Summary
Mantle cell lymphoma (MCL) is characterized by translocation t(11;14)(q13;q32), aggressive clinical behaviour, and poor patient outcomes following conventional chemotherapy. New treatment approaches are needed that target novel biological pathways. All trans retinoic acid (ATRA) is a key retinoid that acts through nuclear receptors that function as ligand-inducible transcription factors. The present study evaluated cell killing effects of ATRA-enriched nanoscale delivery particles, termed nanodisks (ND), on MCL cell lines. Results show that ATRA-ND induced cell death more effectively than naked ATRA (dimethyl sulphoxide) or empty ND. ATRA-ND induced reactive oxygen species (ROS) generation to a greater extent than naked ATRA. The antioxidant, N-acetylcysteine, inhibited ATRA-ND induced apoptosis. Compared to naked ATRA, ATRA-ND enhanced G1 growth arrest, up-regulated p21and p27, and down regulated cyclin D1. At ATRA concentrations that induced apoptosis, expression levels of retinoic acid receptor-α (RARα) and retinoid X receptor-γ (RXRγ) were increased. Compared to naked ATRA, ATRA-ND significantly stimulated transcriptional activity of RARA in a model carcinoma cell line. Furthermore, the RAR antagonist, Ro 41-5253, inhibited ATRA-ND induced ROS generation and prevented ATRA-ND induced cell growth arrest and apoptosis. In summary, incorporation of ATRA into ND enhanced the biological activity of this retinoid in cell culture models of MCL.
Keywords: Nanodisks, Mantle Cell Lymphoma, ATRA, Reactive Oxygen Species, Apoptosis
Introduction
Mantle cell lymphoma (MCL), a subtype of non-Hodgkin lymphoma, arises from the uncontrolled proliferation of a subset of pregerminal centre cells located in the mantle region of secondary follicles (Bertoni & Ponzoni, 2007). MCL is a B-cell malignancy characterized by dysregulation of various oncogenes. The disease pursues a relatively aggressive course, is resistant to long term remission, and is associated with a poor prognosis (Amin et al, 2003). Because of its rapid progression and unresponsiveness to treatment, MCL poses a major challenge to clinicians and researchers. Except for allogeneic stem cell transplantion, no curative therapy exists.
Several investigations have provided evidence that retinoids, such as all trans retinoic acid (ATRA), are useful agents in cancer therapy as they exhibit a central role in cell growth, differentiation, and apoptosis (Soprano et al, 2004; Altucci & Gronemeyer, 2001). Indeed, ATRA is one of the first examples of targeted therapy in human cancer. Its beneficial actions have been well documented in the treatment of acute promyelocytic leukemia (Adamson, 1996). In addition, Kaposi sarcoma, juvenile chronic myeloid leukemia and high-risk neuroblastoma respond to retinoid-based therapies (Jimenez-Lara et al, 2004). ATRA binding to nuclear hormone receptors (e.g. retinoic acid receptor [RAR], retinoid X receptor [RXR]) transactivates target genes, leading to cell growth arrest or apoptosis (Guidoboni et al, 2005; Kitareewan et al, 2008; Altucci et al, 2007). Insofar as MCL cells express retinoid receptors (Guidoboni et al, 2005), we hypothesized that ATRA would exert anti-proliferative effects and thus may have a role as a treatment option.
The present study pursued a novel approach to deliver an ATRA payload to MCL cells in culture. This water insoluble bioactive lipid was stably incorporated into nanoscale lipid particles, termed nanodisks (ND) (Redmond et al, 2007; Ryan, 2008). ND are comprised of a disk-shaped phospholipid bilayer whose periphery is stabilized by amphipathic apolipoproteins. It was hypothesized that, following cellular uptake and/or liberation of ATRA from ND, this retinoid would interact with intracellular binding proteins and, ultimately, nuclear hormone receptors, leading to target gene transactivation, cell growth arrest and/or apoptosis. Indeed, we found that compared to naked ATRA, ATRA-ND elicited enhanced apoptosis and growth arrest in MCL cells, and that this was mediated by RARs and by generation of reactive oxygen species (ROS).
Methods and/or materials
Cell Lines and reagents
Granta and NCEB cells were a gift from Dr. Steven Bernstein (University of Rochester, NY); Jeko cells were obtained from Dr. Steven Rosen (Northwestern University, Chicago, IL); 2',7’-dichlorodihydrofluorescein diacetate (H2DCFDA) was purchased from Molecular Probes, Inc. (Carlsbad, CA), propidium iodide (PI) was from Biosource (Camarillo, CA); ATRA from Sigma Chemical Co. (St. Louis, MO); Ro-41-5253 from Enzo Life Sciences (Plymouth Meeting, PA); and fluorescein isothiocyanate (FITC)-annexin-V Apoptosis Kit from Invitrogen (Carlsbad, CA). The following antibodies were used: RAR and RXR receptor subtypes (Abcam, Cambridge, MA, USA), p21 and p27 (BD Pharmingen, San Diego, CA, USA), p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), poly ADP ribose polymerase (PARP), caspases 9 and 3 (Cell Signaling, Danvers, MA, USA), cyclin D1 (Santa Cruz Biotechnology), and GAPDH (Chemicon, Temecula, CA, USA).
Preparation of ATRA-ND
ATRA-ND were prepared as previously described (Ryan, 2008) using recombinant human apolipoprotein A-I Ryan et al, 2003) as the scaffold protein. Empty ND, lacking ATRA, were prepared in the same manner with the exception that ATRA was omitted from the formulation. In various experiments ATRA alone was presented to cells using dimethylsulfoxide (DMSO) as vehicle (naked ATRA).
Cell Culture and incubations
MCL cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 1% sodium pyruvate and in the presence of penicillin, streptomycin, and glutamine at 37 °C in a humidified atmosphere of 95% air / 5% CO2. Medium was changed after 24 h and every 2–3 days thereafter. Cell viability was measured by trypan blue exclusion.
Reactive oxygen species determination
ROS production was measured by flow cytometry (Singh et al, unpublished observation; Evens et al, 2005). Cells were seeded at a density of 0.5 × 106 cells/well in 24-well plates and treated with ATRA-ND (20 µM) or naked ATRA (20 µM) for 4 h. Cells were then stained with 5 µM H2DCFDA and 2 µg PI per well and incubated for 30 min at 37 °C in a humidified CO2 incubator. Cells were washed with phosphate-buffered saline (PBS) and ROS measured by flow cytometry with a Beckman Coulter EPICS XL-MCL Cytometer. All experiments were performed in triplicate.
Apoptosis measurement
Cellular apoptosis was measured by flow cytometry (Singh et al, 2009; Evens et al, 2005). In brief, cells were incubated with ATRA-ND, naked ATRA or empty ND for 48 h and 72 h, washed with ice cold PBS and resuspended in binding buffer containing 2.5 µl FITC-annexin V and 5.0 µl PI for 15 min at 37 °C in a CO2 incubator. Subsequently, flow cytometry measurements were made on a Beckman Coulter EPICS XL-MCL Cytometer. All experiments were performed in triplicate.
