Abstract
BACKGROUND:
Hypertensive diabetic patients, when compared with essential hypertensive patients, have a higher left ventricular mass index (LVMI) and an impaired cardiac diastolic function (CDF). Autonomic neuropathy (AN) could contribute to this finding.
OBJECTIVE:
To evaluate the relationship between AN tests, and LVMI and CDF in normotensive patients with type 2 diabetes mellitus (DM2) and without AN symptoms or left ventricular hypertrophy.
METHODS:
In 21 normotensive patients with DM2 (group 1) and 16 control subjects (group 2), LVMI and CDF were evaluated using atrial deceleration time, isovolumic relaxation time, E wave, A wave and E/A wave ratio. AN tests performed included a deep breathing test, Valsalva manoeuvre and lying-to-standing test.
RESULTS:
Groups did not differ in clinical and echocardiographic characteristics. None of the patients in either group presented with left ventricular hypertrophy. In group 1, there were correlations between the deep breathing test and LVMI (r=−0.6; P<0.01) and between the deep breathing test and E/A wave ratio (r=0.4; P<0.05). No correlations were found in the control group.
CONCLUSION:
In DM2 patients, AN tests correlated with LVMI and CDF before left ventricular hypertrophy, hypertension, impaired CDF and diabetic AN symptoms were present. The present study suggests that AN tests could be regularly performed in DM2 patients. Any abnormalities in tests should be followed by a cardiac evaluation.
Keywords: Diabetes mellitus type 2, Diabetic autonomic neuropathy, Diabetic cardiomyopathy
Proposed causes of diabetic cardiomyopathy include metabolic abnormalities (hyperglycemia and changes in myocardial lipid metabolism), hypertension (HPT) and autonomic neuropathy (AN) (1–4). Diabetes mellitus (DM) is associated with HPT and coronary atherosclerosis (5), which can reduce myocardial performance.
There is an attractive hypothesis to explain the pathogenesis of diabetic cardiomyopathy. AN has been associated with a high cardiac mortality rate (6,7), and autopsy studies have found low concentrations of noradrenaline in diabetic patients with cardiomyopathy (8). In addition, Kahn et al (9) compared patients with and without AN, and found that patients with AN had reduced levels of plasma catecholamines; this finding was related to abnormalities in diastolic heart function.
We previously reported that good glycemic control in patients with type 2 DM (DM2) and HPT could reverse left ventricular hypertrophy (LVH) (10,11). Unfortunately, we did not evaluate 24 h blood pressure (BP) in the present study. AN has been associated with hyperglycemia (6,7) and a small decrease in nocturnal BP (12,13). Therefore, a reasonable hypothesis is that AN is a means through which hyperglycemia causes preclinical myocardial abnormalities.
The purpose of the present study was to evaluate the relationship between AN tests, and left ventricular mass index (LVMI) and cardiac diastolic function (CDF) in normotensive patients with DM2 and without AN symptoms or LVH.
METHODS
Patients
Thirty-seven subjects were recruited from the Endocrinology Division at the Federal University of Pará (Belém, Brazil). Patients were divided into two groups – group 1 (21 normotensive patients with DM2) and group 2 (16 healthy volunteers recruited from the original population). In both groups, patients were considered to be normotensive according to standard criteria (14). Patients with congestive heart failure, angina pectoris, heart valve disease with hemodynamic effect, previous stroke, history of heart disease, evidence of chronic alcoholism or any symptoms of AN were not included in the study. All individuals presented with normal values of serum creatinine and 24 h proteinuria. In addition, group 2 underwent an oral glucose tolerance test to exclude the possibility of DM. All patients underwent the following tests: echocardiography with Doppler, AN tests (deep breathing test, Valsalva manoeuvre and lying-to-standing test), fasting plasma glucose, glycated hemoglobin, serum creatinine and 24 h proteinuria. Futhermore, diabetic patients calculated their long-term glycemic control. DM was diagnosed according to standard criteria (15). DM2 patients were identified as those with disease onset at 30 years of age or older with no need of insulin treatment. They were treated only with diet or diet plus oral antidiabetic agents (sulphonylureas, metformin or acarbose). None of the patients were treated with insulin during the study. The present study was approved by the Institutional Ethics Committee.
