Abstract
There is growing concern that estrogenic environmental compounds that act as endocrine disrupting chemicals might potentially have adverse effects on hormone-sensitive organs such as the breast. This concern is further fueled by evidence indicating that natural estrogens, specifically 17 β-estradiol (E2), are important factors in the initiation and progression of breast cancer. We have developed an in vitro- in vivo model in which we have demonstrated the carcinogenicity of E2 in the human breast epithelial cells MCF-10F. Hypermethylation of NRG1, STXBP6, BMP6, CSS3, SPRY1 and SNIP were found at different progression stages in this model. The utilization of this powerful and unique model has provided a tool for exploring whether bisphenol A (BPA) and butyl benzyl phthalate (BBP) have relevance in the initiation of breast cancer. These studies provide first hand evidence that the natural estrogen 17 β-estradiol and xenoestrogenic substances like BPA are able to induce neoplastic transformation in human breast epithelial cells.
Keywords: estrogen, BPA, BBP, cell transformation, breast cancer, epigenetic changes, neuregulin
Introduction
Breast cancer is an estrogen dependent malignancy whose incidence is steadily increasing in most western societies and industrialized countries. Each year 44,000 women die of breast cancer, making it the leading cause of cancer deaths among American women that do not smoke and among those aged 40 to 55 years. Breast cancer is uncommon among women younger than 30 years of age but the incidence increases sharply with age but slows somewhat between ages 45 and 50, around the time of menopause (Pike et al., 1983).
The elevated incidence of breast cancer in women has been associated with prolonged exposure to high levels of estrogens as that occurring with early onset of menarche and late menopause (Henderson et al., 1991; Bernstein and Ross, 1993). In obese menopausal women, the adipose tissue becomes the major source of estrogens that contribute to increase the breast cancer risk (Harris et al., 1992; Huang et al., 1997). The relationship between estrogen and breast cancer is supported by epidemiological data that demonstrated that women who receive hormone replacement therapy (HRT) are more likely to develop breast cancer than those who have never used HRT (1997; Rossouw et al., 2002; Beral, 2003; Chlebowski et al., 2003; Li et al., 2003; Bakken et al., 2004; Colditz, 2005). Studies of blood estrogen levels among postmenopausal women have shown that higher levels are associated with increased risk of subsequent breast cancer (Colditz, 1998; Key et al., 2002; Friel et al., 2005). The Million Women Study (MWS) showed a significant increase in the RR of breast cancer in women taking estrogens (conjugated equine estrogens or estradiol orally) (RR 1.30), although the increase was greater for women taking estrogens combined with synthetic progestins (RR= 2.00) (Beral, 2003). The risk increased with duration of use of estrogen alone (RR10 years, 1.37) and estrogen plus progestin (RR10 years 2.31).
Xenoestrogens: bisphenol A (BPA) and butyl benzyl phthalate (BBP)
Xenoestrogens are part of a group of synthetic and naturally occurring agents termed endocrine disruptors because of their capacity to perturb normal hormonal actions. It has been suggested that some endocrine disrupters may contribute to the development of hormone-dependent cancers, such as breast and endometrial cancers (Jobling et al., 1995; Sonnenschein and Soto, 1998).
Bisphenol A (BPA) is a xenoestrogen that has been widely used since the 1950s as a monomer that is polymerized to manufacture polycarbonate plastic and epoxy resins and found as environmental contaminant. Human exposure occurs when BPA leached from common items such as plastic-lined food and beverage cans, as well as and from some dental sealants (Brotons et al., 1995; Olea et al., 1996). BPA thus leaches into food and beverages through the normal use of tin cans and polycarbonate plastic containers, and the rate of leaching increases when polycarbonate is scratched and discolored (Brede et al., 2003; Burridge, 2003; Howdeshell et al., 2003). The BPA released from polycarbonate plastics increases at high or low pH and at high temperatures. Bisphenol A also is used as an additive in many other products, with global capacity at >6 billion pounds per year (Burridge, 2003). Evidence of the estrogenic effects of BPA has been reported in several studies showing that it activates estrogen receptors (ER) alpha and beta (Routledge et al., 2000; Matthews et al., 2001) and stimulates MCF-7 breast cancer cell growth (Krishnan et al., 1993). Although BPA mimics 17 β-estradiol (E2) by competitively binding and activating endogenous ERs, its affinity is at least 10,000-fold less than E2 for both ERα and ERβ (Maruyama et al., 1999), suggesting that other mechanisms could be responsible for its biological effect. There is some uncertainly as to the level of BPA exposure and the risk it presents, but evidence suggests that it can disrupt normal reproductive tract development in male and female rodents (Takao et al., 1999; Gupta, 2000; Ramos et al., 2001; Suzuki et al., 2002). BPA exposure during perinatal periods has been shown to inhibit testosterone synthesis in adult rats (Akingbemi et al., 2004) and promote feminization of Xenopus laevis tadpoles (Levy et al., 2004). There is significant exposure of pregnant women and their fetus to BPA, as indicated by its presence in maternal plasma (3.1ng/ml), fetal plasma (2.3 ng/ml; approximately 10 nM) and placental tissues (1–104.9 ng/g) (Schonfelder et al., 2002). The BPA levels were even higher in the amniotic fluid during the fetal reproductive tract differentiation (8 ng/ml) (Ikezuki et al., 2002). BPA was detected in 95% of the urine samples tested in the United States at concentrations ≥0.1 μg/L (Calafat et al., 2005). Urinary levels of BPA and its conjugates have been found in various populations in Southeast Asia (Matsumoto et al., 2003; Ouchi and Watanabe, 2002; Yang et al., 2003). In 48 female Japanese college students, BPA glucuronide was detected in all the urine samples at concentrations ranging from 0.2 to 19.1 ng/ml, with a median level of 1.2 μg/L (0.77 μg/g creatinine) (Ouchi and Watanabe, 2002). The largest BPA non occupational exposure assessment reported urinary levels of 9.54 μg/L (8.91 μg/g creatinine) in a group of 73 adult Koreans (53.4% female) (Yang et al., 2003).
Butyl benzyl phthalate (BBP) is another endocrine disruptor that has been reported to be an estrogenic compound and a partial agonist for ER (Jobling et al., 1995; Zacharewski et al., 1998; Andersen et al., 1999). It is used as a plasticizer, and is widely used in food wraps and cosmetic formulations. The International Program of Chemical Safety (IPCS) concluded that BBP exposure to the general population is based almost entirely on food intake, because the concentrations of BBP in air, drinking water, and soil are very low making intakes from these routes essentially negligible. Adult intake of BBP has been estimated at 2μg/kg body weight/day and exposure to infants and children could be up to three-fold higher (Ipcs, 1999). The results of several in vivo studies indicate an anti-androgen-like activity of BBP or its major metabolite following in utero exposure. Studies in rats have described that exposure to 500 or 1000 mg/kg bw/day of BBP or 250 or 375 mg/kg bw/day of its major metabolite monobenzyl phthalate on days fifteen to seventeen of pregnancy induced significant alterations in the reproductive system of male offspring, including undescended testes and decrease in the ano-genital distance (Ema and Miyawaki, 2002; Ema et al., 2003). When administered during sexual differentiation, BBP also causes male reproductive tract malformation of the external genitalia, sex accessory glands, epididymides and testes (Gray et al., 2000). The BBP estrogenic activity has been demonstrated by MCF-7 cell proliferation assays and its in vitro binding to the ER (Jobling et al., 1995; Harris et al., 1997; Zacharewski et al., 1998; Andersen et al., 1999; Hong et al., 2005). BBP was found to be weakly estrogenic; it reduced in vitro binding of 17 β-estradiol to the rainbow trout ER at a high concentration (0.01–10 mg/L) (Jobling et al., 1995).