Western blotting
Cells were washed with PBS, centrifuged, and cell pellets treated with radio-immunoprecipitation assay buffer containing protease inhibitors. Cell lysates were centrifuged and supernatants stored at −80 °C. An aliquot of each supernatant was used for protein determination (Bradford, 1976). Fifty µg protein of each sample was subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to a nitrocellulose membrane and probed with specified antibodies. Immune complexes were visualized by enhanced chemiluminescence using high performance chemiluminescence film (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Cell cycle analysis
MCL cells were incubated with ATRA-ND (10 µM), naked ATRA (10 µM), and medium alone for 24 h. Cells were then fixed in 70% ethanol, washed twice in PBS, resuspended in 0.5 ml DNA extraction buffer, incubated for 5 min at room temperature and centrifuged. The pellet was resuspended in 1 ml PBS containing PI (50 µg/ml) and ribonuclease A (200 µg/ml) and incubated at 37°C for 20 min in the dark. The percentage of cells in G1, S, and G2 was evaluated using ModFit LT for Win32 software (Verity Software House, Topsham, ME).
Luciferase reporter assay
Luciferase reporter assays were performed as described previously (Kambhampati et al, 2003). Briefly, to assess retinoic acid-responsive elements (RARE)-driven transcription, MCF-7 cells were transfected with a β-galactosidase expression vector and a RARE-luciferase plasmid (Minnucci et al, 1994) (kindly provided by Dr. Saverio Minucci) using the Superfect transfection reagent (QIAGEN Inc. Valencia, CA). Forty-eight hours after transfection, triplicate cultures were either left untreated or treated with ATRA (10 µM) or empty-ND or ATRA-ND (10 µM) for 24 h. The cells were then washed twice with cold PBS; and after cell lysis, luciferase activity was measured following the protocol of Promega (Madison, WI).
RT-PCR of RARA and RXRG mRNA
Granta cells were treated for 24 h with 20 µM ATRA-ND, 20 µM naked ATRA, empty-ND, or medium alone. Total RNA was isolated by the Qiagen method and dissolved in RNase-free water. One µg RNA was used for reverse transcription using Invitrogen’s one-step superscript Taq DNA polymerase system. PCR amplification was performed at 94 °C for 2 min, 62 °C and 68 °C for 40 cycles. Anti-sense and sense primers for RARA RARB, RARG, RXRA, RXRB and RXRG (Szabova et al, 2003) and GAPDH (Bao-Lei et al, 2006) (IDT Technologies, Coralville, IA) were used and DNA was identified by 1% agarose gel electrophoresis.
Statistical analysis
Data from ROS measurements in live cells were analyzed and expressed as mean fluorescence, and data from apoptosis assays were expressed as a percentage of apoptotic cells (annexin V positive and PI positive) over total cells. Statistical significance was performed by one-way ANOVA and Newman-Keuls multiple comparison test (GraphPad Software, Inc., San Diego, CA) to assess the effects of drugs on apoptosis or ROS production. P values less than 0.01 were considered statistically significant. All experiments were performed in triplicate.
Results
ATRA induced apoptosis in MCL cell lines
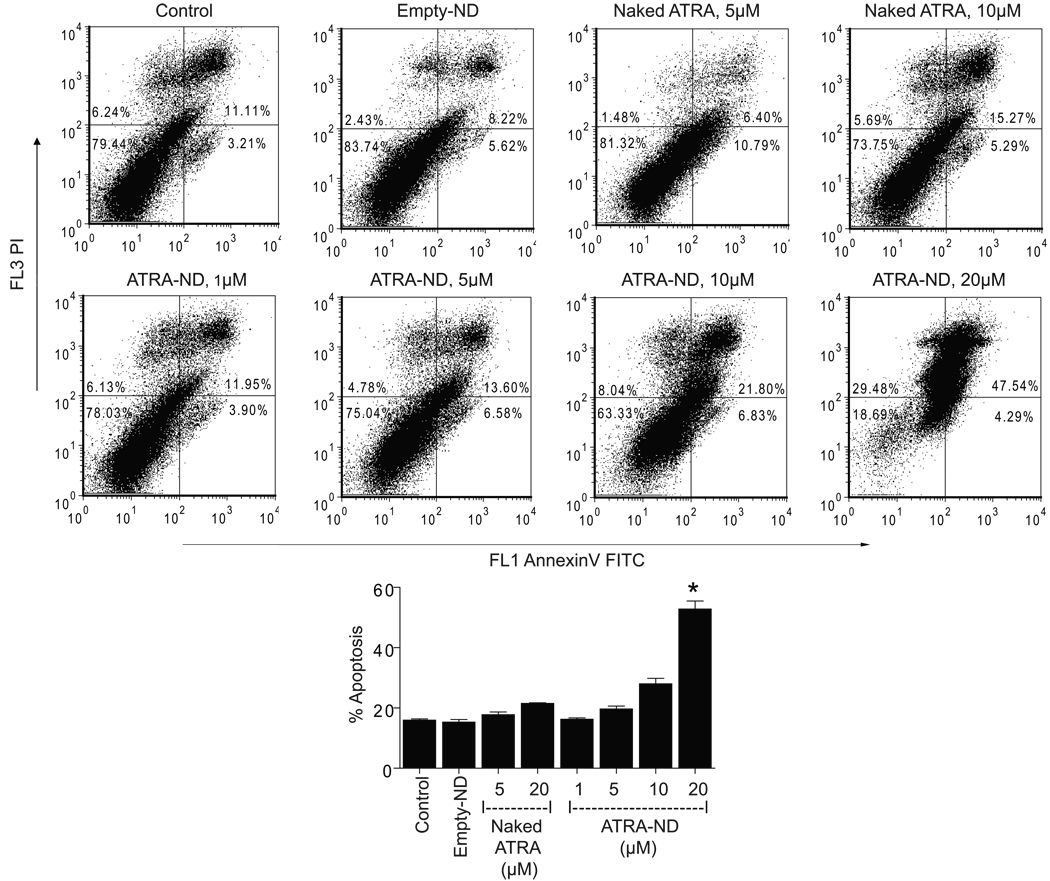
The response of cultured Granta cells to incubations with medium alone (control), empty ND, naked ATRA or ATRA-ND was analyzed by flow cytometry (Fig 1). Compared to medium alone (control), empty ND had no effect on the number of apoptotic cells while naked ATRA showed a slight increase above baseline at 20 µM. By contrast, ATRA-ND produced a dose-dependent increase in apoptosis, with about 50% apoptosis at 20 µM. The finding that ATRA-ND at 10 µM induced a greater apoptotic response than naked ATRA at 20 µM suggests packaging ATRA into ND enhanced the biological activity of this retinoid. We also examined two additional MCL cell lines (NCEB and Jeko, data not shown). In NCEB cells, the apoptotic response to ATRA-ND was similar to that seen in Granta cells. In Jeko cells, whereas naked ATRA induced a greater apoptotic response at 48 h, their effects were similar at 72 h.
Fig. 1.
ATRA-ND cause significant apoptosis in MCL cells. Flow cytometry dot plots of Granta cells exposed to medium alone (control), empty ND, naked ATRA or ATRA-ND at 37 °C in a 5% CO2 atmosphere for 72 h are shown. Early and late apoptotic percentages (AnnexinV/propidium iodide [PI] positive) from each dot plot were combined to estimate total apoptosis (summarized in the bar graph). Values shown are the mean ± SD (n = 3). *p<0.001 vs. Naked ATRA (20 µM).
Mechanism of apoptosis
To investigate the cell death pathway involved, cleavage of caspases -9 and -3, and PARP, were determined after incubation with naked ATRA, ATRA-ND, and empty-ND. As shown in Fig 2, compared to medium alone, no changes were observed in the protein levels of caspase-9 or caspase-3 upon exposure to empty ND in any of the three cell lines examined. In Granta cells, naked ATRA induced a lesser degree of caspase and PARP cleavage than ATRA-ND. The enhanced response to ATRA presented as a component of ND was even greater in NCEB cells. In Jeko cells, the extent of caspase and PARP cleavage was similar with either ATRA-ND or naked ATRA.
