Abstract
House mice offer a powerful system for dissecting the genetic basis of phenotypes that isolate species in the early stages of speciation. We used a series of reciprocal crosses between wild-derived strains of Mus musculus and M. domesticus to examine F1 hybrid male sterility, one of the primary phenotypes thought to isolate these species. We report four main results. First, we found significantly smaller testes and fewer sperm in hybrid male progeny of most crosses. Second, in some crosses hybrid male sterility was asymmetric and depended on the species origin of the X chromosome. These observations confirm and extend previous findings, underscoring the central role that the M. musculus X chromosome plays in reproductive isolation. Third, comparisons among reciprocal crosses revealed polymorphism at one or more hybrid incompatibilities within M. musculus. Fourth, the spermatogenic phenotype of this polymorphic interaction appears distinct from previously described hybrid incompatibilities between these species. These data build on previous studies of speciation in house mice and show that the genetic basis of hybrid male sterility is fairly complex, even at this early stage of divergence.
Keywords: Hybridization, polymorphism, reproductive isolation, speciation
Identifying the specific mutations that isolate incipient species in nature remains a central problem in evolutionary biology. In animals, one common form of isolation occurs when reproduction between two populations results in hybrid offspring with reduced fertility or viability independent of external factors (i.e., intrinsic postzygotic isolation). According to the simple genetic model developed by Bateson (Bateson 1909), Dobzhansky (Dobzhansky 1937), and Muller (Muller 1942), intrinsic postzygotic isolation often evolves due to incompatible mutations at interacting genes (i.e., Dobzhansky-Muller [D–M] incompatibilities). For example, consider two populations with alternative genotypes fixed at two interacting loci, AAbb and aaBB, where aabb was the ancestral genotype. Reproduction between these two populations will produce an AaBb F1 hybrid genotype that may have reduced fitness because A and B have never been tested together by natural selection. There is now considerable empirical support for this model (Coyne and Orr 2004), including multiple studies that have implicated specific genes involved in hybrid sterility (Ting et al. 1998) or inviability (Wittbrodt et al. 1989; Barbash et al. 2003; Presgraves et al. 2003; Brideau et al. 2006; Harrison and Burton 2006).
Despite these fundamental insights, we still know little about the genetic details of reproductive isolation during the earliest stages of speciation. This is because many genetic studies of speciation have relied upon crosses between divergent species that do not hybridize in nature (e.g., Barbash et al. 2003; Presgraves et al. 2003; Brideau et al. 2006). In such cases it is impossible to determine if phenotypes contributing to isolation in the laboratory were ever relevant to gene flow between natural populations (Harrison 1990; Orr and Presgraves 2000). Furthermore, several of the predictions of the D–M model depend critically upon the degree of functional divergence between two hybridizing genomes. For example, under one common model of speciation the number of D–M incompatibilities is expected to increase much faster than linearly with time (Orr 1995). When considering very divergent species, it is likely that many incompatible mutations arose long after the completion of reproductive isolation in nature (Orr 1995). Whether the genetic details of postisolation incompatibilities are representative of the processes that directly contributed to speciation remains an open question.
During the early stages of speciation, reproductive isolation might exhibit patterns not seen at later stages of divergence. For example, consider the general result that F1 hybrid sterility or inviability usually manifests in the heterogametic sex first (i.e., Haldane’s rule; Haldane 1922; Coyne and Orr 1997; Laurie 1997; Orr 1997). A primary explanation for Haldane’s rule is the exposure of epistatic interactions between recessive sex-linked and dominant autosomal D–M incompatibilities in the heterogametic sex (i.e., the dominance theory; Turelli and Orr 1995, 2000). For taxa with heterogametic males, including mammals and Drosophila, this typically involves X-autosome interactions. Even at low levels of divergence Haldane’s rule is often observed regardless of the direction of the intercross (Coyne and Orr 1989a, 1997), presumably because X-linked incompatibilities have accumulated independently in both lineages. However, if the early stages of isolation involve X-linked incompatibilities in one lineage only, then F1 hybrid male dysfunction may initially be asymmetric and depend upon the maternal origin of the X.
The strength of reproductive isolation may also depend on whether individual incompatibilities are fixed between diverging populations. Most theoretical treatments of the D–M model assume the instantaneous fixation of incompatible mutations between populations (e.g., Orr 1995; Turelli and Orr 1995; Orr and Orr 1996). On the other hand, several examples of intraspecific variation in the degree of postzygotic isolation between plant (e.g., Stebbins 1958; Christie and Macnair 1987; Sweigart et al. 2007) and animal species (e.g., Gordon 1927; Patterson and Stone 1952; Forejt and Iványi 1975; Wade and Johnson 1994; Reed and Markow 2004; Kopp and Frank 2005; Shuker et al. 2005; Vyskocilová et al. 2005; Demuth and Wade 2007) have been described. Although only a few studies have examined natural variation in the context of specific loci (Forejt and Iványi 1975; Christie and Macnair 1987; Vyskocilová et al. 2005; Sweigart et al. 2007), all of these data are consistent with polymorphism of D–M incompatibilities.
House mice provide a particularly powerful system for studying the early stages of speciation, with considerable genetic and genomic resources (Dietrich et al. 1996; Mouse Genome Sequencing Consortium 2002; Su et al. 2004; Shifman et al. 2006). House mice are comprised of at least three closely related lineages (Mus domesticus, M. musculus, and M. castaneus) that have diverged from a common ancestor within the last 0.5 million years ago (MYA; She et al. 1990; Boursot et al. 1993). This recent divergence is reflected in the literature by the alternative taxonomic treatment of the three lineages as subspecies of M. musculus (i.e., M. m. domesticus, M. m. musculus, and M. m. castaneus). The most studied pair from the standpoint of speciation is M. domesticus and M. musculus. The native range of M. domesticus occurs in Western Europe, North Africa, and the Middle East, whereas M. musculus is found in Eastern Europe and Northern Asia (Boursot et al. 1993). The two species form a narrow hybrid zone along ~ 2000 km in Europe (Boursot et al. 1993; Sage et al. 1993). Although the exact phenotypes governing reproductive isolation across the hybrid zone are unresolved, some hybrid populations have elevated parasite loads (Sage et al. 1986; Moulia et al. 1991, 1993) and reduced testis size (Britton-Davidian et al. 2005). In the hybrid zone, the X chromosome shows reduced introgression relative to autosomal loci across multiple transects (Tucker et al. 1992; Dod et al. 1993; Munclinger et al. 2002; Macholán et al. 2007), suggesting a large contribution of the X chromosome to reproductive isolation.
Complementary to hybrid zone studies, several laboratory experiments support the notion that M. domesticus and M. musculus are isolated by hybrid male sterility (Table 1). Two sets of D–M incompatibilities have been described between M. domesticus and M. musculus: one set of dominant autosomal epistatic interactions (≥ 3 major sterility factors) that include one or more tightly linked loci on chromosome 17 (Hybrid sterility 1 or Hst1; Forejt and Iványi 1975; Forejt et al. 1991; Vyskocilová et al. 2005) and one set of X-autosome interactions (Storchová et al. 2004; Britton-Davidian et al. 2005; see also Oka et al. 2004, 2007). Interestingly, some of the underlying D–M incompatibilities involved in hybrid male sterility, including Hst1, are apparently not fixed within these species (Forejt and Iványi 1975; Vyskocilová et al. 2005). However, many of these studies are compromised by the use of classic laboratory inbred strains to represent M. domesticus (Table 1). Although primarily of M. domesticus origin (> 80%), the genomes of most classic inbred strains include substantial genetic contributions from both M. musculus and M. castaneus (Wade et al. 2002; Frazer et al., 2007; Yang et al., 2007). The result is a collection of introgressed hybrid genomes (Wade and Daly 2005) shaped by epistatic selection against D–M incompatibilities (Payseur and Hoekstra 2005) and strong artificial laboratory selection (Petkov et al. 2005). Thus, the species origin, existence of natural polymorphism, and biological relevance of D–M incompatibilities remain ambiguous in crosses involving classic inbred strains.
Table 1.
Summary of experiments on F1 hybrid male fertility in house mice.
| No. | Experiment type | HMS1 | Polymorph.2 | References | |
|---|---|---|---|---|---|
| Classic inbred×wild-derived (directional) | |||||
| 1 | ♀Classic inbreds (C57BL/10, A/Ph, BALB/c, DBA/1, AKR/J, C3H/Di, CBA/J, P/J, I/St, F/st) | × ♂M. musculus (wild isolates) | Yes | Maternal and paternal | (Forejt and Iványi 1975) |
| 2 | ♀Classic inbred (C57BL/10) | × ♂M. musculus (PWD, PWK, PWB) | Yes | No | (Forejt 1981) |
| 3 | ♀Classic inbred (BALB/c) | × ♂M. musculus (PWD, PWK) | Yes | No | (Chubb and Nolan 1987) |
| 4 | ♀Classic inbred (A/J, BALB, C57BL/6, AKR, 129, CBA, GRS, SM, C3H) | × ♂M. musculus (NJL) | Yes | Maternal | (Yoshiki et al. 1993) |
| 5 | ♀Classic inbred (C57BL/6) | × ♂M. m. molossinus3 (MSM/Ms) | No | N/A | (Oka et al. 2004) |
| Classic inbred×wild-derived (reciprocal) | |||||
| 6 | ♀Classic inbred (C57BL/10) | × ♂M. musculus (wild isolates) | Yes | Paternal | (Vyskocilová et al. 2005) |
| 7 | ♀M. musculus (wild isolates) | × ♂classic inbred (C57BL/10) | Yes | Maternal | (Vyskocilová et al. 2005) |
| Wild-derived×wild-derived (reciprocal) | |||||
| 8 | ♀M. musculus (PWK) | × ♂M. domesticus (WLA) | Yes | No | (Britton-Davidian et al. 2005) |
| 9 | ♀M. domesticus (WLA) | × ♂M. musculus (PWK) | No | N/A | (Britton-Davidian et al. 2005) |
| 104 | ♀M. musculus (MDH) | × ♂M. domesticus (DDO) | Yes | Yes – unresolved | (Alibert et al. 1997; Britton-Davidian et al. 2005) |
| 114 | ♀M. domesticus (DDO) | × ♂M. musculus (MDH) | Yes | Yes – unresolved | (Alibert et al. 1997; Britton-Davidian et al. 2005) |
| 12 | ♀M. musculus (wild isolates) | × ♂M. domesticus (wild isolates) | No | N/A | (Vanlerberghe et al. 1986) |
| 13 | ♀M. domesticus (wild isolates) | × ♂M. musculus (wild isolates) | No | N/A | (Vanlerberghe et al. 1986) |
Hybrid male sterility for F1 males for a given cross, based on some observed reduction male fertility-related parameters.