In conclusion, BPA and BBP have estrogenic activity although the concentrations required for the effects in vitro and in rodent models were extraordinarily greater than the estimated human exposure. The study of the BPA and BBP effects in human breast epithelial cells might yield important information on whether these compounds have the potential to contribute to breast cancer initiation.
In vitro- in vivo model of estrogen carcinogenicity in human breast epithelial cells
As indicated in the previous sections, there is overwhelming epidemiological, clinical and experimental evidence that estrogens are initiators of breast cancer, and that xenoestrogens may also influence the susceptibility or be involved in the initiation or progression of the disease. We have been pioneers in obtaining convincing experimental evidence that 17 β-estradiol (E2) and its catechol metabolites 4-hydroxyestradiol (4-OH-E2) and 2-hydroxyestradiol (2-OH-E2) are capable of transforming MCF-10F, a human breast epithelial cell (HBEC) line that is ERα-negative and ERβ positive (Russo et al., 2002; Russo et al., 2003). The expression of phenotypes indicative of neoplastic transformation were accompanied by genomic alterations such as loss of heterozygosity and mutations at specific loci in chromosomes 13 and 17 (Fernandez et al., 2006a).
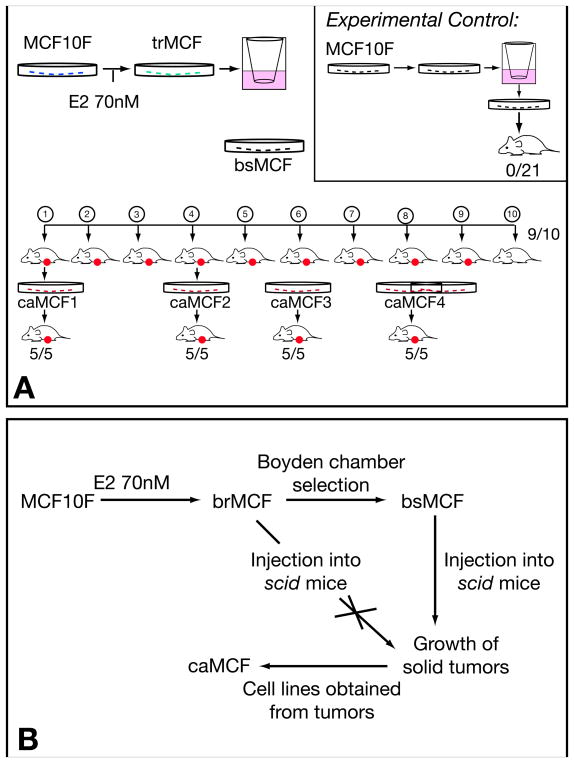
MCF-10F cells were treated with 0.007nM, 70nM or 3.6 μM E2 for 24 hours, twice a week for two weeks. At the end of the second week, the cells were assayed for survival efficiency, ductulogenic capacity and colony efficiency (CE) in agar methocel, all phenotypes indicative of cell transformation (Russo et al., 2003). The cells’ ductulogenic capacity, which expresses the ability of the cells to grow forming three-dimensional structures, was quantitatively evaluated in cells plated in a collagen matrix. Control MCF-10F cells formed duct-like structures that were lined by a single or double layer of low cubical epithelium in collagen and did not form colonies in agar methocel. Estradiol treated cells lost their ability to produce ductules in a dose dependent manner and the number of solid masses increased parallel to the formation of colonies in agar methocel, expressing phenotypes similar to those induced by the carcinogen benzo[a]pyrene (BP) (Russo et al., 2003). The cells treated with either dose of E2 formed colonies in agar methocel that did not differ in size among groups however, the CE in agar methocel increased with increasing doses of E2. These phenotypes were not abrogated when the cells were treated with Tamoxifen or the pure antiestrogen ICI182780 (Russo et al., 2003). Cells were analyzed by flow cytometry revealing that the percentage of cells in the S phase was increased in E2 treated cells in a dose dependent fashion. The effect of E2 on the invasive capabilities of MCF-10F cells was evaluated using Boyden chambers (Lareef et al., 2005). These chambers are fitted with an 8 μm pore size membrane coated with a thin layer of matrigel basement membrane matrix that occludes the pores, blocking non-invasive cells from migrating. Invasive cells are able to detach from and invade through the matrigel matrix to pass through the pores. MCF-10F cells treated with 70nM E2 (also called trMCF) in their 9th passage were placed in the chamber wells and the cells that crossed the basement membrane were collected, expanded and designed as bsMCF (Figure 1A). When the bsMCF cells were injected in the mammary fat pad of severe combined immunodeficient (SCID) mice (Russo et al., 2006), they formed tumors in 9/10 animals within forty-five days of injection. The tumors induced by bsMCF cells were poorly differentiated adenocarcinomas; they were ERα and progesterone receptor (PR) negative and expressed basic keratins of high molecular weight, E-Cadherin, CAM5.2, and vimentin. Cells were isolated from the tumors and grown in vitro giving origin to caMCF cells. The caMCF1, caMCF2, caMCF3 and caMCF4 cell lines were isolated from four single xenograft tumors grown in four animals and all of them produced tumors when they were injected to SCID mice (Russo et al., 2006; Huang et al., 2007) (Figure 1A). This in vitro-in vivo model of estrogen transformed human breast epithelial cells developed in our laboratory represent different stages of tumor initiation and progression, MCF-10F being the normal stage; trMCF cells, the transformed stage; bsMCF cells, the invasive stage and caMCF cells the tumorigenic stage (Figure 1B).
Figure 1. In vitro-in vivo model of estrogen induced transformation of the human breast epithelial cell MCF-10F.
A) Esquematic representation of the experiments performed in SCID mice. MCF-10F was treated with 70nM E2 giving origin to trMCF cells; the trMCF cells were placed on matrigel Boyden chambers and the cells that pass through the membrane were collected, expanded and injected into the fat mammary pad of SCID mice; these cells were designated bsMCF. The bsMCF cells produced tumors in nine out of ten mice. Cells were isolated from the tumors originating caMCF1, caMCF2, caMCF3 and caMCF4, and all of these cells produced tumors when they were injected in the mammary gland of SCID mice. Experimental control: MCF-10F cells were placed in Boyden chambers and the cells that passed through the membranes were collected, expanded and injected into SCID mice; none of these cells produced tumors. (Modified from: Russo et al.: FASEB J 20:1622–1634, 2006). B) Different stages in the in vitro-in vivo model of cell transformation: MCF-10F (normal stage), trMCF (cells transformed by estradiol), bsMCF (invasive stage) and caMCF (tumorigenic stage). The trMCF cells did not form tumors in SCID mice although the invasive bsMCF cells formed tumors that gave origin to caMCF cell lines. (Modified from: Huang et al.: Cancer Res 67 11147–11157, 2007).
The transforming potential of E2 was also evaluated on primary breast epithelial cells. Primary breast cells form duct-like structures in collagen matrix, contrary to tumor cell lines, which form spherical masses. Primary breast epithelial cells were isolated from tissues obtained from three reduction mammoplasties performed for cosmetic reasons. The mammary tissues were digested with collagenase and hyaluronidase, and the organoids were maintained in low-Ca 2+ media as it was described (Soule et al., 1990). The three primary cell cultures were treated with 70 nM E2 twice a week, for two weeks, and as a control cells were treated with DMSO (vehicle in which E2 was dissolved). After the treatments, the ductulogenic capacity of the cells was evaluated: 5,000 cells were suspended in 400 μl 89.3% (PureCol) collagen matrix (Inamed Biomaterials, Fremont, CA) and plated on a 400 μl pre-coated collagen well using a 24 well chamber plate. After three weeks in collagen, the E2 treated cells formed some spherical masses while no solid masses were observed in the controls (Table I). This is the first report indicating that E2 is able to induce phenotypes of cell transformation in primary breast epithelial cells.