Fig. 2.
Effect of ATRA-ND on caspase-3, caspase-9 and PARP levels in MCL cells. Granta, NCEB, and Jeko cells were incubated with medium alone (control), empty ND, naked ATRA (20 µM) and ATRA-ND (20 µM) for 24 h. Following incubation, cell homogenates were separated by SDS-PAGE, transferred to nitrocellulose and probed with antibodies directed against caspase 9, caspase 3, PARP and GAPDH, respectively. For all 3 cell lines, there is decreased caspase 3 and 9 protein after ATRA-ND compared with naked ATRA or medium alone, indicating cleavage of caspase 3 and 9, and increased PARP cleavage with ATRA-ND.
ATRA induced ROS generation in MCL cells
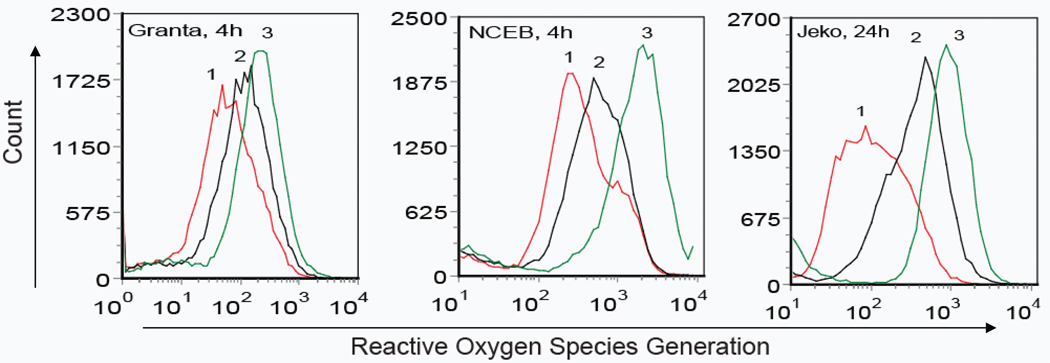
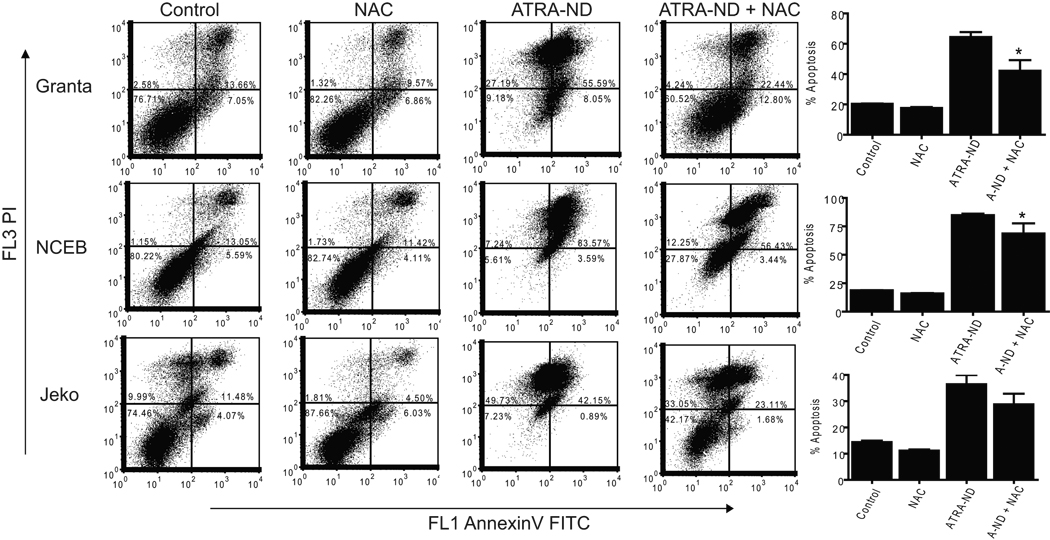
As the apoptotic events noted above may involve enhanced ROS production (Delia et al, 1997; Oridate et al, 1997), the effect of ATRA exposure on ROS generation was determined for each of the three MCL cell lines (Fig 3A). Compared to medium alone, cells treated with naked ATRA showed a modest increase in ROS generation. By contrast, after 4 h exposure, ATRA-ND induced a significant increase in ROS in both Granta and NCEB cells. In Jeko cells, ATRA-ND-induced ROS generation at 24 h was greater than that resulting from the same concentration of naked ATRA. To investigate the role of ROS generation and apoptosis, cells were incubated with N-acetylcysteine (NAC), which restores glutathione and thereby functions as an intracellular antioxidant. NAC reduced ATRA-ND stimulated apoptosis in all three MCL cell lines (Fig 3B), in support of the premise that ATRA-induced apoptosis is mediated partly by ROS production.
Fig. 3.
ATRA-mediated ROS generation in MCL cells. (A) Granta, NCEB, and Jeko cells were treated with medium alone (Peak 1), 20 µM naked ATRA (Peak 2) or 20 µM ATRA-ND (Peak 3) for 4 h (Granta, NCEB) or 24 h (Jeko) at 37 °C. ROS production in live cells was measured by flow cytometry as described in Materials and Methods In all 3 cell lines, there is an increase in ROS after ATRA-ND (peak 3) compared with naked ATRA or medium alone. (B) Incubations as in A, except an additional incubation of 20 µM ATRA-ND (A-ND) plus 10 mM N-acetylcysteine (NAC) was included. After 48 h, cells were analyzed for apoptosis as described in Materials and Methods. Early and late apoptotic percentages (AnnexinV/PI positive) from each dot plot were combined to represent total apoptosis (summarized in the bar graphs). In all 3 cell lines, there is an increase in apoptosis after ATRA-ND, which is abrogated by NAC. Values shown are the mean ± SD (n = 3). *p<0.01 Vs. ATRA-ND
Effect of ATRA on cell cycle arrest and cell cycle regulator proteins
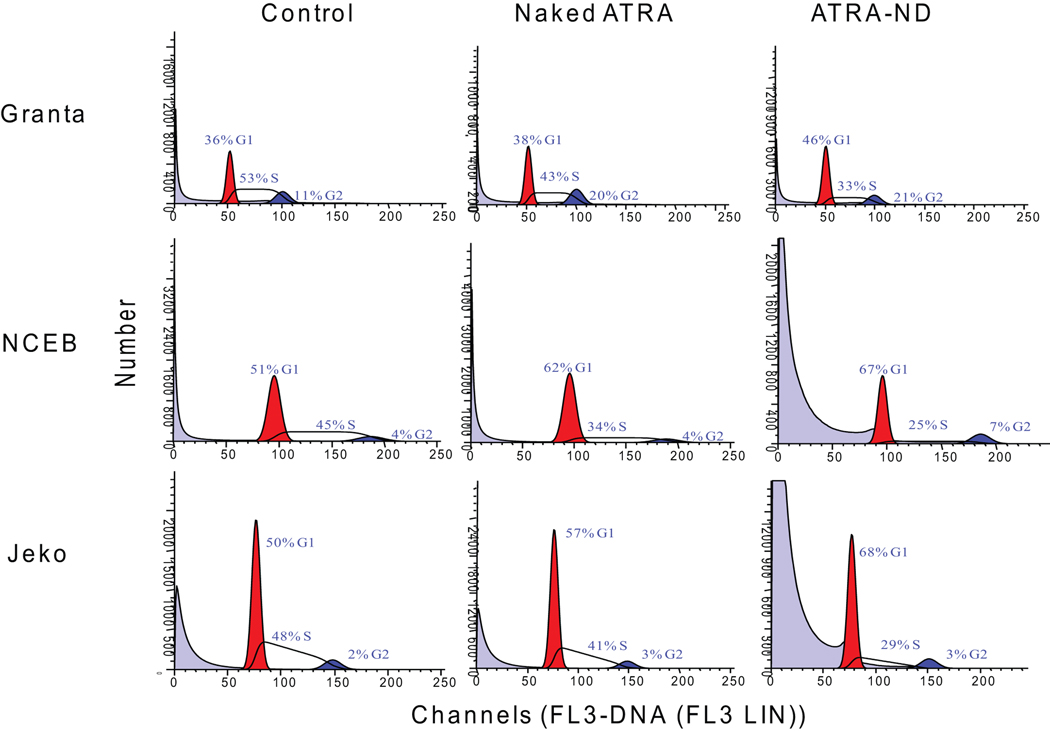
We next analyzed the effects of naked ATRA versus ATRA-ND on the cell cycle. In each of three MCL cell lines examined (Fig 4A); ATRA-ND induced a greater degree of G1 cell cycle arrest than naked ATRA. An increased G1 cell population was detected upon incubation with ATRA-ND (10 µM for 24 h). There was a progressive decrease in S-phase cells and an increase in G1 growth arrest.
