Abstract
Muscarinic acetylcholine receptors (mAChRs) have long been viewed as viable targets for novel therapeutic agents for the treatment of Alzheimer’s disease (AD) and other disorders involving impaired cognitive function. More recent evidence indicates that mAChR activators might also have utility in treating psychosis and other symptoms associated with schizophrenia and other central nervous system (CNS) disorders. Efforts to develop mAChR subtype-selective agonists have been hampered by difficulty in achieving high selectivity for individual mAChR subtypes important for CNS function (M1 and M4) and adverse effects due to activation of peripheral mAChRs (especially M2 and M3). Major advances have now been achieved in the discovery of allosteric agonists and positive allosteric modulators of M1 and M4 that show greater selectivity for individual mAChR subtypes than do previous mAChR agonists. Early studies indicate that these allosteric mAChR activators have properties needed for optimization as potential clinical candidates and have robust effects in animal models that predict efficacy in the treatment of AD, schizophrenia and related disorders.
Introduction
Acetylcholine (ACh)-containing neurons originating in the medial septum and the diagonal band of Broca provide one of the most crucial neuromodulatory inputs to the forebrain in humans and other mammals [1]. These cholinergic projections make synaptic contact with widespread but highly specific targets in a variety of forebrain regions including the basal ganglia, cortex and hippocampus. In addition, there are several other crucial cholinergic systems in the central nervous system (CNS) including cholinergic interneurons in the striatum, nucleus accumbens, olfactory tubercle and Islands of Calleja, in addition to cholinergic projection neurons in the substantia innominata, magnocellular preoptic field and nucleus basalis [1–4]. These nuclei send projections throughout the neocortex and limbic areas and have a crucial role in information processing and multiple forms of learning and memory [3,5,6]. In addition, cholinergic projections have been implicated in a variety of other CNS functions including nociception, regulation of sleep–wake cycles, motor control and arousal. Based on this broad influence of cholinergic pathways in the CNS, agents that regulate cholinergic transmission have been proposed to have potential efficacy in a wide variety of CNS disorders including chronic and neuropathic pain, sleep disorders, epilepsy, schizophrenia, Alzheimer’s disease (AD), Parkinson’s disease and other movement disorders [5–9].
Therapeutic potential of M1 for the treatment of AD Based on the broad influence of cholinergic systems in the CNS, it is surprising that there have not been greater advances in the development of therapeutic agents that target cholinergic signaling. Efforts to develop agents that enhance cholinergic transmission for ameliorating the loss of cognitive function in patients with AD and other memory disorders have been partially successful, and clinical trials with tacrine and other acetylcholinesterase(AChE) inhibitors have established dose-related improvements in measures of cognitive performance and quality of life [3,10]. However, efficacy of these drugs in enhancing cognition is limited, in part because of central and peripheral side effects that are due to generalized activation of multiple cholinergic systems and receptor subtypes.
Evidence indicates that cholinergic transmission in the forebrain regions and cholinergic involvement in learning and memory are mediated primarily by muscarinic ACh receptors (mAChRs) [1,6]. It is possible that selective agonists of mAChR subtypes that are most crucial for regulation of processes involved in cognitive function could provide an advantage to AChE inhibitors for the treatment of AD and related disorders. The mAChRs are members of the family A G-protein-coupled receptors (GPCRs) and include five subtypes termed M1–M5. M1, M3 and M5 couple to Gq and activate phospholipase (PL)C, whereas M2 and M4 couple to Gi/o and associated effector systems (Figure 1) such as ion channels and adenylyl cyclase [6,11]. Evidence indicates that the most prominent adverse effects of AChE inhibitors and other cholinergic agents are mediated by activation of peripheral M2 and M3 mAChRs and include bradycardia, gastrointestinal distress, excessive salivation and sweating [7,9]. By contrast, M1 has been viewed as the most likely subtype for mediating the effects on cognition, attention mechanisms and sensory processing. Because of this, considerable effort has been focused on developing selective M1 agonists for the treatment of AD [5,6]. Unfortunately, these efforts have been largely unsuccessful because of an inability to develop compounds that are highly selective for the M1 mAChR. Because of this, mAChR agonists that have been tested in clinical studies induce the same adverse effects of AChE inhibitors by activation of peripheral mAChRs. To fully understand the physiological roles of individual mAChR subtypes and to further explore the therapeutic utility of mAChR ligands in AD and other disorders, it will be crucial to develop molecules that are highly selective activators of M1 and other individual mAChR subtypes.
Figure 1.
