Abstract
Background
Understanding mollicutes is challenging due to their variety and relationship with host cells. Invasion has explained issues related to their opportunistic role. Few studies have been done on the Ureaplasma diversum mollicute, which is detected in healthy or diseased bovine. The invasion in Hep-2 cells of four clinical isolates and two reference strains of their ureaplasma was studied by Confocal Laser Scanning Microscopy and gentamicin invasion assay.
Results
The isolates and strains used were detected inside the cells after infection of one minute without difference in the arrangement for adhesion and invasion. The adhesion was scattered throughout the cells, and after three hours, the invasion of the ureaplasmas surrounded the nuclear region but were not observed inside the nuclei. The gentamicin invasion assay detected that 1% of the ATCC strains were inside the infected Hep-2 cells in contrast to 10% to the clinical isolates. A high level of phospholipase C activity was also detected in all studied ureaplasma.
Conclusions
The results presented herein will help better understand U. diversum infections, aswell as cellular attachment and virulence.
Background
In 1956, mycoplasma and cell cultures were first associated in laboratory contamination [1]. This contamination affects research by invalidating results in diagnosis. However interference by these bacteria in mammalian non phagocytic cell cultures has been used to study mollicute biology [2].
The opportunism of Mollicutes is a challenging subject. These microbes are diverse enough to explain their relationship variety with the host cells [3]. The adhesion seems crucial for their pathogenicity [4]. In addition, some mollicutes have been detected inside non naturally phagocytic cells. In fact, the intracellular location is well protected from the immune system and some antibiotics [3]. The use of non-phagocytic cells to study mollicutes has been of great interest mainly since Mycoplasma fermentans was initially considered a cofactor in the pathogenesis of AIDS [5]. Other mycoplasmas showed this same characteristic when inoculated in non-phagocytic cells such as M. fermentans [6], M. pneumoniae [7], M. genitalium [8] and M. gallisepticum [9].
Ureaplasma diversum is a bovine-originated mollicute, first isolated in 1969 and considered a non-pathogenic species. Although detected in healthy animals, it is currently considered a pathogenic species due to its strong association with cattle diseases such as placentitis, fetal alveolitis, abortion and birth of weak calves [10]. As with most animal mycoplasmosis, the cause of Ureaplasma-associated reproductive disease is multifactorial [11]. In bulls, this ureaplasma is an important pathogen of the genital tract, involved in such diseases as lowered sperm motility, seminal vesiculitis, and epididymitis [12]. Nevertheless, little is known about the virulence and pathogenic mechanisms of this mollicute.
Because the invasion of U. diversum in not known, we inoculated this mollicute in Hep-2 cells and observed this infection through Confocal Laser Scanning Microscopy (CLSM) and used a gentamicin invasion assay.
Results
U. diversum adhesion and invasion on Hep-2 cells observed by CLSM
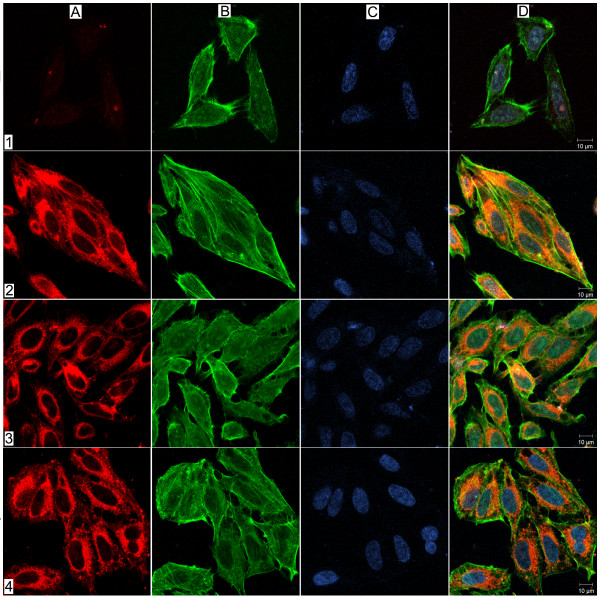
The images of infected cells were from the apical surface to the basolateral region and differentiated the actin filaments in green, from the blue luminescence of nuclei. Therefore the ureaplasmas were detected in red luminescence, discriminating their arrangements in the serial sections of the infected cells. The Dil solution did not show ureaplasmal cytotoxicity (data not presented) and allowed for differentiating the Hep-2 cells from ureaplasmal arrangements. Non-infected Hep-2 cells did not exhibit distinct intracellular Dil fluorescence. The images obtained showed adhesion and invasion of U. diversum in Hep-2 cells (figure 1). After one minute of infection, a few ureaplasmal cells were detected scattered and inside the Hep-2 cells (figure 1.1). After 30 minutes of infection, the ureaplasma persists, scattered, but increased inside the Hep-2 cells (Figure 1.2). At 3, 8 and 12 hours of infection, the microorganisms were detected mostly surrounding the perinuclear regions (Figure 1.3 and 1.4). The studied microorganisms showed no differences in their distribution when adhered to or inside the cytoplasm after 12 hours of infection. Ureaplasmal infection produced no cytopathic effects in Hep-2 cells in the studied period.