Fig. 4.
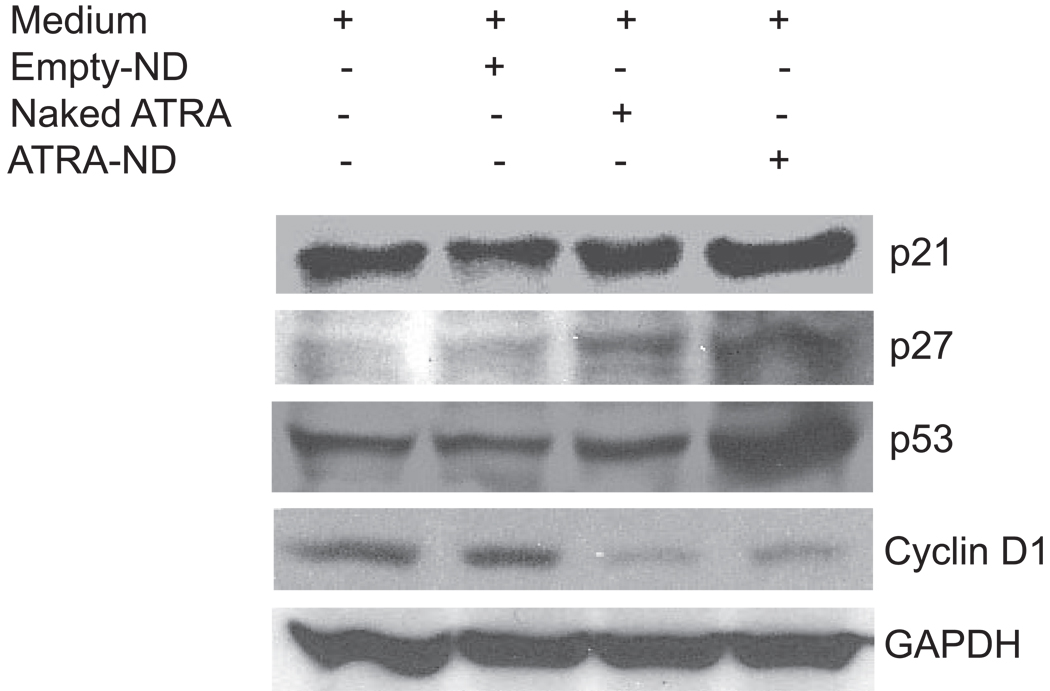
(A). Effect of ATRA on cell cycle status of MCL cells. MCL cells were incubated for 24 h with medium alone, naked ATRA (10 µM) and ATRA-ND (10 µM). Following incubation, cells were analyzed for DNA content as described in Materials and Methods. The percentage of cells in G1, S, and G2 were evaluated using ModFit LT for Win32 software (Verity Software House, Topsham, ME). G1 (red), G2 (blue), and S (hatched). ATRA-ND results in more G1 arrest compared with naked ATRA and medium alone (control). (B) Western Blot analysis of cell cycle regulator protein expression. Whole cell lysates were obtained from Granta cells incubated for 24 h with medium alone (control), empty ND, naked ATRA (20 µM) and ATRA-ND (20 µM) and probed with antibodies directed against p21, p27, p53, cyclin D1 and GAPDH. ATRA-ND results in an increase in p21, p53 and p27 and a decrease in cyclin D1 in all three cell lines.
Cyclin-dependent kinases (CDKs) regulate checkpoints that integrate mitogenic and growth inhibitory signals during cell cycle transitions. CDK inhibitors bind to CDKs and inhibit kinase activity, leading to cell cycle arrest. P21 and p27 negatively regulate CDKs, and because p21 is induced by the tumor suppressor p53 in response to DNA damage, the expression level of these proteins were examined in Granta cells as a function of ATRA exposure. Compared to untreated control cells, no changes in the level of these proteins were seen following incubation with empty-ND. Whereas naked ATRA induced a modest increase in p21, p27 and p53, much larger increases were induced by ATRA-ND (Fig 4B). Since cyclin D1 is induced by the chromosomal translocation t(11;14)(q13;q32), which characterizes MCL, its levels were also determined. Compared to medium alone or empty ND-treated cells, both naked ATRA and ATRA-ND incubation led to a decrease in cyclin D1 expression (Fig 4B). To extend these findings, the levels of cyclins D2 and D3 in Granta cells were determined. Compared to incubation with medium alone or empty-ND, naked ATRA and ATRA-ND induced a clear increase in cyclins D2 and D3 (data not shown). These data suggest ATRA-dependent cyclin D1 downregulation is accompanied by a compensatory increase in cyclins D2 and D3.
Expression of RARs and RXRs in MCL cells
Since the diversity of retinoic acid-induced signaling is associated with RAR and RXR subtypes, we examined retinoid receptor levels and mRNA expression. While empty ND had no effect on RAR α or RXRγ protein levels, ATRA exposure resulted in increased levels of these proteins. In Granta and Jeko cells, ATRA-ND induced a greater increase in RARα protein than naked ATRA, while both forms of ATRA elicited similar increases in NCEB cells. ATRA-ND induced greater expression of RXRγ in each of the three cell lines (Fig 5A).
Fig. 5.
(A) Effect of ATRA on RAR and RXR subtype protein expression. Whole cell lysates were obtained from Granta, NCEB, and Jeko cells incubated for 24 h with medium alone (control), empty ND, naked ATRA (20 µM) or ATRA-ND (20 µM) and immunodetection was performed using antibodies directed against RAR and RXR receptor subtypes, and GAPDH as an internal control. Immune complexes were visualized by enhanced chemiluminescence. ATRA-ND incubation results in an increase in RARα and RXRγ protein compared with control (medium) and naked ATRA. (B) RT-PCR of RAR and RXR subtype mRNA. Granta cells were incubated with medium alone (Control), naked ATRA (20 µM) or ATRA-ND (20 µM) for 24 h. Following incubation, total RNA was isolated as described in Materials and Methods. 1.0 µg RNA was used for amplification.
Subsequently, the relationship between retinoid receptor mRNA and ATRA exposure was evaluated in Granta cells. RT-PCR analysis of various receptor subtypes (Fig 5B) revealed a differential expression of RAR and RXR subtype mRNAs. Compared to naked ATRA, ATRA-ND elicited greater RARA and RXRG mRNA expression while naked ATRA and ATRA-ND elicited a similar induction of RARB, RARG, RXRaA and RXRB.