Schematic illustration of structure and effector systems of the mAChR subtypes. M1, M3 and M5 typically couple to Gaq and associated effector systems such as PLC. M2 and M4 typically couple to Gai/o and associated effectorsystems such as inhibition of adenylyl cyclase (AC) and ion channels, although each receptor subtype can also couple to other signaling pathways. The orthosteric binding site for ACh has been well characterized and resides in the seven transmembrane domain of the receptor. Allosteric ligands bind to other sites on the receptor to either activate (allosteric agonists) or modulate (positive and negative allosteric-site ligands) receptor function. Abbreviations: IP3, inositol (1,4,5)-trisphosphate; ? denotes that exact allosteric binding sites have not been defined.
Discovery of subtype-selective allosteric modulators of mAChRs
Previous attempts to develop agonists that are highly selective for individual mAChR subtypes have failed because of the high conservation of the orthosteric ACh-binding site and difficulty developing compounds that are truly subtype specific. Although there have been reports of subtype-selective M1 agonists in the patent and primary literature, subsequent studies across multiple systems revealed that previous orthosteric agonists are not highly selective when evaluated across multiple systems. A reason for this is that selectivity of these agents is based on functional selectivity or selectivity based on efficacy rather than selective binding to individual mAChR subtypes. Because of this, a weak partial agonist at multiple mAChR subtypes might seem to be selective in cell lines in which there is little or no receptor reserve but might have robust agonist activity at these same mAChR subtypes in systems that have high receptor reserve. Because high receptor reserve is common for mAChRs in native tissues, these agents often activate multiple mAChR subtypes in animal models and humans [6,9,11]. A more recent example, AF267, was suggested to have selective agonist activity at M1 but has similar profiles to previous orthosteric agonists and activates multiple mAChR subtypes (i.e. M1, M3 and M5) [12]. These studies do not rule out the possibility that highly selective orthosteric agonists of individual mAChR subtypes will eventually be developed but do raise a need for also considering other strategies for selectively activating members of this receptor family.
An alternative approach to targeting the highly conserved orthosteric ACh site is to develop compounds that act at allosteric sites on mAChRs that are removed from the orthosteric site and might be less highly conserved. This approach is proving to be highly successful in developing selective ligands for multiple GPCR subtypes (for reviews, see Refs [13,14]). In the case of mAChRs, a major goal has been to develop allosteric ligands that selectively increase the activity of M1 or M4. Allosteric activators can include allosteric agonists, which act at a site removed from the orthosteric site to directly activate the receptor in the absence of Ach, in addition to positive allosteric modulators (PAMs), which do not activate the receptor directly but potentiate activation of the receptor by the endogenous orthosteric agonist ACh. Also, it is possible for a single molecule to have both allosteric potentiator and allosteric agonist activity [13,14].
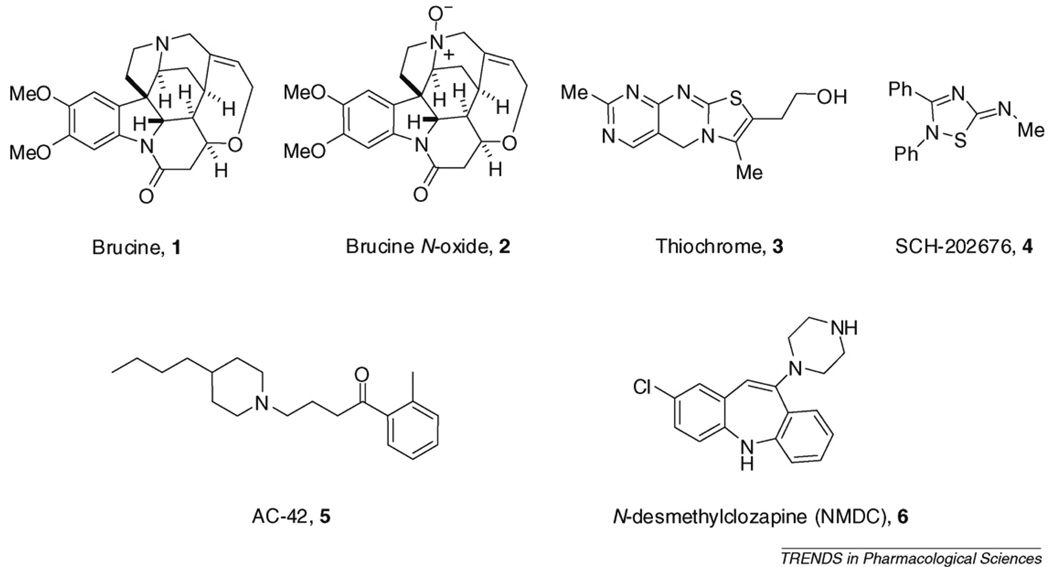
Early work by several laboratories [15–18] identified a diverse array of chemotypes that possess PAM activity at the M1 and M4 mAChR subtypes (Figure 2). The first mAChR PAM reported was brucine (structure 1; Figure 2) [15,18], which acts as an allosteric potentiator at M1 and induces a relatively small increase in the affinity of M1 for carbachol (CCh) or ACh. Brucine is a weak M1 PAM requiring high micromolar concentrations for activity and induces only a twofold increase in ACh affinity at the M1 receptor. However, brucine is relatively selective for M1 and this provided an important proof of concept that it will be possible to develop selective PAMs for this important mAChR subtype. Interestingly, the corresponding N-oxide of brucine (structure 2 in Figure 2) increased ACh affinity
Figure 2.
