10.1016/j.bmcl.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, et al. Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol. Pharmacol. 2007;71:1389–1398. doi: 10.1124/mol.106.032425. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Goudet C, Pin JP, Conn PJ. N-{4-Chloro-2-[(1, 3-dioxo-1, 3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol. Pharmacol. 2008;73:909–918. doi: 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- 55.de Paulis T, et al. Substituent effects of N-(1, 3-diphenyl-1 H-pyrazol-5-yl) benzamides on positive allosteric modulation of the metabotropic glutamate-5 receptor in rat cortical astrocytes. J. Med. Chem. 2006;49:3332–3344. doi: 10.1021/jm051252j. [DOI] [PubMed] [Google Scholar]
- 56.Epping-Jordan MP, et al. In vivo characterization of mGluR5 positive allosteric modulators as novel treatments for schizophrenia and cognitive dysfunction. Neuropharmacol. 2005;49:243. [Google Scholar]
- 57.Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav. Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to trat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- 59.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Rev. Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 60.Schoepp DD, Marek GJ. Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr. Drug Target CNS Neurol. Disord. 2002;1:215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- 61.Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophre nia: moving beyond monoamine antagonists. Mol. Interv. 2008b;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- 62.Grillon C, Cordova J, Levine LR, Morgan CA., 3rd Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist ( LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology (Berl.) 2003;168:446–454. doi: 10.1007/s00213-003-1444-8. [DOI] [PubMed] [Google Scholar]
- 63.Patil ST, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 64.Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R, 4S, 5S, 6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4, 6-dicarboxylic acid ( LY404039) J. Pharmacol. Exp. Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 65.Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J. Pharmacol. Exp. Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- 66.Johnson MP, et al. Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2, 2, 2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J. Med. Chem. 2003;46:3189–3192. doi: 10.1021/jm034015u. [DOI] [PubMed] [Google Scholar]
- 67.Lorrain DS, et al. Group II mGlu receptor activation suppresses norepinephrine release in the ventral hippocampus and locomotor responses to acute ketamine challenge. Neuropsychopharmacology. 2003;28:1622–1632. doi: 10.1038/sj.npp.1300238. [DOI] [PubMed] [Google Scholar]
- 68.Schaffhauser H, et al. Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol. Pharmacol. 2003;64:798–810. doi: 10.1124/mol.64.4.798. [DOI] [PubMed] [Google Scholar]
- 69.Pinkerton AB, et al. Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 3: Identification and biological activity of indanone containing mGlu2 receptor potentiators. Bioorg Med. Chem. Lett. 2005;15:1565–1571. doi: 10.1016/j.bmcl.2005.01.077. [DOI] [PubMed] [Google Scholar]
- 70.Cube RV, et al. 3-(2-Ethoxy-4-{4-[3-hydroxy-2-methyl-4-(3-methylbutanoyl)phenoxy] butoxy}phenyl)propanoic acid: a brain penetrant allosteric potentiator at the metabotropic glutamate receptor 2 (mGluR2) Bioorg Med. Chem. Lett. 2005;15:2389–2393. doi: 10.1016/j.bmcl.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 71.Galici R, et al. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J. Pharmacol. Exp. Ther. 2006;318:173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- 72.Rowe BA, et al. Transposition of three amino acids transforms the human metabotropic glutamate receptor (mGluR)-3 positive allosteric modulation site to mGluR2, and additional characterization of the mGluR2 positive allosteric modulation Site. J. Pharmacol. Exp. Ther. 2008 Apr 22; doi: 10.1124/jpet.108.138271. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 73.Poisik O, et al. Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus. Neuropharmacology. 2005;49:135–145. doi: 10.1016/j.neuropharm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Bennyworth M, et al. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- 75.Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003a;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 76.