Indicates if polymorphism in F1 HMS was observed and could be traced to the maternal or paternal line.
M. musculus molossinus (MSM/Ms) is a wild-derived inbred strain from natural populations in Japan that are of hybrid origin (M. musculus×M. castaneus; Yonekawa et al. 1988).
MDH and DDO are wild-derived outbred strains with low levels of natural introgression. DDO is fixed for a nonstandard karyotype (2n = 34).
Data from crosses involving exclusively wild-derived mice are more limited, and most of these have used outbred mice. In some cases, crosses between the two species produce fully fertile hybrid males (Vanlerberghe et al. 1986), whereas others show reduced hybrid male fertility with variation in both the strength and direction of sterility (Alibert et al. 1997; Britton-Davidian et al. 2005). The genetic basis of this variation is very difficult to discern with outbred wild mice. The most comprehensive study of hybrid male sterility using wild mice was performed by Britton-Davidian et al. (2005), who used both outbred and inbred strains (crosses 8–11, Table 1). The inbred strains consisted of one from each species (PWK representing M. musculus and WLA representing M. domesticus). In crosses between these strains, they found asymmetric F1 male sterility consistent with an X-linked locus in M. musculus interacting with one or more autosomal loci in M. domesticus. Britton-Davidian et al. (2005) did not cross PWK to other strains and thus could not assess the generality of this pattern. Asymmetric sterility has not been observed broadly in other experiments using wild mice (Vanlerberghe et al. 1986; Alibert et al. 1997; Britton-Davidian et al. 2005) and/or classic inbred strains (Vyskocilová et al. 2005).
Here, we examine patterns of hybrid male fertility between M. musculus and M. domesticus using a series of reciprocal crosses between multiple wild-derived inbred and outbred strains of mice. Our goals are to test the generality of several previous findings across different genetic backgrounds, to describe the number of different sets of interacting loci, and to develop these findings in the context of specific wild-derived inbred strains that can subsequently be used for mapping of genes involved in mouse speciation. We address four primary questions: (1) Do F1 hybrid males show reduced fertility in crosses involving wild-derived inbred lines? (2) Is hybrid male sterility asymmetric? (3) Are D–M incompatibilities polymorphic within a single species? (4) At what developmental stage does hybrid sterility arise?
Materials and Methods
ANIMALS AND HUSBANDRY
Breeding colonies for each of four wild-derived inbred strains were established from individuals purchased from the Jackson Laboratory (Bar Harbor, ME). Within M. domesticus we used the strains M. domesticusLEWES/EiJ and M. domesticusWSB/EiJ (hereafter domesticusLEWES and domesticusWSB). Both were isolated from natural populations in eastern North America (Delaware and Maryland, USA, respectively) and represent a recent range expansion of M. domesticus associated with human migration from Western Europe. Within M. musculus we used two strains isolated from different localities outside of the hybrid zone within the Czech Republic, M. musculusCZECHII/EiJ and M. musculusPWK/PhJ (hereafter musculusCZECH and musculusPWK). All four strains have the standard house mouse karyotype (2n = 40). Detailed information on the known history of each strain is available from the Jackson Laboratory (www.jax.org). In addition, we founded an outbred colony of M. musculus derived from mice collected by J. Piálek in the eastern portion of the Czech Republic during 2003–2004 (vicinity of Studenec, 49°11′N, 16°03′E). Starting with eight initial founding pairs, we maintained four breeding pairs per generation using a crossing scheme designed to maximize inbreeding avoidance (Wright 1921). All crosses using outbred mice involved males from the fourth or fifth laboratory generation since collection. The expected inbreeding coefficient after five generations of breeding under our scheme is f = 0.0625, assuming the founding mice were unrelated. Because some of the founders were collected from the same locality, the actual inbreeding coefficient may be higher. Mice were maintained at the University of Arizona Central Animal Facility in accordance with IACUC regulations. Breeding pairs were housed two per cage and pregnant females were isolated and caged individually prior to giving birth.
EXPERIMENTAL DESIGN
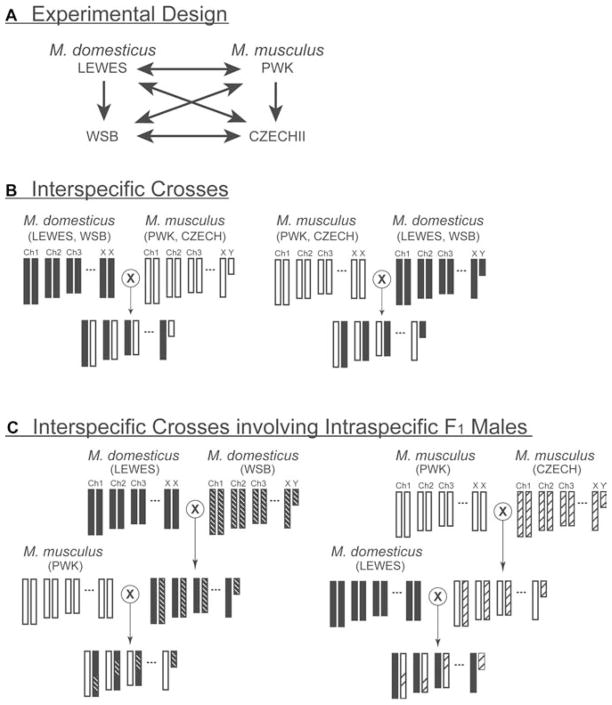
We conducted two kinds of crosses. First, we performed control crosses between the two wild-derived inbred strains within each species to establish null expectations for patterns of F1 male fertility in each species. These controls are important for removing the effects of inbreeding depression. For M. musculus we crossed female musculusPWK with male musculusCZECH, and for M. domesticus we crossed female domesticusLEWES with male domesticusWSB. Second, we performed 11 different types of interspecific crosses. With two strains per species a total of eight pairwise combinations are possible (Fig. 1A, B). We performed two additional crosses involving inbred females from each species (musculusPWK, domesticusLEWES) mated to intraspecific F1 males to evaluate the segregation of hybrid sterility including the potential role of the Y chromosome (Fig. 1C). Finally, to extend the generality of our findings, we performed a series of interspecific crosses between inbred domesticusLEWES females and outbred M. musculus males.
Figure 1.
Experimental crossing scheme and genetic composition of F1 hybrid males. (A) Inter- and intraspecific crossing design, reciprocal crosses are indicated with double headed arrows. (B) Schematic of reciprocal interspecific crosses with M. musculus chromosomes colored white and M. domesticus chromosomes colored black. (C) Interspecific crosses involving an inbred female crossed to an intraspecific F1 male. Recombinant genotypes for M. domesticus (LEWES and WSB) and M. musculus (PWK and CZECH) are distinguished with crosshatch shading.
Interspecific crosses involving musculusPWK are potentially informative regarding the presence of the Hst1 sterility system in the domesticusLEWES and domesticusWSB strains. Hst1-related sterility was described based on crosses between classic inbred strains and wild-caught M. musculus (Table 1). Sterility is caused by interactions between one or more tightly linked loci on chromosome 17 (Hst1; Forejt and Iványi 1975; Forejt et al. 1991) and several other loci (as yet uncharacterized). The Hst locus contains alleles in both classic inbred strains (sterile allele, Hst1s; fertile allele, Hst1f) and wild-derived M. musculus (sterile allele, Hstws; fertile allele, Hstwf). Sterility occurs in male F1 hybrid mice that are heterozygous Hst1s/Hstws. The musculusPWK strain is derived from wild-caught M. musculus mice and is thought to be fixed for the sterility-ensuring Hstws allele (Forejt 1981). Crosses between male musculusPWK and some classic inbred strains (e.g., C57BL/10, BALB/c) yield sterile males characterized by spermatogenic arrest at the pachytene spermatocyte stage (Forejt 1981; Chubb and Nolan 1987). Thus, if the Hst1s allele and related interacting loci are present in domesticusLEWES or domesticusWSB, then sterile sons with early meiotic arrest should result when crossed with male musculusPWK. This assumes that the musculusPWK strain in our experiment retained Hst1-related sterility described in earlier experiments (Forejt 1981; Chubb and Nolan 1987).
A minimum of four crosses was attempted per experimental treatment. Male offspring were weaned at approximately 20 days postpartum and housed in sibling groups of up to four. To reduce potential variance in male fertility associated with social dominance interactions, males were separated into individual cages at 40 days and maintained in isolation until being sacrificed.
QUANTIFICATION OF MALE FERTILITY PARAMETERS
We considered multiple male reproductive phenotypes including testis weight, sperm count, sperm motility, and seminal vesicle weight. In mice, testis weight is highly correlated with sperm count and provides a good measure of the overall reproductive status whereas seminal vesicle weight is highly sensitive to serological levels of testosterone (Forejt and Iványi 1975). Throughout we use the terms “sterility” and “fertility” to reflect general patterns in multiple fertility-related phenotypes. Nevertheless, hybrid male sterility is a complex phenotype and in some cases males with dramatically reduced gamete production will nevertheless be capable of siring some offspring (see below).
All males were sacrificed at an age of 60 days unless noted. In mice, spermatogenesis starts at birth (Eddy 2002), weaning occurs ~20 days postpartum (Pelikán 1981), and males are capable of inseminating females by the age of 50 days (J. M. Good, pers. obs.; L. Drickamer, unpubl. data). For each male, testes and seminal vesicles were immediately dissected and placed in sealed Eppendorf tubes until they could be weighed. We also collected body weight and standard size measurements including length of total body, tail, right hind foot, and right ear.
