Abstract
BACKGROUND:
Familial hypertrophic cardiomyopathy (FHC) is a Mendelian disorder usually caused by mutations in any one of more than 12 genes, most of which encode sarcomere proteins. The disease exhibits extensive genetic heterogeneity, and it is important to identify mutations that result in adverse symptoms and/or lethality in affected individuals. An analysis of disease-causing mutations has been initiated in the Indian population to determine prevalent mutations.
METHODS:
FHC was detected using echocardiography and by analysis of clinical symptoms and family history. The disease-causing mutation was identified using polymerase chain reaction DNA sequencing.
RESULTS:
The p.R787H mutation was identified in the MYH7 gene in two FHC families. Sequence and structure analysis suggested impaired binding of the mutant protein to the myosin essential light chain.
CONCLUSIONS:
Although the mutation results in variable clinical symptoms in the affected individuals, probably owing to the effect of modifier genes and/or environmental factors, it does not appear to be a lethal mutation.
Keywords: Beta-cardiac myosin heavy chain 7 gene, Familial hypertrophic cardiomyopathy, Hypertrophy, Mutation
Familial hypertrophic cardiomyopathy (FHC) is an autosomal-dominant cardiac disorder characterized by left ventricle hypertrophy and myocyte rearrangement (1–3). It is usually caused by mutations in any one of several sarcomere genes, resulting in clinical heterogeneity (4,5). Mutations in MYH7 and MYBPC3 account for approximately 50% to 70% of all FHC cases (6). Structural studies have demonstrated that the MYH7 mutations cluster predominantly in four important domains located in the head and head-rod junction of the protein: the actin binding surface; the nucleotide binding pocket; near the two reactive cysteines located toward the end of the globular head; and in the alpha-helical tail near the essential light chain (ELC) binding site (7–9). Because many patients present with late onset of the disease, identification of a disease-causing mutation can result in early diagnosis and better patient management. In addition, given the extensive genetic heterogeneity, it is imperative to identify those mutations that result in a lethal phenotype to design effective management strategies for patients with a high risk of sudden cardiac death. A few studies in this area have been performed in India and it is important to identify common mutations, if any, in the Indian population (4). In the present study, we performed clinical and molecular genetic analysis of FHC in two unrelated Indian families.
METHODS
Patients and samples
All patients were from the state of Andhra Pradesh in India and were selected from the two collaborating hospitals (Usha Mullapudi Cardiac Centre and Care Hospital, Hyderabad, India). The study was approved by the respective ethics committees of the two hospitals as per the revised Declaration of Helsinki (2004). Clinical diagnosis of FHC was primarily based on a left ventricle wall thickness of greater than 12 mm (measured by echocardiography), electrocardiography and family history. Patient blood samples (as well as blood samples from family members and control subjects) were collected following informed consent.
Mutation detection
DNA was isolated from blood samples as previously described (10). Screening for mutations in MYH7 (exons 3 to 23) and all exons of MYBPC3 was performed as described previously using polymerase chain reaction (PCR) DNA sequencing (4). The sequence of the primers and corresponding annealing temperatures for PCR amplification of exon 21 of MYH7 are given in Table 1. The status of the angiotensin-converting enzyme gene (ace) polymorphism was determined by using two sets of primers as previously described (11,12).
TABLE 1.
Primer sequences and corresponding annealing temperatures
| Gene/exon | Forward primer | Reverse primer | Annealing temperature (°C) |
|---|---|---|---|
| MYH7/E21 | TCCCCACCATCTCTTTCCCTCGTA | TCCTGACACTGCCCCTGAACCA | 58 |
Sequence and structure analysis
Multiple sequence alignment (MSA) of more than 100 protein sequences belonging to the myosin protein family was performed using ClustalW (European Bioinformatics Institute, United Kingdom) as described previously (13). Using this MSA, position-specific residue preference scores were calculated using the following formula:
Where is the position-specific preference score for an amino acid i at a position k in MSA; is the frequency of amino acid residue j at position k and S(i,j) is the pairwise substitution score of amino acid iwith amino acid residue j taken from the Point Accepted Mutation 250 matrix. A conceptual model of arginine to histidine substitution in human MYH7 and its effect on protein interaction was deduced from the x-ray structures of the chicken skeletal muscle myosin homologues 1W7J and 1OE9 (14,15).