Role of RARα in ATRA-ND induced ROS generation, cell cycle arrest, and apoptosis
We next sought to determine the role of RARs in ATRA-ND induced generation of ROS, cell cycle arrest, and apoptosis in cultured MCL cells. Studies were performed with the RAR antagonist, Ro 41-5253. ATRA-ND induced generation of ROS was inhibited by co-incubation with Ro 41-5253 (Fig 6A). To assess the role of RARs in ATRA-ND induced G1 growth arrest, cells were incubated with ATRA-ND alone or ATRA-ND plus Ro 41-5253. While ATRA-ND induced G1 growth arrest in each of three MCL cell lines examined, growth arrest was prevented in cells treated with ATRA-ND plus Ro 41-5253 (Fig 6B). In addition, we determined whether RARs play a role in ATRA-ND induced apoptosis. At 2 µM, Ro 41-5253 effectively blocked ATRA-ND induced apoptosis in each of the MCL cell lines (Fig 6C).
Fig. 6.
Effect of the RAR antagonist Ro 41-5253 on ATRA-ND induced ROS generation, cell cycle progression, and apoptosis in MCL Cells. (A) Granta, NCEB, and Jeko cells were incubated with medium alone (control), ATRA-ND (20 µM) or ATRA-ND plus Ro 41-5253 (2 µM) for 4 h. Following incubation, ROS generation was measured as described in Materials and Methods. The red peak represents control, the blue peak is ATRA-ND, and green peak represents ATRA-ND plus Ro 41-5253. There is an increase in ROS production (blue peak) that is inhibited by the Ro 41-5253 (green peak) (B) Cells were incubated with medium alone (control), ATRA-ND (10 µM), Ro 41-5253 (2 µM) and ATRA-ND plus Ro 41-5253 for 24 h. Following incubation, cells were analyzed for DNA content as described in Materials and Methods. DNA content was quantified by PI staining and flow cytometry. G1 (red), G2 (blue), and S (hatched). (C) MCL cells were incubated with medium (control), Ro 41-5253 (2 µM), ATRA-ND (20 µM) or ATRA-ND plus Ro 41-5253 at 37 °C in a 5% CO2 atmosphere for 48 h. Early and late apoptotic percentages (AnnexinV/PI positive) from each dot plot were combined to estimate total apoptosis (summarized in the bar graph). Ro-41-5253 blocks ATRA-ND induced apoptosis in all three cell lines. Values shown are the mean ± SD (n = 3). *p<0.001 Vs. ATRA-ND.
Increased transcriptional activity of ATRA-ND compared with free ATRA
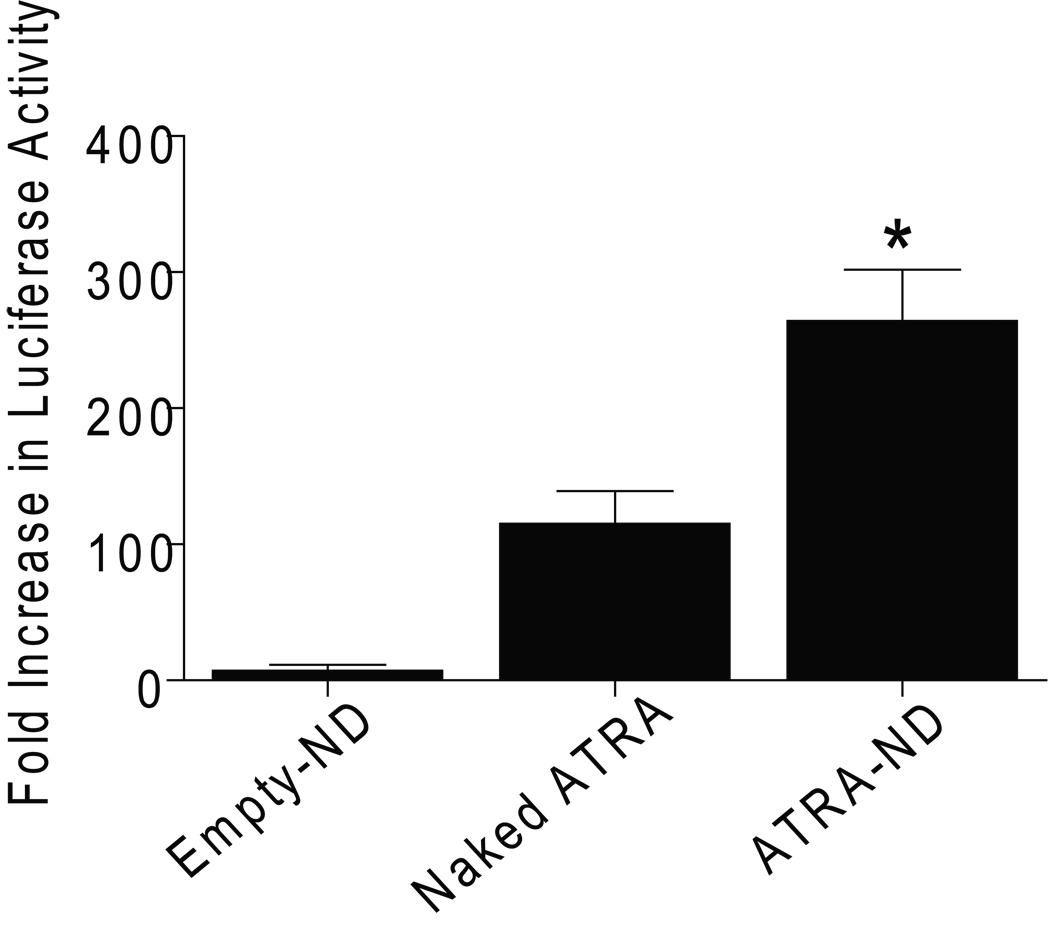
To compare the responsiveness of RARA to ATRA-ND and naked ATRA in another cancer cell model we used MCF-7 breast cancer cells. We measured the effects of NDs on the transcription of RARA in MCF-7 breast cancer cells, which are also sensitive to the growth inhibitory effects of retinoic acid (van der Leede et al, 1997). We tested the effects of empty-ND, naked ATRA, and ATRA-ND on RARα by measuring their ability to stimulate the transcriptional activity of RARA in MCF-7 cells transfected with luciferase reporter genes. The transactivation of RARA induced by ATRA-ND was significantly higher than the naked ATRA. Empty-ND had no effect compared with medium alone (control) (Fig 7).
Fig. 7.
Effect of ATRA on RARA transcription. MCF-7 cells were transfected with an RARE-luciferase construct. Forty eight hours after transfection, cells were treated for 24 h with 10 µM ATRA-ND, 10 µM naked ATRA, empty-ND, or medium alone (control) and luciferase activity was measured. Data are expressed as fold increase in response to each treatment over control untreated samples. Each experiment was performed in triplicate and values shown are mean ± SE (n = 3). *p<0.01 Vs. Naked ATRA.