We evaluated sperm motility and counts using a Makler counting chamber (Sefi-Medical Instruments, Haifa, Israel) and a light microscope at 200× magnification. Sperm suspensions were created by placing the caudal epididymides in a prewarmed (37°C) watch glass with 1 mL of modified Phosphate Buffered Saline (Modified Dulbecco’s solution; www.jax.org/cryo/media.html). The paired caudal epididymides were cut into pieces with a razor blade, covered with parafilm, and allowed to incubate at 37°C for 10 m. Following incubation the epididymides were removed with forceps and the solution was gently mixed with a 200 mL pipettor using a wide-bore tip. To estimate sperm motility, we transferred 5 μl of sperm suspension to the Makler chamber and immediately counted the number of motile and immotile sperm. Overlaid onto the cover slip of the Makler chamber is a 1 mm2 10 × 10 grid. We arbitrarily chose one row of 10 squares, recording the total number of motile sperm summed across squares with a 10 sec observation time per square. We then recorded the total number of nonmotile sperm per row and calculated the proportion of motile sperm. To estimate overall sperm counts, 200 mL of the incubated sperm suspension was transferred to a 0.6-mL tube and heat shocked at 60°C for 5 min to stop motility. This aliquot was then gently mixed and 5 μl was transferred to the Makler chamber. The average number of sperm heads was determined across five rows where each row represents an estimate of sperm concentration in million per 1 mL. In general, these methods were characterized by high precision. For example, we found relatively low variance among 10 independent estimates of sperm count from the same male (mean = 16.5 × 106 sperm, SD = 1.1).
For a subset of males we examined fecundity based on litter size following pairing with musculusPWK females. F1 males (> 60 days postpartum) were caged with a single female and each male was paired successively with two separate females. Females were separated when pregnant or after 20 days. Litter size was recorded within 5 days postpartum. We chose musculusPWK females for this assay because they consistently produce larger litters than the other three inbred strains.
TESTIS HISTOLOGY
Testis histological cross-sections were examined in four intraspecific males (N = 2, ♀ musculusPWK × ♂ musculusCZECH; N = 2, ♀ domesticusLEWES × ♂ domesticusWSB) and 10 interspecific males (N = 2, ♀ musculusPWK × ♂ domesticusLEWES; N = 2, ♀ domesticusLEWES × ♂ musculusPWK; N = 1, ♀ musculusCZECH × ♂ domesticusLEWES; N = 1, ♀ domesticusLEWES × ♂ musculusCZECH; N = 2, ♀ musculusCZECH × ♂ domesticusWSB; N = 2, ♀ domesticusWSB × ♂ musculusCZECH). For each male, we transferred a single whole testis into Bouin’s solution (Ricca Chemical) immediately following sacrifice. Following 24 h of fixation in Bouin’s solution, testes were progressively dehydrated with 30-min ethanol washes (×2 25%, ×2 50%, ×4 70%, ×2 100%). Dehydrated testes were embedded in paraffin, cut into 5-μm cross-sections, stained using periodic acid-Schiff (PAS) according to standard protocols. Histological cross-sections were examined and classified as normal or abnormal based on standard criteria for the progression of mouse spermatogenesis (Russell et al. 1990). All specimens were analyzed blind with respect to their genotype and sample classifications were independently verified.
MOLECULAR ANALYSES
Male fertility within both M. domesticus and M. musculus can be strongly influenced by the t allele (Silver 1985). To detect the presence of the t bearing haplotype in crosses involving outbred M. musculus males we performed a length polymorphism PCR assay using a previously developed protocol for the Tcp-1 locus (Planchart et al. 2000). This assay is diagnostic of t haplotypes versus wild-type alleles but does not discriminate among different t haplotypes. DNA was extracted from fresh tissue using a Purgene DNA isolation kit (Gentra Systems, Minneapolis, MN).
Results
INTRASPECIFIC COMPARISONS
We collected data on several characters related to male fertility for a total of 136 inter- and intraspecific F1 males (all 60-day old) from 59 litters. We first considered F1 males from crosses between two inbred strains within each species (Table 2). Consistent with known species differences, M. domesticus males were significantly larger than M. musculus males for both body (Wilcoxon P = 0.0091) and testis weight (Wilcoxon P = 0.0140). For our combined intraspecific data we found a strong positive association between body weight and testis weight (r2 = 0.58, F 1,18 = 24.94, P < 0.0001), seminal vesicle weight (r2 = 0.56, F 1,18 = 22.76, P = 0.0002), and sperm count (r2 = 0.34, F 1,18 = 9.46, P = 0.0065) but a weak negative association between body weight and sperm motility (r2 = 0.23, F 1,18 = 5.47, P < 0.0311). To correct for size-related differences, we also report testis and seminal vesicle weight (mg) relative to body weight (g) (i.e., Relative Testis Weight, RTW; Relative Seminal Vesicle Weight, RSVW).
Table 2.
Mean reproductive parameters for M. musculus, M. domesticus, and their F1 hybrids.
| Cross | Male progeny | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of males | No. of litters | Body weight g (SD) | Testis weight1 mg (SD) | RTW2 mg/g (SD) | RSVW3 mg/g (SD) | Motility % (SD) | Sperm Count 1×106 (SD) | |
| Intraspecific (control) crosses | ||||||||
| ♀ musculusPWK× ♂ musculusCZECH | 10 | 5 | 13.2 (0.9) | 67.8 (8.3) | 5.1 (0.6) | 4.3 (0.8) | 92.5 (4.0) | 14.3 (3.2) |
| ♀ domesticusLEWES× domesticusWSB | 10 | 6 | 14.7 (1.3) | 81.1 (11.3) | 5.5 (0.5) | 4.6 (0.6) | 87.3 (6.7) | 17.3 (6.0) |
| Combined intraspecific crosses | 20 | 11 | 13.9 (1.3) | 74.4 (11.8) | 5.3 (0.6) | 4.5 (0.7) | 89.9 (6.0) | 15.8 (4.9) |
| Interspecific crosses | ||||||||
| ♀ musculusCZECH× ♂ domesticusLEWES | 9 | 4 | 14.0 (0.8) | 39.6 (4.8)▾ | 2.8 (0.2)▾ | 4.7 (1.1) | n.a. | 2.1 (2.6)▾ |
| ♀ musculusCZECH× ♂ domesticusWSB | 8 | 4 | 17.6 (1.8)▴ | 51.5 (3.0)▾ | 2.9 (0.3)▾ | 5.1 (0.7) | n.a. | 0.1 (0.4)▾ |
| ♀ musculusPWK× ♂ domesticusLEWES | 12 | 5 | 15.1 (1.4) | 49.5 (5.3)▾ | 3.3 (0.2)▾ | 4.6 (0.7) | n.a. | 1.4 (2.0)▾ |
| ♀ musculusPWK× ♂ domesticusWSB | 10 | 4 | 15.1 (1.2) | 50.1 (7.5)▾ | 3.3 (0.4)▾ | 4.0 (0.8) | n.a. | 0.9 (0.6)▾ |
| ♀ domesticusLEWES× ♂ musculusCZECH | 7 | 4 | 15.4 (1.1) | 39.3 (4.2)▾ | 2.6 (0.3)▾ | 5.0 (1.7) | n.a. | 1.4 (0.9)▾ |
| ♀ domesticusLEWES× ♂ musculusPWK | 11 | 4 | 17.1 (0.9)▴ | 83.4 (2.3)▾ | 4.9 (0.3) | 5.9 (0.8)▴ | 87.0 (5.7) | 12.8 (2.6) |
| ♀ domesticusWSB× ♂ musculusCZECH | 8 | 3 | 17.7 (1.4)▴ | 34.5 (5.8)▾ | 2.0 (0.3)▾ | 5.6 (0.9)▴ | n.a. | 0.0 (0.1)▾ |
| ♀ domesticusWSB× ♂ musculusPWK | 8 | 4 | 16.3 (0.6)▴ | 68.0 (5.8) | 4.2 (0.4)▾ | 5.1 (0.8) | 91.9 (2.9) | 14.3 (3.4) |
▴ or ▾, significantly larger or smaller versus the combined intraspecific crosses based on a Wilcoxon rank sum test and a Bonferroni-corrected α = 0.0063.
Single average testis weight in milligrams.
Relative testis weight in milligrams of testis per gram of body weight.
Relative seminal vesicle weight in milligrams of seminal vesicle per gram of body weight.
INTERSPECIFIC COMPARISONS
Most interspecific F1 hybrid males had strongly reduced male reproductive parameters. Half of the crosses between M. domesticus and M. musculus produced males that were significantly larger than males from intraspecific control crosses (Table 2). In contrast, six of the eight interspecific crosses produced males with severely reduced testis weights and sperm counts. Both crosses involving a male musculusPWK produced reproductively normal hybrid males and will be discussed in more detail below. In the six crosses yielding sterile males the average hybrid RTW ranged between 38% (♀ domesticusWSB × ♂ musculusCZECH) and 62% (♀ musculusPWK × ♂ domesticusWSB) of the average for the combined intraspecific control set. Likewise, approximately 40% (22/54) of the males from these six crosses contained no sperm in their caudal epididymides. Because sperm numbers were low or absent, reliable estimates of sperm motility in progeny from these crosses were not possible. General qualitative differences between groups given in Table 2 remained consistent when considering older males (> 60-day old). For example, the strong asymmetrical reduction in testis weights and sperm counts observed in the reciprocal cross between musculusPWK and domesticusLEWES was still apparent in males 110 days and older (N = 44, data not shown). We observed approximately equal sex ratios for interspecific crosses (39 litters, 198 offspring, 51.0% male) with no significant heterogeneity across cross types (χ2 = 7.82, P = 0.55, df = 9).
Hybrid male sterility was partially asymmetric
Crosses involving female musculusPWK and male M. domesticus (domesticusWSB or domesticusLEWES) produced male progeny with significantly reduced testis weights and sperm counts (Fig. 2B, Table 2). In contrast, the reciprocal crosses involving a male musculusPWK produced reproductively normal hybrid males (Fig. 2C, Table 2). Males sired from reciprocal crosses share the same autosomal genotype but differ in the species origin of the sex chromosomes (Fig. 1B). In these crosses, reduced fertility was observed when hybrid males had a M. domesticus Y chromosome and a M. musculus X chromosome. Thus, at least one set of hybrid incompatibilities involved one or both of the sex chromosomes. As part of an ongoing research program, we have introgressed the musculusPWK X chromosome onto the domesticusLEWES autosomal background over several backcross generations. In these backcrosses, male sterility segregates with the M. musculus X chromosome (J. M. Good, unpubl. data).
Figure 2.