Figure 1.
Infection of U. diversum in HEp-2 cells. LSCM optical sections showing internalization of U. diversum in HEp-2 cells after 1 minute (1), 30 minutes (2), 3 hours (3) and 12 hours (4) post-infection. Ureaplasmas were labeled with Vibrant Dil (in red, A), HEp-2 actin filaments stained with phalloidin-FITC (in green, B) and Hep-2 nuclei stained with TO-PRO-3 (in blue, C). In D, merging images A, B, and C. One minute after infection, ureaplasmas were observed inside HEp-2 cells, and after 30 minutes the presence of ureaplasmas inside cells increased. After 3, 8 and 12 hours of infection, ureaplasmas were observed throughout cells cytoplasm.
Disposal of U. diversum in the infected HEp-2 cells
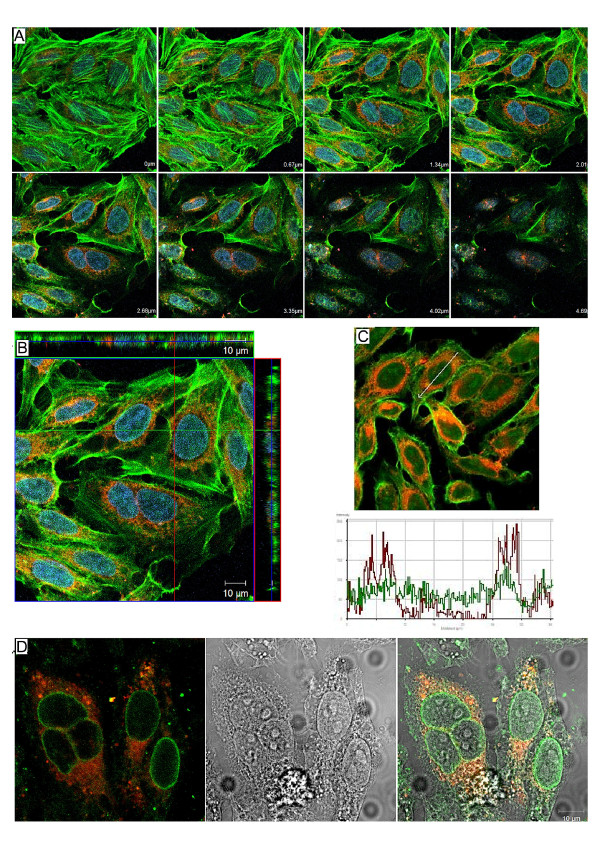
Figure 2 shows disposition of ureaplasma in the studied infection. In figure 2A, optical slices from basal to apical regions of cells, including sections with the nucleus in the plane of the focus were also obtained. The ureaplasmas were detected in different sections of the Hep-2 cell cytoplasm but not inside the nucleus. The orthogonal sections after 3 hours of infection showed a red fluorescence from apical to basolateral regions and throughout the cytoplasm and perinuclear spaces. In figure 2B, images of the tri-dimensional distribution of Hep-2 cells three hours after infection were focused. As shown in figure 2A, red fluorescence was detected throughout the cytoplasm and perinuclear spaces.
Figure 2.