Table I. Primary human breast epithelial cells (HBEC) treated with 17 β-estradiol (E2) plated on collagen.
Primary cells were plated in collagen after treatment with E2. As control, the cells were treated with DMSO (vehicle in which E2 was dissolved). The means of spherical masses (±SD) in 4 wells are indicated.
| Primary cells designation | Treatment | Number of spherical masses on collagen |
|---|---|---|
| HSM3 left | DMSO | 0 |
| 70 nM E2 | 2.75±0.5 | |
| 308 Left | DMSO | 0 |
| 70 nM E2 | 15.5±1.7 | |
| 307 left | DMSO | 0 |
| 70 nM E2 | 4.25±1.2 |
BPA and BBP induce neoplastic transformation of human breast epithelial cells in vitro
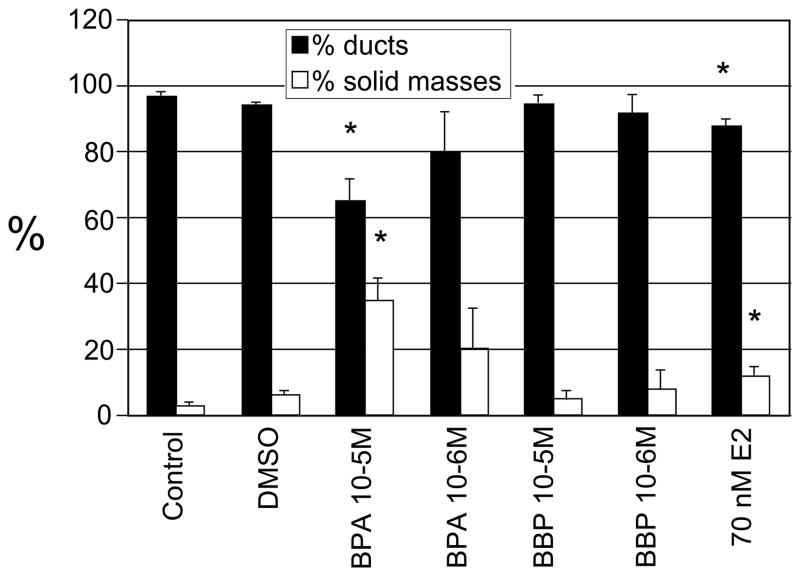
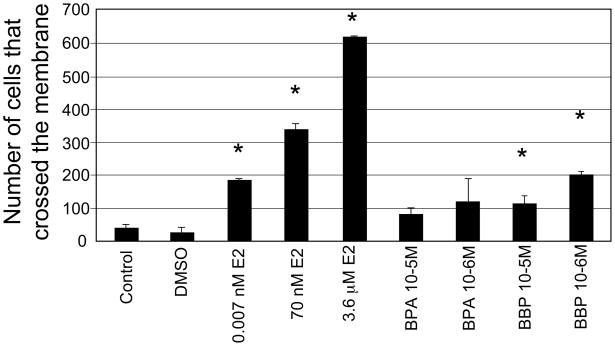
The human breast epithelial cells MCF-10F were treated with 10−3M, 10−4M, 10−5M and 10−6M BPA or BBP continuously for two weeks with fresh media added every day. These doses have already been tested by other authors in HBEC (Hong et al., 2005). As controls, MCF-10F cells were not treated and maintained in the regular media (control group) or treated with 0.284% DMSO (vehicle). The cells treated with 10−3M BPA and BBP died on the second day of treatment. The concentration of 10−4M BPA and BBP was also toxic for MCF-10F cells: the cells died on the 4th and 6th day of treatment, respectively. These data indicated that 10−3M and 10−4M BPA and BBP are toxic for MCF-10F cells being BPA more toxic than BBP. When the treatments finished, the cells were tested for the expression of transformed phenotypes using the collagen assay. For this assay 3,000 cells were plated per well and at the end of the observation period (8 days), the duct-like structures and solid masses formed on 3-D culture were counted (Figure 2). The cells formed a high percentage of duct-like structures in collagen, although there was a significant decrease of ducts in the cells treated with BPA (10−5M) compared to the control and DMSO groups. MCF-10F treated with 10−5M and 10−6M BPA formed a high percentage of solid masses (27 and 20%, respectively) and differences were statistically significant between 10−5M BPA and control/DMSO groups. BBP did not increase the number of solid masses. Interestingly, the number of solid masses after 10−5M BPA treatment was even higher than in cells treated with 70nM E2 and the differences were significant (p<0.01) (Figure 2). The ductules formed by the cells treated with 10−5M BPA were wider and shorter similar to the ones formed by the cells treated with 70nM E2 (Figure 3). Evidence for the estrogenic effects of BPA have been reported in several studies showing that BPA activates estrogen receptors alpha (ERα) and beta (ERβ) although with at least 10,000-fold less affinity than E2 (Maruyama et al., 1999). Ten passages after treatment, the invasive capacity of the cells treated with BPA and BBP was evaluated using the Boyden chambers (Figure 4). The cells that invaded through the Matrigel membrane were counted under a light microscope and, values of chemo invasion were expressed as the number of cells that migrated to the lower chamber. The cells treated with BBP (10−5M and 10−6M) showed a higher invasive capacity compared to the control and the differences were statistically significant (p value ≤ 0.01) (Figure 4). MCF-10F cells treated with E2 at different concentrations (0.007nM, 70nM and 3.6 μM) had higher invasion capacity compared to the control (Figure 4). Furthermore, the invasion capacity of MCF-10F cells treated with 10−6M BBP was similar to that of cells treated with the 0.007nM E2; the differences were not significant between these groups (p> 0.05) (Figure 4).
Figure 2. Percentage of ducts and solid masses in collagen matrix after BPA, BBP and E2 treatments.
MCF-10F cells were treated with 10−5M and 10−6M BPA or BPA continuously for fifteen days. As controls the cells were treated with DMSO (vehicle) or grown in regular media. Also, MCF-10F cells were treated with 70nM E2 for 24 hours, twice a week, for two weeks. Student t-test was used for comparison between treatments and control and a p≤0.01 was accepted as statistically significant. The number of tubules and solid masses grown in four wells were counted. The mean (±SD) and the statistically significant differences from the control (*) are indicated.
Figure 3. Ducts and solid masses formed by MCF-10F cells treated with BPA or BBP.
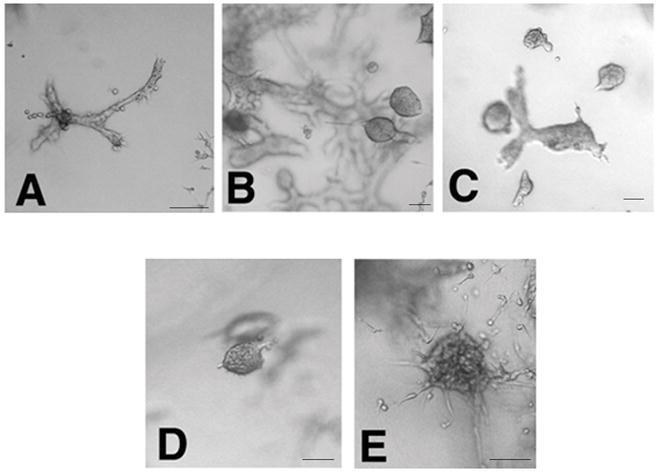
MCF-10F treated with A) DMSO; B) BPA 10−5M; C) BPA 10−6M; D) BBP10−5M; E) BBP 10−6M. The bars at the bottom right corners of each picture represent 100 μm.
Figure 4. Invasion assay.