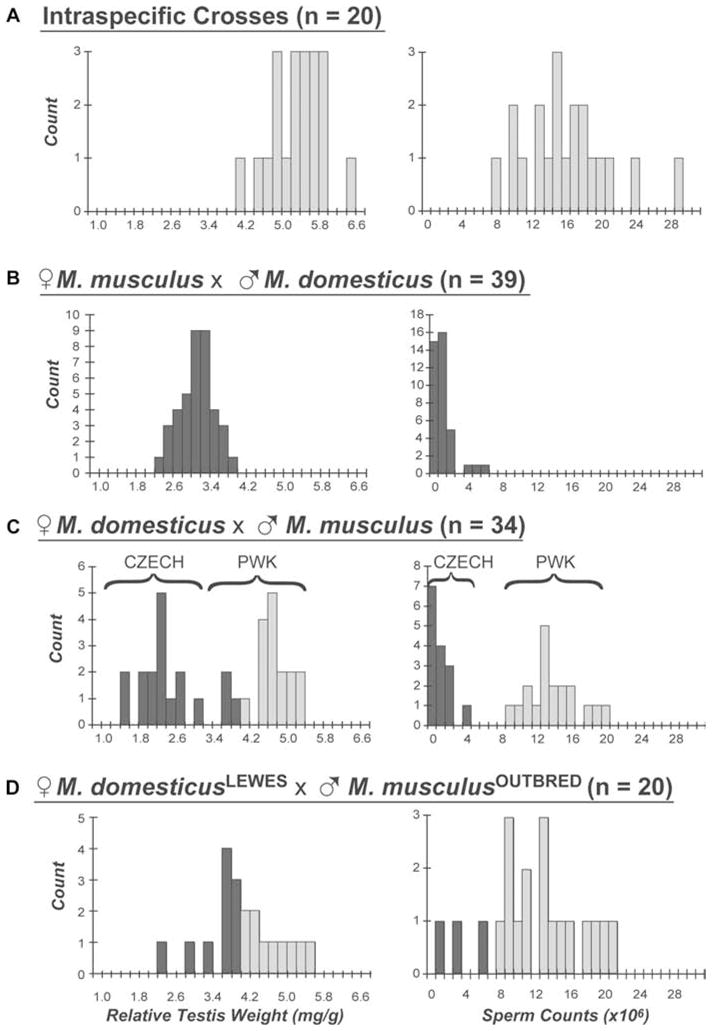
Distributions of relative testis weights (RTW) and sperm counts for male progeny from (A) the combined intraspecific crosses, (B) the interspecific crosses between female M. musculus and male M. domesticus, (C) the interspecific crosses between female M. domesticus and male M. musculus with the strain of the sire indicated (PWK or CZECH), and (D) the interspecific crosses between female M. domesticusLEWES and seven outbred M. musculus males. Shading in (B), (C), and (D) indicate values within the observed range of the combined intraspecific crosses (light) or values outside of these distributions (dark).
To test if hybrid sterility was due to a common set of incompatibilities that are fixed across strains of M. domesticus, we crossed musculusPWK females to M. domesticusLEWES×WSB F1 males. Consistent with a common set of incompatibilities, all 13 males demonstrated significantly reduced reproductive parameters (Table 3). However, given the sample size we cannot exclude a more complicated scenario involving multiple independent loci within each strain.
Table 3.
Mean reproductive parameters for interspecific crosses involving females of each species mated to intraspecific F1 males.
| Cross | Progeny | ||||
|---|---|---|---|---|---|
| No. of males | No. of litters | RTW1 mg/g (SD) | Sperm count 1×106 (SD) | No. of recovered males | |
| ♀ musculusPWK× ♂ domesticusLEWES×WSB | 13 | 3 | 2.9 (0.8) | 1.8 (2.8) | 02 |
| ♀ domesticusLEWES × ♂ musculusPWK×CZECH | 10 | 3 | 3.2 (0.8) | 6.4 (4.3) | 53 |
Relative testis weight in milligrams of testis per gram of body weight.
Males with testis weight, RTW, and sperm counts all higher than the range of values observed for male progeny from crosses between ♀ musculusPWK× ♂ domesticusLEWES and ♀ musculusPWK× ♂ domesticusWSB.
Males with testis weight, RTW, and sperm counts all higher than the range of values observed for male progeny from crosses between ♀ domesticusLEWES× ♂ musculusCZECH.
Asymmetric fertility was also apparent in patterns of fecundity. Five crosses between ♀ musculusPWK and F1 males from the ♀ domesticusLEWES × ♂ musculusPWK cross produced an average of 6.4 offspring per litter (± 1.7 SD) whereas five crosses between ♀ musculusPWK and F1 males from the female ♀ musculusPWK × ♂ domesticusWSB yielded an average of 2.4 offspring per litter (± 2.9 SD). Although small sample sizes preclude significance (Wilcoxon P = 0.0555), this result is qualitatively consistent with our expectations given the differences in testis weight and sperm count between these groups (Table 2).
One or more sterility factors were polymorphic in M. musculus
Hybrid fertility was dramatically reduced in interspecific crosses involving either male or female musculusCZECH (i.e., a symmetric reduction in reproductive parameters), whereas normal fertility occurred in intercrosses involving a male musculusPWK (i.e., an asymmetric reduction in reproductive parameters; Table 2; Fig. 2C). At a minimum, symmetric F1 sterility requires either multiple sets of sex-linked incompatibilities or a single set of exclusively autosomal interactions. Given the existence of X-linked incompatibilities in crosses involving female musculusPWK, these musculusCZECH data indicate that at least two different sets of incompatibilities underlie hybrid male sterility in our crosses. Further, one or more incompatibilities involved in one set were not present in musculusPWK and thus are polymorphic within M. musculus.
In principle, F1 male sterility in crosses between female M. domesticus and male musculusCZECH could be due to either autosomal or Y-linked M. musculus incompatibilities. To explore this in more detail, we crossed domesticusLEWES females to M. musculusPWK × CZECH F1 males. Hybrid males from these crosses will have a musculusCZECH Y, a domesticusLEWES X, and a heterozygous autosomal background with domesticusLEWES alleles combined with a random complement of musculusCZECH or musculusPWK alleles (Fig. 1C). If F1 sterility maps to the musculusCZECH Y chromosome and has a fairly simple genetic basis, we would expect all males from this cross to continue to have significantly reduced fertility. Contrary to this, we observed considerable variance in male reproductive parameters with five of 10 males showing at least partial recovery of fertility, defined as testis weights and sperm counts all exceeding the range observed in the sterile ♀ domesticusLEWES × ♂ musculusCZECH cross (Table 3). These data are consistent with one or more sterility factors on the musculusCZECH autosomal background.
The dramatic difference in hybrid fertility observed between interspecific crosses involving the two different M. musculus genotypes raises the question of the status of this apparent polymorphism in natural populations of M. musculus. To begin to address this issue we analyzed 20 hybrid males descended from crosses between female domesticusLEWES and a total of seven out-bred M. musculus sires derived from wild mice collected in eastern Czech Republic. Six sires were from the fifth generation of our outbred colony whereas the seventh sire was from the fourth generation. Many of the hybrid males had reproductive parameters within the “normal” range of parameters characteristic of the control intraspecific crosses and the ♀ domesticusLEWES × ♂ musculusPWK interspecific cross, whereas a subset showed fairly severe reductions in RTW and/or sperm counts (Fig. 2D). Eighteen of the 20 hybrid males we considered were heterozygous for t haplotypes based on the Tcp-1 PCR diagnostic (Planchart et al. 2000). In contrast, t alleles were completely absent from all crosses involving wild-derived inbred strains (as expected). Two observations argue that segregation of t haplotypes does not explain the reproductive variance among hybrid males we observed. First, of the two wild-type males, one was reproductively normal whereas the other had reduced testis weight and low sperm counts. Second, we observed both normal and sterile t bearing individuals segregating within the same pedigree (i.e., with the same t allele) arguing against the potential influence of different variants of the t locus. These data are thus consistent with results from crosses involving wild-derived inbred lines in suggesting that hybrid sterility factors are polymorphic within natural populations of M. musculus.
Reduced hybrid fertility involved primarily postmeiotic disruption of spermatogenesis
PAS-stained histological cross-sections were assessed to determine the extent of spermatogenesis and any abnormal features (Fig. 3). Spermatogenesis appeared normal in all four intraspecific males (N = 2, ♀ domesticusLEWES × ♂ domesticusWSB; N = 2, ♀ musculusPWK × ♂ musculusCZECH; Fig. 3A) and the two interspecific males from a ♀ domesticusLEWES × ♂ musculusPWK cross (Fig. 3B), consistent with the observation of normal testis weights and sperm counts in these mice (Table 2). In contrast, reduction in spermatogenesis was evident in the remaining eight males analyzed from interspecific crosses (N = 2, ♀ musculusPWK × ♂ domesticusLEWES; N = 1, ♀ musculusCZECH × ♂ domesticusLEWES; N = 1, ♀ domesticusLEWES × ♂ musculusCZECH; N = 2, ♀ musculusCZECH × ♂ domesticusWSB; N = 2, ♀ domesticusWSB × ♂ musculusCZECH). In these eight males we observed no gross abnormalities in the structure or frequency of primary spermatocytes and many normal-appearing round spermatids with intact acrosomes. However, disruptions in postmeiotic spermiogenesis were present in all eight samples, including fewer than normal elongating and condensing spermatids, abnormal spermatid head morphology and clusters of abnormally swollen and pycnotic cells consistent with necrotic or apoptotic cell death and phagocytosis by Sertoli cells (see Fig. 3C, D for examples). We observed some variation in the degree of disruption among individuals of the same genotype. For example, we observed variation in the degree of spermatogenic disruption for the two males from a cross between ♀ musculusPWK × ♂ domesticusLEWES. One male was characterized by a strong reduction in the number of elongating and condensing spermatids and the occurrence of abnormal head morphology. Small clusters of abnormally pycnotic cells were also observed in multiple tubule lumens of this male (Fig. 3C). For the other male, spermatogenesis appeared more normal albeit with diminished numbers of maturing spermatids. We also observed variation in the degree of disruption across tubules within individual interspecific males. In these instances, all tubules appeared abnormal but some were characterized by more advanced stages of normal development. One of the hybrid males (♀ domesticusLEWES × ♂ musculusCZECH) with severely abnormal histology was 90-day old, again confirming that the spermatogenic abnormalities we observed were not strongly age dependent.