RESULTS
Genetic analysis and correlation with clinical data
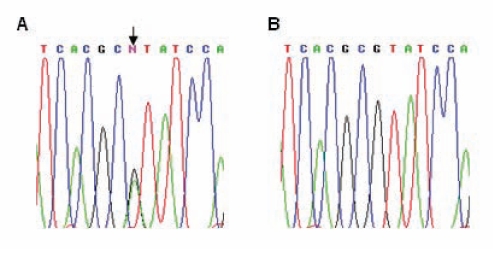
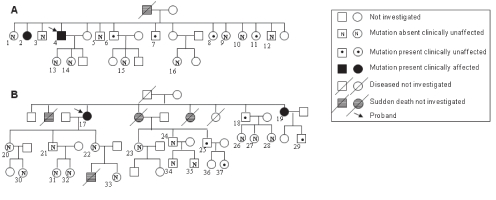
Because mutations in MYH7 and MYBPC3 account for approximately 50% to 70% of FHC patients in various populations (6), the patients were screened by direct PCR DNA sequencing of the coding region and splice consensus sequences for the two genes. For MYH7, only the first 23 exons were screened because most mutations are reported to occur within these exons. The p.R787H mutation (CGT to CAT in exon 21; Figure 1A) was detected in the MYH7 gene in two non-related patient samples. The mutation was confirmed by bidirectional DNA sequencing, and was not detected in 100 unrelated control samples from the local population. There was no other mutation in MYH7 or MYBPC3 in the two patients. Next, blood samples were collected from family members of the two probands following informed consent. Six members each from family 1 (of 16 members screened) and family 2 (of 21 members screened) harboured the mutation (Figure 2). Of 12 members who harboured the mutation (from the two families), only 10 presented themselves for clinical examination. As has been reported for several other MYH7 mutations, a wide range of clinical heterogeneity was detected among the family members harbouring the mutation (Table 2). The interventricular septum (IVS) diameter ranged from 0.7 cm to 2.2 cm (mean 1.28 cm); only three members exhibited an abnormal IVS diameter and clinical symptoms (a penetrance of 30%). Two of the three symptomatic members exhibited mild to severe mitral regurgitation. To analyze the cause for genetic heterogeneity, the status of the ace insertion/deletion polymorphism was determined among members of both families. The homozygous deletion genotype (D/D) has been shown to be associated with adverse clinical symptoms and sudden death. Only two members harboured the D/D genotype who, interestingly, neither exhibited clinical symptoms nor harboured any abnormality in their IVS. The three members who exhibited clinical symptoms, including an abnormal IVS diameter, harboured the homozygous insertion or heterozygous deletion/insertion genotype (Table 2). In addition, the clinical heterogeneity did not correlate with age or sex (Table 2).
Figure 1).
Identification of the MYH7 p.R787H mutation (arrow). A and B show electropherograms of sequencing reactions performed using the forward primer for exon 21 of MYH7 on a proband of family 1 and a control sample, respectively
Figure 2).
Family pedigree for family 1 (A) and family 2 (B). Only those members who agreed to be tested and provided a blood sample are numbered
TABLE 2.
