Abstract
Objective
To develop simulator goggles that produce disease-specific characteristics of selected low vision conditions for use in pharmacy education.
Methods
Individual sets of simulator goggles were developed for glaucoma, cataracts, macular degeneration, diabetic retinopathy, and retinitis pigmentosa. Students rated the presence and severity of disease-specific characteristics after wearing each pair of goggles while manipulating medication-related materials.
Results
One hundred students completed the study. Characteristic symptoms for each disease state were experienced at a moderate to severe level (p < 0.0001). Subjects indicated a high level of agreement among symptom ratings for each disease (Kendall's coefficient = 0.82).
Conclusions
Low vision simulator goggles reliably produced the characteristics of selected conditions experienced in a medication management environment. Further studies are needed to identify suitable patient-centered learning activities using these goggles.
Keywords: low vision, simulation, simulator goggles
INTRODUCTION
Nearly 135 million people around the world1 and as many as 14 million Americans are currently affected by low vision, visual impairment that cannot be corrected by standard glasses, contact lenses, medicine, or surgery, and which hampers performance of daily activities.1-5 The conditions primarily affect people over the age of 65 and are usually a result of age-related eye diseases such as cataracts, glaucoma, diabetic retinopathy, or macular degeneration, but may be caused by genetic diseases such as retinitis pigmentosa.1,5 Low vision results in loss of visual acuity, loss of visual field, and poor detail discrimination.6,7 In addition to affecting activities of daily living, low vision also interferes with performance of instrumental activities of daily living, including management of medications.8 Therefore, low vision is a predictor for risk of at-home medication errors.9-10
The number of Americans with vision impairment is expected to increase dramatically with the aging of the US population.3,5,11,12 In 2003 the Secretary of Health and Human Services tasked the Food and Drug Administration with a study to investigate the availability and use of prescription drug information in formats suitable for visually impaired individuals. A large percentage of this population did not have access to prescription drug labeling and usage information as a result of their visual impairments.11 Consequently, the document Guidelines for Prescription Labeling and Consumer Medication Information for People with Vision Loss was published in 2008. The Guidelines explicitly state that “a concerted effort on the part of pharmacists and pharmacies is needed to address the problem”12(p1) of lack of access to prescription information due to vision loss. Therefore, pharmacy educators must ensure that future members of the profession best suited to address these issues are aware of the specific problems experienced by individuals with different low vision diseases.
Considering these issues, how do we prepare student pharmacists to help this growing patient population? Both the Accreditation Council for Pharmacy Education (ACPE) and the American Association of Colleges of Pharmacy (AACP) encourage design of doctor of pharmacy curricula to include strategies that develop active, self-directed, independent learners who connect material learned in the classroom with real-world practice, with simulation among the recommended methods.13-15
Historically, most published educational research studies on simulation have given little consideration to how well the simulators reproduce the given real-life conditions.16 However, research in the field of low vision education suggests that both the purpose of the simulator and the specific characteristics required in the simulation are necessary considerations to achieve effective teaching using simulators.17,18 Therefore, if we are to develop effective low vision simulation activities for use in pharmacy education, ensuring that the simulators are outcome based and produce the symptoms experienced by low vision patients while managing medications is important.
Researchers in health care professional education programs, including pharmacy, have studied multifactorial aging simulations that include visual impairments as a means of increasing empathy and generalized awareness of disability characteristics.19-29 However, none have addressed the specific medication-related impairment differences among diseases causing low vision. Before activities using these simulations can be described and evaluated, ensuring that the simulators replicate specific characteristics of the diseases in the situations in which the difficulties will be experienced is essential.
Low vision simulator goggles were used first in the geriatric elective course at the University of Louisiana at Monroe (ULM) College of Pharmacy, Aging and Drug Use in the Elderly, in February 2005. The course focused on topics affecting medication use in older adults, including age-related eye diseases and low vision. The goggle simulators used in the first offerings of this activity were created based on recommendations of colleagues who worked with low vision patients and which were developed by low vision researchers in the 1970s.18 “Cup goggles,” purchased from a local welding supply store, were altered to simulate the conditions of glaucoma, cataracts, macular degeneration, diabetic retinopathy, and retinitis pigmentosa. Students wore each simulator and manipulated medication-related materials such as pills and prescription vials, and discussed the specific medication management difficulties experienced with each disease simulation. Following these initial offerings, students commented that they were able to “look around” the obstructions of the macular degeneration and diabetic retinopathy simulators. However, patients with these diseases cannot “look around” the obstructions in their vision. Students also remarked that they were able to minimize the disabilities of macular degeneration, glaucoma, and retinitis pigmentosa by closing one eye, as it decreased the effect created by 2 focal points, 1 in each of the 2 goggle lenses. Patients with these diseases cannot minimize disabilities by closing one eye. Therefore, students did not experience an accurate representation of the symptoms as they relate to medication management in these disease states.