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 77.Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- 78.Johnson MP, et al. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology (Berl.) 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- 79.Govek SP, et al. Benzazoles as allosteric potentiators of metabotropic glutamate receptor 2 (mGluR2): efficacy in an animal model for schizophrenia. Bioorg Med. Chem. Lett. 2005;15:4068–4072. doi: 10.1016/j.bmcl.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 80.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nature Rev. Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- 81.Poewe WH, Lees AJ, Stern GM. Treatment of motor fluctuations in Parkinson’s disease with an oral sustained-release preparation of L-dopa: clinical and pharmacokinetic observations. Clin. Neuropharmacol. 1986;9:430–439. doi: 10.1097/00002826-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv. Neurol. 2003;91:9–18. [PubMed] [Google Scholar]
- 83.Bradley SR, et al. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia Nigra pars reticulata. J. Neurosci. 2000;20:3085–3094. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valenti O, et al. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J. Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez S, et al. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J. Neurosci. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marino MJ, et al. Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson’s disease treatment Proc. Natl Acad. Sci. USA 200310013668–13673. Discovery of a novel mGluR4 PAM and demonstration that this compound has anti-parkinsonian activity in animal models [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Niswender CM, et al. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol. Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maj M, et al. (−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology. 2003;45:895–906. doi: 10.1016/s0028-3908(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 89.Jones P, Xiang Z, Conn PJ. Metabotropic glutamate receptors mGluR4 and mGluR8 regulate transmission in the lateral olfactory tract-piriform. Neuropharmacol. doi: 10.1016/j.neuropharm.2008.06.043. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valenti O, Mannaioni G, Seabrook GR, Conn PJ, Marino MJ. Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J. Pharmacol. Exp. Ther. 2005;313:1296–1304. doi: 10.1124/jpet.104.080481. [DOI] [PubMed] [Google Scholar]
- 91.Ayala JE, Niswender CM, Luo Q, Banko JL, Conn PJ. Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated. Neuropharmacol. 2008;54:804–814. doi: 10.1016/j.neuropharm.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Battaglia G, et al. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. J. Neurosci. 2006;26:7222–7229. doi: 10.1523/JNEUROSCI.1595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br. J. Pharmacol. 2003;138:1026–1030. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nature Rev. Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 95.Clader JW, Wang Y. Muscarinic receptor agonists and antagonists in the treatment of Alzheimer’s disease. Curr. Pharm. Des. 2005;11:3353–3361. doi: 10.2174/138161205774370762. [DOI] [PubMed] [Google Scholar]
- 96.Bymaster FP, Felder C, Ahmed S, McKinzie D. Muscarinic receptors as a target for drugs treating schizophrenia. Curr. Drug Targets. CNS Neurol. Disord. 2002;1:163–181. doi: 10.2174/1568007024606249. [DOI] [PubMed] [Google Scholar]
- 97.Shekhar A, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 98.Greenlee W, et al. Muscarinic agonists and antagonists in the treatment of Alzheimer’s disease. Farmaco. 2001;56:247–250. doi: 10.1016/s0014-827x(01)01102-8. [DOI] [PubMed] [Google Scholar]
- 99.Langmead CJ, et al. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-21. Br. J. Pharmacol. 2008;154:1104–1115. doi: 10.1038/bjp.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spalding TA, et al. Discovery of an ectopic activation site on the M(1) muscarinic receptor. Mol. Pharmacol. 2002;61:1297–1302. doi: 10.1124/mol.61.6.1297. [DOI] [PubMed] [Google Scholar]
- 101.Sur C, et al. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity. Proc. Natl Acad. Sci. USA. 2003;100:13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lazareno S, et al. Allosteric effects of four stereoisomers of a fused indole ring system with 3H-N-methylscopolamine and acetylcholine at M1-M4 muscarinic receptors. Life Sci. 1999;64:519–526. doi: 10.1016/s0024-3205(98)00596-7. [DOI] [PubMed] [Google Scholar]