Cardiovascular An tests
Tests were conducted in both groups as proposed by Ewing et al (16) and Valensi et al (17). They were based on the response of the heart to the Valsalva manoeuvre (Valsalva ratio), variation in heart rate (RR interval) during deep breathing (deep breathing test) and response of BP to the change from a decubitus position to a standing position (lying-to-standing test). The first two tests reflect parasympathetic integrity, and the last reflects sympathetic integrity.
Deep breathing test:
Increases in heart rate (RR interval) while breathing were measured during the deep breathing test. The heart rate usually depends on parasympathetic nervous system integrity. Patients with diabetic AN have a considerable reduction or even complete absence of increases in heart rate. Patients stayed in the supine position, were quiet and took six deep breaths in 1 min (5 s for inspiration and 5 s for expiration) while an electrocardiogram was recorded, using a marker to indicate the end of each inspiration and expiration. Increases in heart rate during the breathing were recorded (normal heart rate increase was defined as at least 15 beats/min, ‘borderline’ as 11 beats/min to 14 beats/min, and abnormal as 10 beats/min or less).
Valsalva ratio (cardiac response to valsalva manoeuvre):
During the effort period, BP falls and the heart rate should rise. After resting, the BP rises, overtaking its normal rest value, and the heart rate decreases. The test consisted of forced exhalation and maintaining a pressure of 40 mmHg for 15 s while an electrocardiogram was recorded. It was performed three times in the space of 1 min, with the patient resting between each test. The results were expressed as a Valsalva ratio, which is the ratio between the highest RR interval after the manoeuvre (reflecting bradycardia following the relaxation) and the lowest RR interval during the manoeuvre (which reflects tachycardia during exercise). The test was performed three times and the mean value for the ratio was used. Valsalva ratios of 1.21 or greater were defined as normal, 1.11 to 1.20 as ‘borderline’ and 1.10 or less as abnormal.
Lying-to-standing test:
The lying-to-standing test was performed as previously described (16–18). The reproducibility of these methods has been demonstrated in diabetic patients.
The coefficients of variation are 9.2%, 12.6% and 6.4% for the Valsalva manoeuvre, deep breathing test and lying-to-standing test, respectively (15).
Individuals of both groups received a score according to values obtained by the cardiovascular AN tests. The values were scored as follows: 0 – all tests resulted in normal values; 1 – only one test resulted in abnormal values; 2 – two tests resulted in abnormal values; and 3 – all tests resulted in abnormal values. Individuals who obtained ‘borderline’ values received a score of 0.5 for the corresponding test.
Echocardiography
M-mode, two-dimensional echocardiographic and cardiac Doppler studies were performed using a commercially available Doppler echocardiography unit (ACUSON P50; Siemens AG, USA) equipped with a 2.5 MHz mechanical transducer. The studies were performed with patients in the partial left lateral supine position. M-mode measurements were performed according to the recommendations of the American Society of Echocardiography (19). Left ventricular mass (LVM) was calculated as previously recommended by Devereux et al (20). The LVMI was calculated by dividing LVM by the body surface area. LVH was present if LVMI was at least 134 g/m2 in men and at least 110 g/m2 in women (20,21). All examinations were analyzed blindly by one independent echocardiographer. Transmitral blood flow signals were obtained above the mitral valve in an apical four-chamber view. The following measurements were made on consecutive cardiac cycles: peak flow velocity of early left ventricular filling (peak E), peak flow velocity of late (atrial) left ventricular filling (peak A), early deceleration time, isovolumic relaxation time and the ratio between early and late diastolic flow velocity peaks (E/A ratio) (normal values: peak E, less than 50 cm/s; peak A, less than 80 cm/s, deceleration time, less than 240 ms; isovolumic relaxation time, less than 110 ms; and E/A ratio, at least 1). All measurements of diastolic function were performed while the patient had a heart rate within the normal range (60 beats/min to 100 beats/min) (22,23).
Other measurements
To evaluate previous long-term glycemic control, the average of all fasting blood glucose and glycated hemoglobin values available before the study was calculated. Glycated hemoglobin was evaluated by high-performance liquid chromatography. Averages of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides were also calculated.