Early PAMs and allosteric agonists of the mAChRs.
1.5-fold at the M4 receptor. Equilibrium and nonequi- librium radioligand-binding studies with brucine, the mAChR antagonist radioligand [3H]-NMS and unlabeled ACh were consistent with a ternary allosteric model in which both the orthosteric and allosteric ligands bind to the receptor simultaneously and modify the affinities of each other.
Discovery of brucine was followed by the discovery of thiochrome (structure 3; Figure 2) as an M4 PAM [17] and SCH-202676 (structure 4; Figure 2) as a PAM of multiple GPCR subtypes including M1 [16,19]. Thiochrome was especially interesting in that it provided M4 subtype selectivity. This compound increases the affinity of ACh three-to fivefold at M4 and increased ACh potency in activating M4 in functionalassays but has no effect on ACh affinity at M1, M2 or M5 [17]. Interestingly, thiochrome has no effect on the equilibrium binding of [3H]-NMS to the five human mAChRs (M1–M5) but inhibits the [3H]-NMS dissociation from M1–M4 [17]. This indicates that the selectivity of thiochrome for M4 is due to selective cooperativity rather than selective interactions with this single mAChR sub- type.
In addition to allosteric potentiators, early efforts led to discovery of highly selective allosteric agonists of M1. For instance, AC-42 (structure 5; Figure 2) [20] was identified as the first M1 agonist that seems to act by binding to an allosteric site rather than the orthosteric ACh site. AC-42 fully activates M1 and is highly selective for M1 relative to other mAChR subtypes (Figure 2). As with allosteric poten- tiators, this selectivity is likely to be achieved by targeting a site distinct from the ACh-binding site. Thus, mutations that render M1 insensitive to ACh do not alter the activity of AC-42, but the activity of AC-42 can be eliminated by mutations in transmembrane domains one and seven that do not alter activation by ACh [20]. N-desmethlyclozapine (structure 6; Figure 2) is another M1 agonist that demonstrates a mode of interaction with the M1 receptor that differs from that of classic orthosteric ligands and might be mechanistically similar to AC-42 [21].
Unfortunately, brucine, AC-42 and other early mAChR allosteric agonists and PAMs lacked the pharmacological profiles and physicochemical properties required to be useful tools to probe the effect of allosteric activation of mAChRs in more complex native systems. For instance, these compounds have off-target activities at other receptors and have relatively low potencies at M1 or M4. This, combined with poor solubility in physiological buffer systems, prevented their use for studies in tissue or animal model systems. However, discovery of these early compounds provided an important advance in establishing the potential of developing more selective activators of M1 and other mAChR subtypes by targeting allosteric sites.
Discovery of highly selective and systemically active M1 PAMs
Over the past two years, several novel selective M1 agonists and allosteric potentiators have been identified. These compounds are providing important new tools to evaluate the potential utility of selective activators of M1 for treatment of AD and other CNS disorders. For instance, TBPB (structure 7; Figure 3) [12,22,23] and 77-LH-28-1 (structure 8; Figure 3) [24,25] have been reported as highly selective agonists of M1 relative to other mAChR subtypes (Figure 3). Both of these compounds are systemically active and are proving to be useful or in vivo studies of M1 activation. TBPB selectively activates M1 in cell lines and has no agonist activity at any other mAChR subtypes. Mutations that reduce the activity of orthosteric agonists have no effect on the response to TBPB, and Schild analysis of the blockade of TBPB effects with the orthosteric antagonist atropine reveals that TBPB does not interact with the orthosteric site in a competitive manner. These data are consistent with the predictions of an allosteric ternary complex model for the actions of two molecules that interact with distinct sites [13,14] and are consistent with the hypothesis that TBPB acts as an allosteric M1 agonist [12]. However, extensive molecular pharmacology and mutagenesis studies will be required to fully establish the mechanism of TBPB action and verify that this com- pound activates M1 by actions at an allosteric site that is completely distinct from the orthosteric site.
Figure 3.
M1 allosteric agonists.
