Summary
Chemokines are soluble factors shown to play important roles in regulating immune cell recruitment during inflammatory responses and defense against foreign pathogens. De-regulated expression and activity of several chemokine signaling pathways have been implicated in cancer progression, including: CCL2, CCL5, CXCL1 and CXCL12. While studies in the past have focused the role of these chemokine signaling pathways in regulating immune responses, emerging studies show that these molecules regulate diverse cellular processes including angiogenesis, and regulation of epithelial cell growth and survival. New evidence indicates that chemokines are critical for cancer progression and indicate complex and diverse functions in the tumor microenvironment. This review will focus on the contributions of chemokine signaling in regulating cancer microvironment and discuss the utility of targeting or delivering chemokines in cancer therapeutics.
Keywords: chemokine, inflammation, cancer, microenvironment, chemokine antagonist
I. Introduction
Chemotactic cytokines, also referred as to as chemokines, have long been recognized as critical mediators of the inflammatory response by regulating recruitment of cells from both the innate and adaptive immune systems to the site of injury or infection (Zabel et al, 2006; De Paepe et al, 2008). Early work on chemokines involved studying the effects of Platelet Factor-4 (also known as CXCL4) on the movement of neutrophils and monocytes (Deuel et al, 1981) through chemotaxis, the process by which cells migrate in response to a concentration gradient of chemokine. Since then, chemokines have also been shown regulate other biological processes including angiogenesis, embryonic implantation, and germ and stem cell migration during embryonic development (Rostene et al, 2007; Salamonsen et al, 2007; Beider et al, 2008; Li and Ransohoff, 2009). It is clear that chemokines not only regulate cellular migration of immune cells, but also the migration, proliferation and survival signals in multiple cell types. While chemokine receptors and ligands are expressed in amphibians and mammals, expression of chemokine ligands and receptors is highly conserved between humans and mice, making the mouse model an advantageous and extensively used system to study chemokine function in vivo and in vitro (DeVries et al, 2006; Zlotnick et al, 2006). The mouse model has been used to study the role of chemokines in normal physiologic responses and also during inflammatory diseases contributed in part by deregulated expression or activitiy of chemokines (Gillitzer and Goebeler, 2001; De Paepe et al, 2008). These studies have lead to promising developments in the treatment of inflammatory diseases such as rheumatoid arthritis (Vital and Emery, 2008). More recently, in vitro and in vivo studies have implicated several inflammatory chemokines including: CCL2, CCL5, CXCL1 and CXCL12 in the progression of various cancers. While development of chemokine receptor antagonists to inhibit chemokine signaling is currently a promising avenue in cancer therapeutics, the complex functions and mechanisms of chemokine signaling in the cancer microenvironment may complicate the effectiveness of these agents. It is therefore important to investigate and understand the functions of inflammatory chemokine signaling in cancer progression at the molecular, cellular and whole organism levels. This review will focus on the current knowledge regarding the role of inflammatory chemokine signaling in cancer progression and the current status of chemokines in cancer therapy and prognosis.
II. Structure and signal transduction
Chemokines are a large family of proteins of which over 40 ligands have been identified. The chemokines have been subdivided into classes depending on the spacing of the cysteine amino acid residues at the NH2 terminus; an X denotes a non- cysteine amino acid residue separating the otherwise conserved cysteine motif. Current classes of the chemokine family are referred to as CC, CXC and CX3C (Tables 1A-C). While the CXC class of chemokines have been shown regulate recruitment of neutrophils and T cells, the CC chemokine regulate both T cells, B cells and recruitment of bone marrow derived cells including-monocytes and dendritic cells (Laing and Secombes, 2004; DeVries et al, 2006).
Table 1.
Systemic and alternative nomenclature for human and mouse chemokineligands and receptors.
| A. CXCL family | |||
|---|---|---|---|
| Chemokine ligand Systemic name | Human | Mouse | Chemokine receptors |
| CXCL1 | GRo-a, MGSA, SCYB1 | Gro1, Mgsa, KC, Scyb1 |
  Duffy Duffy
|
| CXCL2 | GRo-B, MGSA-b, SCYB2 | GROb, MIP-2a, KC, Scyb2 |
 Duffy Duffy
|
| CXCL3 | Gro-g/MGSA-g, SCYB3 | GRO, MIP-2, KC, Scyb3 | CXCR2 |
| CXCL4 | PF4, SCYB4 | PF4, Scyb4 | unknown |
| CXCL5 | ENA-78, SCYB5 | GCP-2, LIX, Scyb5 |
 Duffy Duffy
|
| CXCL6 | GCP-2, SCYB6 | GCP-2, Scyb6 | CXCR2 |
| CXCL7 | NAP-2, SCYB7 | unknown |
 Duffy Duffy
|
| CXCL8 | IL-8, SCYB8 | unknown |
 , ,  Duffy Duffy
|
| CXCL9 | MIg, SCYB9 | Mig, Scyb9 | CXCR3 |
| CXCL10 | IP-10, SCYB10 | IP-10, CRG-2, Scyb10 | CXCR3 |
| CXCL11 | I-TAC, SCYB11 | I-TAC, Scyb11 | CXCR3 |
| CXCL12 | SDF-1 a/b, SCYB12 | SDF-1, PBSF, Scyb12 | CXCR4 |
| CXCL13 | BCA-1, SCYB13 | BLC, Scyb13 | CXCR5 |
| CXCL14 | BRAK, bolekine, MIP-2g, SCYB14 | Scyb14 | unknown |
| CXCL15 | Lungkine, WECHE, SCYB15 | Lungkine, WECHE, Scyb15 | unknown |
| CXCL16 | SR-PSOX | Zmynd15 | CXCR6 |
| B. CCL family | |||
|---|---|---|---|
| Chemokine ligand Systemic name | Human | Mouse | Chemokine receptors |
| CCL1 | I-309, SCYA1 | TCA-3, P500, Scya1 | |
| CCL2 | MCP-1/MCAF, TDCF, SCYA2 | JE. Scya2 |
Duffy, 
|
| CCL3 | MIP-1a, SCYA3 | MIP-1a, Scya3 |
 
|
| CCL4 | SCYA2, SCYA4, MIP1B | MIP-1b, Scya2, Scya4 |
 
|
| CCL5 | RANTES, SCYA5 | RANTES, Scya5 |
 Duffy, Duffy, 
|
| CCL6 | Mrp-1, SCYA6 | C10/MRP-1, Scya6 |
 CCR3, CCR3, 
|
| CCL7 | MCP-3, MARC, FIC, SCYA7 | MARC, Scya7, mcp3 |
 , ,  , CCR3, , CCR3, 
|
| CCL8 | MCP-2, SCYA8 | mcp2, Scya8, HC14 |
 , ,  , CCR3 , CCR3
|
| CCL9/10 | unknown | MRP-2, CCF18, MIP-1g, Scya9 |
CCR3, 
|
| CCL11 | Eotaxin, SCYA11 | Scya11 | CCR3 |
| CCL12 | SCYA12 | MCP-5, Scya12 | CCR2 |
| CCL12 | MCP-4, SCYA13, NCC-1 | unknown |
 CCR3 CCR3
|
| CCL14 | HCC-1, NCC2, SCYA14 | unknown |
 , CCR3 , CCR3
|
| CCL15 | HCC-2, MIP-1d, MIP-5, SCYA15 | unknown |
 CCR3 CCR3
|
| CCL16 | HCC-4, LEC, LCC-1, SCYA16 | unknown |
 , , 
|
| CCL17 | TARC, SCYA17 | TARC, ABCD-2, Scya17 |
 
|
| CCL18 | DC-CK, PARC, AMAC-1, SCYA18 | unknown | unknown |
| CCL19 | MIP-3b, ELC, exodus-3, SCYA19 | MIP-3b/ELC, exodus-3, Scya19 | CCR7 |
| CCL20 | MIP-3a, LARC, exodus-1, SCYA20 | Scya20 | CCR6 |
| CCL21 | 6CKine, SLC, exodus-2, SCYA21 | 6CKinase, SLC, exodus-2, TCA-4 | CCR7 |
| CCL22 | MDC, STCP-1, SCYA22 | ABCD-1, Scya22 |
 
|
| CCL23 | MPIF-1, CKb8, SCYA23 | unknown | CCR1 |
| CCL24 | Eotaxin2, MPIF-2, SCYA24 | MPIF2, Scya24 | CCR3 |
| CCL25 | TECK, SCYA25 | TECK, Scya25 | CCR9 |
| CCL26 | Eotaxin-3, SCYA26 | eotaxin-3, Scya26 | CCR3 |
| CCL27 | CTACK/ILC, SCYA27 | unknown | CCR10 |
| CCL28 | MEC, CCK1, SCYA28 | MEC, Scya28 | CCR3/CCR1 |
| C. XCL and CX3CL families | |||
|---|---|---|---|
| Chemokine ligand Systemic name | Human | Mouse | Chemokine receptors |
| XCL1 | lymphotactin/SCM-1a/ATAC | lymphotactin | XCR1 |
| XCL2 | SCM-1b | unknown | XCR1 |
| CX3CL1 | fractaline | neurotactin/ABCD-3 | CX3CR |
Chemokines signal to seven transmembrane G coupled receptors at the N terminus, resulting in phosphorylation of serine/threonine residues at the C-terminus, conformational changes to the receptor and activation of a heterotrimeric G protein complex bound to the receptor intracellular domain. Activation involves GTP binding to Ga subunit leading to disassociation from its Gb and g subunit partners, and subsequent activation of downstream signaling pathways (Figure 1). These signaling pathways, including: PI-3 Kinase, Rho family of GTPases and MAPK regulate cellular processes such as proliferation, motility and gene expression of matrix metalloproteinases and cytokines (Ganju et al, 1998; Bug et al, 2002; Chinni et al, 2006; Ou et al, 2006). It should be noted that chemokines receptors also activate signaling pathways independent of G proteins, including p38MAPK (Goda et al, 2006) and JAK/Stat (Vila-Coro et al, 1999) to regulate cellular processes such as migration and gene transcription.