The cells that migrated through the membrane were counted after the different treatments: MCF-10F cells treated with 10−5M BPA; 10−6M BPA; 10−5M BBP, 10−6M BBP and, MCF-10F treated with E2 (0.007nM, 70nM or 3.6 μM). As controls, cells were grown in regular media or media with DMSO. Student t-test was used for comparison between treatments and control and a p≤0.01 was accepted as statistically significant. Three replicates were made for each treatment. The mean (±SD) and the statistically significant differences from the control (*) are indicated.
Also, we have treated primary human breast epithelial cells with BPA continuously for two weeks and preliminary data indicated that the cells treated with 10−6M BPA express alterations in the ductulogenic pattern in the collagen matrix.
Genomic and epigenetic changes in the in vitro-in vivo model of cell transformation induced by 17 β-estradiol
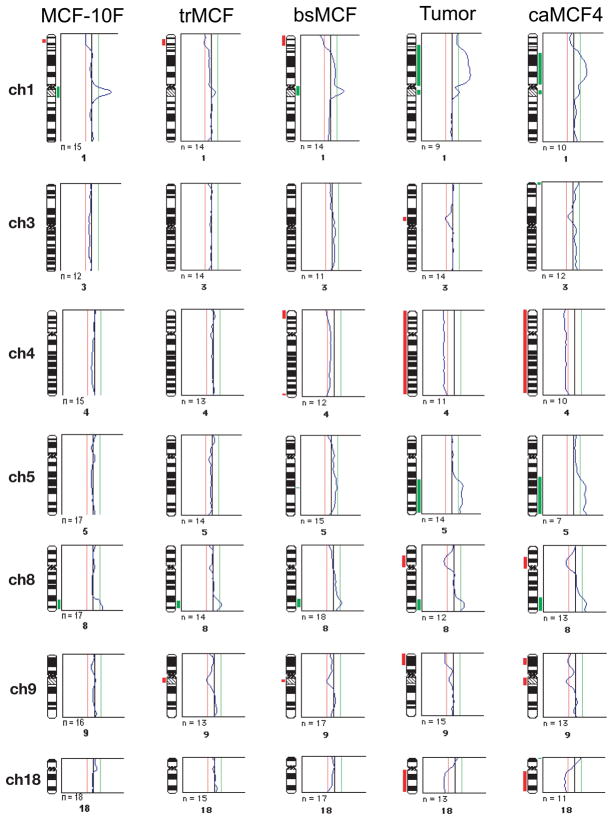
The in vitro-in vivo model described in the previous section represents different stages of cancer progression, MCF-10F being the normal stage; trMCF, the transformed stage; bsMCF, the invasive stage; and a more advance stage represented by caMCF (Figure 1B). These phenotypes correlated with gene dysregulation during the progression of the transformation. The highest number of dysregulated genes was observed in caMCF, being slightly lower in bsMCF, and lowest in trMCF. This order was consistent with the extent of chromosome aberrations (caMCF > bsMCF ≫> trMCF) that were studied using comparative genomic hybridization (CGH) (Figure 5). For CGH, the DNA hybridization and digital image analysis were performed as previously described (Fernandez et al., 2005). Gain or losses are progressive at the different stages of the in vitro-in vivo model; there are few gains or losses in trMCF and, more in the invasive bsMCF and the tumorigenic caMCF cells (Figure 5). Only small chromosome gains were observed in trMCF and most alterations were observed in bsMCF and caMCF cells (Figure 5). Chromosomal amplifications were found in 1p36.12-pter, 5q21.1-qter in both bsMCF and caMCF. Losses of the complete chromosome 4 and 8p11.21–23.1 were found in bsMCF and caMCF cells (Figure 5). In caMCF cells, additional losses were found in 3p12.1–14.1, 9p22.1-pter and 18q11.21-qter. Also a chromosomal amplification in 13q21.31-qter was found in caMCF using high density SNP arrays (Huang et al., 2007). Functional profiling of dysregulated genes revealed progressive changes in the integrin signaling pathway, inhibition of apoptosis, acquisition of tumorigenic cell surface markers and epithelial-mesenchymal transition (EMT). In tumorigenic cells, the levels of E-cadherin, EMA, and various keratins were low and CD44E/CD24 were negative, whereas SNAI2, vimentin, S100A4, FN1, HRAS, TGFβ1, and CD44H were high.
Figure 5. Comparative genomic hybridization (CGH) analysis of the in vitro-in vivo model of cell transformation induced by E2.
The threshold was set at 0.8 and 1.2 for losses and gains respectively; the mean values of individual ratio profiles were calculated from at least 7 metaphase spreads and averaged values were plotted as profiles alongside individual chromosome ideograms. The chromosomal imbalances are shown in a histogram which represents the DNA gains (in green) and losses (in red) as an incidence curve along each chromosome. Only chromosomes with changes are represented. Also, the gains and losses found in the tumor (Tumor) that gave origin to caMCF4 are represented.
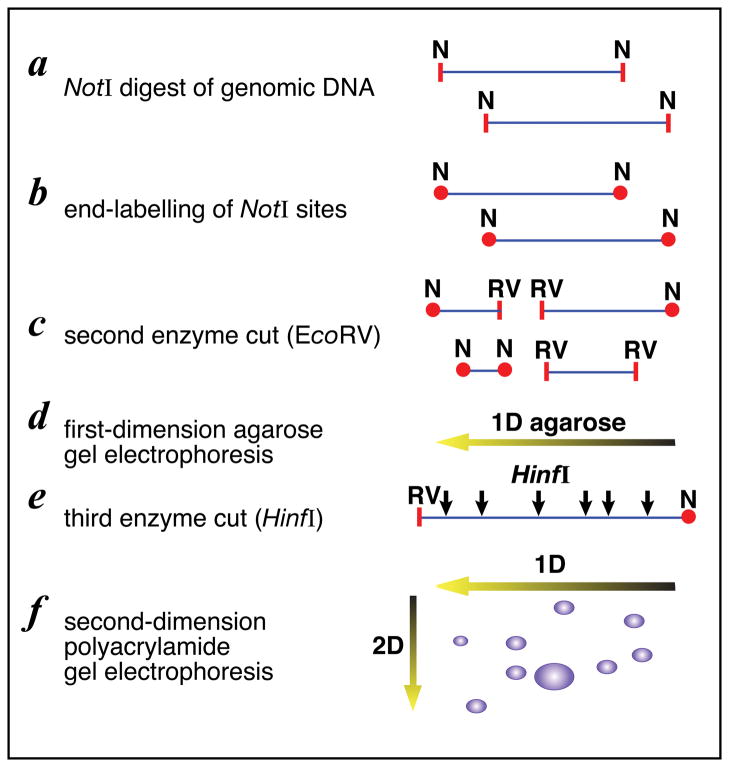
Global CpG island methylation at the different stages in the in vitro-in vivo model of cell transformation was studied using restriction landmark genomic scanning (RLGS) (Fernandez et al., 2006b). RLGS is based on DNA digestion with the restriction enzyme NotI which is methylation-sensitive because it is only able to cut unmethylated DNA (Figure 6). The sites of differential methylation in trMCF, bsMCF and caMCF cells were compared with MCF-10F methylation profile. A total of thirty-eight genes were identified as hypermethylated and four genes were hypomethylated when compared trMCF, bsMCF and caMCF with the control MCF-10F cells (Table II). The data revealed that the methylation pattern of different genes related to ductulogenesis and branching, estrogen metabolism, apoptosis and proliferation was altered (Table II). This is the first demonstration that changes in DNA methylation are involved in the early and late stages of breast cancer progression indicating that from the thirty-eight hypermethylated genes, seventeen of them were potentially involved in the branching process: HOXA9, HOXB5, EPB49, FGF14, SYNE1, FOXD1, BMP6, SPRY1, TM4SF9, SNIP, STXBP6, TLL1, CSS3, OTP, TBR1, NRG1 and ITGA11 (Table II). Real time RT-PCR to study gene expression was important in corroborating that hypermethylation is associated with the silencing of these genes. By using this validation method differences in expression were confirmed for SPRY1, NRG1, STXBP6, BMP6, CSS3, and SNIP among cells in different stages (Table III). SPRY1, NRG1, STXBP6, BMP6, CSS3 and SNIP expressions were down regulated at different stages in the in vitro-in vivo model when compared to the expression in MCF-10F cells (Table III). The trMCF showed low expression of NRG1, STXBP6 and BMP6 compared to MCF-10F, and increased CSS3 (4.79 fold) and SNIP (1.89 fold) expressions. The invasive bsMCF cells showed low expression of SPRY1, NRG1, STXBP6, BMP6, CSS3 and SNIP. In caMCF, no expression of NRG1 and CSS3 was detected and these cells had low expression of BMP6, SPRY1 and SNIP however, they showed higher expressions of STXBP6 (Table III). The expression studies did not correlate with the RLGS results for HOXA9, HOXB5, EPB49, FGF14, SYNE1, FOXD1, TLL1, OTP, TBR1, TM4SF9 and ITGA11; these genes showed the same level of expression at the different stages.