Interestingly, TBPB induces M1-dependent potentiation of N-methyl-d-aspartic acid (NMDA)-receptor currents in hippocampal pyramidal cells, an action that is thought to be important for the cognition-enhancing effects of mAChR activation. By contrast, TBPB does not reduce inhibitory or excitatory synaptic transmission in these neurons; these are thought to be mediated by M2 and M4 mAChRs, respectively [12]. These findings verify the ability of TBPB to activate M1 and the selectivity of TBPB for M1 relative to other mAChR subtypes in a native system. Interestingly, early studies in cell lines indicate that TBPB induces an increase in processing of the amyloid precursor protein towards the non-amyloidogenic pathway and decreased amyloid b production in vitro [12]. This is consistent with previous extensive studies indicating that mAChR activation has favorable effects on amyloid pre- cursor protein processing in animal models and perhaps in humans [5,26] and provides further support to the hypothesis that selective activation of M1 might have disease- modifying effects in the treatment of AD in addition to its potential efficacy in acutely enhancing cognition in patients suffering from this disorder. TBPB is systemically active and crosses the blood–brain barrier, making this a useful tool for studies of cognition-enhancing effects of M1- selective agonists. However, TBPB did not have any of the peripheral adverse effects seen with other less selective mAChR agonists when evaluated in a modified Irwin battery in rodents and compared with oxotremorine; this indicates that the in vitro mAChR selectivity profile is mirrored in vivo [12]. These encouraging results provide strong support for the utility of M1 allosteric agonists in activating this crucial mAChR subtype in the CNS without inducing the adverse effects associated with less selective mAChR agonists.
A second systemically active M1 agonist, 77-LH-28-1 was discovered as a structural analog of AC-42 [24,25]. 77-LH-28-1 is highly selective for M1 relative to other mAChR subtypes but does have weak agonist activity at M3 [24]. Interestingly, in contrast to effects of atropine on the response to TBPB, the orthosteric antagonist scopola-mine induces parallel rightward shifts in the 77-LH-28-1 concentration–response relationship [24]. Consistent with this, recent radioligand-binding studies reveal that 77-LH-28-1 binds to the orthosteric site in a competitive manner [27]. However, extensive mutagenesisstudies combined with functional studies and analysis of the effects of 77-LH-28-1 on binding of an orthosteric antagonist ligand are consistent with an allosteric mode of agonist action of this compound. These studies also raise the possibility that 77- LH-28-1 might be what has been referred to as a ‘bi-topic’ ligand that binds to a site that overlaps with the orthos- teric site but also includes an allosteric site that modulates orthosteric-site affinity [27]. Electrophysiological studies indicate that 77-LH-28-1 increases hippocampal activity as assessed by increases in single-unit firing of hippocampal CA1 pyramidal cells in vitro and in vivo and induction of synchronous network activity in the gamma and/or theta frequency bands in the hippocampus [24]. Interestingly, 77-LH-28-1 selectively activates the coupling of M1 to Gaq and Gas signaling without activating the coupling of M1 to Gai in Chinese hamster ovary cells [25]; this indicates that this compound might differentially activate different responses to M1 activation. Such differential effects of allosteric agonists on different responses to M1 activation could prove to be crucially important in determining the in vivo and ultimately therapeutic potential of allosteric M1 agonists and raise the need to fully evaluate these novel models in animal models and ultimately in clinical studies.
Findings that TBPB and 77-LH-28-1 might differentially alter activities of orthosteric site ligands indicate that these compounds might act at by different mechanisms to activate M1. Mutagenesis and binding studies also indicate that AC-42 and 77-LH-28-1 interact with the receptor in a similar manner [27]. This is interesting in light of the structural similarities between AC-42 and 77-LH-28-1 and the fact that these compounds are structurally distinct from TBPB. On the surface, these data might indicate that multiple allosteric sites are present on M1 (as was previously demonstrated for class C GPCRs such as metabotropic glutamate mGlu5 receptors [13,14,28]). However, it is also possible that different allosteric agonists interact with overlapping sites and that there is no strict allosteric ‘pharmacophore’ that is clearly related to selective interaction at this site. The latter point can be addressed within the TBPB series, in which subtle structural changes have been shown to erode mAChR subtype selectivity to provide non-selective mAChR agonists; this indicates that close structural similarity does not ensure selective allosteric-site interactions [29,30].
In addition to the discovery of novel M1-selective allosteric agonists, exciting progress has been made in the discovery of novel M1 PAMs that serve as allosteric potentiators of this receptor. For instance, multiple M1 allosteric potentiators (structures 9–12; Figure 4) have recently been identified in a high-throughput functional-screening campaign [31]. These molecules belong to several structurally distinct chemical scaffolds and include compounds that are selective for M1 relative to other mAChR subtypes. None of the compounds identified had agonist activity but each behaved as a pure PAM and induced parallel leftward shifts in the ACh concentration–response relationships. None of the novel M1 PAMs competes for binding at the orthosteric ACh-binding site. The two most selective compounds, VU0090157 (structure 11; Figure 4) and VU0029767 (structure 12; Figure 4), were studied in detail and induced progressive shifts in ACh affinity at M1 that are consistent with their effects in a functional assay; this indicates that they mechanistically enhance M1 activity by increasing ACh affinity. However, these compounds were strikingly different in their ability to potentiate responses at a mutant M1 receptor that exhibits decreased affinity for ACh and in their ability to affect responses of the allosteric M1 agonist TBPB. Furthermore, VU0090157 induced similar potentiation in M1 activation of PLC and PLD activity, whereas VU0029767 potentiated activation of PLC but not PLD signaling. This provides another example of an ability of novel M1 PAMs to differentially regulate coupling of the receptor to different signaling pathways. Based on this, it is possible that different M1 PAMs will have different actions on different responses to M1 activation in native systems.