The objective of this study was to develop simulator goggles that produce disease-specific characteristics of selected low vision conditions for use in pharmacy education. The goggles were modified based on student comments and with considerations of the purpose of the simulators and the specific characteristics required in simulations involving medication management.30
METHODS
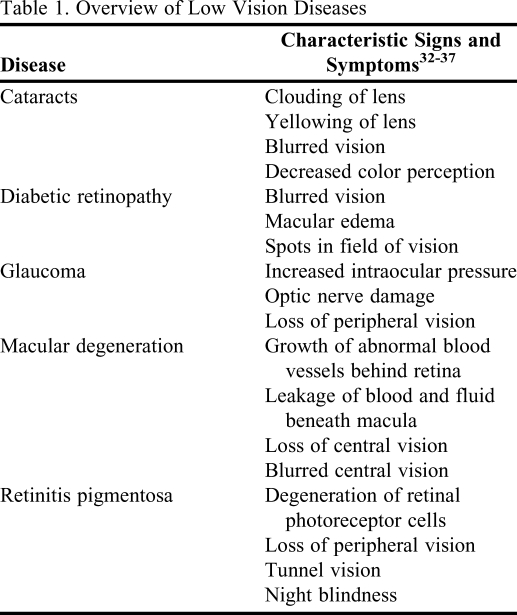
The goggles were designed to simulate advanced-stage characteristics of each disease resulting in conditions of low vision, defined as visual acuity of 20/70 or worse, or a visual field less than 40 degrees,31 and which would be experienced by a patient when managing medications (Table 1). Each pair of cup goggles included 2 clear and 2 dark plastic inserts. The clear inserts were used for the glaucoma, cataract, macular degeneration, and diabetic retinopathy goggles, but no inserts were used for the retinitis pigmentosa goggles. A discussion of each simulator goggle design follows, and a list of materials needed to make the simulators appears in Table 2.
Table 1.
Overview of Low Vision Diseases
Table 2.
Materials List to Make Simulator Goggles
a eg, Blitz #22400
Glaucoma
The original simulators for glaucoma were created with all but a single pinhole spot in the center of each insert painted black. However, this simulator design made it difficult to simulate symptoms of glaucoma in both eyes simultaneously because there were 2 focal points in the simulator, 1 in each goggle lens. In the modified glaucoma simulators, a half-inch diameter half-circle along the inner edge of each insert was covered with masking tape, and the uncovered areas were painted with flat black spray paint. The tape was removed after the paint dried, and the inserts were replaced into the goggle frames with the clear portion of each lens along the inner edge of the goggle frames near the nosepiece. By painting all but this half-inch spot on the inner edge of the inserts, the viewer's peripheral vision was blocked completely, creating 1 focal point and a visual field less than 40 degrees.
Cataracts
Using a coarse, natural-bristled paintbrush, the inserts for the cataract goggles were painted with clear fingernail polish over the entire lens. The natural bristles smeared the polish, creating a blurred effect. Multiple layers of polish were applied, allowing each layer to dry between coats. The layering process was repeated until the Snellen Eye Chart for 20/70 vision was indiscernible by the researchers at a distance of 20 feet while wearing the goggles. After the final layer of clear polish dried, the entire insert was colored with a yellow permanent marker to create the yellowish-brownish discoloration of the lens. This goggle design was not modified from the original model.
Macular Degeneration
In the original macular degeneration simulators for this activity, a large black spot was drawn in the center of each insert with a black permanent marker. However, these simulators also made it difficult to simulate symptoms of the disease in both eyes; and since the spot was in the center of the lens with clear space on both sides, students also were able to look around the spot. To create the modified macular degeneration inserts, a crescent moon shape about 2 inches long and a half inch wide at the widest point along the outer edge of each insert was first covered with masking tape. The uncovered areas were painted with flat black spray paint, and the tape was removed after the paint dried. The inserts were replaced into the goggle frames with the clear crescent-shaped portion of each lens along the outer edge of the goggle frames. By painting all but the outer edge of the inserts, the viewer could see only 1 large obstruction and would have a difficult time trying to “cheat.” A visual field less than 40 degrees was also created.