Figure 1.

G protein dependent signal transduction through chemokinereceptors: CXCR4 signaling in lymphocytes as a model
The ability of multiple chemokines to bind to the same receptor and the ability of a single chemokine to bind to multiple receptors (Table 1) create the possibility of redundant signaling. In vitro studies have shown that chemokines stimulate migration through common signaling pathways such as G coupled protein dependent mechanisms (Cotton and Claing, 2009). However, there is also evidence supporting the possibility of unique functions for each chemokine ligand/receptor pair. First, chemokine ligands exhibit different binding affinities to the same receptor. For example, chemokines exhibit a greater affinity for CXCR2 versus CXCR1 (Devalaraja and Richmond, 1999; Rajagopalan and Rajarathnam, 2006). In addition, different ligands which bind to the same receptor exert different biological effects in certain cell types. For example, CCL3, CCL4 and CCL5 have been shown stimulate migration of activated T cells, but only CCL5 can stimulate migration of resting T cells (Taub et al, 1993). Moreover, knockout mouse studies of chemokine receptors and ligands such as CCL2 and CCR2 do not show compensatory upregulation of other ligands (Boring et al, 1997; Huang et al, 2001). The purpose of the multiple ligand/receptor binding pairs remains under investigation, but these studies indicate that unique roles for each chemokine/receptor pair may serve as a mechanism to regulate cellular responses to chemokine signaling.
Other multiple mechanisms also exist to regulate chemokine signaling. Continuous chemokine signaling has been shown to lead to receptor desensitization, internalization through b arrestin and clathrin dependent mechanisms, resulting in downregulation of chemokine signaling (Aramori et al, 1997; Oppermann, 2004). In addition, the D6 and Duffy receptors which bind multiple ligands (Table 1), do not appear to activate signaling pathways, indicating that these receptors may act to sequester chemokine ligands as additional regulatory mechanisms for down regulation of chemokine signaling (Locati et al, 2005; Comerford et al, 2007). In fully developed mammalian organisms, these mechanisms are vital to controlling immune responses during inflammation to restore normal tissue homeostasis.
III. Role of chemokine signaling in inflammation and cancer
A. Phenotypes of knockout mouse models
Chemokines appear to play dual roles in the inflammatory process. On the one hand, targeted deletion of CC and CXC chemokine receptors or ligands in mice leads to increased susceptibility to viral or bacterial infections and decreased clearance of pathogens due to diminished immune cell recruitment. On the other hand, targeted deletion of chemokines and chemokine receptors have been shown to alleviate the phenotypes of inflammatory diseases including rheumatoid arthritis, multiple sclerosis, autoimmune encephalitis and macular degeneration (Table 2).
Table 2.
Targeted deletion of inflammatory chemokineand chemokinereceptors in mice
| Gene | phenotype | citation |
|---|---|---|
| CCL2 | diminished macrophage and TH1 T cell responses inexperimental autoimmune encephalomyelitis (EAE), no compensatory upregulation of CCR2 binding ligands | Huang et al, 2001 |
| CCR2 | decreased formation of lung granulomas induced by mycobacterium bovis, similar phenotype in EAE as ccl2-/-, decreased monocyte recruitment to inflamed tissues | Boring et al, 1997; Fife et al, 2000; Tsou et al, 2007 |
| CCL5 | defects in T cell proliferation, an overall reduction in T cell activation and recruitment in cutaneous delayed-type hypersensitivity assays | Makino et al, 2002 |
| CCR5 | decreased NK cell mobilization in mice infected with herpes simplex virus 2 leading to decreased survival, increased NK cell infiltration in experimental mouse model of colitis correlating with increased resistance to disease | Yamaoka et al, 1998; Andres et al, 2000; Thapa et al, 2007 |
| CXCL1 | artherosclerotic lesions leads to a reduction in macrophage recruitment associated with decreased lesion formation | Boisvert et al, 2006 |
| CXCR2 | decreased neutrophil recruitment and delayed wound healing responses, abnormal granulocyte differentiation, mice develop splenomegaly | Cacalano et al, 1994; Devalaraja et al, 2000 |
| CXCL12 | perinatal lethality, defects in B cell development in in fetal liver and bone marrow, reduction in myeloid progenitors in bone marrow, defects in cardiac development | Nagasawa et al, 1996 |
| CXCR4 | perinatal lethality due to multiple defects including decreased bone marrow cell and B cell development abnormal cerebellum morphology | Ma et al, 1998 |
Chronic chemokine signaling is associated with macrophage and T cell accumulation at the inflammatory site, and studies indicate that chronic activation of macrophages may lead to alterations in normal tissue architecture, abnormal angiogenesis and DNA damage due to excess secretion of reactive oxygen species (ROS); (Gillitzer and Goebeler, 2001; Moll et al, 2009).
While the causes for chronic chemokine signaling are still under investigation, gene mutations in chemokine receptors in immune cells (Hernandez et al, 2003) and increased expression of cytokines which regulate chemokine expression at the site of inflammation (Firestein, 2004) may be important contributing factors.
These studies indicate that that loss of control over chemokine signaling results in long range consequences with damaging changes in the tissue microenvironment. Emerging studies indicate that de-regulation of the activity and expression of chemokine ligands and receptors in cancer may also alter the tumor microenvironment with long term consequences.
B. Expression patterns in cancer
Many studies indicate a significant correlation of elevated expression of chemokines and signaling receptors with poor cancer prognosis and lymph node metastases in various cancers as determined by immunohistochemistry and RNA in situ analyses (Table 3). Interestingly, the same chemokine receptors that show chronic signaling inflammatory diseases are upregulated in cancer, including CCL2, CCL5, CXCL1 and CXCL12.
Table 3.