Figure 6. Restriction Landmark Genomic Scanning (RLGS).
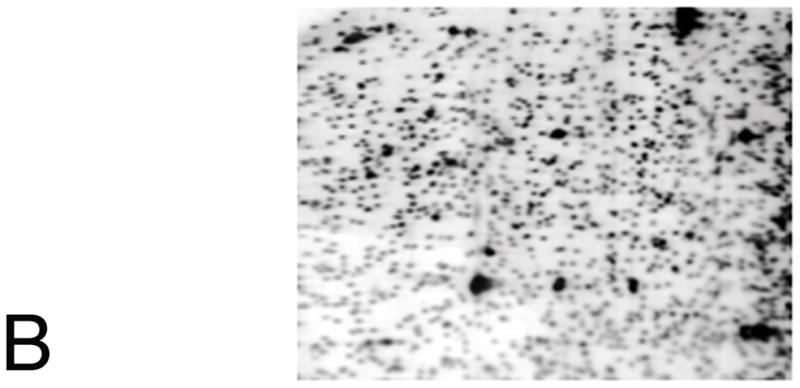
A) The DNA is cut with the methylation-sensitive NotI. The recognition sequence for NotI, GC’GGCCGC, has two CpG sites and NotI will not cut if the site is methylated. The overhangs of cut sites are filled with radiolabeled dCTP and dGTP. Afterwards, the fragments were digested with EcoRV to get fragments into a resolvable size and, the labeled fragments were separated in first dimension 0.8% agarose tube gel. The gel was treated with HinfI to cut the DNA into smaller fragments and the tube gel was placed perpendicularly on a top of 5% polyacrylamide gel and separated in second dimension. B) Autoradiogram of the RLGS polyacrylamide gel. Sites of differential methylation are identified by comparison with a control profile; when comparing control and treated, hypomethylation would appear as greatly increased intensity of RLGS spots; RLGS spot loss is due to hypermethylation of the NotI site. Each virtual spot is pre-defined by a specific sequence in a database.
Table II.
Hypermethylated and hypomethylated genes in the in vitro- in vivo model of cell transformation induced by 17β-estradiol.
| Hypermethylated Genes | Chromosome | CpG location | Gene Description |
|---|---|---|---|
| MAPK11 | 22q13.33 | body | mitogen-activated protein kinase 11 |
| HOXA9 | 7p15.2 | 5′ | homeo box A9 |
| MAF | 16q23.1 | 5′+ body | v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian) |
| KCNC1 | 11p15.1 | 5′ | potassium voltage-gated channel, |
| EPB49 | 8p21.3 | Body | erythrocyte membrane protein band 4.9 |
| SYNE1 | 6q25.2 | 5′ | spectrin repeat containing, nuclear envelope 1 |
| KCNK9 | 8q24.3 | 5′ | potassium channel, subfamily K, member 9 |
| FOXD1 | 5q12–q13 | 5′ | forkhead box D1 |
| AB020689 | 4q31.21 | 5′ | Hypothetical protein KIAA0882 |
| ITGA11 | 15q23 | 5′ | integrin, alpha 11 |
| GRM6 | 5q35 | 5′ | glutamate receptor, metabotropic 6 |
| STXBP6 | 14q11.2 | 5′ | syntaxin binding protein 6 (amisyn) |
| KCNIP2 | 10q24.32 | 5′ | Kv channel interacting protein 2 |
| HR | 8p21.3 | 5′ | hairless homolog (mouse) |
| NRG1 | 8p21.1 | 5′ | neuregulin 1 |
| SULT4A1 | 22q13.2 | 5′ | sulfotransferase family 4A, member 1 |
| TM4SF9 | 4q23 | 5′ | transmembrane 4 superfamily, member 9 |
| CSS3 | 5q23.3 | 5′ | chondroitin sulfate synthase 3 |
| HOXB5 | 17q21.32 | 3′ | homeo box B5 |
| TLL1 | 4q32.3 | 5′ | tolloid-like 1 |
| OTP | 5q14.1 | 5′ | orthopedia homolog (Drosophila) |
| PDE6D | 2q37.1 | 5′ | phosphodiesterase 6D, cGMP-specific, |
| FGF14 | 13q33.1 | 5′ | fibroblast growth factor 14 |
| ASB18 | 2q37.3 | Body | ankyrin repeat and SOCS box-containing 18 |
| TBR1 | 2q24.2 | 3′ | T-box, brain, 1 |
| ABR | 17p13.3 | 5′ | active BCR-related gene |
| GNAL | 18p11.21 | 5′ | guanine nucleotide binding protein, |
| C9orf58 | 9q34.13 | 5′+body | chromosome 9 open reading frame 58 |
| SNIP | 17q21.2 | Body | Hypothetical protein KIAA1684 |
| AK096941 | Chr5 | 5′ | hypothetical gene supported by AK096941 |
| AB018254 | 8p23.3 | 5′ | Hypothetical protein KIAA0711 |
| KCNA2 | 1p13.3 | 5′ | potassium voltage-gated channel, |
| SGPP2 | 2q36.1 | Body | sphingosine-1-phosphate phosphotase 2 |
| ITPK1 | 14q32.12 | 5′+body | inositol 1,3,4-triphosphate 5/6 kinase |
| SPRY1 | 4q28.1 | 5′ | sprouty homolog 1, |
| AB014526 | 4q33 | 5′ | Hypothetical protein KIAA0626 |
| LOC375323 | 3p25.3 | 5′ | lipoma HMGIC fusion partner-like protein 4 |
| BMP6 | 6p24.3 | 5′ | bone morphogenetic protein 6 |
| Hypomethylated genes | |||
| CAST | 5q15–q21 | 5′ | calpastatin |
| ALX3 | 1p13.3 | 5′ | aristaless-like homeobox 3 |
| BACH2 | 6q15 | 5′ | BTB and CNC homology 1 |
| POGZ | 1q21.3 | 5′ | pogo transposable element with ZNF domain |
Table III. Expression studies in the in vitro-in vivo model of cell transformation.