Distribution of U. diversum in infected HEp-2 cells. LSCM images showing the internalization of U. diversum in HEp-2 cells. Ureaplasmas stained by Dil (in red), actin filaments stained by phalloidin-FITC (green) and cells nuclei stained by TO-PRO-3 (in blue). A and B: Z-series of optical slices (A) and orthogonal projection (B) showing the presence and distribution of ureaplasmas inside HEp-2 cell. C: Image and graphic representation of HEp-2 cells after 12 hours post-infection. The arrow in confocal image indicates the cell in which the ureaplasma (in red) and actin (in green) was analyzed, and the detection of actin and ureaplasmas throughout this cell is represented in the graphic. D: Infected HEp-2 cells submitted to immunofluorescence with anti-lamin antibody (in green), showing ureaplasmas (in red) in the perinuclear region, but not inside the cell nuclei. All the images show ureaplasmas distributed throughout the HEp-2 cytoplasms, and concentrated in the perinuclear region, surrounding the nuclei.
Figure 2C is the graphic representation obtained with the software Imaris 3.1.3 (Bitplane AG) that confirms the perinuclear localization of ureaplasma. In addition, the Hep-2 cells were treated with RNAase for 30 min in all periods of infection and incubated with the goat anti-lamin antibodies (diluted 1:800 overnight) washed and exposed for 3 hours to anti-goat immunoglobulin (anti-goat FITC, diluted 1:100). The ureaplasma could be observed close to the nuclear lamin (Figure 2D); however, intranuclear ureaplasmas were not confirmed. The nuclear envelope lamina is a supramolecular protein assembly associated with the nucleoplasmic surface of the inner nuclear membrane. This delimitation was important to determine the presence of ureaplasmas in the perinuclear regions, but not inside the cell nuclei.
Gentamicin invasion assay
The UB medium promoted the growth of studied ureaplasmas. The exposure of inoculum size of ureaplasmas used for gentamicin allowed no recovery in UB medium. However the ureaplasma of infected Hep-2 cells incubated with gentamicin and trypsinized allowed recovery of this microorganism. In this assay, it was possible to determine that the clinical isolates of ureaplasma revealed to be more concentrated in Hep-2 cells than reference strains. This quantification was determined by 10-fold dilutions of ureaplasma obtained after gentamicin assay in UB medium and expressed as Changing Color Units/ml (CCU/ml). Therefore, the internalization of studied ureaplasma in Hep-2 was confirmed and quantified in this assay. Gentamycin is impermeable to mammalian cells in the concentration used: it kills only the extra cellular ureaplasma but not the internalized bacteria. The rates of invasion were expressed as the percentage of CCU obtained after antibiotic exposure relative to the initial inoculum (frequency of invasion). The calculated p-value < 2.2e-16, test for equality of proportions with continuity correction, R project, Vienna, Austria allow for concluding that approximately 1% of the initial inoculum had survived the gentamicin treatment in type-strains and about 10% in clinical isolates. The ATCC strain has a high passage in UB medium. No differences were observed in frequency of invasion between high and low passages clinical isolates (p-value < 2.2e-16).
Phospholipase C activity
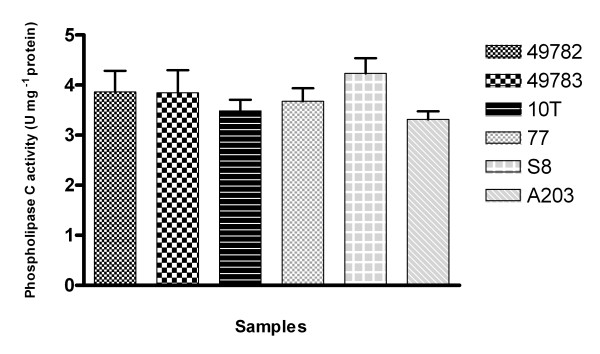
The ureaplasmas were initially cultured at 37°C for 24 hours in one ml of UB broth with pNPPC. The supernatants were evaluated at a wavelength of 405 nm (OD405) in a Multiskan Microplate Reader (Flow Laboratories, Mississauga, Ontario, Canada). The phospholipase C activity was found in the studied ureaplasma and all produced high levels of this enzyme. The average activity was 2,476 to 3,396 pNPPC hydrolysis (U mg-1 protein) (figure 3). This was the highest level that allowed detection of this compound in the present study. The phospholipase C activity also measured in sonicated ureaplasmas cells. The average activity was 0,783 to 0,821 pNPPC hydrolysis (U mg-1 protein). These results showed that most activity is related to secreted enzyme. No differences were detected between the reference strains and clinical isolates. However this activity could be associated with a feature for invasion of ureaplasma.