Protein expression of commonly found chemokinesand chemokine receptors in cancer epithelium.
| Breast | colorectal | brain | melanoma | prostate | non-small cell lung | ovarian | thyroid | pancreatic | renal cell | |
|---|---|---|---|---|---|---|---|---|---|---|
| CCL2 | +1 | + | +6 | + | vl | nd | - | nd | + | nd |
| CCR2 | nd | vl | +6 | nd | + | + | nd | nd | vl | nd |
| CCL5 | +1,2 | nc | nd | + | + | + | nd | +9 | + | nd |
| CCR5 | +3 | nc | +7 | + | nd | + | nd | nd | nd | nd |
| CXCL1 | +4 | + | +6,7 | + | + | nd | + | nd | nd | + |
| CXCR2 | nd | + | +7 | + | + | + | + | +10 | + | + |
| CXCL12 | v11,4 | + | +7 | nd | + | nd | + | nd | + | + |
| CXCR4 | +1,5 | + | +6 | + | + | + | + | +9 | + | + |
(-): reduced expression, (+): increased expression, nd: not determined, vl: very low or undetectable; nc: no change
invasive ductal carcinoma
estrogen receptor negative (ER negative)/progesterone receptor negative stage II breast cancer
infiltrating ductal carcinoma and infiltrating lobular carcinoma
ER negative breast cancer
invasive lobular carcinoma
glioblastoma
glioma,
astrocytoma
papillary thyroid carcinoma
medullary thyroid carcinoma
Epithelial specific expression of chemokines and chemokine receptors have been validated by protein and RNA expression analysis of breast, renal, prostate cancer cell lines (Schrader et al, 2002; Vaday et al, 2006; Wente et al, 2008). In addition, decreased expression of Duffy and D6 decoy receptors in breast cancer inversely correlate with lymph node metastases and increased survival rates, indicating the cancer cells showed impaired ability to down regulate chemokine signaling (Ou et al, 2006; Wu et al, 2008). These studies associate increased expression of chemokines and decreased expression of decoy receptor with invasive cancer. However, recent studies show loss of CCL2 in ovarian cancer (Arnold et al, 2005) and reduced CCR2 expression in patients with myeloma (Van de Broek et al, 2006) indicate a possible tumor suppressive role for chemokine signaling which may in part be tissue dependent. Studies have also found associations between the patterns of chemokine expression and levels of immune cells in the primary tumor, as demonstrated by studies of CCL2. In breast cancer, increased CCL2 expression is associated with increased levels of tumor associated macrophages, which have been found to correlate with the invasive phenotype and poor cancer prognosis (Valkovic et al, 1998; Ueno et al, 2000). However, in cervival cancer, loss of CCL2 expression is associated with decreased levels of macrophages in cervical cancer, and correlates with poor cancer prognosis (Kleine-Lowinski et al, 1999). Taken together, these studies demonstrate a tissue dependent pattern of expression and a tissue dependent role for chemokines and immune cells in cancer progression.
Recent studies have shown that the surrounding tumor stroma also show significant changes to chemokine expression. In particular, increased expression of CXCL1 was consistently observed in the stroma of multiple types of breast cancers by microarray analysis of tumor associated stroma, correlating with lymph node metastases, invasiveness and poor patient survival (Finak et al, 2008). Recent studies have shown that CXCL1 and CXCR2 may play a role in regulating replicative senescence in fibroblasts through a p53 dependent mechanism as a possible means to suppress tumor formation. Furthermore, gene mutations in components of the CXCL1 signaling pathway may allow tumor cells to escape this tumor suppressive mechanism (Acosta et al, 2008). In addition to CXCL1, increased expression of CXCL12 have been reported in the stroma of basal and invasive mammary ductal adenocarcinoma, head and neck cancer, papillary thyroid carcinoma and squamous cell carcinoma by SAGE, differential display and immunohistochemistry studies (Frederick et al, 2000; Shellenberger et al, 2004).
The increased expression of these chemokines in the stroma correlates with tumor size and lymph node invasion (Kleer et al, 2008; Oliveira-Neto et al, 2008). These studies indicate that stromal specific expression of chemokines may be an important factor to consider when determining cancer prognosis.
While changes in protein or RNA expression of chemokine receptors and ligands are strongly associated with progression of various cancer types, few studies of serum levels and polymorphisms have currently yielded significant links to cancer. Elevated serum levels of CCL5 correlate with poor patient prognosis in ovarian cancer (Tsukishiro et al, 2006) and breast cancer (Niwa et al, 2001). While serum levels of CCL5 had no significant association with patient survival in gastric cancer, high serum levels of CCL5 correlated significantly with invasive disease.
Serum level studies have involved comparisons between healthy vs. cancer patient samples, as well as malignant vs. benign samples. The differences in results in serum level studies may be due to differences in patient samples in terms age, disease stage, and whether or not disease was treated prior to sample collection. Future comparative analyses must consider such variables, as well as the possibility that changes in chemokine levels may be restricted to the local tumor environment. Therefore the prognostic value of serum analyses might be lower than analysis of expression within the tumor/stroma itself.
C. Regulation of chemokine expression
As a number of studies have reported alterations in the expression of chemokines and chemokine receptors in various cancers, current studies are underway to determine the possible causes for the changes in expression during cancer progression. Certain environmental and soluble factors have been shown to induce expression of chemokines and chemokine receptors in epithelial and mesenchymal cells. Hypoxic regulated factors and hepatocyte growth factor (HGF) were shown to induce expression of CXCR4 in MCF-7 cells and MDA-MB-231 cells (Matteucci et al, 2007), while TNF-a enhanced CXCR4 expression in ovarian cancer cells (Kulbe et al, 2005). Multiple cytokines and growth factors including, TNF-a, IL-6, MSP, LMP1, CD40 were shown to induce expression of CCL2 and CCL5 in epithelial cells, macrophages, fibroblasts and endothelial cells (Biswas et al, 1998; Buettner et al, 2007). The possibility of genetic mutations as a possible cause for abnormal chemokine expression is currently under investigation. Loss of heterozygosity of CCL2 in cervical carcinoma at 17q11.2 has been associated with diminished progression, increased survival, and macrophage accumulation (Zijlmans et al, 2006). Gene variants of chemokines and receptors have been detected in one study in the form of base substitutions or base deletions: CCL5 -403(G>A), CXCL12 +801(G>A), CCR2 V64I (G>A), CCR5 (Delta32), though only CXCL12 +801(G>A) has been found to be associated with increased prostate cancer risk (Petersen et al, 2008). In addition, the polymorphism CCL5 -403(G/A) has been associated with increased susceptibility of prostate cancer (Saenz-Lopez et al, 2008).
Studies of CXCL1 signaling have suggested that inactivating point mutations to CXCR2 (CXCR2G354W allele) in lung adenocarcinoma may be a means to escape CXCL1 induction of cellular senescence, and consequently tumor suppression (Acosta et al, 2008)
While these few studies have detected a significant association with cancer risk or invasiveness, the functional significance to these gene variants remain largely unclear. Further studies should be conducted to investigate and understand the functional mechanisms of these gene variants during cancer progression.
In summary, studies analyzing differences in local expression of chemokines, compared to those measuring chemokine levels in the serum, offer a stronger, more consistent association is between cancer prognosis and changes in patterns of chemokines ligands and receptors in the primary tumor and metastatic lesions. Currently, the genetic link between chemokine expression patterns and cancer remains unclear. As a number of soluble factors can regulate expression of chemokine ligands and receptors, it is more likely that alterations in expression of these regulators combined with possible genetic mutations may contribute to de-regulated expression of chemokines and their receptors.
D. Role of chemokines in tumor progression in the mouse model
The use of transgenic and transplantion mouse models of cancer have proven invaluable in understandng the functions and significance of chemokine signaling during disease progression. A tumor promoting role for inflammatory chemokines is best demonstrated in studies of breast cancer. Over expression of CXCL1 in breast cancer cells results in increased tumor growth and lung metastasis when these over expressing cells are grafted in the mammary fat pads of nude mice (Li and Sidell, 2005; Vazquez-Martin et al, 2007). In addition, siRNA mediated knockdown of CXCR4 expression in mouse and human mammary carcinoma cells inhibits tumor growth and metastatic spread in nude mice (Smith et al, 2004; Liang et al, 2005). CCL2 knockout mice bred to HER2/neu transgenic mice produce progeny exhibiting slower mammary tumor growth and longer tumor latency (Conti et al, 2004). While these studies indicate that expression of chemokines in the tumor epithelial is sufficient to promote breast cancer progression in mice, mesenchymal stem cells have been shown to enhance cancer progression in nude mice through CCL5, CXCL12, CXCR4 and CCL2 dependent mechanisms (Karnoub et al, 2007) indicating these chemokine also regulate breast cancer progression through mediation of stromal: epithelial interactions.