The expressions of different genes were studied by real time RT-PCR. The values express fold induction compared to MCF-10F. The real time RT-PCR was made in triplicate for each sample/primer and the standard deviations are indicated.
| SPRY1 | NRG1 | STXBP6 | BMP6 | CSS3 | SNIP | |
|---|---|---|---|---|---|---|
| MCF-10F | 1±0 | 1±0 | 1±0 | 1±0 | 1±0 | 1±0 |
| trMCF | 1.17±0.03 | 0.37±0.03 | 0.11±0.02 | 0.62±0.08 | 4.79±1.34 | 1.89±0.14 |
| bsMCF | 0.35±0.04 | 0.004±0.01 | 0.39±0.07 | 0.75±0.007 | 0±0 | 0.22±0.03 |
| Tumor cell lines: | ||||||
| caMCF1 | 0.41±0.06 | 0±0 | 4.27±0.15 | 0.33±0.03 | 0±0 | 0.18±0.05 |
| caMCF2 | 0.40±0.04 | 0±0 | 2.96±0.26 | 0.36±0.02 | 0±0 | 0.10±0.01 |
| caMCF3 | 0.71±0.19 | 0±0 | 4.52±0.26 | 0.20±0.02 | 0±0 | 0.19±0.02 |
| caMCF4 | 0.36±0.18 | 0±0 | 6.7±0.07 | 0.21±0.01 | 0±0 | 0.34±0.02 |
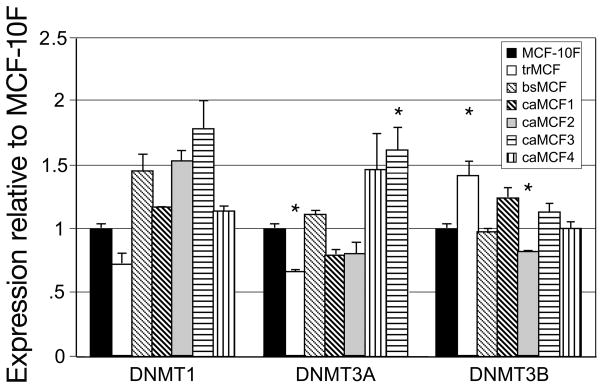
There are three active DNA methyltransferase (DNMTs) and DNA hypermethylation was correlated with their high expressions in tumors. Increased expression of the DNMT1 was found in colon tumors and leukemia (Smiraglia and Plass, 2002) and its expression appears to increase progressively with advancing stages of both colon and lung cancers (Lee et al., 1996). DNMT3A and DNMT3B expressions has been reported to be elevated in acute myeloid leukemia (Melki et al., 1998) and in solid tumors (Belinsky et al., 1996). The DNMTs expressions were studied in the in vitro-in vivo model of cell transformation using real time RT-PCR. The trMCF and caMCF4 cells showed higher expression of DNMT3B and DNMT3A respectively compared to MCF-10F (p<0.01) (Figure 7). DNMT1 did not show significantly differences at the different stages of the in vitro-in vivo model.
Figure 7. DNMT1, DNMT3A and DNMT3B expressions in the in vitro- in vivo model of cell transformation.
The expression of the different DNA methyltransferases was studied by real time RT-PCR in trMCF, bsMCF and caMCF cells and compared to the expression in MCF-10F. The real time RT-PCR was done in triplicate for each sample/primer/probe. Student t-test was used for comparison between treatments and control and a p≤0.01 was accepted as statistically significant. The mean (±SD) and the statistically significant differences from the control MCF-10F (*) are indicated.
Controlling the epigenetic mechanism of gene silencing
In contrast to mutations, which are essentially irreversible, methylation changes are reversible, raising the possibility of developing therapeutics based on restoring the normal methylation state to cancer-associated genes. The drugs 5-aza-2′-cytidine (5-aza-C) and 5-aza-2′-deoxycytidine (5-aza-dC) have proven valuable for basic studies of DNA methylation and transcriptional silencing. Using these compounds and the histone deacetylase inhibitor trichostatin A (TSA) we have confirmed that the genes identified by RLGS were epigenetically silenced. SPRY1, NRG1, STXBP6, BMP6, CSS3 and SNIP showed increased expression after 5-aza-dC and/or TSA treatments, indicating that they are epigenetically regulated (unpublished data).
The NRG1, or heregulin alpha (HRG), was down-regulated very early in the transformation process (trMCF) and its expression remains low in the invasive and tumorigenic cells (bsMCF and caMCF), indicating that the epigenetic effect can be maintained in multiple cell generations (Figure 8). NRG1 has been shown to induce the growth and differentiation of epithelial cells and promotes branching morphogenesis (Yang et al., 1995). NRG1 is a ligand for ErbB3 and ErbB4 and regulates both cell proliferation and terminal differentiation in the mammary gland (Sartor et al., 2001; Long et al., 2003; Tidcombe et al., 2003). We studied NRG1 expression in nine human breast tissue samples and we found that its expression was low in 6/6 samples of invasive ductal carcinoma while it was high in 3/3 samples of the normal breast tissue (Table IV). Recently it was shown that NRG1 was hypermethylated in tumor samples while it was unmethylated in normal breast tissue and the authors proposed that NRG1 was a tumor suppressor gene (Chua et al., 2008). NRG1 was found to be heavily methylated in 76.5% breast cancer cell lines that had no NRG1 expression and, relatively unmethylated in normal breast cell lines and cancer cell lines expressing this gene (Chua and Paw, 2006).
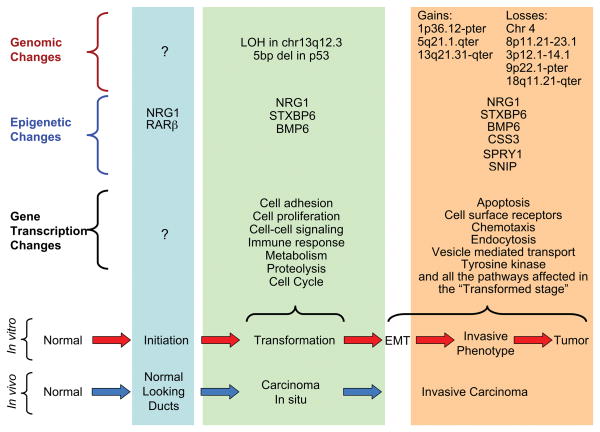
Figure 8. Molecular pathway of neoplastic transformation of breast epithelial cells.
In the lower portion of the figure, the phenotypes of breast cancer progression in vivo are compared with those in the in vitro model. The genomic, epigenetic and gene transcription changes are listed and compared among the different stages of cancer progression. In the epigenetic changes, the hypermethylated genes at the different stages are indicated. In the transformation stage, loss of heterozygosity (LOH) in chromosome 13q12.3 and a 5 base-pair deletion in p53 exon 4 were detected. In the invasive and tumor stages, several gains and losses have been described. EMT: epithelial-mesenchymal transition.
Table IV. NRG1 expression in normal and tumor breast samples.