Figure 4.
M1 positive allosteric modulators.
In a preliminary report, researchers reported an encouraging advance in the discovery of benzyl quinolone carboxylic acid (BQCA; structure 13; Figure 4) as a highly selective and efficacious allosteric potentiator for M1 [32,33]. As with VU0090157 and VU0029767, BQCA has no direct agonist activity but induces a robust leftward shift of the concentration–response relationship of ACh at activating M1. BQCA has no activity at M2, M3 or M4. Interestingly, BQCA is systemically active and fully reverses the cognitive impairment induced by scopolamine in a contextual fear-conditioning model of episodic-like memory [32]. In addition, BQCA induces changes in electroencephalography oscillations in a manner that is consistent with a potential cognition-enhancing effect [33]. These preliminary findings provide exciting new data in support of the hypothesis that it will be possible to develop highly selective M1 PAMs that have potential for cognition-enhancing effects in animal models.
It is important to note that, at present, the mechanisms underlying the selectivities of M1-selective allosteric agonists are not fully established. The initial rationale driving this approach is that allosteric ligands bind to evolutionarily less-conserved allosteric sites on the M1 receptor, as opposed to the highly conserved orthosteric (ACh) binding site and might, therefore, bind selectively to allosteric sites on individual mAChR subtypes. This hypothesis has been clearly established for metabotropic glutamate receptors where selective binding of allosteric ligands to individual subtypes has been clearly established as the basis for the selectivity of some ligands [13]. Also, the finding that M1 PAMs do not bind to the orthosteric site on M1 or any other mAChR subtypes and selectively increase ACh affinity at the orthosteric site of M1 but not M2–M5 is consistent with this hypothesis. However, this does not rule out the possibility that these compounds bind to allosteric sites on multiple mAChR subtypes with similar affinities but that selectivity is conferred by selective cooperativity with orthosteric-site agonists [13,14], as has been suggested for selective PAM activity of thiochrome on M4 [17]. If this is the case, it is possible that selectivity of these compounds will vary depending on the system in which their effects are measured. Thus, it will be crucial to develop a clear understanding of the molecular basis for the observed selectivity in future studies. Optimally, this should be addressed with radioligands that act at the allosteric but not the orthosteric sites. However, studies involving detailed analysis of effects on binding of orthosteric-site ligands in addition to mutagenesis studies can also shed light on this important issue.
Allosteric modulators of mAChRs have efficacy as novel antipsychotic agents of the orthosteric mAChR agonists that have been devel- oped in clinical trials for the treatment of AD, an M1/M4 agonist termed xanomeline advanced furthest in clinical development [34,35]. Although potential cognition-enhan- cing effects of this compound in Phase III trials were encouraging, the most surprising finding in the xanome- line trial was that this M1/M4 agonist had robust thera- peutic effects on psychotic symptoms and behavioral disturbances associated with AD [34,35]. Xanomeline induced robust dose-dependent reductions in vocal out- bursts, suspiciousness, delusions, hallucinations, mood swings and other behavioral disturbances. This is consist- ent with a growing body of clinical evidence that AChE inhibitors have efficacy in treating behavioral disturbances and psychotic symptoms in patients suffering from AD in addition to Lewy body dementia, Parkinson’s disease dementia, and vascular dementia [36–42]. More recently, xanomeline [43] was shown to induce a robust improvement in positive and negative symptoms and also provide improvements in some measures of cognitive function in schizophrenic patients. Interestingly, the response was superior to that seen with traditional antipsychotics, and therapeutic effects were seen in less than one week, as opposed to the multi-week delay for traditional anti-psychotics. Together, these studies provide strong clinical validation of mAChR agonists as novel therapeutic agents used for the treatment of psychosis and behavioral disturbances in patients suffering from a broad range of disorders including schizophrenia, AD and other neurodegenerative disorders.
Although the exact mAChR subtype responsible for the effects of xanomeline in schizophrenia patients agents is not certain, xanomeline is an M1/M4-preferring agonist and multiple animal studies indicate that one or both of these mAChR subtypes is likely to be responsible for the clinical efficacy of this compound [6,7,44–49]. Thus, it will be important to rigorously evaluate the potential roles of both of these receptor subtypes in animal models that predict efficacy in the different symptom clusters associated with schizophrenia (i.e. positive, negative and cognitive symptoms). Interestingly, the M1-selective allosteric agonist TBPB has activity in multiple animal models used to predict the efficacy in treatment of positive symptoms of schizophrenia [12]. These include the reversal of amphetamine-induced hyperlocomotor activity and disruption of prepulse inhibition, in addition to changes in c-fos expression that are almost identical to changes induced by atypical antipsychotic agents. These data indicate that selective activators of M1 might mimic some of the effects of xanomeline in animal models that are relevant to the clinical efficacy of the less selective mAChR agonist.