The same chemokines shown to play tumor promoting roles in breast cancer affect other types of cancer differently. Targeted deficiency in expression of CCR2 in the XK14-HPVR(2) mouse model of cervical carcinoma does not significantly affect tumor angiogenesis or end stage progression even though decreased numbers of macrophages have been observed in the primary tumor. The modest phenotype may be due to increased presence of tumor associated neutrophils observed in the CCR2 deficient mice; these cells have been proposed to exert tumor promoting effects to balance out any tumor suppressive effects caused by CCR2 deficiency (Pahler et al, 2008). In addition, CCL2 over expression in colon carcinoma cells and rat gliosarcoma cells suppress tumor formation when these tumor cells are injected into rodents (Hoshino et al, 1995). This anti-tumor phenotype correlates with increased accumulation and activation of macrophages at the site of injection in immunocompromised mice (Rollins and Sunday, 1991) and increased T cell responses in immunocompetent mice (Manome et al, 1995). Moreover, over expression of CCL5 in the thymoma cell lines EL4 or EG 7 has been shown to inhibit tumor growth when injected in mice, characterized by increased recruitment of T cells, natural killer (NK) cells, and dendritic cells. These studies indicate that enhanced chemokine signaling in cancer in part drives immune cell recruitment to the primary tumor. The ability of immune cells to mount an anti-tumor response may in part be tissue type dependent, and dependent on the mouse model used, as immune cells appear to be more effective in preventing establishment of the primary tumor in mice with intact immune systems as opposed to immunocompromised mice. In addition, studies have reported tumor-promoting roles for antigen presenting cells including macrophages and dendritic cells in immunocompromised mouse models and transgenic mouse models of cancer, which may in part be regulated by signals in the tumor microenvironment (Pollard, 2004; Melief, 2005; Allavena et al, 2008).
E. Role of chemokine signaling in epithelial and tumor associated stromal cells
Studies have revealed that chemokines regulate growth and migratory signals in multiple types of epithelial cells. Induction of chemotaxis has been observed in a number of carcinoma cell lines including those of breast, prostate, melanoma and lung (Prest et al, 1999; Woodward et al, 2002, Loberg et al, 2006; Huang et al, 2008). Chemokines were also shown to regulate growth of certain carcinoma cell lines. CXCL12, CCL2, CCL5 and CXCL1 were shown to stimulate proliferation of melanoma, glioma, and prostate cancer cell proliferation (Payne and Cornelius, 2002; Darash-Yahana et al, 2004; Loberg et al, 2006; Vaday et al, 2006). These same chemokines appear to mainly stimulate migratory signals in breast cancer cells; however, CXCL12 has been shown to stimulate proliferation of breast cancer cells (Allinen et al, 2004). These studies indicate that the abilty of chemokines to stimulate cell proliferation may be cell type specific.
In addition to signaling in epithelial cells, chemokines have also been shown to regulate the functions of various mesenchymal cell types. The ability of chemokines to stimulate chemotactic responses of T cells and antigen presenting cells using in vitro assays is well established (Zabel et al, 2006). Additional studies have shown that CCL2, CXCL1, CCL5, CXCL12 function as potent angiogenic factors, stimulating endothelial cell migration and tube formation, as well as angiogenesis in corneal and chick cam angiogenesis assays (Goede et al, 1999; Salcedo et al, 2000; Azenshtein 2002; Dhawan and Richmond, 2002). Recent studies have also shown that chemokines can also regulate migration of lung fibroblasts through a CXCR2 dependent mechanism (Wislez et al, 2006); however the significance of chemokines on the regulation of fibroblast behavior currently remains unclear. These studies demonstrate that chemokines can regulate the functions of multiple types of mesenchymal cells with important implications on the functions on these cells in the tumor microenvironment.
Expression of chemokines in the tumor-associated stroma indicates that chemokines may regulate stromal: epithelial interactions in the primary tumor microenvironment. This hypothesis is supported by studies of carcinoma cells interactions with various stromal cell types. Increased expression of CXCL1 in melanoma-associated fibroblasts indicated that CXCL1 may regulate paracrine signaling interactions between fibroblasts and melanoma cancer cells to promote cancer cell proliferation and migration (Gallagher et al, 2005). In lung adenocarcinoma cells that showed increased expression of CXCR2, co-culture with lung stromal cells including macrophages, endothelial cells and fibroblasts increased carcinoma cell proliferation and enhanced tumor growth in mice, through a CXCL1/CXCR2 dependent mechanism (Zhong et al, 2008).
Studies have also shown that stromal: epithelial interactions regulated by chemokines signaling may also have important implications in metastatic spread. To study the mechanisms of bone metastases during prostate cancer, human prostate cancer cells were co-cultured with normal human bone fibroblasts in a 3-D co-culture model. These experiments resulted in long term changes in gene expression patterns in the bone fibroblasts including increased expression of CCL5, CXCL5 and CXCL15. Moreover, co-culture of these carcinoma-associated bone fibroblasts enhanced tumor progression of a benign prostate cell line in mice, associated with increased chemokine expression (Sung et al, 2008), indicating that carcinoma cells can exert long term tumor promoting changes in stromal cells associated with enhanced chemokine signaling. In other studies, increased expression of CCL2 in human bone marrow endothelial cells was shown to enhance prostate cancer cell proliferation and migration associated with increased Rac1 signaling when co-cultured in vitro (van Golen et al, 2008) with important implications in the mechanism of transendothelial migration of prostate cancer cells to the bone. In other studies, mesenchymal stem cells co-cultured with human breast cancer cell lines including: MCF-7, T47D and MDA-MB-231 cells enhanced cancer cell migration in vitro through CCL5, CCL2 and CXCL12 dependent mechanisms with implications in bone metastases as well as invasiveness of the primary tumor (Karnoub et al, 2007; Corcoran et al, 2008; Molloy et al, 2009). Furthermore, CXCL12 and its receptor CXCR4 have been shown to regulate homing of receptor expressing cancer cells to tissues where non-malignant stromal cells express CXCL12 (Muller et al, 2001). These studies demonstrate that tumor cell metastasis is not random, but guided by the expression of chemokine receptors and adhesion molecules on the neoplastic cells.
IV. Current use of chemokines in cancer therapy
As the importance of chemokine expression becomes more recognized in the growth, invasion and metastasis of cancer, it also becomes necessary to develop inhibitors of these chemokines or their related receptors. There have been many studies performed using various inhibitors of chemokines and chemokine receptors and recently have shown efficacy in the treatment of cancer of solid tissues in preclinical models. Currently, a few of these studies have proceeded to clinical trials towards patient therapy.
One promising avenue in the treatment of metastatic disease in solid tumors is in the targeting of the CXCL12 and its receptor CXCR4, which have been shown to regulate homing of cancer cells to distant sites as best demonstrated in breast cancer (Muller et al, 2001). One of the most widely studied compounds is AMD3100 which is thought to specifically block CXCR4 signaling (Burger et al, 1999). This compound has shown efficacy in murine models as it delays pulmonary metastases of mammary carcinoma cells (Smith et al, 2004), reduces dissemination of ovarian cancer cells (Kajiyama et al, 2008) and reduces gastric cancer tumor growth (Yasumoto et al, 2006). While the mechanism of action for AMD3100 is currently under investigation, AMD3100 has been shown to inhibit CXCL12 stimulated migration of breast (Cabioglu et al, 2005), ovarian (Scotton et al, 2002) and gastric cancer cells (Ohira et al, 2006) as well as decrease the invasiveness of prostate cancer cells (PC3 cell line) (Zhang et al, 2008). This compound has been also been tested in vitro studies with other cell types including, pancreatic, colorectal, osteosarcoma and, malignant melanoma (Burger and Peled, 2009). These studies indicate that AMD3100 inhibits tumor progression, in part by inhibiting tumor epithelial cell proliferation, migration and invasion. Recent studies have shown that AMD3100 has mobilizes hematopoietic cells into the peripheral blood (Azab et al, 2009). The increased mobilization renders leukemia and myeloma cells more sensitive to chemotherapeutic intervention by decreasing their association with the bone marrow (Nervi et al, 2008). Thus, the potential benefits of AMD3100 are currently being evaluated in combination with other chemotherapies in patients with blood cancers (Cashen et al, 2008; Stewart et al, 2009). However, the effects of AMD3100 on hematopoietic cells in solid tumors remain unclear. Given the contribution of hematopoietic cells to the progression of solid tumors (Pollard, 2004; Karnoub et al, 2007), the functional consequences of AMD3100 in the tumor microenvironment should be further investigated.