The NRG1 expression was studied using real time RT-PCR in normal and tumor breast samples. Assays were done in triplicate. The values express NRG1 fold induction (±SD) compared to NRG1 expression in sample # 1
| Breast tissue identification Sample # | Relative NRG1 expression | |
|---|---|---|
| 1 | Normal tissue | 1±0.13 |
| 4 | Normal tissue | 2.44±0.25 |
| 7 | Normal tissue | 1.53±0.11 |
| 4582 T | Invasive carcinoma | 0.027±0.023 |
| 99–101 T | Invasive carcinoma | 0.011±0.00016 |
| 99–223 T | Invasive carcinoma | 0.0045±0.001471 |
| 00– 126 T | Invasive carcinoma | 0.01±0.001 |
| 99– 190 T | Invasive carcinoma | 0.003±0.0009 |
| 99–218 T | Invasive carcinoma | 0.015±0.0011 |
BMP6 (bone morphogenetic protein 6) showed reduced expression in the transformed (trMCF), invasive and tumor stages (bsMCF and caMCF) (Figure 8). BMP proteins regulate growth, differentiation and apoptosis of various cells including epithelial cells, and play critical roles during embryogenesis and the morphogenesis of various organs. Expression of BMP6 was reduced in 18/44 breast carcinoma samples compared with tumor-free resection margins lines (Clement et al., 1999). STXBP6 (amisyn) showed reduced expression in the transformed and invasive cells (trMCF and bsMCF); this protein binds components of the SNARE complex that has a central role in vesicle targeting and fusion in eukaryotic cells; it has been shown that epimorphin, one of the SNARE proteins, directs epithelial morphogenesis (Hirai et al., 1998). The CSS3 (chondroitin sulfate synthase 3 or CHSY2) showed increased expression in the transformed cells (trMCF) and, it was not expressed in the invasive nor in the tumorigenic stages (bsMCF and caMCF) suggesting its hypermethylation at these stages (Figure 8); CSS3 is a glycosyltransferase that transfers glucuronic acid (GlcUA) to the non-reducing end of the elongating chondroitin polymer; some chondroitin sulfate proteoglycans (CSPG) provide high osmotic pressure and water retention, and others modulate not only cell adhesion to extracellular matrix, cell migration, cell proliferation, and morphogenesis, but also cytokine signals (Schwartz et al., 2002). SNIP was down regulated in the invasive and tumor cells (bsMCF and caMCF) (Figure 8); SNIP is a signaling molecule involved in the linkage of actin cytoskeleton to the extracellular matrix during cell migration, cell invasion and cell transformation (Bouton et al., 2001). SPRY1, sprouty homolog 1 expression was low in the invasive and tumor cells (bsMCF and caMCF) (Figure 8). SPRY proteins were found to be endogenous inhibitors of the Ras/mitogen-activated protein kinase pathway that play an important role in the remodeling of branching tissues. It has been shown that Spry1 and Spry2 were down-regulated consistently in breast cancers (Lo et al., 2004). Spry1 and 2 were expressed specifically in the luminal epithelial cells of breast ducts, with higher expression during stages of tissue remodeling when the epithelial ducts are forming and branching (Lo et al., 2004). These findings suggest that Sprys might be involved as a modeling counter balance and surveillance against inappropriate epithelial expansion. The Sprouty proteins are increasingly being recognized as deregulated in various types of cancer; therefore, the finding that this gene can be epigenetically regulated in our HBEC model of cell transformation is especially relevant.
Therefore, the genomic and epigenetic changes triggered by estrogen exposure that lead normal cells to tumorigenesis confirm the role of this steroid hormone in cancer initiation (Figure 8).
Relevance of neoplastic transformation of human breast epithelial cells in vitro by estrogens and xenoestrogens
Altogether our data indicate that BPA as well as E2 are able to induce neoplastic transformation of human breast epithelial cells. Importantly we have also demonstrated that MCF-10F cells treated with E2 formed tumors when they were injected in SCID mice. The model not only mimics the primary breast tumors but also provides a unique system for understanding genomic changes that lead to specific neoplastic phenotypes (Figure 8). More importantly, this model may be used to test the functional role of specific genomic and epigenetic changes that take place during transformation, invasion and tumorigenesis. Our data also support the concept that E2 can act as a carcinogenic agent without ERα, although we cannot rule out the possibility that ERβ, or other receptors not yet identified, could play a role in the early stages of cell transformation, invasion and tumorigenesis. Although this model is extremely valuable in furthering our understanding of estrogen induced carcinogenesis in MCF-10F cells, an immortalized cell line, we also presented evidence that primary breast epithelial cells are also transformed by the natural estrogen and xenoestrogens as well. More importantly we present first evidence that epigenetic changes are involved in the early stages of cancer initiation by altering ductulogenesis (Figure 8). Epigenetic changes of different genes arise early at the “initiation” stage. Data supporting this hypothesis came from the analysis of morphologically normal tissue surrounded invasive carcinomas where hypermethylation of NRG1 and RAR β was detected (unpublished data). DNA mutations and other epigenetic modifications appear in the “transformation” stage (carcinoma in situ). We postulate that the “initiation” and “transformation” (carcinoma in situ) stages could be reverted to a “normal” stage by drugs that are capable of modifying the methylation pattern of affected genes. In the “invasion” and “tumor” stages (invasive carcinomas) important chromosomal gains and losses appear (Figure 8). Furthermore, we found that BPA was able to induce transformation of the human breast epithelial cells MCF-10F. After treatment with BPA, the cells produced less number of tubules in collagen and an increased number of solid masses and this compound also increased the invasion capacity of the cells. The treatment with BBP did not increased solid mass formation although it increased the invasion capacity of the cells.
Acknowledgments
Grant R21 ES015894 and U01 ES/CA 12771 from the NIH, USA, supported this work. The authors thank Kara Snider and Patricia A. Russo for the technical work. The Restriction Landmark Genomic Scanning (RLGS) was done in collaboration with Dr. Christopher Plass from Ohio State University and the analysis of metaphase-CGH was performed by Dr. Binaifer Balsara from the Research Cytogenetic and Genomic Facility of the Fox Chase Cancer Center.
References
- 1.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 350(9084):1047–59. [PubMed] [Google Scholar]
- 2.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145(2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 3.Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, et al. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999;107(Suppl 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken K, Alsaker E, Eggen AE, Lund E. Hormone replacement therapy and incidence of hormone-dependent cancers in the Norwegian Women and Cancer study. Int J Cancer. 2004;112(1):130–4. doi: 10.1002/ijc.20389. [DOI] [PubMed] [Google Scholar]
- 5.Belinsky SA, Nikula KJ, Baylin SB, Issa JP. Increased cytosine DNA-methyltransferase activity is target-cell-specific and an early event in lung cancer. Proc Natl Acad Sci U S A. 1996;93(9):4045–50. doi: 10.1073/pnas.93.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15(1):48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 8.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 2003;20(7):684–9. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 9.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103(6):608–12. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burridge E. Eur Chem News. 2003 April 14–20;:17. [Google Scholar]
- 11.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113(4):391–5. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. Jama. 2003;289(24):3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 13.Chua Y, Paw E. The NEUREGULIN1 gene and breast cancer. Breast Cancer Res. 2006;8 (Suppl 2):5. [Google Scholar]
- 14.Chua YL, Pole JCM, Chin SF, Ellis IO, Caldas C, O’Hare MJ, et al. NRG1 is frequently silenced by methylation in breast cancer and is strong candidate for the 8p tumor suppressor gene. Breast Cancer Res. 2008;10 (Suppl 2):11. doi: 10.1038/onc.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement JH, Sanger J, Hoffken K. Expression of bone morphogenetic protein 6 in normal mammary tissue and breast cancer cell lines and its regulation by epidermal growth factor. Int J Cancer. 1999;80(2):250–6. doi: 10.1002/(sici)1097-0215(19990118)80:2<250::aid-ijc14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998;90(11):814–23. doi: 10.1093/jnci/90.11.814. [DOI] [PubMed] [Google Scholar]
- 17.Colditz GA. Estrogen, estrogen plus progestin therapy, and risk of breast cancer. Clin Cancer Res. 2005;11(2 Pt 2):909s–17s. [PubMed] [Google Scholar]