An important breakthrough for selective activation of M4 occurred with the discovery of VU0010010 (structure 14; Figure 5) as a highly selective M4 PAM with no activity at any other mAChR subtype [50]. VU0010010 is an allosteric potentiator and does not activate M4 directly but dramatically potentiates the response of the receptor to ACh. Extensive in vitro pharmacological characterization reveals that VU0010010 binds to an allosteric site to increase the affinity of M4 for ACh and increase the efficiency of coupling of M4 to G proteins, and that it does not bind to, or functionally modulate, any other mAChR subtype. VU0010010 induces a selective potentiation of M4-mediated depression of transmission at excitatory synapses in hippocampal CA1 pyramidal cells, but it has no effect on responses that are thought to be mediated by M1 or M2. More recently, two related compounds were reported, VU0152099 and VU0152100 (structures 15 and 16; Figure 5), that are also highly selective for M4, readily cross the blood–brain barrier, and have pharmacokinetic properties making them highly suitable for behavioral studies [51]. Interestingly, VU0152100 and VU0152099 reverse amphetamine-induced hyperlocomotion in rats [51], which indicates that M4 PAMs have efficacy in at least one model used to predict antipsychotic efficacy. Chan and colleagues [52] reported the discovery of a similar, but structurally distinct, M4 PAM termed LY2033298 (structure 17; Figure 5). This compound exhibits highly selective M4 potentiator activity similar to that of VU0010010. Interestingly, LY2033298 has also been shown to possess in vivo efficacy in reducing the conditioned avoidance response, which is another rodent paradigm predictive of antipsychotic drug efficacy. Collectively, the studies with M1 and M4 allosteric modulators clearly demonstrate the potential of targeting allosteric sites for developing highly selective activators of individual mAChR subtypes and indicate that both M1 and M4 might provide viable targets for the development of novel anti- psychotic agents. Because rat preclinical models are employed, an important point with respect to these M4 PAMs concerns species differences. Uniformly VU0152099, VU0152100 and LY2033298 are approxi- mately tenfold more potent on human M4 than rat M4 with fold-shifts of comparable or greater magnitude, and mAChR subtype-selectivity remains intact.
Figure 5.
M4 positive allosteric modulators.
A final point that is interesting to note is that, to date, there have been no reports of negative allosteric modulators of M1 or M4, which could potentially provide a novel therapeutic approach for the treatment of disorders such as Parkinson’s disease, dystonia or epileptic disorders. Moreover, unlike the metabotropic glutamate receptors [53,54], there have not yet been any reports of mAChR PAMs being converted into mAChR negative allosteric modulators (NAMs) or neutral modulators. However, based on successes in discovery of NAMs for other GPCR subtypes, it is likely that allosteric modulators might also provide a viable approach to the discovery of selective allosteric antagonists for individual mAChR subtypes.
Conclusions
Exciting new data from animal and clinical studies indicate that ligands at mAChR subtypes might provide a novel approach to the treatment of multiple CNS disorders. This includes compelling evidence that selective increases in the activity of M1 and M4 could provide a novel approach to the treatment of AD and schizophrenia and perhaps have efficacy in treating psychosis and cognitive impairment in other neurodegenerative diseases. Major advances have been made in establishing selective allosteric agonists and PAMs of both M1 and M4 as an alternative approach to orthosteric agonists for the development of selective receptor activators. These molecules have now been optimized for use in animal models, and early studies indicate that they have effects in animal models that predict efficacy in the treatment of these disorders. In addition to potential clinical utility of selective mAChR activators, abundant clinical and animal studies indicate that highly selective antagonists of specific mAChR subtypes might have utility in the treatment of other CNS disorders including dystonia, Parkinson’s disease, epilepsy and others [5–9]. In each case, subtype selectivity will be a key to achieving clinical efficacy in the absence of adverse effects. For other GPCRs, it has been possible to discover allosteric modulators that serve as either activators or inhibitors (i.e. NAMs) of receptor function [12]. Thus, it might be possible to discover selective allosteric antagonists of mAChR subtypes. Indeed, a recent high-throughput screening campaign that focused on M1 PAMs was also successful in identifying NAMs of mAChR subtypes [31]. These exciting advances are providing a fundamental advance in our approaches to regulating mAChRs as drug targets and might provide a novel approach to treatment of AD and schizophrenia in addition to other CNS disorders.