Diabetic Retinopathy
To create the diabetic retinopathy inserts, the entire surface of the lenses were painted with multiple layers of clear fingernail polish using a coarse, natural-bristled paintbrush to create a blurred effect, allowing the polish to dry between coats. As with the cataract goggles, the layering process was repeated until the Snellen Eye Chart for 20/70 vision was indiscernible. Next, several large asymmetrically shaped spots were drawn in a scattered pattern over the inserts with a black permanent marker to replicate the spots created by leaking blood vessels in the field of vision. The only modification of this goggle design was to enlarge the spots, making it difficult to focus on anything by looking around the obstructions.
Retinitis Pigmentosa
To simulate retinitis pigmentosa, the inserts that came with the goggles were removed and hinged oil spouts were inserted into the circular frames. Because early comments indicated that students were able to minimize retinitis pigmentosa symptoms by closing one eye, the spouts were positioned such that the hinges could be manipulated horizontally, allowing students to point the 2 nozzles inward to create a single focal point and a visual field less than 40 degrees for this study.
The ULM Institutional Review Board granted approval for this study. A survey instrument was created to query the degree to which visual acuity, visual field, or color discrimination symptoms were experienced. Three items were rated using a Likert scale of 0 to 10 for each pair of goggles. The items consisted of both characteristic symptoms that should be experienced and uncharacteristic symptoms that should not be experienced to test for disease-specific symptom validity. The survey instrument was examined for face and content validity by a group of faculty members prior to administration, and pre-tested in a group of students who suggested minor changes in terminology to improve understanding. One hundred student pharmacists at the ULM College of Pharmacy enrolled in the study. Written informed consent was obtained prior to the activity. Subjects had no previous experience with low vision simulators.
To keep subjects from identifying the specific disease states and characteristic symptoms, each pair of goggles was numbered, and students were not informed of the disease state associated with each pair of goggles. A set of control goggles with clear, unaltered lenses was also used in the study to screen rater reliability. Students wore each pair of goggles and manipulated pills, medication vials, prescription labels, and patient information leaflets. Students were instructed to read the text on prescription labels or patient information leaflets when evaluating symptoms related to visual field or acuity, and to examine various nonprescription drug products to evaluate color discrimination. Identical materials were used for all students. Students were told not to consider the frames of the goggles as an obstruction, nor the chain connecting the 2 goggle cups as “spots” in the field of vision, as these factors were consistent for all goggles. Students were also instructed to use an alcohol wipe to clean the inside of each pair of goggles after each use to reduce potential transmission of ocular pathogens. (Note: When the goggles using clear lens inserts were assembled, the painted sides of the inserts were positioned facing outward so the paint or polish would not be removed when students cleaned the goggles after each use). After each simulation, students responded to the corresponding section of the questionnaire.
Symptom ratings were categorized as none (0), mild (1-4), moderate (5-7), or severe (8-10). On an a priori basis, acceptable simulation of a given disease state was defined as a median symptom rating of moderate or above for characteristic symptoms and mild or below for uncharacteristic symptoms. The agreement of the symptom levels among multiple raters was tested with Kendall's coefficient of concordance for ordinal response. Data were analyzed nonparametrically due to the ordinal nature of the scale and the non-normal data distribution as revealed by the Shapiro-Wilk test of normality. Wilcoxon signed-rank tests were used to compare the median characteristic and uncharacteristic symptom ratings against the threshold values defined above. Symptom ratings of subjects with and without corrective lenses were examined with a Mann-Whitney test to evaluate differences between the 2 groups. Data were analyzed with SAS version 9.1 for Windows, using an alpha level of 0.05.