- 18.Ema M, Miyawaki E. Effects on development of the reproductive system in male offspring of rats given butyl benzyl phthalate during late pregnancy. Reprod Toxicol. 2002;16 (1):71–6. doi: 10.1016/s0890-6238(01)00200-3. [DOI] [PubMed] [Google Scholar]
- 19.Ema M, Miyawaki E, Hirose A, Kamata E. Decreased anogenital distance and increased incidence of undescended testes in fetuses of rats given monobenzyl phthalate, a major metabolite of butyl benzyl phthalate. Reprod Toxicol. 2003;17(4):407–12. doi: 10.1016/s0890-6238(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez SV, Russo IH, Lareef M, Balsara B, Russo J. Comparative genomic hybridization of human breast epithelial cells transformed by estrogen and its metabolites. Int J Oncol. 2005;26(3):691–5. [PubMed] [Google Scholar]
- 21.Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int J Cancer. 2006a;118(8):1862–8. doi: 10.1002/ijc.21590. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez SV, Wu YZ, Russo IH, Plass C, Russo J. The role of DNA methylation in estrogen-induced transformation of human breast epithelial cells. Proc Am Assoc Cancer Res. 2006b;47:1590. [Google Scholar]
- 23.Friel PN, Hinchcliffe C, Wright JV. Hormone replacement with estradiol: conventional oral doses result in excessive exposure to estrone. Altern Med Rev. 2005;10(1):36–41. [PubMed] [Google Scholar]
- 24.Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 25.Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224(2):61–8. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- 26.Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105(8):802–11. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer (1) N Engl J Med. 1992;327(5):319–28. doi: 10.1056/NEJM199207303270505. [DOI] [PubMed] [Google Scholar]
- 28.Henderson BE, Ross RK, Pike MC. Toward the primary prevention of cancer. Science. 1991;254(5035):1131–8. doi: 10.1126/science.1957166. [DOI] [PubMed] [Google Scholar]
- 29.Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, Bissell MJ. Epimorphin functions as a key morphoregulator for mammary epithelial cells. J Cell Biol. 1998;140(1):159–69. doi: 10.1083/jcb.140.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong EJ, Ji YK, Choi KC, Manabe N, Jeung EB. Conflict of estrogenic activity by various phthalates between in vitro and in vivo models related to the expression of Calbindin-D9k. J Reprod Dev. 2005;51(2):253–63. doi: 10.1262/jrd.16075. [DOI] [PubMed] [Google Scholar]
- 31.Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111(9):1180–7. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Fernandez SV, Goodwin S, Russo PA, Russo IH, Sutter TR, et al. Epithelial to mesenchymal transition in human breast epithelial cells transformed by 17beta-estradiol. Cancer Res. 2007;67(23):11147–57. doi: 10.1158/0008-5472.CAN-07-1371. [DOI] [PubMed] [Google Scholar]
- 33.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. Jama. 1997;278(17):1407–11. [PubMed] [Google Scholar]
- 34.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–41. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 35.IPCS. Concise International Chemical Assessment Document 17, Butyl benzyl phthalate. Geneva: World health Organization, International Programme on Chemical Safety; 1999. [Google Scholar]
- 36.Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103(6):582–7. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132(6):2279–86. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 39.Lareef MH, Garber J, Russo PA, Russo IH, Heulings R, Russo J. The estrogen antagonist ICI-182-780 does not inhibit the transformation phenotypes induced by 17-beta-estradiol and 4-OH estradiol in human breast epithelial cells. Int J Oncol. 2005;26 (2):423–9. [PubMed] [Google Scholar]
- 40.Lee PJ, Washer LL, Law DJ, Boland CR, Horon IL, Feinberg AP. Limited up-regulation of DNA methyltransferase in human colon cancer reflecting increased cell proliferation. Proc Natl Acad Sci U S A. 1996;93(19):10366–70. doi: 10.1073/pnas.93.19.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy G, Lutz I, Kruger A, Kloas W. Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ Res. 2004;94(1):102–11. doi: 10.1016/s0013-9351(03)00086-0. [DOI] [PubMed] [Google Scholar]
- 42.Li CI, Malone KE, Porter PL, Weiss NS, Tang MT, Cushing-Haugen KL, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. Jama. 2003;289(24):3254–63. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 43.Lo TL, Yusoff P, Fong CW, Guo K, McCaw BJ, Phillips WA, et al. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Res. 2004;64(17):6127–36. doi: 10.1158/0008-5472.CAN-04-1207. [DOI] [PubMed] [Google Scholar]
- 44.Long W, Wagner KU, Lloyd KC, Binart N, Shillingford JM, Hennighausen L, et al. Impaired differentiation and lactational failure of Erbb4-deficient mammary glands identify ERBB4 as an obligate mediator of STAT5. Development. 2003;130(21):5257–68. doi: 10.1242/dev.00715. [DOI] [PubMed] [Google Scholar]
- 45.Maruyama S, Fujimoto N, Yin H, Ito A. Growth stimulation of a rat pituitary cell line MtT/E-2 by environmental estrogens in vitro and in vivo. Endocr J. 1999;46(4):513–20. doi: 10.1507/endocrj.46.513. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto A, Kunugita N, Kitagawa K, Isse T, Oyama T, Foureman GL, et al. Bisphenol A levels in human urine. Environ Health Perspect. 2003;111(1):101–4. doi: 10.1289/ehp.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol. 2001;14(2):149–57. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 48.Melki JR, Warnecke P, Vincent PC, Clark SJ. Increased DNA methyltransferase expression in leukaemia. Leukemia. 1998;12(3):311–6. doi: 10.1038/sj.leu.2400932. [DOI] [PubMed] [Google Scholar]
- 49.Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104(3):298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouchi K, Watanabe S. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780(2):365–70. doi: 10.1016/s1570-0232(02)00547-0. [DOI] [PubMed] [Google Scholar]
- 51.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. ’Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303(5920):767–70. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 52.Ramos JG, Varayoud J, Sonnenschein C, Soto AM, Munoz De Toro M, Luque EH. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol Reprod. 2001;65(4):1271–7. doi: 10.1095/biolreprod65.4.1271. [DOI] [PubMed] [Google Scholar]
- 53.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 54.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J Biol Chem. 2000;275(46):35986–93. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 55.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, et al. 17-Beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20(10):1622–34. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 56.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol. 2003;87(1):1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 57.Russo J, Lareef MH, Tahin Q, Hu YF, Slater C, Ao X, et al. 17Beta-estradiol is carcinogenic in human breast epithelial cells. J Steroid Biochem Mol Biol. 2002;80(2):149–62. doi: 10.1016/s0960-0760(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 58.Sartor CI, Zhou H, Kozlowska E, Guttridge K, Kawata E, Caskey L, et al. Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol Cell Biol. 2001;21(13):4265–75. doi: 10.1128/MCB.21.13.4265-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):A703–7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz NB, Domowicz M. Chondrodysplasias due to proteoglycan defects. Glycobiology. 2002;12(4):57R–68R. doi: 10.1093/glycob/12.4.57r. [DOI] [PubMed] [Google Scholar]
- 61.Smiraglia DJ, Plass C. The study of aberrant methylation in cancer via restriction landmark genomic scanning. Oncogene. 2002;21(35):5414–26. doi: 10.1038/sj.onc.1205608. [DOI] [PubMed] [Google Scholar]
- 62.Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. 1998;65(1–6):143–50. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 63.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50(18):6075–86. [PubMed] [Google Scholar]
- 64.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, et al. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16(2):107–16. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 65.Takao T, Nanamiya W, Nagano I, Asaba K, Kawabata K, Hashimoto K. Exposure with the environmental estrogen bisphenol A disrupts the male reproductive tract in young mice. Life Sci. 1999;65(22):2351–7. doi: 10.1016/s0024-3205(99)00502-0. [DOI] [PubMed] [Google Scholar]
- 66.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci U S A. 2003;100(14):8281–6. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang M, Kim SY, Lee SM, Chang SS, Kawamoto T, Jang JY, et al. Biological monitoring of bisphenol a in a Korean population. Arch Environ Contam Toxicol. 2003;44(4):546–51. doi: 10.1007/s00244-002-2124-0. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G, et al. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995;131(1):215–26. doi: 10.1083/jcb.131.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zacharewski TR, Meek MD, Clemons JH, Wu ZF, Fielden MR, Matthews JB. Examination of the in vitro and in vivo estrogenic activities of eight commercial phthalate esters. Toxicol Sci. 1998;46(2):282–93. doi: 10.1006/toxs.1998.2505. [DOI] [PubMed] [Google Scholar]