Discussion
MCL is an aggressive malignant lymphoma with moderate chemosensitivity and poor prognosis and remains one of the most difficult therapeutic challenges. Complete response rates to chemotherapy range from 20% to 40%, with median survivals of 2 to 3 years. Emerging anticancer drugs, such as retinoids, have been shown to exert dramatic biological effects in acute promyelocytic leukaemia (APL). In addition, retinoic acid isomers have also been shown to inhibit the proliferation of primary MCL cells (Guidoboni et al, 2005). ATRA was first introduced as a differentiating agent in the treatment of APL and is still regarded as first-line drug against APL. It reaches a maximum plasma concentration of about 2 µM to induce complete remission (Wang & Chen, 2000). In leukemic cell lines (HL-60), however, ATRA or 9-cis-RA induced reduced proliferation at higher doses (5–20 µM) and in a dose-dependent manner (Orlandi et al, 2003). Similar to HL-60 cells, we demonstrated apoptotic effects of ATRA in MCL cell lines using this same dose range. Based on the observation that ATRA in DMSO has the capacity to treat APL (Jingo et al, 2001) and that ATRA-ND cause cell death in HepG2 cells, we hypothesized that ATRA delivered by ND would be more effective than ATRA in DMSO in causing apoptosis in MCL cells. In the present study, MCL cell lines were treated with ATRA in DMSO (naked ATRA) or in nanodisks (ATRA-ND) where increased apoptosis was observed as a function of increasing doses of ATRA-ND while naked ATRA was less effective. These data are in agreement with Sundaresan et al. (1997) who reported an induction of apoptosis after treatment with ATRA in both freshly obtained patient cells and B-cell lymphoma cells. These authors tested both free ATRA and ATRA in liposomes and found that liposomal ATRA elicited a better response than free ATRA in terms of tumour growth inhibition and apoptosis induction. In addition to lymphoma cells, in vitro cell viability assays with human HepG2 hepatoma cells (Redmond et al., 2007) demonstrated enhanced cell growth inhibition activity for ATRA-ND compared to naked ATRA. Furthermore, in MCF-7 breast cancer cells, which are sensitive to the growth inhibitory effects of retinoic acid, ATRA-ND exposure induced a significant increase in RARA transcript (Fig. 7). Thus, it may be concluded that packaging ATRA in lipid particles enhances the biological effectiveness of this retinoid. In this regard, ND have potential advantages over liposomes as an ATRA delivery vehicle, including nanoscale size (<20 nm diameter), full water solubility, exposure of both faces of the bilayer to the external environment and the presence of protein as an intrinsic structural component (Ryan, 2008). This latter feature facilitates incorporation of targeting sequences via protein engineering of the apolipoprotein component (Iovannisci et al, 2009).
Our data show that the exposure of Granta and NCEB cells to ATRA-ND triggered cleavage of caspases and PARP, suggesting activation of the intrinsic pathway, consistent with studies in acute lymphoblastic leukemia cells (Faderl et al, 2003). Characteristic apoptotic events may also involve enhanced ROS production (Green & Reed, 1998), because ROS are reported to be involved in retinoid-induced apoptosis in human leukemic and cervical cells (Delia et al, 1997; Oridate et al, 1997). The present study provides evidence that ATRA-ND induce significant generation of ROS in MCL cells. This conclusion was supported by our experiments with N-acetylcysteine, which increased intracellular glutathione as an endogenous anti-oxidant and partially blocked ATRA-ND-induced apoptosis.
Our data on cell cycle events indicate that ATRA-ND treatment, compared to naked ATRA or empty ND, resulted in a marked increase in the cell population in the G1 phase of the cell cycle in Granta, NCEB, and Jeko cells. This increase in G1 phase was accompanied by a corresponding decrease in the S phase. These data suggest that ATRA-ND inhibits the G1-S transition and that this effect was greater with ATRA-ND than naked ATRA. Using a specific siRNA to knockdown p21, Yu et al (2008) have demonstrated that p21 is a critical regulator of cell growth and that its activation is required in growth inhibition by ATRA. In addition, Tanaka et al (2007) have demonstrated that p21 is a transcriptional target of retinoid receptors, and that the expression of RAR and RXR in cancers is crucial in inducing p21-mediated cell growth arrest. In addition to p21, we also found that MCL cells treated with ATRA-ND showed a significant increase in p27 and p53 proteins, suggesting that they play a key role in cell cycle arrest and apoptosis (Dierov et al, 1999; Izban et al, 2000). Chiarle et al. (2000, 2001) reported that p27 levels in mantle cells were regulated by proteasome-mediated degradation and that low expression of p27 was a negative risk factor for MCL patients. It is conceivable that ATRA-ND may affect the proteasome activity responsible for p27 degradation.
Cell cycle control and differentiation are deregulated in many cancers (Clarke & Allan, 2009). The t(11:14) abnormality associated with MCL results in translocation of CCND1 (BCL1), a member of the cyclin gene family, from chromosome 11 to 14. This translocation leads to dysregulation of CCND1 and plays a role in overexpression of CCND1 mRNA (Ott et al, 1996). High levels of CCND1 mRNA are consistently found in malignant cells with the t(11:14) abnormality (Alkan et al, 1995). ATRA-ND treatment of Granta cells induced a significant decrease in cyclin D1, in keeping with the observed cell growth arrest. In addition, ATRA-ND treated Granta cells displayed increased levels of cyclins D2 and D3. These findings suggest that upregulation of cyclins D2 and D3 may be compensatory with respect to the decrease in cyclin D1. Spinella et al. (1999) also reported a decline in cyclin D1 after treatment with retinoic acid and Baldassarre et al. (2000) found increased cyclin D3 in human embryonal carcinoma cells treated with retinoic acid. In other studies, Fu et al. (2005) suggested an upregulation of D2 or D3 substitutes for D1 in MCL cells while Mozos et al. (2009) reported an increase in cyclins D2 and D3 in cyclin D1 deficient MCL cells. When cyclin D1 was knocked down with lentiviral shRNA, cyclin D2 levels increased (Klier et al., 2008). It is conceivable that the decline in cyclin D1 in ATRA-ND treated Granta cells may involve the ubiquitin-proteasome pathway. This pathway, which is recognized to exist in MCL cells, plays a major role in degradation of proteins involved in the cell cycle, proliferation, and apoptosis (Bogner et al, 2006). Consistent with this interpretation, Spinella et al. (1999) found that retinoids accelerate ubiquitination and subsequent degradation of cyclin D1 via a process that is blocked by proteasome inhibitors. Given the major role of the ubiquitin-proteasome pathway in the degradation of proteins involved in cell cycle progression and apoptosis, it is plausible that this pathway may be operative in ATRA-induced cyclin D1 degradation in Granta cells.
The present study evaluated the ability of ATRA-ND to activate RARs or RXRs in Granta cells and noted that, while these transcripts were nearly undetectable in untreated cells, their levels increased with ATRA exposure. ATRA-ND increased the mRNA levels of RARA and RXRG and their respective protein levels. This finding is similar to studies with B-cell lymphoma reported by Sundaresan et al (1997). In malignant myeloid leukemic cells, RAR subtypes and RXRα are expressed to a variable degree, and ATRA-induced RARβ expression (Lehmann et al, 2001). The potential role of RAR α in ATRA-ND induced cell death is demonstrated by inhibition of ROS production, cell cycle arrest, and apoptosis caused by Ro 41-5253. In other studies, selective RARα antagonists have been shown to reverse the effect of retinoids on cell growth inhibition in NHL-B cells (Sundaresan et al. (1997), mouse B cells (Lehmann et al, 1992), and Burkitt lymphoma cells (Cariati et al, 2000). Whereas results from the present study suggest RARα mediates ATRA-ND induced effects on ROS, cell growth, and apoptosis in MCL cells, it is conceivable that other retinoid receptors may also play a role.