Footnotes
Conflict-of-interest statement
P.J.C. has received compensation over the past two years as a consultant from: Merck and Co., Johnson and Johnson, Hoffman La Roche, GlaxoSmithKline, Lundbeck Research USA, Epix Pharmaceuticals, Invitrogen Life Technologies, Evotec Inc., Addex Pharmaceuticals, Michael J. Fox Foundation, Seaside Therapeutics, Cephalon Inc., AstraZeneca USA, NeurOp Inc., Forest Research Institute, LEK Consulting, The Frankel Group, Prestwick Chemical Co., Millipore Corp., Genentech, IMS Health, Primary Insight and Otsuka. P.J.C. has received honoraria as a speaker from: University of Toronto, American Society for Bone and Mineral Research, University of Alabama Birmingham, University of Michigan, Southern Research Inst., Harvard Medical School and the University of North Carolina. P.J.C. receives research support that includes salary support from the National Institutes of Health, Michael J. Fox Foundation, Seaside Therapeutics and Vanderbilt University.
References
- 1.Brown TA, Zador AM. Hippocampus. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford University Press; 1990. pp. 346–388. [Google Scholar]
- 2.Cooper J, et al. The Biochemical Basis of Neuropharmacology. Oxford University Press; 1996. [Google Scholar]
- 3.Hasselmo ME. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisani A, et al. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Fisher A. M1 muscarinic agonists target major hallmarks of Alzheimer’s disease - the pivotal role of brain M1 receptors. Neurodegener. Dis. 2008;5:237–240. doi: 10.1159/000113712. [DOI] [PubMed] [Google Scholar]
- 6.Langmead CJ, et al. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Bymaster FP, et al. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur. J. Neurosci. 2003;17:1403–1410. doi: 10.1046/j.1460-9568.2003.02588.x. [DOI] [PubMed] [Google Scholar]
- 8.Katzenschlager R, et al. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst. Rev. 2003 doi: 10.1002/14651858.CD003735. CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wess J, et al. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 10.Barten DM, Albright CF. Therapeutic strategies for Alzheimer’s disease. Mol. Neurobiol. 2008;37:171–186. doi: 10.1007/s12035-008-8031-2. [DOI] [PubMed] [Google Scholar]
- 11.Felder CC, et al. Therapeutic opportunities for muscarinic receptors in the central nervous system. J. Med. Chem. 2000;43:4333–4353. doi: 10.1021/jm990607u. [DOI] [PubMed] [Google Scholar]
- 12.Jones CK, et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor reduces amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. 2008;28:10422–10433. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conn PJ, et al. Allosteric modulators of GPCRs as a novel approach for treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.May LT, et al. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 15.Jakubik J, et al. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol. Pharmacol. 1997;52:172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- 16.Lanzafame A, Christopoulos A. Investigation of the interaction of a putative allosteric modulator, N-(2,3-diphenyl-1,2,4-thiadiazole-5-(2H)-ylidene) methanamine hydrobromide (SCH-202676), with M1 muscarinic acetylcholine receptors. J. Pharmacol.Exp. Ther. 2004;308:830–837. doi: 10.1124/jpet.103.060590. [DOI] [PubMed] [Google Scholar]
- 17.Lazareno S, et al. Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol. Pharmacol. 2004;65:257–266. doi: 10.1124/mol.65.1.257. [DOI] [PubMed] [Google Scholar]
- 18.Lazareno S, et al. Subtype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol. Pharmacol. 1998;53:573–589. doi: 10.1124/mol.53.3.573. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi AB, et al. SCH-202676: an allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol. Pharmacol. 2001;59:30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- 20.Spalding TA, et al. Discovery of an ectopic activation site on the M1 muscarinic receptor. Mol. Pharmacol. 2002;61:1297–1302. doi: 10.1124/mol.61.6.1297. [DOI] [PubMed] [Google Scholar]
- 21.Sur C, et al. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones CK, et al. TBPB is a highly selective M1 allosteric muscarinic receptor agonist in vitro and produces robust antypsychotic-like effects in vivo. Neuropsychopharmacology. 2006;31:S117. [Google Scholar]
- 23.Kinney GG. Muscarinic receptor activiation for the treatment of schizophrenia. Neuropsychopharmacology. 2006;31:S26. [Google Scholar]
- 24.Langmead CJ, et al. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br. J. Pharmacol. 2008;154:1104–1115. doi: 10.1038/bjp.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas RL, et al. G protein coupling and signaling pathway activation by M1 muscarinic acetylcholine receptor orthosteric and allosteric agonists. J. Pharmacol. Exp. Ther. 2008;327:365–374. doi: 10.1124/jpet.108.141788. [DOI] [PubMed] [Google Scholar]