Statistical analysis
All normally distributed values are presented as mean ± SD and all other values are presented as median (range). In comparison with the non-normally distributed variables, the Mann-Whitney U test was used to test the differences between the two groups. For all normally distributed variables, the Student’s t test was used for comparison between the two groups. For correlation analysis, correlation coefficients (Pearson or Spearman) were calculated. A two-tailed P<0.05 was considered to be statistically significant. All calculations were made with a commercially available program, SigmaStat 1.0 (Jandel Scientific Software, USA).
RESULTS
Clinical characteristics of both groups are presented in Table 1. Group 1 and group 2 did not differ in age, body mass index or sex. In group 1, the average fasting blood glucose was 9.61±3.3 mmol/L and median DM duration was five years (range one to 25 years). Laboratory characteristics are also presented in Table 1.
TABLE 1.
Clinical parameters of diabetic patients (group 1) and controls (group 2)
| Parameter | Group 1 (n=21) | Group 2 (n=16) | P |
|---|---|---|---|
| Age, years | 54±8 | 48±11 | NS |
| Sex, women/men, n/n | 12/9 | 10/6 | NS |
| Body mass index, kg/m2 | 28±4 | 26±3 | NS |
| Duration of DM, years | 5 (1–25) | – | – |
| Fasting blood glucose, mmol/L | 9.61±3.3 | – | – |
| Glycated hemoglobin, % | 9.0±1.4 | – | – |
| Total cholesterol, mmol/L | 5±0.82 | – | – |
| HDL-C, mmol/L | 1.18±0.18 | – | – |
| LDL-C, mmol/L | 3±0.79 | – | – |
| VLDL-C, mmol/L | 0.77±0.36 | – | – |
| Triglycerides, mmol/L | 2.07±1.43 | – | – |
Data presented as mean ± SD or median (range) unless otherwise indicated. DM Diabetes mellitus; HDL-C High-density lipoprotein cholesterol; LDL-C Low-density lipoprotein cholesterol; NS Not significant; VLDL-C Very low-density lipoprotein cholesterol
Echocardiographic data are expressed in Table 2. No difference was found in LVMI, or systolic and diastolic function between group 1 and group 2. No patients in either group presented with LVH.
TABLE 2.
Echocardiographic results of diabetic patients (group 1) and controls (group 2)
| Group 1 (n=21) | Group 2 (n=16) | P | |
|---|---|---|---|
| Left ventricular mass, g | 137±31 | 154±23 | NS |
| Left ventricular mass index, g/m2 | 78±18 | 88±8 | NS |
| Deceleration time, ms | 195±25 | 203±38 | NS |
| Isovolumic relaxation time, ms | 92±18 | 98±13 | NS |
| E wave, cm/s | 63±16 | 63±16 | NS |
| A wave, cm/s | 56±21 | 50±19 | NS |
| E/A wave ratio | 1.1 (0.8–2.5) | 13 (0.9–2.2) | NS |
| Ejection fraction, % | 70±8 | 73±2 | NS |
Data presented as mean ± SD or median (range). NS Not significant
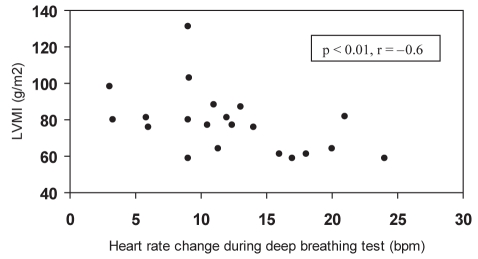
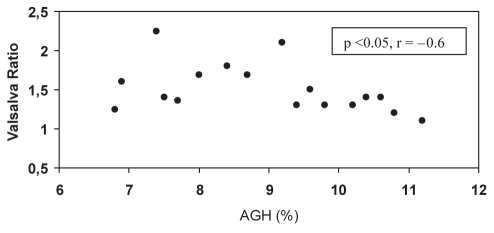
AN data are shown in Table 3. In group 1, patients presented borderline values in the deep breathing test and a tendency toward higher AN scores versus group 2. In group 1, there was a correlation between the deep breathing test and LVMI (r=−0.6, P<0.01) (Figure 1) and the deep breathing test and E/A wave ratio (r=0.4, P=0.05). The Valsalva ratio was correlated with average glycated hemoglobin (r=−0.6, P<0.05) (Figure 2). There were no correlations among AN, LVMI, and systolic and diastolic function in group 2.