RESULTS
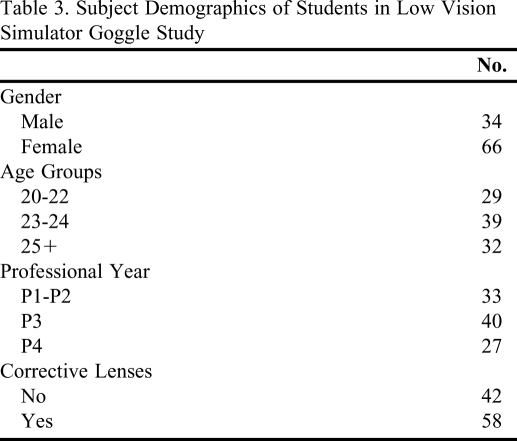
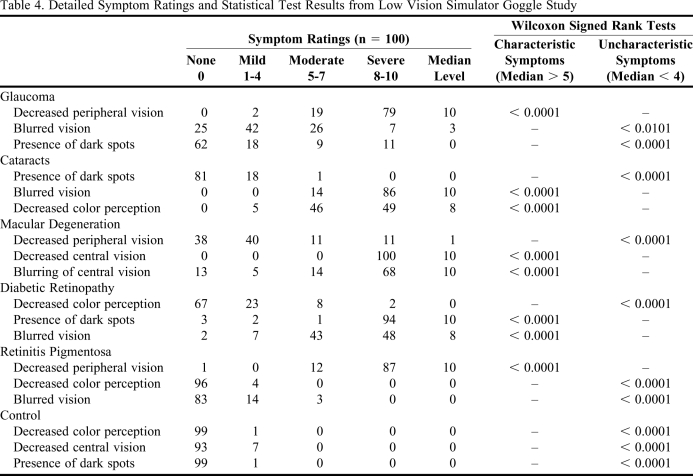
All enrolled subjects completed the study. Subject demographics appear in Table 3. The subjects had a high level of agreement in the rating of visual effect symptoms produced by the goggles (Kendall's coefficient = 0.82). The characteristic symptoms for each goggle were greater than a rating of 5 (moderate), and uncharacteristic symptoms were lower than a rating of 4 (mild). Detailed information on the symptom ratings and Wilcoxon signed-rank tests appears in Table 4. When comparing symptom ratings from subjects with corrective lenses with those from subjects without corrective lenses, a difference was detected for the presence of dark spots in the goggles for glaucoma (p < 0.0412) and diabetic retinopathy (p < 0.0126).
Table 3.
Subject Demographics of Students in Low Vision Simulator Goggle Study
Table 4.
Detailed Symptom Ratings and Statistical Test Results from Low Vision Simulator Goggle Study
DISCUSSION
With millions of people affected by low vision at an increased risk for medication errors, pharmacists must be aware of the medication management difficulties faced by this group of patients. However, there are many different visual impairments, each with characteristics that affect medication management in different ways. To decrease this growing risk for medication errors, pharmacists should be cognizant of the characteristics of different low vision diagnoses and the particular medication management difficulties experienced by each group.
To prepare future pharmacists to address the special needs of low vision patients, teaching activities must provide them with opportunities to experience the symptoms, emotions, and challenges of these patients. Only then will they have the knowledge, skills, and attitudes required to tailor medication management assistance to individuals in this large segment of the population. However, ensuring that the characteristics of the teaching methods are accurate and applicable to the setting is critical for establishing the effectiveness of the tool. A search of the literature failed to identify any studies which describe low vision simulator designs that reliably produce characteristics of various low vision diseases in a medication management setting. After informed reflection and a review of the literature, the simulation goggles described are the first to be considered specifically for use in pharmacy education. Use of these simulators can provide students with unique learning experiences, giving them the advantage of “seeing” things through the eyes of the patient. In addition to promoting growth of students toward being active and self-directed learners, low vision goggle simulation activities can be used also to challenge students to use their creativity, critical thinking, and problem-solving skills to solve the medication management problems of these individuals, as recommended by AACP and ACPE.13,14
Although some significant differences were noted among the subjects with and without corrective lenses, the differences did not affect the study's findings. Differences in ratings for dark spots in the glaucoma and diabetic retinopathy goggles existed for subjects with corrective lenses compared to those without corrective lenses. The symptom was uncharacteristic for glaucoma, with the median score of both groups being 0. This difference may be explained by slightly higher symptom ratings in the corrective lenses group; however, the median rating was below the threshold of 4 for uncharacteristic symptoms. The symptom was expected for the diabetic retinopathy goggles. The difference in this group was probably due to some ratings in the corrective lens group that were below 10, while all ratings in the non-corrective lens group were 10. Also, the median response for both groups was 10, which was above the threshold of 8 for characteristic symptoms.