Figure 3.
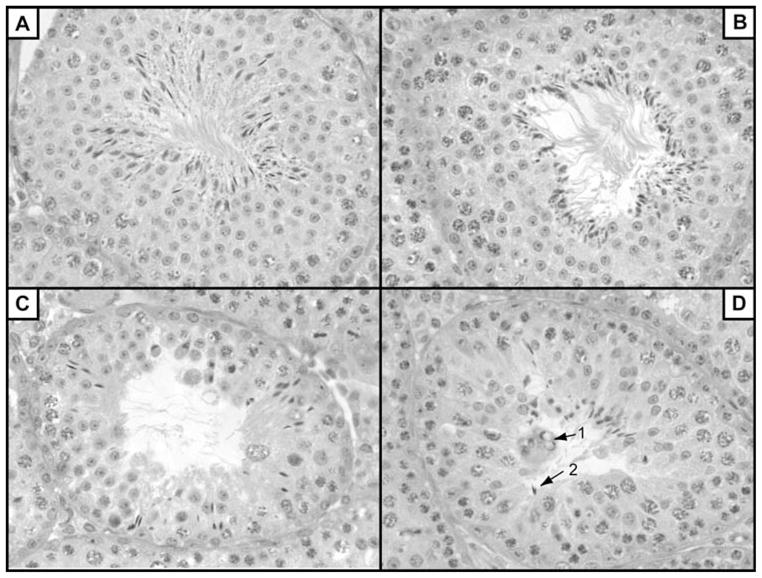
Histological cross-sections from testes. Panels A and B show seminiferous tubules from males with normal spermatogenesis: (A) M. musculus F1 male (♀ musculusPWK × ♂ musculusCZECH), (B) interspecific F1 male (♀ domesticusLEWES × ♂ musculusPWK). Panels C and D are of seminiferous tubules from interspecific F1 males with disrupted spermatogenesis: (C) ♀ musculusPWK × ♂ domesticusLEWES and (D) ♀ domesticusLEWES × ♂ musculusCZECH. In panels C and D, note the diminished numbers of germ cells overall, and poor organization of the seminiferous epithelium with clumps of vesiculated cells (D1) and abnormal sperm head morphology (D2).
Discussion
Using a series of reciprocal crosses between wild-derived inbred strains of M. musculus and M. domesticus, we found evidence for multiple genetic factors underlying hybrid male sterility. Many hybrid males showed reduced fertility. Reduced hybrid fertility was asymmetric in some crosses, consistent with a large effect of the M. musculus X chromosome. Reciprocal crosses also revealed polymorphism within M. musculus for one or more loci necessary for reduced hybrid male fertility. Hybrid male sterility was due primarily to postmeiotic disruptions of spermatogenesis. When combined with previous results, these data suggest a fairly complex genetic basis to hybrid male sterility in this system. Below we discuss our findings in relation to other studies on hybrid male sterility in house mice. We also discuss the broader significance of asymmetry and polymorphism in the evolution of reproductive isolation.
ASYMMETRY, X-LINKED STERILITY, AND THE INITIATION OF HALDANE’S RULE
The X chromosome often plays a central role in the evolution of postzygotic reproductive isolation (i.e., the large X-effect; Dobzhansky 1936; Coyne and Orr 1989b; Tao et al. 2003). One of the most striking aspects of our data was the strong asymmetry in hybrid male sterility in reciprocal crosses between musculusPWK and either M. domesticus strain (Table 2, Fig. 2), suggesting hybrid incompatibilities originate on one or both of the sex chromosomes. Asymmetric F1 sterility has been shown in one other cross, also involving musculusPWK (Britton-Davidian et al. 2005), and our results demonstrate that this pattern is consistent across multiple wild-derived M. domesticus genotypes. Two lines of evidence suggest that the observed asymmetric reduction in hybrid fertility was due to interactions involving the M. musculus × chromosome. First, we have introgressed the musculusPWK X chromosome onto the domesticusLEWES autosomal background as part of an ongoing research program to map X-linked hybrid male sterility genes. In these backcrosses, male sterility segregates with the M. musculus X chromosome (J. M. Good, unpubl. data; see also Britton-Davidian et al. 2005). Second, hybrid male sterility is caused by substitution of a wild-derived M. musculus X chromosome onto the genomic background of the classic inbred strain C57BL/6 (Storchová et al. 2004). It remains to be seen if the underlying genetic architecture across these examples is the same.
In the hybrid zone, the sex chromosomes show significantly reduced cline widths (i.e., reduced introgression) relative to the autosomes in most transects (Vanlerberghe et al. 1986; Tucker et al. 1992; Dod et al. 1993; Munclinger et al. 2002; Macholán et al. 2007). Significantly reduced cline widths for the X and Y chromosomes are commonly assumed to reflect selection against sex-linked hybrid incompatibilities in the hybrid zone (Tucker et al. 1992; Dod et al. 1993; Payseur et al. 2004; Macholán et al. 2007) and our data on hybrid male sterility are consistent with this interpretation for the X chromosome. Interestingly, genomic regions with very narrow cline widths are also often highly asymmetric in cline shape (Payseur et al. 2004). However, asymmetric patterns of gene flow across the hybrid zone are seen at many X-linked and autosomal loci and are probably strongly influenced by demography (Macholán et al. 2007; Teeter et al., in press). It is unclear whether some of these asymmetries in the hybrid zone are also caused by epistatic interactions.
Dominance theory posits that Haldane’s rule reflects the exposure of interactions between recessive X-linked and dominant autosomal incompatibilities in the heterogametic sex (Muller 1942; Turelli and Orr 1995, 2000). X-autosome incompatibilities in F1 hybrids are different from dominant interactions between autosomal loci in that they depend on the direction of the cross (Fig. 1B). If the initiation of sterility or inviability involves an X-autosome incompatibility, the first mutation in the evolution of F1 postzygotic isolation will necessarily present asymmetric problems in hybrid males. Thus, asymmetry in the reciprocal crosses between musculusPWK and either M. domesticus strain (Table 2, Fig. 2) likely reflects the relatively recent divergence of these species.
Haldane’s rule for sterility evolves quite rapidly relative to inviability (Wu 1992; True et al. 1996; Coyne and Orr 1997; Sawamura et al. 2000; Tao et al. 2003) and likely has multiple causes in species in which males are the heterogametic sex (Wu and Davis 1993; Wu et al. 1996; Presgraves and Orr 1998). For example, the rapid evolution of hybrid male sterility may occur as a byproduct of intense sexual selection on male reproductive genes and/or an inherent developmental sensitivity of spermatogenesis (Wu and Davis 1993). Both processes may contribute to the evolution of hybrid male sterility in mice. Multiple mating is common in mice, creating a potential arena for intense postcopulatory sexual selection in the form of sperm competition (Dean et al. 2006). Consistent with this there is abundant evidence that genes involved in male reproduction evolve rapidly in mice (Mouse Genome Sequencing Consortium 2002; Winter et al. 2004; Torgerson et al. 2005) and are frequently the target of positive selection (Torgerson et al. 2002; Good and Nachman 2005; Torgerson and Singh 2006). Moreover, gene knockout and mutagenesis models disproportionately cause male sterility suggesting mouse spermatogenesis is exceptionally sensitive to genetic disruptions (Escalier 2001; Handel et al. 2006).
POLYMORPHISM OF DOBZHANSKY–MULLER INCOMPATIBILITIES
The large amount of variation in the degree of hybrid male sterility observed across a range of crosses has long suggested polymorphism within mice for hybrid sterility factors (Table 1). In an important set of experiments, Forejt and colleagues used a series of crosses between classic inbred females and wild-derived M. musculus males to describe the Hst1 sterility system (Forejt and Iváanyi 1975; Forejt 1981; Forejt et al. 1991; Trachtulec et al. 1994, 2005). In these crosses, epistatic interactions between three or more loci caused early meiotic arrest in hybrid males (Forejt and Iványi 1975; Forejt 1981). Polymorphism for sterility factors was observed in both species and was shown to represent allelic variation in both classic inbred strains (sterile allele, Hst1s; fertile allele, Hst1f) and wild-derived M. musculus (sterile allele, Hstws; fertile allele, Hstwf). The Hstws and Hstwf alleles have since been shown to be polymorphic in multiple populations of M. musculus (Forejt and Iványi 1975; Vyskocilová et al. 2005). We also found evidence of polymorphism within M. musculus at one or more hybrid sterility factors; however, the variation we observe is likely distinct from Hst1 (see below for a detailed discussion).
Understanding how D–M incompatibilities are fixed within natural populations is central to determining which evolutionary forces are most important for the evolution of reproductive isolation (Schluter 2000; Coyne and Orr 2004; Funk et al. 2006). Data on polymorphic male sterility in house mice add to a growing list of species that show intraspecific variation in the strength of intrinsic postzygotic isolation in both plants (Stebbins 1958; Christie and Macnair 1987; Sweigart et al. 2007) and animals (Gordon 1927; Patterson and Stone 1952; Forejt and Iványi 1975; Wade and Johnson 1994; Reed and Markow 2004; Kopp and Frank 2005; Shuker et al. 2005; Vyskocilová et al. 2005). Transient polymorphism is a necessary state of mutations fixed by genetic drift or positive selection; therefore, the documentation of polymorphic reproductive isolation in and of itself does not speak to the prevalence of either force during speciation. Nevertheless, because targets of positive directional selection are less likely to be sampled during the polymorphic state, the frequent occurrence of polymorphic D–M incompatibilities could suggest that nondirectional evolutionary forces play an important role early in speciation (e.g., genetic drift or balancing selection).
Some examples of polymorphic reproductive isolation, including house mice, involve genetic variation for hybrid sterility or inviability segregating within single populations (Forejt and Iványi 1975; Reed and Markow 2004; Kopp and Frank 2005; Shuker et al. 2005; Vyskocilová et al. 2005; Sweigart et al. 2007). The Hst sterile and fertile alleles (Hstws and Hstwf) are polymorphic within multiple localities of M. musculus in the Czech Republic and appear to segregate at intermediate frequencies (Forejt and Iványi 1975; Vyskocilová et al. 2005). These data remain one of the clearest examples of naturally segregating variation at a specific D–M incompatibility locus. Likewise, sterility factors segregating between musculusCZECH and musculusPWK reflect variation in reproductive isolation sampled over a small geographic scale. Our intercrosses involving outbred M. musculus males from a third locality in the Czech Republic also yielded hybrid male offspring with fertility ranging from normal to severely reduced (Fig. 2D). Although the frequency and overall geographic spread of the underlying D–M incompatibilities remains to be determined, polymorphism at loci involved in reproductive isolations seems to be a common phenomenon in house mice.