TABLE 3.
Autonomic neuropathy (AN) data for diabetic patients (group 1) and controls (group 2)
| Group 1 (n=21) | Group 2 (n=16) | P | |
|---|---|---|---|
| Deep breathing test, beats/min | 11±5 | 15±9 | NS |
| Valsalva ratio | 1.51±0.3 | 1.46±0.3 | NS |
| Lying-to-standing test, mmHg | 10±8 | 8±7 | NS |
| AN score | 1 (0–2.5) | 0.5 (0–2) | NS |
Data presented as mean ± SD or median (range). NS Not significant
Figure 1).
Correlation between deep breathing test and left ventricular mass index (LVMI) in normotensive patients with diabetes mellitus type 2. bpm Beats per minute
Figure 2).
Correlation between average glycated hemoglobin (AGH) and Valsalva ratio in normotensive patients with diabetes mellitus type 2
DISCUSSION
The present study demonstrated a correlation between AN tests, and LVMI and CDF in normotensive patients with DM2. Positive AN tests occurred even before LVH, impaired CDF and diabetic AN symptoms were present. This correlation was not found in the control group. Additionally, in diabetic individuals, AN tests were correlated with average glycated hemoglobin. Our data suggest that AN could precede LVH and be a contributing factor to preclinical cardiac abnormalities in normotensive patients with DM2.
It is well known that LVH and CDF abnormalities are considered to be relevant findings in preclinical diabetic cardiomyopathy (24). Some studies (11,25) have demonstrated that a higher frequency of LVH in patients with DM occurs particularly when HPT is also present. Our study found no increased LVM in normotensive diabetic patients compared with controls, reinforcing the hypothesis that HPT, when associated with DM, increases the hyperglycemic damage in the myocardium (25). Nicolino et al (26) have also described similar LVM in normotensive diabetic patients when compared with normal subjects. Grossman et al (25) showed that, when compared with essential hypertensive patients, diabetic hypertensive patients had a higher LVMI, independent of office BP levels. The fact that our study did not demonstrate these differences in diabetic patients with normal BP suggests that when HPT is not present, it becomes difficult to show higher LVMI in diabetic patients versus controls.
We have previously reported that better glycemic control in hypertensive patients with DM2 can reduce the degree of LVH independently of BP levels (11). We also recently showed that there was no difference in average diurnal systolic and diastolic BP when comparing diabetic hypertensive patients with essential hypertensive patients (10). However, patients with DM2 demonstrated worse diastolic function, higher LVMI and increased average nocturnal systolic BP. Because we know that AN is associated with hyperglycemia (6,7,27) and a small decrease in nocturnal BP (12,13), a reasonable hypothesis is that AN is a means through which hyperglycemia can cause preclinical myocardial abnormalities.
Vanninen et al (28), studying diabetic patients without cardiovascular disease, showed a correlation between heart rate variability during the deep breathing test and the sum of septum thickness and the thickness of the left ventricle posterior wall, but not between heart rate variability and LVMI. However, they did not describe whether these patients individually had LVH. Therefore, we believe that our study is the first to demonstrate a correlation between AN tests and LVMI in normotensive patients with DM2 and without LVH.
A correlation between heart rate variability during breathing and left ventricular filling has been described (28). In addition, echocardiographic studies (9,29–31) in diabetic patients without clinical evidence of cardiovascular disease have shown abnormalities in heart relaxation and diastolic ventricular filling. In our study, diabetic patients showed a positive correlation between the variation in heart rate during breathing (deep breathing test) and E/A wave ratio, suggesting an influence of AN on CDF.
Abnormalities in left ventricular filling have been previously more related to AN in diabetes of long duration (9,32,33). Nicolino et al (26) compared diabetic patients without heart disease with normal controls. Diabetic patients were divided into two groups according to the presence or absence of HPT. The group with normotensive diabetic patients had evidence of diastolic dysfunction when compared with normal controls. However, the mean age of patients in this group was 64±9.1 years, 20% were insulin dependent and the mean duration of diabetes in the study was 9.1 years. In the present study, patients did not use insulin, the mean age was 54±8 years and median duration of diabetes was five years. These differences could be the reason we have not been able to demonstrate worse diastolic function in our normotensive diabetic patients compared with normal individuals. In addition, our patients did not have LVH that could produce change in CDF.