- 26.Caccamo A, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Lebon G, et al. Mutagenic mapping suggests a novel binding mode for selective agonists of M1 muscarinic acetylcholine receptors. Mol. Pharmacol. 2008 doi: 10.1124/mol.108.050963. http://molpharm.aspetjournals.org 10.1124/mol.108.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, et al. N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol. Pharmacol. 2008;73:909–918. doi: 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- 29.Bridges TM, et al. Synthesis and SAR of analogues of the M1 allosteric agonist TBPB. Part I. Exploration of alternative benzyl and privileged structure moieties. Bioorg. Med. Chem. Lett. 2008;18:5439–5443. doi: 10.1016/j.bmcl.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller RN, et al. Synthesis and SAR of analogues of the M1 allosteric agonist TBPB. Part II. Amides, sulfonamides and ureas: the effect of capping the distal basic piperidine nitrogen. Bioorg. Med. Chem. Lett. 2008;18:5444–5449. doi: 10.1016/j.bmcl.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marlo J, et al. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol. Pharmacol. doi: 10.1124/mol.108.052886. http://molpharm.aspetjournals.org 10.1124/ mol.108.052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray W, et al. Allosteric potentiation of the M1 muscarinic receptor provides unprecedented selectivity and a novel therapeutic strategy for the treamtent of alzheimer’s disease. Alzheimers Dement. 2008;4 Suppl. 1:T761. [Google Scholar]
- 33.Wittmann M, et al. In vivo pharmacodynamic effects of BQCA, a novel selective allosteric M1 receptor modulator. Alzheimers Dement. 2008;4 Suppl. 1:T770. [Google Scholar]
- 34.Bodick NC, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 35.Bodick NC, et al. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1997;11 Suppl. 4:S16–S22. [PubMed] [Google Scholar]
- 36.Assal F, Cummings JL. Neuropsychiatric symptoms in the dementias. Curr. Opin. Neurol. 2002;15:445–450. doi: 10.1097/00019052-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Bullock R, Cameron A. Rivastigmine for the treatment of dementia and visual hallucinations associated with Parkinson’s disease: a case series. Curr. Med. Res. Opin. 2002;18:258–264. doi: 10.1185/030079902125000813. [DOI] [PubMed] [Google Scholar]
- 38.Feldman H. Treating Alzheimer’s disease with cholinesterase inhibitors: what have we learned so far? Int. Psychogeriatr. 2002;14 Suppl. 1:3–5. doi: 10.1017/s1041610203008639. [DOI] [PubMed] [Google Scholar]
- 39.Grossberg GT. The ABC of Alzheimer’s disease: behavioral symptoms and their treatment. Int. Psychogeriatr. 2002;14 Suppl. 1:27–49. doi: 10.1017/s1041610203008652. [DOI] [PubMed] [Google Scholar]
- 40.Kaufer D. Beyond the cholinergic hypothesis: the effect of metrifonate and other cholinesterase inhibitors on neuropsychiatric symptoms in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 1998;9 Suppl. 2:8–14. doi: 10.1159/000051193. [DOI] [PubMed] [Google Scholar]
- 41.Mirza NR, et al. Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists. CNS Drug Rev. 2003;9:159–186. doi: 10.1111/j.1527-3458.2003.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosler M. The efficacy of cholinesterase inhibitors in treating the behavioural symptoms of dementia. Int. J. Clin. Pract. 2002 Suppl:20–36. [PubMed] [Google Scholar]
- 43.Shekhar A, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 44.Anagnostaras SG, et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat. Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- 45.Felder CC, et al. Elucidating the role of muscarinic receptors in psychosis. Life Sci. 2001;68:2605–2613. doi: 10.1016/s0024-3205(01)01059-1. [DOI] [PubMed] [Google Scholar]
- 46.Gerber DJ, et al. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl d-aspartate receptor. Curr. Drug Target. CNS Neurol. Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- 48.Messer WS., Jr Cholinergic agonists and the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2002;2:353–358. doi: 10.2174/1568026024607553. [DOI] [PubMed] [Google Scholar]
- 49.Miyakawa T, et al. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirey JK, et al. An allosteric potentiator of M4 mA ChR modulates hippocampal synaptic transmission. Nat. Chem. Biol. 2008;4:42–50. doi: 10.1038/nchembio.2007.55. [DOI] [PubMed] [Google Scholar]
- 51.Brady A, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotion behavior in rats. J. Pharmacol. Exp. Ther. 2008;327:941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan WY, et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Brien JA, et al. A family of highly selective allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5) Mol. Pharmacol. 2003;64:731–741. doi: 10.1124/mol.64.3.731. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S, et al. Synthesis and SAR of a mGluR5 allosteric partial antagonist lead: Unexpected modulation of pharmacology with slight structural modifications to a 5-(phenylethynyl)pyrimidine scaffold. Bioorg. Med. Chem. Lett. 2008;18:4098–4101. doi: 10.1016/j.bmcl.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]