Two very different processes could generate polymorphism of D–M incompatibilities in mice. First, D–M incompatibilities could be polymorphic within either mouse species because of interspecific introgression of previously fixed loci. Reduced interspecific gene flow is considered a hallmark of loci directly involved in hybrid incompatibilities (Rieseberg et al. 1999; Payseur et al. 2004) but recombination within a hybrid zone may break up D–M incompatibilities and enable introgression of underlying loci (Virdee and Hewitt 1994). Polymorphism introduced via interspecific gene flow may extend well beyond the boundaries of the mouse hybrid zone due to human-mediated dispersal (Macholán et al. 2007) and could occur in any wild-derived strains of mice. Indeed, a recent genomic scan of SNP variation among multiple wild-derived inbred strains (including WSB) found multiple putative cases of interspecific introgression (Yang et al., 2007).
Alternatively, polymorphism may reflect naturally segregating variation within species. Genetic drift likely plays an important role in the local fixation of Robertsonian chromosomal rearrangements within some populations of M. domesticus (Nachman and Searle 1995). In many instances these rearrangements appear weakly under-dominant (Searle 1993) and result in reduced fertility in hybrids between populations fixed for alternative karyotypes (Piálek et al. 2001). However, drift seems a less plausible explanation for the maintenance of polymorphic D–M incompatibilities in mice, given the apparent frequency and geographic scale at which polymorphism has been observed (Forejt and Iványi 1975; Vyskocilová et al. 2005). In general, determining the frequency and geographic spread of polymorphic D–M incompatibilities will be critical in evaluating the relative importance of natural selection versus genetic drift in the evolution of hybrid male sterility between M. musculus and M. domesticus.
A COMPLEX GENETIC BASIS TO F 1 REPRODUCTIVE ISOLATION IN MICE
The results presented here suggest that at least three sets of F1 epistatic D–M incompatibilities occur between M. musculus and M. domesticus (Table 4). It is difficult to speculate on the genetic basis of the sterility polymorphism within M. musculus without a direct linkage analysis; however, it is unlikely that the incompatibilities we observed between ♀ M. domesticus (both strains) and ♂ musculusCZECH involve Hst1 for the following reasons. First, we observed primarily postmeiotic problems in all impaired hybrid males (Fig. 3) and this general phenotype is distinct from the early meiotic arrest generated by Hst1 and interacting loci (Forejt 1981; Chubb and Nolan 1987). Second, as described previously, the musculusPWK strain has been described to carry the sterility-ensuring Hstws allele (Forejt 1981; Chubb and Nolan 1987), yet the male progeny produced from crossing female domesticusLEWES or domesticusWSB to male musculusPWK were normal. If the strain of musculusPWK that we used has retained the Hstws allele described in these previous experiments (Forejt 1981; Chubb and Nolan 1987), then both domesticusLEWES and domesticusWSB appear to be missing some component of this epistatic interaction necessary for sterility. Consequently, it follows that the male sterility involved in crosses of female domesticusLEWES or domesticusWSB to male musculusCZECH appears distinct from both the Hst1 and the musculus X chromosome D–M incompatibility systems.
Table 4.
Inferred genetic linkage and number of loci involved in three sets of hybrid incompatibilities involved in F1 male sterility between M. musculus and M. domesticus.
| musculus1 | domesticus | Polymorphic2 | No. of loci3 | Supporting crosses4 |
|---|---|---|---|---|
| Auto (Hstws) | Auto (Hst1) | M, D5 | ≥3 | 1–4, 6–7, 10, 11 |
| X (Hstx1) | Auto | Unresolved | ≥3 | CW, 86 |
| Auto/Y | Auto/X | M | ≥27 | CW |
Genetic linkage of incompatibilities by species, Auto, autosomal; X, X chromosome.
Evidence of polymorphism in musculus (M) or domesticus (D).
Estimated number of sterility factors.
Crosses consistent with inferred incompatibilities. CW, current work, and numbers correspond to crosses in Table 1.
Evidence of polymorphism in domesticus is based on variation among classic inbred strains.
See also Storchová et al. 2004.
Minimum estimate assuming a D–M epistatic incompatibility model.
These arguments suggest that a third set of F1 epistatic D–M incompatibilities occur between M. musculus and M. domesticus. In this set of D–M incompatibilities, one or more of the underlying sterility factors is polymorphic in M. musculus (present in musculusCZECH, absent in musculusPWK). In a recent geographic survey of the Hst1 incompatibility system, Vyskocilová et al. (2005) described three pedigrees of mice (out of seven) where hybrid male sterility was polymorphic and did not segregate with variation at the Hst1 locus. Their experiment involved outbred wild M. musculus. Therefore, reciprocal crosses controlled for genotype could not be produced. It is unclear whether the polymorphism they described involved loci interacting with Hst1, the X-autosome incompatibility system, or a new set of incompatibilities. It is possible that the polymorphism observed in their study was tracking the same sterility factors that are polymorphic between musculusCZECH and musculusPWK.
Overall, hybrid male sterility in house mice appears more complicated than previously thought (Forejt 1996). Currently, we have little information on the total number or independence of loci involved in each of the three proposed set of epistatic interactions that underlie F1 hybrid male sterility between M. musculus and M. domesticus. If the interacting partners in these three putative sets of epistatic interactions were partially nonindependent then the total number of incompatibilities would be reduced. However, the number of hybrid incompatibilities typically increases as a function of experimental resolution (Coyne and Orr 2004) and thus Table 4 likely represents a severe underestimate. Regardless, the observation of polymorphism at multiple loci that have a large impact on F1 hybrid fertility suggests that resolving the overall genetic architecture of hybrid male sterility may require considerable population-level sampling within both M. musculus and M. domesticus (Vyskocilová et al. 2005). The unexpected combination of genetic complexity and polymorphism during incipient mouse speciation highlights the utility of this system for studying the early stages of speciation.
Although our approach has been to focus exclusively on hybrid male fertility, it is likely that other phenotypes also play an important role in reproductive isolation between these species. For example, increased hybrid susceptibility to parasites (Sage et al. 1986; Moulia et al. 1991, 1993), female sterility (Britton-Davidian et al. 2005), and assortative mating have all been described (Smadja and Ganem 2002; Smadja et al. 2004; Ganem et al. 2005). None of these phenotypes appear as strong or as consistent across studies as hybrid male sterility but nevertheless may contribute to overall reproductive isolation between M. musculus and M. domesticus. The existence of several potential isolating mechanisms combined with our increasing appreciation of the genetic complexity underlying male sterility is consistent with recent estimates suggesting many genes are involved in reproductive isolation across the hybrid zone (Macholán et al. 2007).
CONCLUSIONS AND FUTURE DIRECTIONS
The true power of studying speciation in a model genetic system is the potential to experimentally dissect the genetic basis of reproductive isolation. In mice, this goal has been impaired by a lack of genetic studies using inbred strains derived from natural populations. We have shown that F1 hybrid male sterility in mice involves both polymorphic and X-linked loci, broadly consistent with previous results. At least one set of D–M incompatibilities described here appears distinct from previously described X-linked or Hst1-related interactions. Importantly, although reproductive isolation in mice appears to have a fairly complex and variable genetic basis, a surprising amount of genetic complexity in F1 hybrid male sterility was captured by a small subset of wild-derived inbred strains. These strains are widely available and should facilitate the fine-scale genetic dissection of reproductive isolation in this system without reliance on classic laboratory strains.
Acknowledgments
We are grateful to J. Piálek for collecting and providing access to wild M. musculus individuals. We thank S. Bornstein for assistance with histological analyses. C. W. Birky, M. Dean, A. Doyle, and T. Quill provided useful advice on experimental design and methodology. We thank members of the Nachman lab, J. Piálek, B. Payseur, A. Sweigart, J. Feder, R. Storchová, nd two anonymous reviewers for providing critical comments on early versions of the manuscript. We thank B. Gibson, M. Longhi, K. Smith, M. Dean, Dr. M. Rand, and the staff of the University of Arizona Central Animal Facility for assistance with mouse husbandry. This research was supported by an NSF IGERT grant Genomics Initiative (#DGE0114420, JMG), an NSF grant (#DEB0213013, MWN), and two NIH grants (1 RO1 GM074245-01A1, MWN; HD48998, MAH).