In our study, diabetic patients showed borderline values in the deep breathing test and a tendency toward higher AN scores versus controls. In addition, we found a strong correlation between AN tests, and LVMI and CDF in diabetic patients only. This could indicate that even early-stage AN could be related to preclinical cardiac abnormalities in DM2 patients. AN tests have well-established coefficients of variation (18), but in our opinion, an improvement in sensitivity could be useful to identify AN earlier. In our diabetic patients, AN tests correlated with LVMI and CDF even before LVH, impaired CDF and diabetic AN symptoms were present. It suggests that AN tests should be regularly performed in patients with DM2, and that any abnormalities in those tests should be followed by a detailed cardiac evaluation.
REFERENCES
- 1.Fein FS. Diabetic cardiomyopathy. Diabetes Care. 1990;13:1169–79. doi: 10.2337/diacare.13.11.1169. [DOI] [PubMed] [Google Scholar]
- 2.Barbagallo M, Grupta R, Resnick L. Cellular ions in NIDDM: Relation of calcium to hyperglycemia and cardiac mass. Diabetes Care. 1996;19:1393–8. doi: 10.2337/diacare.19.12.1393. [DOI] [PubMed] [Google Scholar]
- 3.Paulson DJ, Crass MF. Myocardial triacyglycerol fatty acid composition in diabetes mellitus. Life Sci. 1980;27:2237–43. doi: 10.1016/0024-3205(80)90390-2. [DOI] [PubMed] [Google Scholar]
- 4.Uusitupa M, Mustonen J, Airaksinen J. Diabetic heart muscle disease. Ann Med. 1990;22:377–86. doi: 10.3109/07853899009147274. [DOI] [PubMed] [Google Scholar]
- 5.Garcia MJ, McNamara PM, Gordon T, Cannell WB. Morbidity and mortality in diabetics in the Framingham population: Sixteen years follow up study. Diabetes. 1964;23:105–11. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 6.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. QJ Med. 1980;49:95–108. [PubMed] [Google Scholar]
- 7.Ewing DJ, Campbell IW, Clarke BF. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann Intern Med. 1980;92:308–11. doi: 10.7326/0003-4819-92-2-308. [DOI] [PubMed] [Google Scholar]
- 8.Neubauer B, Christensen NJ. Norepinephrine, epinephrine and dopamine content of the cardiovascular system in long-term diabetics. Diabetes. 1976;25:6–10. doi: 10.2337/diab.25.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Kahn JK, Zola B, Juni JE, Vinik AL. Radionuclide assessment of left ventricular diastolic filling in diabetes mellitus with and without cardiac autonomic neuropathy. J Am Coll Cardiol. 1986;7:1303–9. doi: 10.1016/s0735-1097(86)80150-4. [DOI] [PubMed] [Google Scholar]
- 10.Felício JS, Ferreira SR, Plavnik FL, et al. Hyperglycemia and nocturnal systolic blood pressure are associated with left ventricular hypertrophy and diastolic dysfunction in hypertensive diabetic patients. Cardiovasc Diabetol. 2006;5:19. doi: 10.1186/1475-2840-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felício JS, Pacheco JT, Ferreira SR, et al. Effect of blood glucose on left ventricular mass in patients with hypertension and type 2 diabetes mellitus. Am J Hypertens. 2000;13:1149–54. doi: 10.1016/s0895-7061(00)01200-0. [DOI] [PubMed] [Google Scholar]
- 12.Mulec H, Blohme G, Kullenberg K, Nyberg G, Bjorck S. Latent overhydration and nocturnal hypertension in diabetic nephropathy. Diabetologia. 1995;38:216–20. doi: 10.1007/BF00400097. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen F, Rossing P, Bang L, et al. On the mechanisms of blunted nocturnal decline in arterial blood pressure in NIDDM patients with diabetic nephropathy. Diabetes. 1995;44:783–9. doi: 10.2337/diab.44.7.783. [DOI] [PubMed] [Google Scholar]