- 103.Jones CK, et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor reduces amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. doi: 10.1523/JNEUROSCI.1850-08.2008. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan WY, et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl Acad. Sci. USA. doi: 10.1073/pnas.0800567105. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shirey JK, et al. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission Nature Chem. Biol 2008442–50. Together with reference 104, important studies identifying allosteric modulation of the M4 muscarinic receptor as a novel approach for the treatment of CNS disorders [DOI] [PubMed] [Google Scholar]
- 106.Brady A, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotion behavior in rats. J. Pharmacol. Exp. Ther. doi: 10.1124/jpet.108.140350. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nawaratne V, et al. New insights into the function of M4 muscarinic acetylcholine receptors gained using a novel allosteric modulator and a DREADD (designer receptor exclusively activated by a designer drug) Mol. Pharmacol. 2008;74:1119–1131. doi: 10.1124/mol.108.049353. [DOI] [PubMed] [Google Scholar]
- 108.Black JW, Leff P. Operational models of pharmacological agonism. Proc. R. Soc. Lond. B Biol. Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 109.Kenakin T. Allosteric agonist modulators. J. Recept Signal Transduct. Res. 2007;27:247–259. doi: 10.1080/10799890701509000. [DOI] [PubMed] [Google Scholar]
- 110.Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation. J. Biol. Chem. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 111.Leppik IE. Antiepileptic drugs in development: prospects for the near future. Epilepsia. 1994;35 Suppl 4:29–40. doi: 10.1111/j.1528-1157.1994.tb05953.x. [DOI] [PubMed] [Google Scholar]
- 112.Matsui H, Lazareno S, Birdsall NJ. Probing of the location of the allosteric site on m1 muscarinic receptors by site-directed mutagenesis. Mol. Pharmacol. 1995;47:88–98. [PubMed] [Google Scholar]
- 113.Gnagey AL, Seidenberg M, Ellis J. Site-directed mutagenesis reveals two epitopes involved in the subtype selectivity of the allosteric interactions of gallamine at muscarinic acetylcholine receptors. Mol. Pharmacol. 1999;56:1245–1253. doi: 10.1124/mol.56.6.1245. [DOI] [PubMed] [Google Scholar]
- 114.Krejci A, Tucek S. Changes of cooperativity between N-methylscopolamine and allosteric modulators alcuronium and gallamine induced by mutations of external loops of muscarinic M(3) receptors. Mol. Pharmacol. 2001;60:761–767. [PubMed] [Google Scholar]
- 115.Buller S, Zlotos DP, Mohr K, Ellis J. Allosteric site on muscarinic acetylcholine receptors: a single amino acid in transmembrane region 7 is critical to the subtype selectivities of caracurine V derivatives and alkane-bisammonium ligands. Mol. Pharmacol. 2002;61:160–168. doi: 10.1124/mol.61.1.160. [DOI] [PubMed] [Google Scholar]
- 116.Voigtlander U, et al. Allosteric site on muscarinic acetylcholine receptors: identification of two amino acids in the muscarinic M2 receptor that account entirely for the M2/M5 subtype selectivities of some structurally diverse allosteric ligands in N-methylscopolamine-occupied receptors. Mol. Pharmacol. 2003;64:21–31. doi: 10.1124/mol.64.1.21. [DOI] [PubMed] [Google Scholar]
- 117.Huang XP, Prilla S, Mohr K, Ellis J. Critical amino acid residues of the common allosteric site on the M2 muscarinic acetylcholine receptor: more similarities than differences between the structurally divergent agents gallamine and bis(ammonio)alkane-type hexamethylene-bis-[dimethyl-(3-phthalimidopropyl)ammonium]dibromide. Mol. Pharmacol. 2005;68:769–778. doi: 10.1124/mol.105.014043. [DOI] [PubMed] [Google Scholar]
- 118.Prilla S, Schrobang J, Ellis J, Holtje HD, Mohr K. Allosteric interactions with muscarinic acetylcholine receptors: complex role of the conserved tryptophan M2422Trp in a critical cluster of amino acids for baseline affinity, subtype selectivity, and cooperativity. Mol. Pharmacol. 2006;70:2181–2193. doi: 10.1124/mol.106.023481. [DOI] [PubMed] [Google Scholar]