Figure 3.
Phospholipase C measured in Ureplasma diversum strains studied. The absorbant was measured at 405 nm after incubation or 24 hours 37°C in UB broth with pNPPC.
Discussion
Adhesion and invasion has been studied in a few mollicutes, most being human-originated species. Adhesion is considered an important feature to pathogenesis of these bacteria, and the invasion, a subsequent event, has been described in phagocytic or non phagocytic cells. Therefore chronic and recurrent mycoplasmosis may be explained in part by the reported failures of antibiotic treatments and immune response escape [3]. Vancini & Benchimol [13] reported M. hominis invasion in Trichomonas vaginalis and escaped from the vacuolization of trichomonad cytosol. This finding adds to understanding the challenging features of mollicute biology and their transmission among the hosts.
Consistent with other studied mollicutes, the infection described herein with U. diversum in Hep-2 cells allowed for identifying this ureaplasma as another mammalian cell invader and may also explain and support prior findings on some ureaplasmal infections in bovines.
CSLM has been used to detect mollicute invasion in non phogocytic cells confirming its advantage in detecting U. diversum invasion. The gentamicin invasion assay also confirmed this finding.
U. diversum was detected in Hep-2 cells one minute after infection. M. penetrans has been observed as early as 20 minutes after infection in HeLa cells [14], while in HEp-2 cells, the invasion occurred after 2 hours of infection [4]. Cell internalization after 20 minutes was also detected for M. genitalium in HeLa cells, and the mycoplasmas remained inside the cells for 7 days [15]. Winner et al. [9] observed penetration of M. gallisepticum in HeLa-229 and CEF cells occurred as early as five minutes after infection, and the intracellular mycoplasmas increased after 2 hours.
Ureaplasmas have not been previously reported as cell invaders and have never been compared in their invasion rate. In the present study, U. diversum showed a hasty invasion in Hep-2 cells.
Mollicute reference strains and the clinical isolates showed that these bacteria may have differences in growth and behavior when inoculated in animals or cell cultures [9,16]. The high passage strains have been described as more adapted to axenic growth in contrast to the low passage clinical isolates that have shown to be more aggressive in experimental infections [17]. Even in erythrocytes, HeLa-229 and CEF cells M. gallisepticum R low strain exhibited the highest invasion frequencies than the high passage strain [9,17]. The authors suggested a loss or switching off of the genetic information in this species for the invasion process in the high passage strains.
In the present study, we observed the same frequency of invasion in the gentamicin invasion assay for the high and low passages clinical strains.
Even when a field isolates, the higher passage ureaplasma may not lose or change yet the genetic expression for the studied invasion. In fact these mollicutes are few studied and quite different, therefore, they may reveal additional features for these bacteria.
Buim (unpublished data) observed that the high (WVU 1853) and low passage isolates (MS1 and MS2) of M. synoviae also showed similar adhesion and invasion into Hep-2 cells and similarly surrounded the nucleus. Ueno et al. [18] observed the same results with high and low passages of M. genitallium infecting HeLa and endometrial human cells.
In this study, both ureaplasma reference strains and clinical isolates were detected inside the cells similarly surrounding the perinuclear regions but not inside the nucleus. The perinuclear arrangement was observed in other mollicutes [9,15,16]. Nevertheless, Ueno et al. [18] detected M. genitalium inside the nucleus after 30 minutes infection. Meseguer et al. [19] observed abnormal fluorescence in nuclear images in infected cultures, but failed to confirm the location of M. pneumonie.
The invasion of mollicutes is not completely established and different mechanisms have been proposed based on the studied mollicute and infected cells. Yavlovich et al. [20,21] showed the dependence of plasminogen-Pg in the invasion process of M. fermentans MF. The Pg treated MF were able to invade HeLa cells in three hours, but not the untreated MF.
The phospholipase C (PLC) is detected in many walled bacteria and is considered a virulence factor for tissue damage. In some mollicutes, PLC was detected [22] and associated with the cell invasion due to membrane and cytoskeleton modification. The mycoplasmal PLC was also associated with a host cell signal transduction cascade and the rearrangement of host cytoskeletal components [2,22].