- 14.Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure The Seventh Report of The Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) Hypertension. 2003;42:1206. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of medical care in diabetes – 2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 16.Ewing DJ, Boland O, Netlson JMM, Cho CG, Clark BF. Autonomic neuropathy, QT interval lengthening, and unexpected deaths in male diabetics patients. Diabetologia. 1991;34:182–5. doi: 10.1007/BF00418273. [DOI] [PubMed] [Google Scholar]
- 17.Valensi P, Sachs RN, Harfouche B, et al. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001;24:339–43. doi: 10.2337/diacare.24.2.339. [DOI] [PubMed] [Google Scholar]
- 18.Valensi PE, Johnson NB, Blanche PM, Extramania F, Motte G, Coumel P. Influence of cardiac autonomic neuropathy on heart rate dependence of ventricular repolarization in diabetic patients. Diabetes Care. 2002;25:918–23. doi: 10.2337/diacare.25.5.918. [DOI] [PubMed] [Google Scholar]
- 19.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantification in M-mode echocardiography measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 20.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1987;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 21.Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: The Framingham Heart Study. Am J Cardiol. 1987;59:956–60. doi: 10.1016/0002-9149(87)91133-7. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura RA, Abel MD, Hatle LK, Tajek AL. Assessment of diastolic function of the heart: Background and current applications of Doppler echocardiography. Part II. Clinical studies. Mayo Clin Proc. 1989;64:181–204. doi: 10.1016/s0025-6196(12)65673-0. [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1997;55:613–8. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed SS, Jaferi GA, Narang RM, Regan TJ. Preclinical abnormality of left ventricular function diabetes mellitus. Am Heart J. 1975;89:153–8. doi: 10.1016/0002-8703(75)90039-3. [DOI] [PubMed] [Google Scholar]
- 25.Grossman E, Rosenthal T, Shemesh J, et al. Left ventricular mass in diabetes-hypertension. Arch Int Med. 1992;152:1001–4. [PubMed] [Google Scholar]
- 26.Nicolino AM, Longobardi G, Furgi G, et al. Left ventricular diastolic filling in diabetes mellitus with and without hypertension. Am J Hypertens. 1995;8:382–9. doi: 10.1016/0895-7061(95)00022-h. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer MA, Weinberg CR, Cook DL, et al. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care. 1984;7:447–53. doi: 10.2337/diacare.7.5.447. [DOI] [PubMed] [Google Scholar]
- 28.Vanninen E, Mustonen J, Vainio P, Länsimies E, Uusitupa M. Left ventricular function and dimensions in newly diagnosed non-insulin-dependent diabetes mellitus. Am J Cardiol. 1992;70:371–8. doi: 10.1016/0002-9149(92)90622-6. [DOI] [PubMed] [Google Scholar]
- 29.Sandersen JE, Brown DJ, Rivellese A, Kohner E. Diabetic cardiomyopathy? An echocardiographic study of young diabetics. Br Med J. 1978;1:404–7. doi: 10.1136/bmj.1.6110.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro LM. Echocardiographic features of impaired ventricular function in diabetes mellitus. Br Heart J. 1991;45:122–8. doi: 10.1136/hrt.47.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Airaksinen J, Kaila J, Ikäheimo M. Impaired left ventricular filling in yong female diabetics: An echocardiographic study. Acta Med Scand. 1984;216:509–16. doi: 10.1111/j.0954-6820.1984.tb05039.x. [DOI] [PubMed] [Google Scholar]
- 32.Araiksinen KEJ, Koistinen MJ, Ikaheimo MJ, et al. Augmentation of atrial contribution to left ventricular filling in IDDM subjects as assessed by Doppler echocardiography. Diabetes Care. 1989;12:159–61. doi: 10.2337/diacare.12.2.159. [DOI] [PubMed] [Google Scholar]
- 33.Uusitupa M, Mustonen J, Laakso M, et al. Impairment of diastolic function in middle-age type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients free of cardiovascular disease. Diabetologia. 1988;31:783–91. doi: 10.1007/BF00277478. [DOI] [PubMed] [Google Scholar]