LITERATURE CITED
- Alibert P, Fel-Clair F, Manolakou K, Britton-Davidian J, Auffray JC. Developmental stability, fitness, and trait size in laboratory hybrids between European subspecies of the house mouse. Evolution. 1997;51:1284–1295. doi: 10.1111/j.1558-5646.1997.tb03975.x. [DOI] [PubMed] [Google Scholar]
- Barbash DA, Siino DF, Tarone AM, Roote J. A rapidly evolving MYB-related protein causes species isolation in Drosophila. Proc Natl Acad Sci USA. 2003;100:5302–5307. doi: 10.1073/pnas.0836927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson W. Heredity and variation in modern lights. In: Seward AC, editor. Darwin and modern science. Cambridge Univ. Press; Cambridge. UK: 1909. pp. 85–101. [Google Scholar]
- Boursot P, Auffray JC, Britton-Davidian J, Bonhomme F. The evolution of house mice. Ann Rev Ecol Syst. 1993;24:119–152. [Google Scholar]
- Brideau NJ, Flores HA, Wang J, Maheshwari S, Wang X, Barbash DA. Two Dobzhansky–Muller genes interact to cause hybrid lethality in Drosophila. Science. 2006;314:1292–1295. doi: 10.1126/science.1133953. [DOI] [PubMed] [Google Scholar]
- Britton-Davidian J, Fel-Clair F, Lopez J, Alibert P, Boursot P. Postzygotic isolation between two European subspecies of the house mouse: estimates from fertility patterns in wild and laboratory-bred hybrids. Biol J Linn Soc. 2005;84:379–393. [Google Scholar]
- Christie P, Macnair MR. The distribution of postmating reproductive isolating genes in populations of the yellow monkey flower, Mimulus guttatus. Evolution. 1987;41:571–578. doi: 10.1111/j.1558-5646.1987.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Chubb C, Nolan C. Mouse hybrid sterility and testicular function. Biol Reprod. 1987;36:1343–1348. doi: 10.1095/biolreprod36.5.1343. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Patterns of speciation in Drosophila. Evolution. 1989a;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Two rules of speciation. In: Otte D, Endler J, editors. Speciation and its consequences. Sinauer Associates; Sunderland, MA: 1989b. pp. 180–207. [Google Scholar]
- Coyne JA, Orr HA. Patterns of speciation in Drosophila revisited. Evolution. 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates, Inc; Sunderland, MA: 2004. [Google Scholar]
- Dean MD, Ardlie KG, Nachman MW. The frequency of multiple paternity suggests that sperm competition is common in house mice (Mus domesticus) Mol Ecol. 2006;15:4141–4151. doi: 10.1111/j.1365-294X.2006.03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth JP, Wade MJ. Population differentiation in the beetle Tribolium castaneum. II. Haldane’s rule and incipient speciation. Evolution. 2007;61:694–699. doi: 10.1111/j.1558-5646.2007.00049.x. [DOI] [PubMed] [Google Scholar]
- Dietrich WF, Miller J, Steen R, Merchant MA, Damron-Boles D, Husain Z, Dredge R, Daly MJ, Ingalls KA, O’Connor TJ, Evans CA, DeAngelis MM, Levinson DM, Kruglyak L, Goodman N, Copeland NG, Jenkins NA, Hawkins TL, Stein L, Page DC, Lander ES. A comprehensive genetic map of the mouse genome. Nature. 1996;380:149–152. doi: 10.1038/380149a0. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Studies on hybrid sterility. II. Localization of sterility factors of sterility factors in Drosophila pseudoobscura hybrids. Genetics. 1936;21:113–135. doi: 10.1093/genetics/21.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics and the origin of species. Columbia Univ. Press; New York: 1937. [Google Scholar]
- Dod B, Jermiin LS, Boursot P, Chapman VH, Nielsen JT, Bonhomme F. Counterselection on sex chromosomes in the Mus musculus European hybrid zone. J Evol Biol. 1993;6:529–546. [Google Scholar]
- Eddy EM. Male germ cell gene expression. Recent Prog Horm Res. 2002;57:103–128. doi: 10.1210/rp.57.1.103. [DOI] [PubMed] [Google Scholar]
- Escalier D. Impact of genetic engineering on the understanding of spermatogenesis. Hum Reprod Update. 2001;7:191–210. doi: 10.1093/humupd/7.2.191. [DOI] [PubMed] [Google Scholar]
- Forejt J. Hybrid sterility gene located in T/t-H-2 supergene on chromosome. In: Reisfeld R, Ferrone S, editors. Current trends in histocompatibility. Immunogenetic and molecular profiles. Vol. 17. Plenum Press; London: 1981. pp. 103–131. [Google Scholar]
- Forejt J. Hybrid sterility in the mouse. Trends Genet. 1996;12:412–417. doi: 10.1016/0168-9525(96)10040-8. [DOI] [PubMed] [Google Scholar]
- Forejt J, Iványi P. Genetic studies on male sterility of hybrids between laboratory and wild mice (Mus musculus L.) Genet Res. 1975;24:189–206. doi: 10.1017/s0016672300015214. [DOI] [PubMed] [Google Scholar]
- Forejt J, Vincek V, Klein J, Lehrach H, Loudová-Micková M. Genetic mapping of the t-complex region on mouse chromosome 17 including the Hybrid sterility-1 gene. Mammal Genome. 1991;1:84–91. doi: 10.1007/BF02443783. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Eskin E, Kang HM, Bogue MA, Hinds DA, Beilharz EJ, Gupta RV, Montgomery J, Morenzoni MM, Nilsen GB, et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- Funk DJ, Nosil P, Etges WJ. Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc Natl Acad Sci USA. 2006;103:3209–3213. doi: 10.1073/pnas.0508653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem G, Ginane C, Ostrowski MF, Orth A. Assessment of mate preference in the house mouse with reference to investigations on assortative mating. Biol J Linn Soc. 2005;84:461–471. [Google Scholar]
- Good JM, Nachman MW. Rates of protein evolution are positively correlated with developmental timing of expression during mouse spermatogenesis. Mol Biol Evol. 2005;22:1044–1052. doi: 10.1093/molbev/msi087. [DOI] [PubMed] [Google Scholar]
- Gordon M. The genetics of a viviparous top-minnow Platypoecilus; the inheritance of two kinds of melanophores. Genetics. 1927;12:253–283. doi: 10.1093/genetics/12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane JBS. Sex ratio and unisexual sterility in animal hybrids. J Genet. 1922;12:101–109. [Google Scholar]
- Handel MA, Lessard C, Reinholdt L, Schimenti J, Eppig JJ. Mutagenesis as an unbiased approach to identify novel contraceptive targets. Mol Cell Endo. 2006;250:201–205. doi: 10.1016/j.mce.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Harrison RG. Hybrid zones: windows on evolutionary process. Oxf Surv Evol Biol. 1990;7:69–128. [Google Scholar]
- Harrison JS, Burton RS. Tracing hybrid incompatibilities to single amino acid substitutions. Mol Biol Evol. 2006;23:559–564. doi: 10.1093/molbev/msj058. [DOI] [PubMed] [Google Scholar]
- Kopp A, Frank AK. Speciation in progress? A continuum of reproductive isolation in Drosophila bipectinata. Genetica. 2005;125:55–68. doi: 10.1007/s10709-005-4787-8. [DOI] [PubMed] [Google Scholar]
- Laurie CC. The weaker sex is heterogametic: 75 years of Haldane’s rule. Genetics. 1997;147:937–951. doi: 10.1093/genetics/147.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macholán M, Munclinger P, Sugerková M, Dufková P, Bímová B, Bozíková E, Zima J, Piálek J. Genetic analysis of autosomal and X-linked markers across a mouse hybrid zone. Evolution. 2007;61:746–771. doi: 10.1111/j.1558-5646.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- Moulia C, Aussel JP, Bonhomme F, Boursot P, Nielsen JT, Renaud F. Wormy mice in a hybrid zone: a genetic control of susceptibility to parasite infection. J Evol Biol. 1991;4:679–687. [Google Scholar]
- Moulia C, Lebrun N, Dallas J, Orth A, Renaud F. Experimental evidence of genetic determinism in high susceptibility to intestinal pinworm infection in mice: a hybrid zone model. Parasitology. 1993;106:387–393. doi: 10.1017/s0031182000067135. [DOI] [PubMed] [Google Scholar]
- Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Muller HJ. Isolating mechanisms, evolution, and temperature. Biol Symp. 1942;6:71–125. [Google Scholar]
- Munclinger P, Boziková E, Sugerková M, Piálek J, Macholán M. Genetic variation in house mice (Mus, Muridae, Rodentia) from the Czech and Slovak Republics. Folia Zool. 2002;51:81–92. [Google Scholar]
- Nachman MW, Searle JB. Why is the house mouse karyotype so variable? Trends Ecol Evol. 1995;10:397–402. doi: 10.1016/s0169-5347(00)89155-7. [DOI] [PubMed] [Google Scholar]
- Oka A, Mita A, Sakurai-Yamatani N, Yamamoto A, Takagi N, Takano-Shimizu T, Toshimori K, Moriwaki K, Shiroishi T. Hybrid breakdown caused by substitution of the X chromosome between two mouse subspecies. Genetics. 2004;166:913–924. doi: 10.1534/genetics.166.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A, Aoto T, Totsuka Y, Takahashi R, Ueda M, Mita A, Sakurai-Yamatani N, Yamamoto H, Kuriki S, Takagi N, Moriwaki K, Shiroishi T. Disruption of genetic interaction between two autosomal regions and the X chromosome causes reproductive isolation between mouse strains derived from different subspecies. Genetics. 2007;175:185–197. doi: 10.1534/genetics.106.062976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HA. The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics. 1995;139:1805–1813. doi: 10.1093/genetics/139.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HA. Haldane’s rule. Ann Rev Ecol Syst. 1997;28:195–218. [Google Scholar]
- Orr HA, Orr LH. Waiting for speciation: the effect of population subdivision on the time to speciation. Evolution. 1996;50:1742–1749. doi: 10.1111/j.1558-5646.1996.tb03561.x. [DOI] [PubMed] [Google Scholar]
- Orr HA, Presgraves DC. Speciation by postzygotic isolation: forces, genes and molecules. BioEssays. 2000;22:1085–1094. doi: 10.1002/1521-1878(200012)22:12<1085::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Stone WS. Evolution in the genus Drosophila. Macmillan and Co; New York: 1952. [Google Scholar]
- Payseur BA, Hoekstra HE. Signatures of reproductive isolation in patterns of single nucleotide diversity across inbred strains of mice. Genetics. 2005;171:1905–1916. doi: 10.1534/genetics.105.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payseur BA, Krenz JG, Nachman MW. Differential patterns of introgression across the X chromosome in a hybrid zone between two species of house mice. Evolution. 2004;58:2064–2078. doi: 10.1111/j.0014-3820.2004.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Pelikán J. Patterns of reproduction in the house mouse. In: Berry RJ, editor. Biology of the house mouse. Academic Press; New York: 1981. pp. 205–229. [Google Scholar]