An important aspect of the present results relates to differences in biological activity observed with different ATRA delivery methods, i.e., naked ATRA versus ATRA-ND. Whereas ATRA solubilized in DMSO had relatively little effect on Granta cells, packaging ATRA into ND greatly enhanced apoptosis and cell cycle arrest. It is conceivable that solubilization of ATRA in the ND hydrophobic milieu effectively concentrates this bioactive lipid and provides a means for more efficient delivery to target cells. Further, in order to demonstrate the functional significance of ATRA packaged in ND, we have shown that ATRA-ND increased RARA transcriptional activity compared with naked ATRA in MCF-7 cells transiently transfected with an RARA luciferase reporter vector. It has yet to be determined if ATRA in ND is more stable or resists degradation during the course of cell incubations or whether ATRA-ND are taken up by the cells via receptor-mediated endocytosis. Further work is required to elucidate the molecular basis of the enhanced biological activity of ATRA when presented to cells as a component of ND and whether the enhanced activity observed in cell culture can be extended to an in vivo setting. Nonetheless, results obtained in this study suggest ATRA-ND represent a potentially effective approach to the treatment of mantle cell lymphoma and should be explored further.
Acknowledgments
This study was supported by grants from the NIH (1R43CA141904, TMF), The Northwestern University Feinberg School of Medicine Department of Medicine Lymphoma Research Fund (LIG), the Meyer Research Fund (LIG), the Philip Bligh Research Fund (LIG), NIH (HL-64159, ROR), NIH (CA121192, LCP), and Merit Review Grant, Department of Veterans Affairs (LCP). The authors gratefully thank Dr. Steven H. Bernstein (University of Rochester, NY) for Granta and NCEB cells, Dr. Steven Rosen (Northwestern University, Chicago, IL) for Jeko cells, and Thanh Son Nguyen (CHORI) for technical assistance.
Footnotes
Authorship
A.T.K.S. designed and performed experiments, analyzed data, prepared figures, and wrote the manuscript; L.I.G. designed experiments, analyzed data, and wrote the manuscript; R.O.R. provided ATRA- and empty-nanodisks for the study, analyzed data, reviewed the paper, and provided critical revisions; T.M.F. analyzed data, reviewed the paper, and provided critical revisions; J.A.B. prepared ATRA- and empty-nanodisks for the study; R.J.A., A.S., and N.S. performed part of the experiments; L.C.P., S.B., S.Y., and A.M.E. contributed to data analysis and review.
Conflict-of-Interest Disclosure
The authors declare no competing financial interests.
References
- Adamson PC. All trans retinoic acid pharmacology and its impact on the treatment of acute promyelocytic leukemia. Oncologist. 1996;1:305–314. [PubMed] [Google Scholar]
- Alkan S, Schnitzer B, Thompson JL, Moscinski LC, Ross CW. Cyclin D1 protein expression in mantle cell lymphoma. Annals of Oncology. 1995;6:567–570. doi: 10.1093/oxfordjournals.annonc.a059245. [DOI] [PubMed] [Google Scholar]
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nature Reviews Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- Altucci L, Leibovitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nature Reviews. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- Amin HM, McDonnell TJ, Medeiros J, Rassidakis GZ, Leventaki V, O'Connor SL, Keating MJ, Lai R. Characterization of 4 mantle cell lymphoma cell lines. Archives of Pathology and Laboratory Medicine. 2003;127:424–431. doi: 10.5858/2003-127-0424-COMCLC. [DOI] [PubMed] [Google Scholar]
- Bao-Lei T, Zhu-Zhong M, Yi S, Jun-Jie Q, Yan D, Hua L, Bin L, Guo-Wei Z, Zhi-Xian S. Knocking down PML impairs p53 signaling transduction pathway and suppresses irradiation induced apoptosis in breast carcinoma cell MCF-7. Journal of Cellular Biochemistry. 2006;97:561–571. doi: 10.1002/jcb.20584. [DOI] [PubMed] [Google Scholar]
- Baldassarre G, Boccia A, Bruni P, Sandomenico C, Barone MV, Pepe S, Angrisano T, Belletti B, Motti ML, Fusco A, Viglietto G. Retinoic acid induces neuronal differentiation of embryonal carcinoma cells by reducing proteasome-dependent proteolysis of the cyclin-dependent inhibitor p27. Cell Growth & Differentiation. 2000;11:517–526. [PubMed] [Google Scholar]
- Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. International Journal of Biochemistry and Cell Biology. 2007;39:1747–1753. doi: 10.1016/j.biocel.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Bogner C, Peschel C, Decker T. Targeting the proteasome in mantle cell lymphoma: A promising therapeutic approach. Leukemia & Lymphoma. 2006;47:195–205. doi: 10.1080/10428190500144490. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cariati R, Zancai P, Quaia M, Cutrona G, Giannini F, Rizzo S, Boiocchi M, Dolcetti R. Retinoic acid induces persistent, RARα-mediated anti-proliferative responses in Epstein Barr virus immortalized B lymphoblasts carrying an activated c-myc oncogene but not in Burkitt’s lymphoma cell lines. International Journal of Cancer. 2000;86:375–384. doi: 10.1002/(sici)1097-0215(20000501)86:3<375::aid-ijc12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Budel LM, Skolnik J, Frizzera G, Chilosi M, Corato A, Pizzolo G, Magidson J, Montagnoli A, Pagano M, Maes B, De Wolf-Peeters C, Inghirami G. Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood. 2000;95:619–626. [PubMed] [Google Scholar]
- Chiarle R, Pagano M, Inghirami G. The cyclin dependent kinase inhibitor p27 and its prognostic role in breast cancer. Breast Cancer Research. 2001;3:91–94. doi: 10.1186/bcr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Allan LA. Cell-cycle control in the face of damage-a matter of life or death. Trends in Cell Biology. 2009;19:89–98. doi: 10.1016/j.tcb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Delia D, Aiello A, Meroni L, Nicolini M, Reed JC, Pierotti MA. Role of antioxidants and intracellular free radicals in retinamide induced cell death. Carcinogenesis. 1997;18:943–948. doi: 10.1093/carcin/18.5.943. [DOI] [PubMed] [Google Scholar]
- Dierov J, Sawaya BE, Prosniak M, Gartenhaus RB. Retinoic acid modulates a bimodal effect on cell cycle progression in human adult T-cell leukemia cells. Clinical Cancer Research. 1999;5:2540–2547. [PubMed] [Google Scholar]
- Evens AM, Lecane P, Magda D, Prachand S, Singhal S, Nelsonx J, Miller RA, Gartenhaus RB, Gordon LI. Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood. 2005;105:1265–1273. doi: 10.1182/blood-2004-03-0964. [DOI] [PubMed] [Google Scholar]
- Faderl S, Lotan R, Kantarjian HM, Harris D, Van Q, Estrov Z. N-(4-Hydroxylphenyl)retinamide (fenretinide, 4-HPR), a retinoid compound with antileukemic and proapoptotic activity in acute lymphoblastic leukemia (ALL) Leukemia Research. 2003;27:259–266. doi: 10.1016/s0145-2126(02)00162-5. [DOI] [PubMed] [Google Scholar]