Other inhibitors to the CXCL12/CXCR4 pathway have shown promising results in pre-clinical models and in early clinical trials. CTCE-9908, which is a peptide analog of CXCL12 and an active inhibitor of the ligand, has shown promising results as a well tolerated drug that stabilized disease in early clinical trials for late stage cancer patients (Hotte et al, 2007; Evans, 2008). In pre-clinical studies, this compound has been used in the treatment of osteogenic sarcoma in mice and has been found to decrease growth, adhesion, migration, and invasion in osteosarcoma cells in vitro (Kim et al, 2008). When these cells were then injected into the tail veins of mice, treatment with CTCE-9908 resulted in a 50% reduction in the number of gross metastatic lung nodules and a marked decrease in micro-metastatic disease. Similar results have also seen with melanoma cells, but only when the cells were pre-treated with the inhibitor before injection (Kim et al, 2008). While CTCE-9908 specifically targets CXCL12 and AMD3100 is inhibits CXCR4, both compounds inhibited metastatic spread of various cancers in preclinical models, underscoring the importance of CXCL12/CXCR4 signaling in metastatic disease.
Another chemokine receptor/ligand pair that has been studied in the growth and metastasis of cancer is CCL5/CCR5. Like CXCL12/CXCR4, this pair of proteins has been implicated in the growth and metastasis of many types of cancers, including breast cancer and multiple myeloma. One study showed that monoclonal antibodies to CCR5 significantly blocked CCL5 signaling in MDA-MB-231 breast cancer cells enhanced by mesenchymal stem cells in vitro. Furthemore, systemic treatment of tumor bearing mice with anti -CCR5 inhibited metastatic spread of MDA-MB-231 cells (Karnoub et al, 2007). These studies indicate that CCR5 antagonists significantly block interactions between tumor epithelial cells and mesenchymal stem cells during tumor progression. In other studies, daily systemic treatment of a functional antagonist to CCL5 (met-RANTES or met-CCL5) slowed tumor growth of 410.4 breast cancer cells transplanted into mice, and also reduced macrophage infiltration into the tumor (Robinson et al, 2003). However, Met-CCL5 was unable to reduce the infiltration of other immune cells such as neutrophils (Robinson et al, 2003), which have also been shown to play tumor promoting roles in breast cancer (Queen et al, 2005). One possibility is that these cells express other chemokine or cytokine receptors that could respond to other ligands in the tumor microenvironment while another possibility is that other CCR5 binding ligands could compensate for lack of CCL5 function. Thus, it is possible that redundant signaling of chemokine receptors could reduce the efficacy of these chemokine antagonists in cancer. Indeed, these factors may be partly responsible for the lack of improvement reported in Phase IIA clinical trials for the treatment of rheumatoid arthritis (Vergunst et al, 2008). These studies indicate that inhibiting stromal: epithelial interactions mediated by CCL5 represent one potential approach to treating metastatic disease. Another CCR5 antagonist, TAK-779 has been found to inhibit CCL5-induced prostate cancer cell invasion in a concentration dependent manner (Vaday et al, 2006), indicating that CCR5 antagonists also affects autocrine signaling in cancer cells. However, the mechanism of this compound and efficacy in other types of cancers in in vitro and in vivo studies still remains unclear. Taken together, these studies indicate that the CCL5/CCR5 represents another potentially significant chemokine signaling pathway to target in cancer therapeutics.
Other chemokine antagonists have also shown potential for clinical application in cancer treatment. For example, the CXCR2-selective antagonist AZ10397767 has been shown to decrease resistance of androgen-independent prostate cancer cells to oxaliplatin through an NF-kB dependent mechanism (Wilson et al, 2008). Co-administration of this inhibitor with TRAIL increased the sensitivity of PC3 cells, and was most likely due to the ability of AZ10397767 to block TRAIL and IL-8-induced upregulation of c-FLIP (Wilson et al, 2008). These studies indicate potential benefits in combining chemokine antagonists with other therapies.
In addition to pharmacologic inhibitors that have been designed to specifically target chemokines and their receptors, recent studies have also found that pharmacologic inhibitors used to target other molecules also act as chemokine inhibitors. For example, Etanercept (Enbrel), a TNF-a inhibitor normally used to treat arthritis, has been shown to significantly decrease systemic levels of CCL2 in patients with metastatic breast cancer (Madhusudan et al, 2004). In addition, tamoxifen treatment has been found to inhibit CCL2 mRNA and protein expression in endometrial cancer cell line (EFE184) (Wang et al, 2006). These studies reveal that therapies intended to target specific molecules also indirectly affect the expression of other molecules. However, these unintended side effects may be exploited towards the treatment of cancer, as CCL2 as well as many other chemokines have been shown to play tumor promoting roles in different cancers (Table 3). It is possible that current drug therapies used to treat inflammatory diseases or particular types of cancers could function as inhibitors of chemokine signaling and be redirected towards the treatment of other cancers. However, this avenue of treatment requires further investigation and understanding how conventional therapies affect expression and function of chemokines at the molecular and cellular levels. In summary, while few chemokine antagonists have proceeded to clinical trials (Table 4), the development of chemokine antagonists for the treatment of cancer remains a promising avenue to explore.
Table 4.
Current use of chemokinesin cancer therapy
| Chemokine Target | Antagonist/Agonist | Cancer | Clinical Trial Status |
|---|---|---|---|
| CXCR4 | AMD3100 | Leukemia and Lymphoma | Phase II |
| CXCL12 | CTCE-9908 | Late stage ovarian, breast, lung, colorectal, melanoma, gastric | Phase I/II |
| CCL5 | Met-CCL5 | Breast Cancer | Pre-clinical |
| CCR5 | TAK-779 | Multiple Myeloma, Prostate | Pre-clinical |
| CXCR2 | AZ10397767 | Prostate | Pre-clinical |
| SB225002 | Esophageal | ||
| CCR2 | MLN1202 | Breast | Pre-clinical |
V. Summary and Conclusions: Future role of chemokines in the clinical setting
It is apparent that chemokine signaling regulates multiple processes during tumor progression including primary tumor growth, tumor angiogenesis and metastatic spread. Based on current studies, chemokines mediate stromal: epithelial interactions in the primary tumor microenvironment to regulate tumor growth and invasion (Figure 2A). In addition, studies also indicate that chemokines also regulate homing of chemokine receptor expressing cancer cells to distant sites in metastatic disease (Figure 2B).
Figure 2.