Molecular and clinical analyses of family members harbouring the MYH7 p.R787H mutation
| Patient* | Sex/age (years) | IVS, cm | LVPW, cm | LVEF, % | MR | ace | Dys | Ang | CP | Syn | P-Syn | SL | HT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Family 1 | |||||||||||||
| 4 | M/50 | 1.5 | 1.2 | 65 | Severe | I/I | No | No | Yes | No | No | No | Yes |
| 2 | F/63 | 0.9 | 1.0 | 65 | Absent | I/I | No | No | No | No | No | No | No |
| 7 | M/38 | 0.9 | 1.3 | 55 | Absent | D/I | No | No | No | No | No | No | No |
| 6 | M/40 | 0.7 | 1.1 | 50 | Absent | I/I | No | No | No | No | No | No | No |
| 8 | F/36 | – | D/I | ||||||||||
| 11 | F/34 | – | I/I | ||||||||||
|
Family 2 | |||||||||||||
| 17 | F/63 | 2.1 | 1.0 | 55 | Absent | I/I | Yes | No | Yes | No | No | Yes | Yes |
| 19 | F/49 | 2.2 | 1.0 | 53 | Mild | I/I | No | No | Yes | No | No | Yes | Yes |
| 18 | M/52 | 1.0 | 1.0 | 68 | Absent | D/I | No | No | No | No | No | No | No |
| 25 | M/33 | 1.0 | 0.9 | 68 | Absent | D/D | No | No | No | No | No | No | No |
| 37 | F/9 | 0.8 | 0.9 | 63 | Absent | D/D | No | No | No | No | No | No | No |
| 29 | M/30 | 1.0 | 1.0 | 63 | Absent | I/I | No | No | No | No | No | No | No |
The numbers in the first column correspond to those given in Figure 2 for each family. ace Angiotensin-converting enzyme genotype; Ang Angina; CP Chest pain; D Deletion; Dys Dyspnea; F Female; HT Hypertension; I Insertion; IVS Interventricular septum diameter; LVEF Left ventricle ejection fraction; LVPW Left ventricle posterior wall thickness; M Male; MR Mitral regurgitation; P-Syn Presyncope; SL Swelling of limbs; Syn Syncope
Sequence and structure analysis
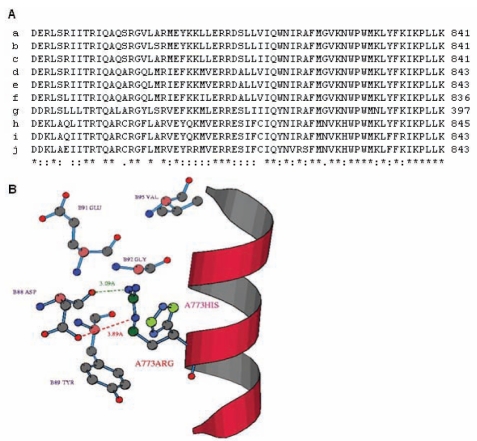
To understand the molecular basis for the pathogenic effect of the MYH7 p.R787H mutation, sequence and structure analyses of the mutant MYH7 protein were performed. MSA of the neck region of human MYH7 (amino acid residues 771 to 841) (16) with myosin chains from other species revealed extensive sequence conservation; the R787 residue was found to be invariant (Figure 3A). A sequence profile was then constructed for myosin chains; ie, the position-specific preferences of the 20 amino acid residues calculated from the MSA of related myosin chains as described in the Methods section. It was observed that the profile score for arginine at position 787 was 0.3, for aspartic acid was −1.8, for lysine was 0.3 and for histidine was −0.6, indicating that any substitution that either reversed the charge or resulted in an aromatic amino acid was not favoured. The R787 residue is present in the neck/hinge region of MYH7, which is predicted to interact with the myosin ELC (16). The crystal structure of human MYH7 is not available, whereas that of the chicken skeletal myosin is available and has been used previously to map MYH7 mutations (9,15). The structure of the chicken homologue was used to deduce the likely structural environment at position 787 in the human MYH7 protein and possible changes occurring due to the mutation. The residue in the chicken protein equivalent to human R787 is R773. It was found that R773 is in close proximity to residues G92, D88, Y89 and E91 of the ELC and its amino group is likely to form an H-bond with the O-group of the D88 residue of the ELC (Figure 3B). In addition, an ionic interaction (salt bridge) is also likely to occur between these two amino acids (Figure 3B). However, these interactions are precluded when arginine is replaced by histidine (Figure 3B). Therefore, the changes in the interactions that occur due to the p.R787H mutation are likely to compromise the binding of MYH7 to the ELC, thereby affecting efficiency of muscle contraction.
Figure 3).