The invading mycoplasmas generate uptake signals that trigger the assembly of highly organized cytoskeletal structures in the host cells. The invasion of M. penetrans is associated with tyrosine phosphorylation of a 145-kDa host cell protein that activate PLC to generate two additional messengers: phosphatidylinositol metabolites and diacylglycerol [23]. These observations support the hypothesis that M. penetrans use phospholipase to cleave membrane phospholipids, thereby initiating the signal transduction cascade. Moreover, the PLC appears to play a role in the escape from the primary vacuole and in gaining access to the cytoplasm [24]. Listeria monocytogenes deficient in PLC are 500-fold less virulent in mice [25].
The studied ureaplasma showed a high PLC activity, without differences between the reference strains and the clinical isolates. This activity explains similar behavior in Hep-2 cells and suggests the role of PLC as a factor for invasion of ureaplasma.
Conclusions
The biological consequences of mycoplasma invasion are not established. Mycoplasma infections are quite variable and the diversity of species and hosts potentiate the complexity of this context. The intracellular location for these bacteria appears to be a comfortable niche for growth, allowing them to be more aggressive and more protected against immune response and antibiotics. Although U. diversum is a little studied species, its intracellular location adds this important feature to the understanding of mollicutes and explains their importance in bovine diseases.
Methods
Ureaplasma diversum and cell lines
Four isolates of U. diversum and two type-strains, ATCC 49782 and 49783, were studied. Isolates 77 and A203 were recovered from the vaginal mucus of a bovine vulvovaginitis (high passage), and the isolates 10T and S8 recovered from frozen bovine semen previously mixed with antibiotics in an artificial insemination center in Brazil (low passage). The isolates were initially identified with culturing characteristics and specie-specific PCR [26].
The Hep-2 (ATCC-USA CCL-23) cell lines were hosts to ureaplasmas in the present study and were previously certified to be free of mycoplasma by culture and PCR [27]. The cells were cultured in 5% of CO2 at 37°C in Minimum Essential Medium (MEM) containing 2 mM L-glutamine and Earl's balanced salts, supplemented with 10% fetal calf serum Cult Lab, São Paulo, Brazil). Twenty-four hours prior to mycoplasma infection, Hep-2 cell monolayers were established for 10-20% confluence on 13 mm glass slides, in 24-well micro plates (TPP - Switzerland), with one ml of MEM medium (Cult Lab, São Paulo, Brazil) for analysis by confocal microscopy. The Hep-2 cells used in the present study were analyzed for presence of mycoplasmas by culture and PCR.
Labeling Mycoplasma cells
The methodology was based on Basemam et al. [28]. The ureaplasmas were first cultured in 2 ml of ureaplasma medium (UB) at 37°C and expanded to 50 ml in the same broth. In a logarithmic growth phase (based in colorimetric changes), the culture was centrifuged at 20,600 g for 30 minutes at 25°C. The pellets were homogenized by washing twice with PBS and incubated with carbocyanine dye solution (Vybrant™ Dil cell-labeling solution-Dil, V-22885, Molecular Probe, Eugene, Oregon, USA). Two-hundred microliters of Vibrant Dil (diluted at 1:200) were added to 105 - 107 mycoplasma cells in one ml of PBS and incubated at 37°C, for 40 minutes. The number of ureaplasma cells was determined by 10-fold dilution in UB medium and expressed as Changing Color Units/ml (CCU/ml). The labeled bacteria were centrifuged for 10 minutes at 20,600 g, at 25°C, washed twice with PBS, gently homogenized and transferred to the monolayer of Hep-2 cells.
Inoculation of ureaplasma on Hep-2 cells [28]
The Hep-2 cells at 60 to 70% confluence corresponding to approximately 106 cells/glass slide were selected for ureaplasmal infection. These cells were initially washed with PBS and inoculated with 105 to 107 of labeled mycoplasmas contained in one ml of MEM with 2% bovine fetal serum. The sets of inoculated cells were incubated at 37°C in 5% CO2 atmosphere for one and 30 minutes, 3, 8 and 12 hours. After each period of infection, the bacterial suspension was gently removed and each well with cell monolayer was washed three times with PBS. The infected Hep-2 cells were then fixed with 3.7% formaldehyde in PBS for 30 minutes at room temperature and washed three times with PBS and treated with 0.05% Triton X-100 for 10 minutes.