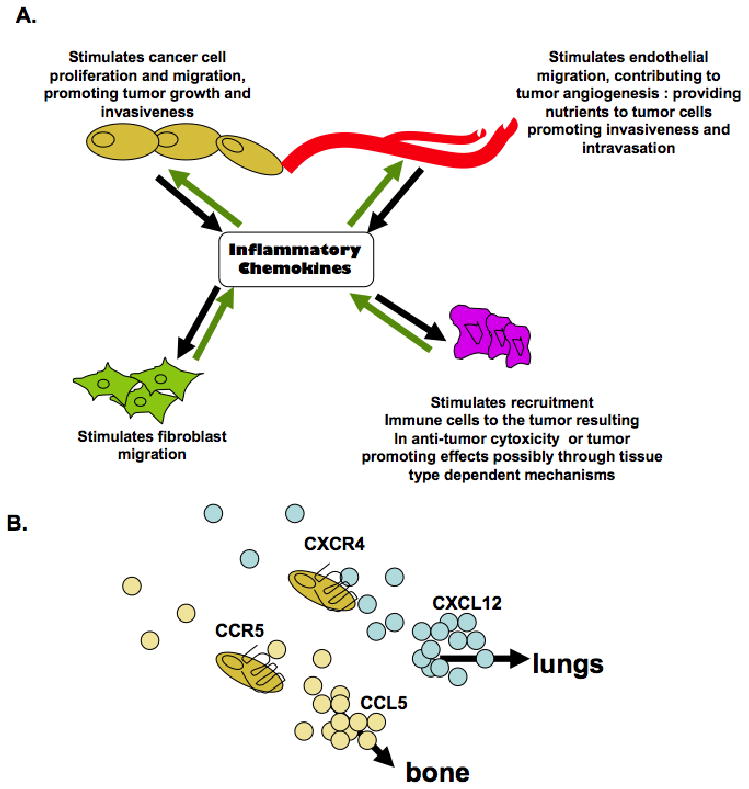
Model for the functional roles of inflammatory chemokinesignaling in cancer. (A) Stromal and epithelial cells secrete inflammatory chemokines, contributing to the bioavailability of chemokinesin the local tisuemicroenvironment. Inflammatory chemokinesregulate autocrineand paracrinesignaling interactions between stromal and epithelial cells to regulate cellular proliferation, migration and invasion. (B) Expression of Inflammatory chemokinesin normal tissues, as demonstrated by CXC112 and CCL5 create a chemokinegradient which regulate homing of chemokinereceptor expressing cancer cells to other organs.
Studies of chemokine expression in the cancer stroma and cancer stage, grade and patient survival and indicate these expression patterns may be indicative of host responses towards the tumor.
Chemokine expression has correlated with the presence of immune cells in some cancers representing either a positive or poor progonosis in cancers (Kleine-Lowinski et al, 1999; Ueno et al, 2000) and may be dependent on the tissue type.
The ability of immune cells to mount anti- tumor response may be in part dependent on the soluble factors expressed in the tumor microenvironment (Lewis and Pollard, 2006). Thus, by regulating the recruitment of immune cells to the primary tumor, the expression of chemokines in cancer may have multi-level consequences on cancer progression.
While further studies should be investigated on the functional consequences of chemokine signaling on immune cells recruitment in cancer, the strong correlations of cancer stromal specific expression of chemokines and tumor malignancy indicate that a practical application for chemokines may be as markers for cancer prognosis.
While studies have shown that chemokine antagonists are effective in treating invasive cancer in mouse models, there are many issues associated with therapeutic targeting of chemokines that have to be addressed and fully understood prior to their use in the treatment of cancer. The possibility of cytotoxic effects in normal tissues is always a consideration in the delivery of chemotherapeutic drugs which target specific molecules (Chari, 2008; Wysocki et al, 2008). This issue is an important consideration particularly for chemokines and their receptors, which are expressed in normal and cancer tissues. The promiscuous binding of ligands to multiple receptors may further complicate the ability of chemokine antagonists to specifically target cancer tissues. While some chemokine ligand/receptor pairs exhibit unique functions, as demonstrated by CCL2/CCR2 signaling, the possibility of redundant signaling may further complicate the effectiveness of chemokine antagonists in cancer.
In summary, while current studies indicate promising roles for chemokines in the clnical setting, the sheer number of chemokines, pleiotropic effects of chemokines, and complex mechanisms of signal transduction add further questions regarding the functions of chemokines in cancer. What signaling pathways do chemokines activate to regulate cellular proliferation and migration, and are these signaling pathways activated in a cell type dependent context? How do chemokines regulate the dynamic interactions between different types of immune cells in the tumor microenvironment? How do chemokines coordinate with other signaling pathways in the tumor microenvironment to regulate tumor progression? Answering these and other questions through further study will lead to a greater understanding of the role of chemokines in tumor progression, and will enable the design of more effective applications for chemokines in the clinical setting.
Acknowledgments
This work was supported by grant number 1K99CA127357-01A2 from the National Cancer Institute and funds from University of Kansas Endowment.
Abbreviations
- c-FLIP
Caspase-8 homologue FLICE-Inhibitory Protein
- LMP1
Latent Membrane Protein 1
- MSP
Macrophage Stimulating Protein
- NK
natural killer
- ROS
reactive oxygen species
- RANTES
Regulated upon Activation Normal T cell Expressed and Secreted
- siRNA
small interfering RNA
- TNF-α
Tumor Necrosis Factor α
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists
References
- Adriaenssens E, Dumont L, Lottin S, Bolle D, Leprêtre A, Delobelle A, Bouali F, Dugimont T, Coll J, Curgy JJ. H19 overexpression in breast adenocarcinoma stromal cell is associated with tumor values and steroid receptor status but independent of p53 and Ki-67 expression. Am J Pathol. 1998;153:1597–1607. doi: 10.1016/S0002-9440(10)65748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Aramori I, Ferguson SS, Bieniasz PD, Zhang J, Cullen B, Cullen MG. Molecular mechanism of desensitization of the chemokine receptor CCR-5: receptor signaling and internalization are dissociable from its role as an HIV-1 co-receptor. EMBO J. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Huggard PR, Cummings M, Ramm GA, Chenevix-Trench G. Reduced expression of chemokine (C-C motif) ligand-2 (CCL2) in ovarian adenocarcinoma. Br J Cancer. 2005;92:2024–2031. doi: 10.1038/sj.bjc.6602596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial IM. The CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009 doi: 10.1182/blood-2008-10-186668. Epub, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beider K, Abraham M, Peled A. Chemokines and chemokine receptors in stem cell circulation. Front Biosci. 2008;13:6820–6833. doi: 10.2741/3190. [DOI] [PubMed] [Google Scholar]
- Biswas P, Delfanti F, Bernasconi S, Mengozzi M, Cota M, Polentarutti N, Mantovani A, Lazzarin A, Sozzani S, Poli G. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91:258–265. [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner M, Meyer B, Schreck S, Niedobitek G. Expression of RANTES and MCP-1 in epithelial cells is regulated via LMP1 and CD40. Int J Cancer. 2007;121:2703–2710. doi: 10.1002/ijc.23018. [DOI] [PubMed] [Google Scholar]
- Bug G, Rossmanith T, Henschler R, Kunz-Schughart LA, Schroder B, Kampfmann M, Kreutz M, Hoelzer D, Ottmann OG. Rho family small GTPases control migration of hematopoietic progenitor cells into multicellular spheroids of bone marrow stroma cells. J Leukoc Biol. 2002;72:837–845. [PubMed] [Google Scholar]
- Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–3667. [PubMed] [Google Scholar]
- Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- Cabioglu N, Summy J, Miller C, Parikh NU, Sahin AA, Tuzlali S, Pumiglia K, Gallick GE, Price JE. CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res. 2005;65:6493–6497. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- Cashen A, Lopez S, Gao F, Calandra G, MacFarland R, Badel K, DiPersio J. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:1253–1261. doi: 10.1016/j.bbmt.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Chari RV. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res. 2008;41:98–107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- Comerford I, Litchfield W, Harata-Lee Y, Nibbs RJ, McColl SR. Regulation of chemotactic networks by ‘atypical’ receptors. Bioessays. 2007;29:237–247. doi: 10.1002/bies.20537. [DOI] [PubMed] [Google Scholar]
- Conti I, Dube C, Rollins BJ. Chemokine-based pathogenetic mechanisms in cancer. In: Chadwick DJ, Goode J, editors. Cancer and Inflammation. Novartis Foundation; 2004. pp. 29–39. [PubMed] [Google Scholar]
- Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, Packman K, Patel PS, Rameshwar P. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE. 2008;3:e2563. doi: 10.1371/journal.pone.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cell Signal. 2009 doi: 10.1016/j.cellsig.2009.02.008. Epub, ahead of print. [DOI] [PubMed] [Google Scholar]
- Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A. Role of high expression levels of CXCR4 in tumor growth, vascularization, metastasis. Faseb J. 2004;18:1240–1242. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- De Paepe B, Creus KK, De Bleecker JL. Chemokines in idiopathic inflammatory myopathies. Front Biosci. 2008;13:2548–2577. doi: 10.2741/2866. [DOI] [PubMed] [Google Scholar]
- Deuel TF, Senior RM, Chang D, Griffin GL, Heinrikson RL, Kaiser ET. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981;78:4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalaraja MN, Richmond A. Multiple chemotactic factors: fine control or redundancy? Trends Pharmacol Sci. 1999;20:151–156. doi: 10.1016/s0165-6147(99)01342-5. [DOI] [PubMed] [Google Scholar]
- DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–415. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72:9–18. [PMC free article] [PubMed] [Google Scholar]
- Evans D. Reuters. Chemokine Therapeutics Corp; 2008. Chemokine Therapeutics Announces Final Results of CTCE-9908 Phase I/II Clinical Trial in Late Stage Cancer Patients. Epub, Press release from. [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- Firestein GS. The T cell cometh: interplay between adaptive immunity and cytokine networks in rheumatoid arthritis. J Clin Invest. 2004;114:471–474. doi: 10.1172/JCI22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick MJ, Henderson Y, Xu X, Deavers MT, Sahin AA, Wu H, Lewis DE, El-Naggar AK, Clayman GL. In vivo expression of the novel CXC chemokine BRAK in normal and cancerous human tissue. Am J Pathol. 2000;156:1937–1950. doi: 10.1016/S0002-9440(10)65067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher PG, Bao Y, Prorock A, Zigrino P, Nischt R, Politi V, Mauch C, Dragulev B, Fox JW. Gene expression profiling reveals cross-talk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res. 2005;65:4134–4146. doi: 10.1158/0008-5472.CAN-04-0415. [DOI] [PubMed] [Google Scholar]
- Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The alpha-chemokine, stromal cell-derived factor-1alpha, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- Goda S, Inoue H, Umehara H, Miyaji M, Nagano Y, Harakawa N, Imai H, Lee P, Macarthy JB, Ikeo T, Domae N, Shimizu Y, Iida J. Matrix metalloproteinase-1 produced by human CXCL12-stimulated natural killer cells. Am J Pathol. 2006;169:445–458. doi: 10.2353/ajpath.2006.050676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765–770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Hatake K, Kasahara T, Takahashi Y, Ikeda M, Tomizuka H, Ohtsuki T, Uwai M, Mukaida N, Matsushima K, Miura Y. Monocyte chemoattractant protein-1 stimulates tumor necrosis and recruitment of macrophages into tumors in tumor-bearing nude mice: increased granulocyte and macrophage progenitors in murine bone marrow. Exp Hematol. 1995;23:1035–1039. [PubMed] [Google Scholar]
- Hotte S, Hirte H, Iacobucci A, Wong D, Korz W, Miller W. Phase I/II study of CTCE-9908, a novel anticancer agent that inhibits CXCR4, in patients with advanced solid cancers. Paper presented at: Molecular Targets and Cancer Therapeutics International Conference.2007. [Google Scholar]
- Huang CY, Fong YC, Lee CY, Chen MY, Tsai HC, Hsu HC, Tang CH. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 2008;77:794–803. doi: 10.1016/j.bcp.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Kim SY, Lee CH, Midura BV, Yeung C, Mendoza A, Hong SH, Ren L, Wong D, Korz W, Merzouk A, Salari H, Zhang H, Hwang ST, Khanna C, Helman LJ. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2008;25:201–211. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, Bloushtain-Qimron N, Chen YH, Carrasco D, Hu M, Yao J, Kraeft SK, Collins LC, Sabel MS, Argani P, Gelman R, Schnitt SJ, Krop IE, Polyak K. Epithelial and stromal cathepsin K and CXCL14 expression in breast tumor progression. Clin Cancer Res. 2008;14:5357–5367. doi: 10.1158/1078-0432.CCR-08-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Lowinski K, Gillitzer R, Kuhne-Heid R, Rosl F. Monocyte-chemo-attractant-protein-1 (MCP-1)-gene expression in cervical intra-epithelial neoplasias and cervical carcinomas. Int J Cancer. 1999;82:6–11. doi: 10.1002/(sici)1097-0215(19990702)82:1<6::aid-ijc2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355–10362. doi: 10.1158/0008-5472.CAN-05-0957. [DOI] [PubMed] [Google Scholar]
- Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol. 2004;28:443–460. doi: 10.1016/j.dci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Li J, Sidell N. Growth-related oncogene produced in human breast cancer cells and regulated by Syk protein-tyrosine kinase. Int J Cancer. 2005;117:14–20. doi: 10.1002/ijc.21074. [DOI] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. The roles of chemokine CXCL12 in embryonic and brain tumor angiogenesis. Semin Cancer Biol. 2009;19:111–115. doi: 10.1016/j.semcancer.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- Loberg RD, Day LL, Harwood J, Ying C, St John LN, Giles R, Neeley CK, Pienta KJ. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–586. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locati M, Torre YM, Galliera E, Bonecchi R, Bodduluri H, Vago G, Vecchi A, Mantovani A. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 2005;16:679–686. doi: 10.1016/j.cytogfr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Madhusudan S, Foster M, Muthuramalingam SR, Braybrooke JP, Wilner S, Kaur K, Han C, Hoare S, Balkwill F, Talbot DC, Ganesan TS, Harris AL. A phase II study of etanercept (Enbrel), a tumor necrosis factor alpha inhibitor in patients with metastatic breast cancer. Clin Cancer Res. 2004;10:6528–6534. doi: 10.1158/1078-0432.CCR-04-0730. [DOI] [PubMed] [Google Scholar]
- Manome Y, Wen PY, Hershowitz A, Tanaka T, Rollins BJ, Kufe DW, Fine HA. Monocyte chemoattractant protein-1 (MCP-1) gene transduction: an effective tumor vaccine strategy for non-intracranial tumors. Cancer Immunol Immunother. 1995;41:227–235. doi: 10.1007/BF01516997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteucci E, Ridolfi E, Maroni P, Bendinelli P, Desiderio MA. c-Src/histone deacetylase 3 interaction is crucial for hepatocyte growth factor dependent decrease of CXCR4 expression in highly invasive breast tumor cells. Mol Cancer Res. 2007;5:833–845. doi: 10.1158/1541-7786.MCR-07-0054. [DOI] [PubMed] [Google Scholar]
- Melief CJ. Cancer immunology: cat and mouse games. Nature. 2005;437:41–42. doi: 10.1038/437041a. [DOI] [PubMed] [Google Scholar]
- Moll NM, Cossoy MB, Fisher E, Staugaitis SM, Tucky BH, Rietsch AM, Chang A, Fox RJ, Trapp BD, Ransohoff RM. Imaging correlates of leukocyte accumulation and CXCR4/CXCL12 in multiple sclerosis. Arch Neurol. 2009;66:44–53. doi: 10.1001/archneurol.2008.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy AP, Martin FT, Dwyer RM, Griffin TP, Murphy M, Barry FP, O'Brien T, Kerin MJ. Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, their potential role in mediating interactions with breast cancer cells. Int J Cancer. 2009;124:326–332. doi: 10.1002/ijc.23939. [DOI] [PubMed] [Google Scholar]
- Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, Dipersio JF. Chemosensitization of AML following mobilization by the CXCR4 antagonist AMD3100. Blood. 2008 doi: 10.1182/blood-2008-06-162123. Epub, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin Cancer Res. 2001;7:285–289. [PubMed] [Google Scholar]
- Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y, Nakanuma Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155–1168. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Neto HH, Silva ET, Leles CR, Mendonca EF, Alencar Rde C, Silva TA, Batista AC. Involvement of CXCL12 and CXCR4 in lymph node metastases and development of oral squamous cell carcinomas. Tumour Biol. 2008;29:262–271. doi: 10.1159/000152944. [DOI] [PubMed] [Google Scholar]