- Fu K, Weisenburger DD, Greiner TC, Dave S, Wright G, Rosenwald A, Chiorazzi M, Iqbal J, Gesk S, Siebert R, De Jong D, Jaffe ES, Wilson WH, Delabie J, Ott G, Dave BJ, Sanger WG, Smith LM, Rimsza L, Braziel RM, Müller-Hermelink HK, Campo E, Gascoyne RD, Staudt LM, Chan WC. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315–4321. doi: 10.1182/blood-2005-04-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Guidoboni M, Zancai P, Cariati R, Rizzo S, Dal Col J, Pavan A, Gloghini A, Spina M, Cuneo A, Pomponi F, Bononi A, Doglioni C, Maestro R, Carbone A, Boiocchi M, Dolcetti R. Retinoic acid inhibits the proliferative response induced by CD40 activation and interleukin-4 in mantle cell lymphoma. Cancer Research. 2005;65:587–595. [PubMed] [Google Scholar]
- Iovannisci DM, Beckstead JA, Ryan RO. Targeting nanodisks via an apolipoprotein - single chain variable antibody chimera. Biochemical and Biophysical Research Communications. 2009;379:466–469. doi: 10.1016/j.bbrc.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izban KF, Alkan S, Singleton TP, His ED. Multiparameter immunohistochemical analysis of the cell cycle proteins cyclin D1, Ki-67, p21, p27, and p53 in mantle cell lymphoma. Archives of Pathology and Laboratory Medicine. 2000;124:1457–1462. doi: 10.5858/2000-124-1457-MIAOTC. [DOI] [PubMed] [Google Scholar]
- Jimenez-Lara AM, Clarke N, Altucci L, Gronemeyer H. Retinoic acid induced apoptosis in leukemic cells. Trends in Molecular Medicine. 2004;10:508–515. doi: 10.1016/j.molmed.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Jingo Y, Wang I, Xia I, Chen GQ, Chen Z, Miller WH, Waxman S. Combined effect of all trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 2001;97:264–269. doi: 10.1182/blood.v97.1.264. [DOI] [PubMed] [Google Scholar]
- Kambhampati S, Li Y, Verma A, Sassano A, Majchrzak B, Deb DK, Parmar S, Giafis N, Kalvakolanu DV, Rahman A, Uddin S, Minucci S, Tallman MS, Fish EN, Platanias LC. Activation of protein kinase C delta by all trans retinoic acid. Journal of Biological Chemistry. 2003;278:32544–32551. doi: 10.1074/jbc.M301523200. [DOI] [PubMed] [Google Scholar]
- Kitareewan S, Blumen S, Sekula D, Bissonnette RP, Lamph WW, Cui Q, Gallagher R, Dmitrovsky E. G0S2 is an all-trans retinoic acid target gene. International Journal of Oncology. 2008;33:397–404. [PMC free article] [PubMed] [Google Scholar]
- Klier M, Anastasov N, Hermann A, Meindl T, Angermeier D, Raffeld M, Fend F, Quintanilla-Martinez L. Specific lentiviral shRNA-mediated knockdown of cyclin D1 in mantle cell lymphoma has minimal effects on cell survival and reveals a regulatory circuit with cyclin D2. Leukemia. 2008;22:2097–2105. doi: 10.1038/leu.2008.213. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Jong L, Fanjul A, Cameron JF, Lu XP, Haefner P, Dawson MI, Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992;258:1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Lehmann S, Paul C, Torma H. Retinoid receptor expression and its correlation to retinoid sensitivity in non-M3 acute myeloid leukemia blast cells. Clinical Cancer Research. 2001;7:367–373. [PubMed] [Google Scholar]
- Minnucci S, Zand DJ, Dey A, Marks MS, Nagata T, Grippo JF, Ozato K. Dominant negative retinoid X receptor beta inhibits retinoic acid-responsive gene regulation in embryonal carcinoma cells. Molecular and Cellular Biology. 1994;14:360–372. doi: 10.1128/mcb.14.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozos A, Royo C, Hartmann E, De Jong D, Baró C, Valera A, Fu K, Weisenburger DD, Delabie J, Chuang SS, Jaffe ES, Ruiz-Marcellan C, Dave S, Rimsza L, Braziel R, Gascoyne RD, Solé F, López-Guillermo A, Colomer D, Staudt LM, Rosenwald A, Ott G, Jares P, Campo E. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 2009;94:1555–1562. doi: 10.3324/haematol.2009.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oridate N, Suzuki S, Higuchi M, Mitchell MF, Hong WK, Lotan R. Involvement of reactive oxygen species in N-(4-hydroxyphenyl) retinamide induced apoptosis in cervical carcinoma cells. Journal of the National Cancer Institute. 1997;89:1191–1198. doi: 10.1093/jnci/89.16.1191. [DOI] [PubMed] [Google Scholar]
- Orlandi M, Mantovani B, Ammar K, Avitabile E, Dal Monte P, Bartolini G. Retinoids and cancer: Antitumoral effects of ATRA, 9-cis RA and the new retinoid IIF on the HL-60 leukemic cell line. Medical Principles and Practice. 2003;12:164–169. doi: 10.1159/000070753. [DOI] [PubMed] [Google Scholar]
- Ott MM, Helbing A, Ott G, Bartek J, Fischer L, Dürr A, Kreipe H, Müller-Hermelink HK. Bcl-1 rearrangement and cyclin D1 protein expression in mantle cell lymphoma. Journal of Pathology. 1996;179:238–242. doi: 10.1002/(SICI)1096-9896(199607)179:3<238::AID-PATH566>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Redmond KA, Nguyen T-S, Ryan RO. All-trans-retinoic acid nanodisks. International Journal of Pharmaceutics. 2007;339:246–250. doi: 10.1016/j.ijpharm.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RO. Nanodisks: Hydrophobic drug delivery vehicles. Expert Opinion on Drug Delivery. 2008;5:343–351. doi: 10.1517/17425247.5.3.343. [DOI] [PubMed] [Google Scholar]
- Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expression and Purification. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annual Review of Nutrition. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- Spinella MJ, Freemantle SJ, Sekula D, Chang JH, Christie AJ, Dmitrovsky E. Retinoic acid promotes ubiquitination and proteolysis of cyclin D1 during induced tumor cell differentiation. Journal of Biological Chemistry. 1999;31:22013–22018. doi: 10.1074/jbc.274.31.22013. [DOI] [PubMed] [Google Scholar]
- Sundaresan A, Claypool K, Mehta K, Lopez-Berestein G, Cabanillas F, Ford RJ., Jr Retinoid mediated inhibition of cell growth with stimulation of apoptosis in aggressive B-cell lymphomas. Cell Growth and Differentiation. 1997;8:1071–1082. [PubMed] [Google Scholar]
- Szabova L, Macejova D, Dvorcakova M, Mostbock S, Blazickova S, Zorad S, Walrand S, Cardinault N, Vasson MP, Rock E, Brtko J. Expression of nuclear retinoic acid receptor in peripheral blood mononuclear cells (PBMC) of healthy subjects. Life Sciences. 2003;72:831–836. doi: 10.1016/s0024-3205(02)02307-x. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Suh KS, lo AM, De Luca LM. p21 is a common transcriptional target of retinoid receptors. Journal of Biological Chemistry. 2007;282:29987–29997. doi: 10.1074/jbc.M701700200. [DOI] [PubMed] [Google Scholar]
- van der Leede BM, van den Brink CE, Pijnappel WW, Sonneveld E, van der Saag PT, van der Burg B. Autoinduction of retinoic acid metabolism to polar derivatives with decreased biological activity in retinoic acid sensitive, but not in retinoic acid resistant human breast cancer cells. Journal of Biological Chemistry. 1997;272:17921–17928. doi: 10.1074/jbc.272.29.17921. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Chen Z. Differentiation and apoptosis induction therapy in acute promyelocytic leukemia. Lancet Oncology. 2000;1:101–106. doi: 10.1016/s1470-2045(00)00017-6. [DOI] [PubMed] [Google Scholar]
- Yu Z, Li W, Lu Q, Wang L, Zhang X, Han P, Chen P, Pei Y. p21 is required for ATRA-mediated growth inhibition of MEPM cells, which involves RAR. Journal of Cellular Biochemistry. 2008;104:2185–2192. doi: 10.1002/jcb.21773. [DOI] [PubMed] [Google Scholar]