Labeling Hep-2 cells
For cytoskeleton visualization of infected and non infected Hep-2 cells, these cells were stained for 30 minutes at 37°C with phalloidin associated with fluoresceine-isothiocyanate (Sigma) diluted at 1:200. This fluorochrome was removed with three washings of PBSA. Then, the cells were treated with RNAase (10 mg/ml) for 30 minutes. The nuclei were stained with TO-PRO-3 (Molecular Probes, dilution 1:500). The preparations of Hep-2 cells and mycoplasmas were mounted with antifading solution (Vecta Shield, Vector Laboratories, Burlingame, CA, USA) on histological slides. The cells were fixed with 3.7% formaldehyde, treated with 0.5% Triton X-100 (10 minutes), exposed to goat anti-B lamin antibody overnight and incubated for 3 hours with anti-goat immuglobulin (1:100, Sigma) conjugated with fluorescein. The cells were washed three times with PBSA and mounted with Vecta Shield on histological slides.
Confocal Laser Scanning Microscopy
The infected and non-infected Hep-2 cells were observed under Confocal Laser Scanning Microscope - CLSM (Carl Zeiss LSM 510, Germany, equipped with Argon laser, 488 nm, and 2 helium/neon 543 nm wavelengths) to visualize the luminescence of fluochromes.
Twenty fields with 8 to 10 infected and non infected cells with ureaplasma in each cytological preparation from each period were examined. A series of optical slices from basal to apical regions of cells, including sections with the nucleus in the plane of the focus were also obtained, and images of the tri-dimensional distribution of intracellular labelled-microorganims were focused. Images of all preparations were documented.
Gentamicin invasion assay
The gentamicin invasion assay was performed to determine the invasion rate of viable ureaplasma inside the eukaryotic cells according to the Yavlovich et al [29]. Previously, the ureplasmas strains used in this study were tested for susceptibility to gentaminin in the concentration utilized in this assay (400 μg/ml). All strains were inhibited by gentamicin. The amount of 104 Hep-2 cells per well were seeded in 24-well micro plates. After 24 hours of incubation at 37°C in 5% CO2, the cell cultures were inoculated with 105 to 107 ureaplasmas (CCU/ml). The infected cells were incubated for three hours, washed three times with PBS and incubated for an additional three hours in MEM (1 ml/well) containing 400 μg/ml gentamicin to eliminate the non internalized ureaplasmas. The antibiotic solution was removed and the infected cells were trypsinized and cultured in UB broth. The remaining ureaplasmas were enumerated by CCU methodology and performed in triplicate. These results were compared with the initial ureaplasmal suspensions.
Phospholipase C activity
Twenty micromoles of P-nitrophenylphosphorylcholine - pNPPC (Sigma) were used as a substrate to detect the phospholipase C activity of ureaplasma. The method is based on the hydrolysis of pNPPC, with the release of the chromogen, p-nitrophenol (NP). The analysis was performed in 96-well microtiter plates (TPP - Switzerland). The ureaplasmas were initially cultured at 37°C for 24 hours in one ml of UB broth with pNPPC. The supernatants were transferred to 96-well microtiter plates and evaluated at a wavelength of 405 nm (OD405) in a Multiskan Microplate Reader (Flow Laboratories, Mississauga, Ontario, Canada). The adjusted OD405 values from each ureaplasmal pNPPC hydrolysis were subtracted from the negative control wells. The negative control was the UB broth and pNPPC without bacteria. All tests were done in triplicate.
Authors' contributions
LMM, PMU, MB and JT: all tests realized in this study. BAC and GMMS: confocal analysis. RLN, MY, RCO, AMSG: bacteria isolation. TAM: performed cell culture. ACBJR: data analysis. All authors read and approved the final manuscript.
Contributor Information
Lucas Miranda Marques, Email: lucasm@usp.br.
Priscilla M Ueno, Email: priscillaueno@hotmail.com.
Melissa Buzinhani, Email: buzinhani@usp.br.
Beatriz A Cortez, Email: biacortez@yahoo.com.br.