- Oppermann M. Chemokine receptor CCR5: insights into structure, function, regulation. Cell Signal. 2004;16:1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Ou ZL, Wang J, Hou YF, Luo JM, Shen ZZ, Shao ZM. Downregulation of Duffy antigen receptor for chemokine (DARC) is associated with lymph node metastasis in human breast cancer. Zhonghua Zhong Liu Za Zhi. 2006;28:586–589. [PubMed] [Google Scholar]
- Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- Petersen DC, Severi G, Hoang HN, Padilla EJ, Southey MC, English DR, Hopper JL, Giles GG, Hayes VM. No association between common chemokine and chemokine receptor gene variants and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:3615–3617. doi: 10.1158/1055-9965.EPI-08-0896. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Prest SJ, Rees RC, Murdoch C, Marshall JF, Cooper PA, Bibby M, Li G, Ali SA. Chemokines induce the cellular migration of MCF-7 human breast carcinoma cells: subpopulations of tumour cells display positive and negative chemotaxis and differential in vivo growth potentials. Clin Exp Metastasis. 1999;17:389–396. doi: 10.1023/a:1006657109866. [DOI] [PubMed] [Google Scholar]
- Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- Rajagopalan L, Rajarathnam K. Structural basis of chemokine receptor function--a model for binding affinity and ligand selectivity. Biosci Rep. 2006;26:325–339. doi: 10.1007/s10540-006-9025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003;63:8360–8365. [PubMed] [Google Scholar]
- Rollins BJ, Sunday ME. Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol Cell Biol. 1991;11:3125–3131. doi: 10.1128/mcb.11.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- Saenz-Lopez P, Carretero R, Cozar JM, Romero JM, Canton J, Vilchez JR, Tallada M, Garrido F, Ruiz-Cabello F. Genetic polymorphisms of RANTES, IL1-A, MCP-1 and TNF-A genes in patients with prostate cancer. BMC Cancer. 2008;8:382. doi: 10.1186/1471-2407-8-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin Reprod Med. 2007;25:437–444. doi: 10.1055/s-2007-991041. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- Schrader AJ, Lechner O, Templin M, Dittmar KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T, Gatzlaff P, Atzpodien J, Buer J, Lauber J. CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Cancer. 2002;86:1250–1256. doi: 10.1038/sj.bjc.6600221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, Bridger G, Balkwill FR. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930–5938. [PubMed] [Google Scholar]
- Shellenberger TD, Wang M, Gujrati M, Jayakumar A, Strieter RM, Burdick MD, Ioannides CG, Efferson CL, El-Naggar AK, Roberts D, Clayman GL, Frederick MJ. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64:8262–8270. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]
- Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- Stewart DA, Smith C, MacFarland R, Calandra G. Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood Marrow Transplant. 2009;15:39–46. doi: 10.1016/j.bbmt.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, Dahut WL, Nelson P, Lee JK, Amin MB, Lyles R, Johnstone PA, Marshall FF, Chung LW. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 2008;68:9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Tsukishiro S, Suzumori N, Nishikawa H, Arakawa A, Suzumori K. Elevated serum RANTES levels in patients with ovarian cancer correlate with the extent of the disorder. Gynecol Oncol. 2006;102:542–545. doi: 10.1016/j.ygyno.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, survival in human breast cancer. Clin Cancer Res. 2000;6:3282–3289. [PubMed] [Google Scholar]
- Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. Prostate. 2006;66:124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- Valkovic T, Lucin K, Krstulja M, Dobi-Babic R, Jonjic N. Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol Res Pract. 1998;194:335–340. doi: 10.1016/S0344-0338(98)80057-5. [DOI] [PubMed] [Google Scholar]
- Van de Broek I, Leleu X, Schots R, Facon T, Vanderkerken K, Van Camp B, Van Riet I. Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematologica. 2006;91:200–206. [PubMed] [Google Scholar]
- van Golen KL, Ying C, Sequeira L, Dubyk CW, Reisenberger T, Chinnaiyan AM, Pienta KJ, Loberg RD. CCL2 induces prostate cancer transendothelial cell migration via activation of the small GTPase Rac. J Cell Biochem. 2008;104:1587–1597. doi: 10.1002/jcb.21652. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Colomer R, Menendez JA. Protein array technology to detect HER2 (erbB-2)-induced ‘cytokine signature’ in breast cancer. Eur J Cancer. 2007;43:1117–1124. doi: 10.1016/j.ejca.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Vergunst CE, Gerlag DM, Lopatinskaya L, Klareskog L, Smith MD, van den Bosch F, Dinant HJ, Lee Y, Wyant T, Jacobson EW, Baeten D, Tak PP. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2008;58:1931–1939. doi: 10.1002/art.23591. [DOI] [PubMed] [Google Scholar]
- Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- Vital EM, Emery P. The development of targeted therapies in rheumatoid arthritis. J Autoimmun. 2008;31:219–227. doi: 10.1016/j.jaut.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Zheng W, Zhang S, Chen X, Hornung D. Expression of monocyte chemotactic protein-1 in human endometrial cancer cells and the effect of treatment with tamoxifen or buserelin. J Int Med Res. 2006;34:284–290. doi: 10.1177/147323000603400307. [DOI] [PubMed] [Google Scholar]
- Wente MN, Mayer C, Gaida MM, Michalski CW, Giese T, Bergmann F, Giese NA, Buchler MW, Friess H. CXCL14 expression and potential function in pancreatic cancer. Cancer Lett. 2008;259:209–217. doi: 10.1016/j.canlet.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008;7:2649–2661. doi: 10.1158/1535-7163.MCT-08-0148. [DOI] [PubMed] [Google Scholar]
- Wislez M, Fujimoto N, Izzo JG, Hanna AE, Cody DD, Langley RR, Tang H, Burdick MD, Sato M, Minna JD, Mao L, Wistuba I, Strieter RM, Kurie JM. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- Woodward JK, Elshaw SR, Murray AK, Nichols CE, Cross N, Laws D, Rennie IG, Sisley K. Stimulation and inhibition of uveal melanoma invasion by HGF, GRO, IL1alpha and TGFbeta. Invest Ophthalmol Vis Sci. 2002;43:3144–3152. [PubMed] [Google Scholar]
- Wu FY, Ou ZL, Feng LY, Luo JM, Wang LP, Shen ZZ, Shao ZM. Chemokine decoy receptor d6 plays a negative role in human breast cancer. Mol Cancer Res. 2008;6:1276–1288. doi: 10.1158/1541-7786.MCR-07-2108. [DOI] [PubMed] [Google Scholar]
- Wysocki PJ, Zolnierek J, Szczylik C, Mackiewicz A. Targeted therapy of renal cell cancer. Curr Opin Investig Drugs. 2008;9:570–575. [PubMed] [Google Scholar]
- Yasumoto K, Koizumi K, Kawashima A, Saitoh Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi Y, Yoshie O, Saiki I. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66:2181–2187. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
- Zabel BA, Zuniga L, Ohyama T, Allen SJ, Cichy J, Handel TM, Butcher EC. Chemoattractants, extracellular proteases, the integrated host defense response. Exp Hematol. 2006;34:1021–1032. doi: 10.1016/j.exphem.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Zhang S, Qi L, Li M, Zhang D, Xu S, Wang N, Sun B. Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J Exp Clin Cancer Res. 2008;27:62. doi: 10.1186/1756-9966-27-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Roybal J, Chaerkady R, Zhang W, Choi K, Alvarez CA, Tran H, Creighton CJ, Yan S, Strieter RM, Pandey A, Kurie JM. Identification of secreted proteins that mediate cell-cell interactions in an in vitro model of the lung cancer microenvironment. Cancer Res. 2008;68:7237–7245. doi: 10.1158/0008-5472.CAN-08-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A. The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol. 2006;208:507–17. doi: 10.1002/path.1918. [DOI] [PubMed] [Google Scholar]
- Zlotnick A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biology. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]