This study has limitations. First, while comparable results may be found in other pharmacy student samples due to the relative similarity of visual characteristics in young adults, the results of this single-site, non-randomized sample may not be generalizable to all student pharmacists. It is also necessary for students who wear glasses to remove their corrective lenses before putting on the cup goggles, thus causing lower visual acuity than students who wear contacts or do not require vision correction. “Cover-all” goggles which fit over glasses would allow students who wear glasses to maintain their vision correction during the simulation. These goggles are slightly more costly ($12 vs $8), yet remain relatively inexpensive. Using cover-all goggles, however, would require modifications in the size and position of the painted obstructions for the glaucoma and macular degeneration goggles, and would prevent the snug insertion of oil spouts into the goggle frames for the retinitis pigmentosa simulators.
CONCLUSIONS
This study demonstrated that each pair of simulator goggles described reliably produced the characteristic low vision symptoms in the setting for which they were designed. Further studies are needed to describe suitable patient-centered learning activities for use in pharmacy education and to identify students' perceptions of the specific medication management difficulties experienced by patients with different low vision diseases.
ACKNOWLEDGMENTS
The authors express special thanks to Kathy Foster and Janet Bernhardt, CLVT, TVI, for low vision mentoring, to Dr. Lamar Pritchard, Dean, ULM College of Pharmacy, 2004-2009, for administrative and financial support of this project, and to Dr. Roxie Stewart, Dr. Lexi Nguyen, and Dr. Hien Nguyen for facilitation of this study.
REFERENCES
- 1. Information for Healthy Vision, Low Vision FAQs, National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/lowvision/content/faq.asp. Accessed May 6, 2010.
- 2.Vitale S, et al. Prevalence of visual impairment in the United States. JAMA. 2006;295(18):2158–2163. doi: 10.1001/jama.295.18.2158. [DOI] [PubMed] [Google Scholar]
- 3.Janiszewski R, Heath-Watson SL, Semidey AY, Rosenthal AM, Do Q. The low visibility of low vision: increasing awareness through public health education. J Visual Impairment Blindness. 2006;100(Suppl):849–861. [Google Scholar]
- 4. Facts and Figures on Americans with Vision Loss. American Foundation for the Blind. http://www.afb.org/Section.asp?SectionID=15&TopicID=413&DocumentID=4900. Accessed May 6, 2010.
- 5. National Plan for Eye and Vision Research, National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/strategicplanning/np_low.asp. Accessed May 6, 2010.
- 6. National Eye Health Education Program, Low Vision Prevalence Data, National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/nehep/programs/lowvision/goals.asp. Accessed May 6, 2010.
- 7. American Academy of Ophthalmology Vision Rehabilitation Committee. Vision Rehabilitation for Adults. San Francisco, CA: American Academy of Ophthalmology; 2007. http://www.guideline.gov/summary/summary.aspx?ss=15&doc_id=11755&nbr=6059. Accessed May 6, 2010.
- 8.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Health. 2004;94(5):823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Comments on Prescription Drug Information Accessibility. American Foundation for the Blind. http://www.afb.org/Section.asp?SectionID=3&TopicID=329&DocumentID=2454. Accessed May 6, 2010.
- 10.Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults. Morbidity and Mortality Weekly Report MMWR. 1999;48(Supp):131–156. [PubMed] [Google Scholar]
- 11. American Foundation for the Blind. Executive summary of The Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Section 107(f). Department of Health and Human Services. http://www.afb.org/Section.asp?SectionID=3&TopicID=329&DocumentID=3629. Accessed May 6, 2010.
- 12. Guidelines for Prescription Labeling and Consumer Medication Information for People with Vision Loss. A Collaborative Project of American Society of Consultant Pharmacists Foundation and American Foundation for the Blind. American Society of Consultant Pharmacists Foundation. http://www.ascpfoundation.org/downloads/Rx-CMI%20Guidelines%20vision%20loss-FINAL2.pdf. Accessed May 6, 2010.
- 13. Accreditation Council for Pharmacy Education. Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Adopted January 15, 2006. http://www.acpe-accredit.org/pdf/ACPE_Revised_PharmD_Standards_Adopted_Jan152006.pdf. Accessed May 6, 2010.