Renata L Neto, Email: relneto@usp.br.
Maurício Yamaguti, Email: yamau@usp.br.
Rosângela C Oliveira, Email: rcoliveira@usp.br.
Ana Márcia S Guimarães, Email: anamarcia@usp.br.
Telma A Monezi, Email: telmaamo@usp.br.
Antonio Carlos R Braga, Jr, Email: acjr@ufba.br.
Gláucia M Machado-Santelli, Email: gmmsante@usp.br.
Jorge Timenetsky, Email: joti@usp.br.
Acknowledgements
This study was supported by FAPESP (grant 06/56855-0). We thank Aricelma P. França for valuable technical assistance.
References
- Robinson LB, Wichelhausen RH. Contamination of human cell cultures by pleuropneumonialike organisms. Science. 1956;124:1147–1148. doi: 10.1126/science.124.3232.1147. [DOI] [PubMed] [Google Scholar]
- Rottem S, Naot Y. Subversion and exploitation of host cells by mycoplasmas. Trends Microbiol. 1998;6:436–440. doi: 10.1016/S0966-842X(98)01358-4. [DOI] [PubMed] [Google Scholar]
- Rottem S. Interaction of mycoplasmas with host cells. Physiol Rev. 2003;83:417–432. doi: 10.1152/physrev.00030.2002. [DOI] [PubMed] [Google Scholar]
- Baseman JB, Tully JG. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis. 1997;3:21–32. doi: 10.3201/eid0301.970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SC, Hayes MM, Kotani H, Pierce PF, Wear DJ, Newton PB, Tully JG, Shih JW. Adhesion onto and invasion into mammalian cells by Mycoplasma penetrans: a newly isolated mycoplasma from patients with AIDS. Mod Pathol. 1993;6:276–280. [PubMed] [Google Scholar]
- Stadtländer CT, Watson HL, Simecka JW, Cassell GH. Cytopathogenicity of Mycoplasma fermentans (including strain incognitus) Clin Infect Dis. 1993;17:S289–301. doi: 10.1093/clinids/17.supplement_1.s289. [DOI] [PubMed] [Google Scholar]
- Balish MF, Santurri RT, Ricci AM, Lee KK, Krause DC. Localization of Mycoplasma pneumoniae cytadherence-associated protein HMW2 by fusion with green fluorescent protein: implications for attachment organelle structure. Mol Microbiol. 2003;47:49–60. doi: 10.1046/j.1365-2958.2003.03282.x. [DOI] [PubMed] [Google Scholar]
- Jensen JS, Orsum R, Dohn B, Uldum S, Worm AM, Lind K. Mycoplasma genitalium : a cause of male urethritis? Genitourin. Med. 1993;69:265–269. doi: 10.1136/sti.69.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner F, Rosengarten R, Citti C. In vitro cell invasion of Mycoplasma gallisepticum. Infect Immun. 2000;68:4238–4244. doi: 10.1128/IAI.68.7.4238-4244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RB, Ruhnke HL, Doig PA, Poitras BJ, Palmer NC. The effects of Ureaplasma diversum inoculated into the dynamic cavity in cows. Theriogenology. 1983;20:367–373. doi: 10.1016/0093-691X(83)90071-7. [DOI] [PubMed] [Google Scholar]
- Sanderson MW, Chenoweth PJ. The role of Ureaplasma diversum in bovine reproduction. Compend Contin Educ Pract Vet. 1999;21:S98–S111. [Google Scholar]
- Mulira GL, Saunders JR, Barth AD. Isolation of Ureaplasma diversum and mycoplasmas from genital tracts of beef and dairy cattle in Saskatchewan. Can Vet J. 1992;33:46–49. [PMC free article] [PubMed] [Google Scholar]
- Vancini RG, Benchimol M. Entry and intracellular location of Mycoplasma hominis in Trichomonas vaginalis. Arch Microbiol. 2008;189:7–18. doi: 10.1007/s00203-007-0288-8. [DOI] [PubMed] [Google Scholar]
- Borovsky Z, Tarshis M, Zhang A, Rottem S. Mycoplasma penetrans invasion of HeLa cells induces protein kinase C activation and vacuolation in the host cells. J Med Microbiol. 1998;47:915–922. doi: 10.1099/00222615-47-10-915. [DOI] [PubMed] [Google Scholar]