- Petkov PM, Graber JH, Churchill GA, DiPetrillo K, King BL, Paigen K. Evidence of a large-scale functional organization of mammalian chromosomes. PLoS Genet. 2005;1:312–322. doi: 10.1371/journal.pgen.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piálek J, Hauffe HC, Rodriguez-Clark KM, Searle JB. Raciation and speciation in house mice from the Alps: the role of chromosomes. Mol Ecol. 2001;10:613–625. doi: 10.1046/j.1365-294x.2001.01209.x. [DOI] [PubMed] [Google Scholar]
- Planchart A, You Y, Schimenti JC. Physical mapping male fertility and meiotic drive quantitative trait loci in the mouse t complex using chromosome deficiencies. Genetics. 2000;155:803–812. doi: 10.1093/genetics/155.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves DC, Orr HA. Haldane’s rule in taxa lacking a hemizygous X. Science. 1998;282:952–954. doi: 10.1126/science.282.5390.952. [DOI] [PubMed] [Google Scholar]
- Presgraves DC, Balagopalan L, Abmayr SM, Orr HA. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature. 2003;423:715–719. doi: 10.1038/nature01679. [DOI] [PubMed] [Google Scholar]
- Reed LK, Markow TA. Early events in speciation: polymorphism for hybrid male sterility in Drosophila. Proc Natl Acad Sci USA. 2004;101:9009–9012. doi: 10.1073/pnas.0403106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg LH, Whitton J, Gardner K. Hybrid zones and the genetic architecture of a barrier to gene flow between two sunflower species. Genetics. 1999;152:713–727. doi: 10.1093/genetics/152.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha AP, Hikin, Clegg ED. Histological and histopathological evaluation of the testis. Cache River Press; Clearwater, FL: 1990. [Google Scholar]
- Sage RD, Atchley WR, Capanna E. House mice as models in systematic biology. Syst Biol. 1993;42:523–561. [Google Scholar]
- Sage RD, Heyneman D, Lim KC, Wilson AC. Wormy mice in a hybrid zone. Nature. 1986;324:60–63. doi: 10.1038/324060a0. [DOI] [PubMed] [Google Scholar]
- Sawamura K, Davis AW, Wu C-I. Genetic analysis of speciation by means of introgression into Drosophila melanogaster. Proc Natl Acad Sci USA. 2000;97:2652–2655. doi: 10.1073/pnas.050558597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D. The ecology of adaptive radiation. Oxford Univ. Press; New York: 2000. [Google Scholar]
- Searle JB. Chromosomal hybrid zones in eutherian mammals. In: Harrison RG, editor. Hybrid zones and the evolutionary process. Oxford Univ. Press; New York: 1993. pp. 309–353. [Google Scholar]
- She JX, Bonhomme F, Boursot P, Thaler L, Catzeflis F. Molecular phylogenies in the genus Mus: comparative analysis of electrophoretic, scnDNA hybridization, and mtDNA RFLP Data. Biol J Linn Soc. 1990;41:83–103. [Google Scholar]
- Shifman S, Bell JT, Copley RR, Taylor MS, Williams RW, Mott R, Flint J. A high-resolution single nucleotide polymorphism genetic map of the mouse genome. PLoS Biol. 2006;4:2227–2237. doi: 10.1371/journal.pbio.0040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuker DM, Underwood K, King TM, Butlin RK. Patterns of male sterility in a grasshopper hybrid zone imply accumulation of hybrid incompatibilities without selection. Proc R Soc Lond B. 2005;272:2491–2497. doi: 10.1098/rspb.2005.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver LM. Mouse t haplotypes. Ann Rev Genet. 1985;19:179–208. doi: 10.1146/annurev.ge.19.120185.001143. [DOI] [PubMed] [Google Scholar]
- Smadja C, Ganem G. Subspecies recognition in the house mouse: a study of two populations from the border of a hybrid zone. Behav Ecol. 2002;13:312–320. [Google Scholar]
- Smadja C, Catalan J, Ganem G. Strong premating divergence in a unimodal hybrid zone between two subspecies of the house mouse. J Evol Biol. 2004;17:165–176. doi: 10.1046/j.1420-9101.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. The inviability, weakness, and sterility of interspecific hybrids. Adv Genet. 1958;9:147–215. doi: 10.1016/s0065-2660(08)60162-5. [DOI] [PubMed] [Google Scholar]
- Storchová R, Gregorová S, Buckiová D, Kyselová V, Divina P, Forejt J. Genetic analysis of X-linked hybrid sterility in the house mouse. Mammal Genome. 2004;15:515–524. doi: 10.1007/s00335-004-2386-0. [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweigart AL, Mason AR, Willis JH. Natural variation for a hybrid incompatibility between two species of Mimulus. Evolution. 2007;61:141–151. doi: 10.1111/j.1558-5646.2007.00011.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, Xhen SN, Hartl DL, Laurie CC. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. I. Differential accumulation of hybrid male sterility effects on the x and autosomes. Genetics. 2003;164:1383–1397. doi: 10.1093/genetics/164.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter KC, Payseur BA, Harris LW, Bakewell MA, Thibodeau LM, O’Brien JE, Krenz JG, Sans-Fuentes M, Nachman MW, Tucker PK. Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Res. doi: 10.1101/gr.6757907. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CT, Tsaur SC, Wu ML, Wu CI. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science. 1998;282:1501–1504. doi: 10.1126/science.282.5393.1501. [DOI] [PubMed] [Google Scholar]
- Torgerson DG, Kulathinal RJ, Singh RS. Mammalian sperm proteins are rapidly evolving: evidence of positive selection in functionally diverse genes. Mol Biol Evol. 2002;19:1973–1980. doi: 10.1093/oxfordjournals.molbev.a004021. [DOI] [PubMed] [Google Scholar]
- Torgerson DG, Singh RS. Enhanced adaptive evolution of sperm-expressed genes on the mammalian X chromosome. Heredity. 2006;96:39–44. doi: 10.1038/sj.hdy.6800749. [DOI] [PubMed] [Google Scholar]
- Torgerson DG, Whitty BR, Singh RS. Sex-specific functional specialization and the evolutionary rates of essential fertility genes. J Mol Evol. 2005;61:650–658. doi: 10.1007/s00239-005-0007-5. [DOI] [PubMed] [Google Scholar]
- Trachtulec Z, Vincek V, Hamvas RMJ, Forejt J, Lehrach H, Klein J. Physical map of mouse chromosome 17 in the region relevant for positional cloning of the Hybrid Sterility-1 gene. Genomics. 1994;23:132–137. doi: 10.1006/geno.1994.1468. [DOI] [PubMed] [Google Scholar]
- Trachtulec Z, Mihola O, Vlcek C, Himmelbauer H, Paces V, Forejt J. Positional cloning of the Hybrid sterility 1 gene: fine genetic mapping and evaluation of two candidate genes. Biol J Linn Soc. 2005;84:637–641. [Google Scholar]
- True JR, Weir BS, Laurie CC. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans. Genetics. 1996;142:819–837. doi: 10.1093/genetics/142.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker PK, Sage RD, Warner J, Wilson AC, Eicher EM. Abrupt cline for sex chromosomes in a hybrid zone between two species of mice. Evolution. 1992;46:1146–1163. doi: 10.1111/j.1558-5646.1992.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Turelli M, Orr HA. The dominance theory of Haldane’s rule. Genetics. 1995;140:389–402. doi: 10.1093/genetics/140.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. Dominance, epistasis and the genetics of postzygotic isolation. Genetics. 2000;154:1663–1679. doi: 10.1093/genetics/154.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe F, Dod B, Boursot P, Bellis M, Bonhomme F. Absence of Y chromosome introgression across the hybrid zone between Mus musculus domesticus and Mus musculus musculus. Genet Res. 1986;48:191–197. doi: 10.1017/s0016672300025003. [DOI] [PubMed] [Google Scholar]
- Virdee SR, Hewitt GM. Clines for hybrid dysfunction in a grasshopper hybrid zone. Evolution. 1994;48:392–407. doi: 10.1111/j.1558-5646.1994.tb01319.x. [DOI] [PubMed] [Google Scholar]
- Vyskocilová M, Trachtulec Z, Forejt J, Piálek J. Does geography matter in hybrid sterility in house mice? Biol J Linn Soc. 2005;84:663–674. [Google Scholar]
- Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat Genet. 2005;37:1175–1180. doi: 10.1038/ng1666. [DOI] [PubMed] [Google Scholar]
- Wade MJ, Johnson NA. Reproductive isolation between two species of flour beetles, Tribolium castaneum and T. freemani: variation within and among geographical populations of T. castaneum. Heredity. 1994;72:155–162. doi: 10.1038/hdy.1994.22. [DOI] [PubMed] [Google Scholar]
- Wade CM, Kulbokas EJ, Kirby AW, Zody MC, Mullikin JC, Lander ES, Lindblad-Toh K, Daly MJ. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- Winter EE, Goodstadt L, Ponting CP. Elevated rates of protein secretion, evolution, and disease among tissue-specific genes. Genome Res. 2004;14:54–61. doi: 10.1101/gr.1924004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittbrodt J, Adam D, Malitschek B, Maueler W, Raulf F, Telling A, Robertson SM, Schartl M. Novel putative receptor tyrosine kinase encoded by the melanoma-inducing Tu locus in Xiphophorus. Nature. 1989;341:415–421. doi: 10.1038/341415a0. [DOI] [PubMed] [Google Scholar]
- Wright S. Systems of mating. II. The effects of inbreeding on the genetic composition of a population. Genetics. 1921;6:124–143. doi: 10.1093/genetics/6.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-I. A note on Haldane’s rule: hybrid inviability versus hybrid sterility. Evolution. 1992;46:1584–1587. doi: 10.1111/j.1558-5646.1992.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Wu C-I, Davis AW. Evolution of postmating reproductive isolation: the composite nature of Haldane’s rule and its genetic bases. Am Nat. 1993;142:187–212. doi: 10.1086/285534. [DOI] [PubMed] [Google Scholar]
- Wu C-I, Johnson NA, Palopoli MF. Haldane’s rule and its legacy: why are there so many sterile males? Trends Ecol Evol. 1996;11:281–284. doi: 10.1016/0169-5347(96)10033-1. [DOI] [PubMed] [Google Scholar]
- Yang H, Bell TA, Churchill GA, Pardo-Manuel deVillena F. On the subspecific origin of the laboratory mouse. Nat Genet. 2007;39:1100–1107. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
- Yonekawa H, Moriwaki K, Gotoh O, Miyashita N, Matsushima Y, Shi LM, Cho WS, Zhen XL, Tagashira Y. Hybrid origin of Japanese mice Mus musculus molossinus: evidence from restriction analysis of mitochondrial DNA. Mol Biol Evol. 1988;5:63–78. doi: 10.1093/oxfordjournals.molbev.a040476. [DOI] [PubMed] [Google Scholar]
- Yoshiki A, Moriwaki K, Sakakura T, Kusakabe M. Histological studies on male sterility of hybrids between laboratory and wild mouse strains. Dev Growth Diff. 1993;35:271–281. doi: 10.1111/j.1440-169X.1993.00271.x. [DOI] [PubMed] [Google Scholar]