- 14.AACP Commission to Implement Change in Pharmaceutical Education. Background paper II: entry-level, curricular outcomes, curricular content and educational process. Am J Pharm Educ. 1993;57(4):377–385. [Google Scholar]
- 15. AACP Commission to Implement Change in Pharmaceutical Education. Background Paper V: Maintaining our commitment to change. American Association of Colleges of Pharmacy, 1995. http://www.aacp.org/resources/historicaldocuments/Documents/BackgroundPaper5.pdf. Accessed May 6, 2010.
- 16.Andrews DH. Relationships among simulators, training devices, and learning: a behavioral view. Educ Technol. 1988;28(1):48–54. [Google Scholar]
- 17.Bozeman LA. The Fidelity of Low Vision Simulator Systems in Clinical and Functional Settings [dissertation] Austin, TX: University of Texas; 1998. [Google Scholar]
- 18.Bozeman LA, Chang CS. Central field loss in object-system and low-vision simulation. Bull Spec Educ. 2006;31:207–220. [Google Scholar]
- 19.Reichman SL, Weaver-Meyers P. Glaucoma and cataracts: a nurse-patient simulation for nursing students. J Nurs Educ. 1984;23(7):314–315. doi: 10.3928/0148-4834-19840901-13. [DOI] [PubMed] [Google Scholar]
- 20.Lorraine V, Allen S, Lockett A, Rutledge CM. Sensitizing students to functional limitations in the elderly: an aging simulation. Fam Med. 1998;30(1):15–18. [PubMed] [Google Scholar]
- 21.Varkey P, Chutka DS, Lesnick TG. The Aging Game: improving medical students' attitudes toward caring for the elderly. J Am Med Dir Assoc. 2006;7(4):224–229. doi: 10.1016/j.jamda.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Plake KS. A course on chronic illness: learning the patient's perspective. Am J Pharm Educ. 2003;67(1) Article 9. [Google Scholar]
- 23.Evans S, Lombardo M, Belgeri M, Fontane P. The geriatric medication game in pharmacy education. Am J Pharm Educ. 2005;69(3) Article 46. [Google Scholar]
- 24.Miller SW. Teaching geriatrics to generation Y. Am J Pharm Educ. 2004;68(3) Article 67. [Google Scholar]
- 25.Kennedy DH, Fanning KD, Thornton PL. The age game: an interactive tool to supplement course material in a geriatrics elective. Am J Pharm Educ. 2004;68(5) Article 115. [Google Scholar]
- 26.Robinson SB, Rosher RB. Effect of the “half-full aging simulation experience” on medical students' attitudes. Gerontol Geriatr Educ. 2001;21(3):3–12. [Google Scholar]
- 27.McVey LJ, Davis DE, Cohen JJ. The “aging game”: an approach to education in geriatrics. JAMA. 1989;262(11):1507–1509. doi: 10.1001/jama.262.11.1507. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman SB, Brand FR, Beatty PG, Hamill LA. Geriatrix: a role-playing game. Gerontologist. 1985;25(6):568–572. doi: 10.1093/geront/25.6.568. [DOI] [PubMed] [Google Scholar]
- 29.Chen JT, LaLopa J, Dang DK. Impact of patient empathy modeling on pharmacy students caring for the underserved. Am J Pharm Educ. 2008;72(2) doi: 10.5688/aj720240. Article 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eye Disease Simulations. National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/health/examples/. Accessed May 6, 2010.
- 31. Key Definitions of Statistical Terms. American Foundation for the Blind. http://www.afb.org/Section.asp?SectionID=15&DocumentID=1280. Accessed May 6, 2010.
- 32. Facts About Glaucoma. National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/health/glaucoma/glaucoma_facts.asp. Accessed May 6, 2010.
- 33. Facts About Cataracts. National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/health/cataract/cataract_facts.asp. Accessed May 6, 2010.
- 34. Facts About Age-Related Macular Degeneration. National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/health/maculardegen/armd_facts.asp. Accessed May 6, 2010.
- 35. Facts About Diabetic Eye Disease. National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/health/diabetic/retinopathy.asp. Accessed May 6, 2010.
- 36. Treatment for Retinitis Pigmentosa. National Eye Institute, National Institutes of Health. http://www.nei.nih.gov/news/pressreleases/rppressrelease.asp. Accessed May 6, 2010.
- 37. Eye on NEI, Snapshot, National Eye Institute. http://www.nei.nih.gov/eyeonnei/snapshot/. Accessed May 6, 2010.