- Dallo SF, Baseman JB. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb Pathog. 2000;29:301–309. doi: 10.1006/mpat.2000.0395. [DOI] [PubMed] [Google Scholar]
- Much P, Winner F, Stipkovits L, Rosengarten R, Citti C. Mycoplasma gallisepticum : Influence of cell invasiveness on the outcome of experimental infection in chickens. FEMS Immunol Med Microbiol. 2002;34:181–186. doi: 10.1111/j.1574-695X.2002.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Vogl G, Plaickner A, Szathmary S, Stipkovits L, Rosengarten R, Szostak MP. Mycoplasma gallisepticum invades chicken erythrocytes during infection. Infect Immun. 2008;76:71–77. doi: 10.1128/IAI.00871-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno PM, Timenetsky J, Centonze VE, Wewer JJ, Cagle M, Stein MA, Krishnan M, Baseman JB. Interaction of Mycoplasma genitalium with host cells: evidence for nuclear localization. Microbiology. 2008;154:3033–3041. doi: 10.1099/mic.0.2008/020735-0. [DOI] [PubMed] [Google Scholar]
- Meseguer MA, Alvarez A, Rejas MT, Sánchez C, Pérez-Díaz JC, Baquero F. Mycoplasma pneumoniae : a reduced-genome intracellular bacterial pathogen. Infect Genet Evol. 2003;3:47–55. doi: 10.1016/S1567-1348(02)00151-X. [DOI] [PubMed] [Google Scholar]
- Yavlovich A, Higazi AA, Rotten S. Plasminogen binding and activation by Mycoplasma fermentans. Infect Immun. 2001;69:1977–1982. doi: 10.1128/IAI.69.4.1977-1982.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavlovich A, Katzenell A, Tarshis M, Higazi AA, Rottem S. Mycoplasma fermentans binds to and invades HeLa cells: involvement of plasminogen and urokinase. Infect Immun. 2004;72:5004–5011. doi: 10.1128/IAI.72.9.5004-5011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Sasaki T, Watanabe T. AIDS-associated mycoplasmas possess phospholipases C in the membrane. Infect Immun. 1995;63:4174–4177. doi: 10.1128/iai.63.10.4174-4177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev J, Borovsky Z, Rosenshine I, Rottem S. Invasion of HeLa cells by Mycoplasma penetrans and induction of tyrosine phosphorylation of a 145 kda host cell protein. FEMS Microbiol Lett. 1995;132:189–194. doi: 10.1111/j.1574-6968.1995.tb07832.x. [DOI] [PubMed] [Google Scholar]
- Meyer DH, Mintz KP, Fives-Taylor PM. Models of invasion of enteric and periodontal pathogens into epithelial cells: a comparative analysis. Crit Rev Oral Biol Med. 1997;8:389–409. doi: 10.1177/10454411970080040301. [DOI] [PubMed] [Google Scholar]
- Marquis H, Doshi V, Portnoy DA. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso MV, Scarcelli E, Grasso LMPS, Teixeira SR, Genovez ME. Ureaplasma diversum and reproductive disorder in Brazilian cows and heifers; first report. Anim Reprod Sci. 2000;63:137–143. doi: 10.1016/S0378-4320(00)00176-7. [DOI] [PubMed] [Google Scholar]
- Van Kuppeveld FP, Logt JTM Van Der, Angulo AF, Van Zoest MJ, Quint WGV, Niesters HGM, Galama JMD, Melchers WJG. Genus - and Species-Specific Identification of Mycoplasmas by 16S rRNA Amplification. Appl Environ Microbiol. 1992;58:2606–2615. doi: 10.1128/aem.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman JB, Lange M, Criscimagna NL, Giron JA, Thomas CA. Interplay between mycoplasmas and host target cells. Microb Pathog. 1995;19:105–116. doi: 10.1006/mpat.1995.0050. [DOI] [PubMed] [Google Scholar]
- Yavlovich A, Tarshis M, Rottem S. Internalization and intracellular survival of Mycoplasma pneumoniae by non-phagocytic cells. FEMS Microbiol Lett. 2004;233:241–246. doi: 10.1111/j.1574-6968.2004.tb09488.x. [DOI] [PubMed] [Google Scholar]