Protein sequence and structure analysis of the MYH7 p.R787H mutation. A Multiple sequence alignment of the amino acids that constitute the neck region of human MYH7 is shown, with the corresponding region of other myosin chains from various species. The multiple sequence alignment was performed using ClustalW (European Bioinformatics Institute, United Kingdom) as previously described (13). The R787 residue is shown in: a Homo sapiens MYH7; b Canis lupus familiaris MYH6; c Equus caballus MYH7; d Mesocricetus auratus MYH6; e Rattus norvegicus MYH6; f Coturnix cotumix japonica MYHC3; g Lethenteron japonicum MHC; h Sus scrofa MHC-1; i Equus caballus MYHC-2A; j Gallus gallus MHC. B The result of structural analysis of the mutation is shown. Interfacial residues occurring within the 5 A0 region of the R773 position (equivalent to R787 in human MYH7) in the chicken MYH7 protein are shown. The figure depicts the probable interactions of R773 with the essential light chain. A probable H-bond (green dotted line) and ionic bond (red dotted line) between the residues R773 of MYH7 and D88 of the essential light chain are represented. The probable side chain conformation of the mutant histidine at position 773 of MYH7 is also shown
DISCUSSION
Recurrent mutations have been suggested to be a rare occurrence in FHC. In the present study, we detected the MYH7 p.R787H mutation in two unrelated families. Our study is the first detailed characterization of the p.R787H mutation; it is important to determine genotype-phenotype correlation for each mutation for better patient management. Although it appears to be a relatively benign mutation, the role of the p.R787H mutation in causing FHC has already been documented; it was first reported from France (6) and later from Spain (17), but no clinical analysis was performed. The 10 people from two families who harboured the mutation exhibited a wide range of clinical heterogeneity. We are aware of the small sample size in the present study and, therefore, cannot draw any definite conclusion regarding the association of hypertrophy with environmental or genetic factors. However, neither sex nor age appeared to correlate with extent of hypertrophy (Table 2). The two individuals who harboured the D/D genotype did not exhibit clinical symptoms, although it should be noted that they were the youngest of all family members carrying the mutation. It is possible that other factor(s) (genetic or environmental) are responsible for modulating the clinical presentation of the disease resulting from this mutation. The fact that there was no cardiac death in any of the affected members with different genetic backgrounds (because they belonged to two different families) and that many individuals carrying the mutation were asymptomatic suggests that the p.R787H mutation is relatively benign.
Structural analysis of FHC-causing mutations is an important area of study for determining the effect of the mutation at the protein level. Although, in a previous study (17), the R787 residue has been ascribed to the myosin head, the crystal structure of chicken skeletal myosin has clearly revealed that it is located in the neck/hinge region, which connects the globular head (primarily responsible for force production) to the tail region. The neck region is predicted to interact with the myosin ELC and regulatory light chains, and this interaction may be important for translation of ATP hydrolysis to movement (9). Mice lacking this domain develop hypertrophic cardiomyopathy (18). Our results indicate that replacement of arginine by histidine at position 787 in MYH7 is likely to weaken its interaction with the myosin ELC. Interestingly, the majority of mutations in the ELC binding interface, including p.I736T (19), p.L766R (20), p.G768R, p.D778G, p.S782R and p.A797T (21), also result in mild symptoms. Similarly, mutations in the ELC, which probably compromise binding to the myosin heavy chain, have also been shown to result in FHC (16).
In the current study, we detected the MYH7 p.R787H mutation in two unrelated families; individuals harbouring the mutation exhibited variable clinical symptoms. Sequence and structure analysis of the MYH7 mutation revealed that the mutant protein might be compromised in its ability to bind ELC efficiently. It is important to identify prevalent mutations in a population, especially those that result in a lethal phenotype. Our results suggest that the MYH7 p.R787H mutation may result in mild clinical symptoms similar to other mutations that occur in the neck region of the protein.
Acknowledgments
We thank the patients, their family members and the control subjects for kindly consenting to participate in the present study. The study was supported by a grant to MDB from the Department of Biotechnology, Government of India.