- 119.Avlani V, May LT, Sexton PM, Christopoulos A. Application of a kinetic model to the apparently complex behavior of negative and positive allosteric modulators of muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2004;308:1062–1072. doi: 10.1124/jpet.103.059840. [DOI] [PubMed] [Google Scholar]
- 120.Jakubik J, Krejci A, Dolezal V. Asparagine, valine, and threonine in the third extracellular loop of muscarinic receptor have essential roles in the positive cooperativity of strychnine-like allosteric modulators. J. Pharmacol. Exp. Ther. 2005;313:688–696. doi: 10.1124/jpet.104.080358. [DOI] [PubMed] [Google Scholar]
- 121.Liaw CW, Grigoriadis DE, Lorang MT, De Souza EB, Maki RA. Localization of agonist-and antagonist-binding domains of human corticotropin-releasing factor receptors. Mol. Endocrinol. 1997;11:2048–2053. doi: 10.1210/mend.11.13.0034. [DOI] [PubMed] [Google Scholar]
- 122.Dragic T, et al. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl Acad. Sci. USA. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gerlach LO, Skerlj RT, Bridger GJ, Schwartz TW. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J. Biol. Chem. 2001;276:14153–14160. doi: 10.1074/jbc.M010429200. [DOI] [PubMed] [Google Scholar]
- 124.Tsamis F, et al. Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 inhibit human immunodeficiency virus type 1 entry. J. Virol. 2003;77:5201–5208. doi: 10.1128/JVI.77.9.5201-5208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosenkilde MM, et al. Molecular mechanism of AMD3100 antagonism in the CXCR4 receptor: transfer of binding site to the CXCR3 receptor. J. Biol. Chem. 2004;279:3033–3041. doi: 10.1074/jbc.M309546200. [DOI] [PubMed] [Google Scholar]
- 126.Maeda K, et al. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virol. 2004;78:8654–8662. doi: 10.1128/JVI.78.16.8654-8662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maeda K, et al. Structural and molecular interactions of CCR5 inhibitors with CCR5. J. Biol. Chem. 2006;281:12688–12698. doi: 10.1074/jbc.M512688200. [DOI] [PubMed] [Google Scholar]
- 128.Hemstapat K, de Paulis T, Chen Y, Brady AE, Grover VK, Alagille D, Tamagnan GD, Conn PJ. A novel class of positive allosteric modulators of mGluR1 interact with a site distinct from that of negative allosteric modulators. Mol. Pharmacol. 2006;70:616–626. doi: 10.1124/mol.105.021857. [DOI] [PubMed] [Google Scholar]
- 129.Dupuis DS, Relkovic D, Lhuillier L, Mosbacher J, Kaupmann K. Point mutations in the transmembrane region of GABAB2 facilitate activation by the positive modulator N, N’-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4, 6-diamine (GS39783) in the absence of the GABAB1 subunit. Mol. Pharmacol. 2006;70:2027–2036. doi: 10.1124/mol.106.028183. [DOI] [PubMed] [Google Scholar]
- 130.Pagano A, et al. The non-competitive antagonists 2-methyl-6-(phenylethynyl)pyridine and 7-hydroxy-iminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester interact with overlapping binding pockets in the transmembrane region of group I metabotropic glutamate receptors. J. Biol. Chem. 2000;275:33750–33758. doi: 10.1074/jbc.M006230200. [DOI] [PubMed] [Google Scholar]
- 131.Knoflach F, et al. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc. Natl Acad. Sci. USA. 2001;98:13402–13407. doi: 10.1073/pnas.231358298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baez M, et al. Molecular mapping of a subtype selective site for positive allosteric modulation of the mGlu2 receptor. Neuropharmacol. Abstr. 2002;43:274–275. [Google Scholar]
- 133.Malherbe P, et al. Mutational analysis and molecular modeling of the allosteric binding site of a novel, selective, noncompetitive antagonist of the metabotropic glutamate 1 receptor. J. Biol. Chem. 2003;278:8340–8347. doi: 10.1074/jbc.M211759200. [DOI] [PubMed] [Google Scholar]
- 134.Petrel C, et al. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca(2+)-sensing receptor. J. Biol. Chem. 2003;278:49487–49494. doi: 10.1074/jbc.M308010200. [DOI] [PubMed] [Google Scholar]
- 135.Miedlich SU, Gama L, Seuwen K, Wolf RM, Breitwieser GE. Homology modeling of the transmembrane domain of the human calcium sensing receptor and localization of an allosteric binding site. J. Biol. Chem. 2004;279:7254–7263. doi: 10.1074/jbc.M307191200. [DOI] [PubMed] [Google Scholar]
- 136.Binet V, et al. The heptahelical domain of GABA(B2) is activated directly by CGP7930, a positive allosteric modulator of the GABA(B) receptor. J. Biol. Chem. 2004;279:29085–29091. doi: 10.1074/jbc.M400930200. [DOI] [PMC free article] [PubMed] [Google Scholar]