REFERENCES
- 1.Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: An introduction to pathology and pathogenesis. Br Heart J. 1994;72:S2–3. doi: 10.1136/hrt.72.6_suppl.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (1) N Engl J Med. 1987;316:780–9. doi: 10.1056/NEJM198703263161305. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (2) N Engl J Med. 1987;316:844–52. doi: 10.1056/NEJM198704023161405. [DOI] [PubMed] [Google Scholar]
- 4.Bashyam MD, Savithri GR, Gopikrishna M, Narasimhan C. A p.R870H mutation in the beta-cardiac myosin heavy chain 7 gene causes familial hypertrophic cardiomyopathy in several members of an Indian family. Can J Cardiol. 2007;23:788–90. doi: 10.1016/s0828-282x(07)70828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashyam MD, Savithri GR, Kumar MS, Narasimhan C, Nallari P. Molecular genetics of familial hypertrophic cardiomyopathy (FHC) J Hum Genet. 2003;48:55–64. doi: 10.1007/s100380300007. [DOI] [PubMed] [Google Scholar]
- 6.Richard P, Charron P, Carrier L, et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–32. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 7.Arad M, Seidman JG, Seidman CE. Phenotypic diversity in hypertrophic cardiomyopathy. Hum Mol Genet. 2002;11:2499–506. doi: 10.1093/hmg/11.20.2499. [DOI] [PubMed] [Google Scholar]
- 8.McNally EM. Beta-myosin heavy chain gene mutations in familial hypertrophic cardiomyopathy: The usual suspect? Circ Res. 2002;90:246–7. [PubMed] [Google Scholar]
- 9.Rayment I, Holden HM, Sellers JR, Fananapazir L, Epstein ND. Structural interpretation of the mutations in the beta-cardiac myosin that have been implicated in familial hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 1995;92:3864–8. doi: 10.1073/pnas.92.9.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashyam MD, Bashyam L, Savithri GR, Gopikrishna M, Sangal V, Devi AR. Molecular genetic analyses of beta-thalassemia in South India reveals rare mutations in the beta-globin gene. J Hum Genet. 2004;49:408–13. doi: 10.1007/s10038-004-0169-9. [DOI] [PubMed] [Google Scholar]
- 11.Lindpaintner K, Pfeffer MA, Kreutz R, et al. A prospective evaluation of an angiotensin-converting-enzyme gene polymorphism and the risk of ischemic heart disease. N Engl J Med. 1995;332:706–11. doi: 10.1056/NEJM199503163321103. [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan V, Zhu Y, Bala K, et al. Association between ACE gene polymorphism and diabetic nephropathy in South Indian patients. JOP. 2001;2:83–7. [PubMed] [Google Scholar]
- 13.Devi AR, Gopikrishna M, Ratheesh R, Savithri G, Swarnalata G, Bashyam M. Farber lipogranulomatosis: Clinical and molecular genetic analysis reveals a novel mutation in an Indian family. J Hum Genet. 2006;51:811–4. doi: 10.1007/s10038-006-0019-z. [DOI] [PubMed] [Google Scholar]
- 14.Coureux PD, Sweeney HL, Houdusse A. Three myosin V structures delineate essential features of chemo-mechanical transduction. EMBO J. 2004;23:4527–37. doi: 10.1038/sj.emboj.7600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coureux PD, Wells AL, Menetrey J, et al. A structural state of the myosin V motor without bound nucleotide. Nature. 2003;425:419–23. doi: 10.1038/nature01927. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol. 2007;292:H1643–54. doi: 10.1152/ajpheart.00931.2006. [DOI] [PubMed] [Google Scholar]
- 17.Laredo R, Monserrat L, Hermida-Prieto M, et al. [Beta-myosin heavy-chain gene mutations in patients with hypertrophic cardiomyopathy] Rev Esp Cardiol. 2006;59:1008–18. doi: 10.1157/13093977. [DOI] [PubMed] [Google Scholar]
- 18.Welikson RE, Buck SH, Patel JR, Moss RL, Vikstrom KL, Factor SM. Cardiac myosin heavy chains lacking the light chain binding domain cause hypertrophic cardiomyopathy in mice. Am J Physiol. 1999;276:H2148–58. doi: 10.1152/ajpheart.1999.276.6.H2148. [DOI] [PubMed] [Google Scholar]
- 19.Perrot A, Schmidt-Traub H, Hoffmann B, Prager M, Bit-Avragim N, Rudenko R. Prevalence of cardiac beta-myosin heavy chain gene mutations in patients with hypertrophic cardiomyopathy. J Mol Med. 2005;83:468–77. doi: 10.1007/s00109-005-0635-7. [DOI] [PubMed] [Google Scholar]
- 20.Mckeown P, Needham E, Mattu R, Jeffery S, Kozielska E, Wilczok T. Lys766Asn mutation in exon 21 of the β-myosin heavy chain gene in a Polish family with hypertrophic cardiomyopathy. Eur Heart J. 1997;18:407. (Abst) [Google Scholar]
- 21.Moolman-Smook J, De Lange W, Corfield V, Brink P. Expression of HCM causing mutations: Lessons learnt from genotype-phenotype studies of the South African founder MYH7 A797T mutation. Med Genet. 2000;37:951–6. doi: 10.1136/jmg.37.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]