Abstract
A conformational change of the prion protein is responsible for a class of neurodegenerative diseases called the transmissible spongiform encephalopathies that include mad cow disease and the human afflictions kuru and Creutzfeldt–Jakob disease. Despite the attention given to these diseases, the normal function of the prion protein in healthy tissue is unknown. Research over the past few years, however, demonstrates that the prion protein is a copper binding protein with high selectivity for Cu2+. The structural features of the Cu2+ binding sites have now been characterized and are providing important clues about the normal function of the prion protein and perhaps how metals or loss of protein function play a role in disease. The link between prion protein and copper may provide insight into the general, and recently appreciated, role of metals in neurodegenerative disease.
Introduction–Prions and Neurodegenerative Disease
It is difficult to think of a protein that has garnered more attention than the prion protein. Named by Nobel Laureate Stanley B. Prusiner, the term “prion” refers to the proteinaceous, infectious particles responsible for mad cow disease, scrapie in sheep and goats, chronic wasting disease in deer and elk, and the human diseases kuru and Creutzfeldt–Jakob disease (CJD).1,2 Among humans, prion diseases are very rare, accounting for approximately one in a million deaths. Nevertheless, each outbreak of the disease–from a single animal with mad cow disease in Canada to infected herds of deer and elk in the Midwest–brings a fresh spate of newspaper articles and widespread discussion of these complex neurodegenerative disorders.
Why the great interest in diseases that seemingly pose little threat to humans? There are several reasons. First, there is well-justified concern that the spread of contaminated food, transplant tissues, or blood products will lead to a widespread outbreak. Several such cases are well-known, including new variant Creutzfeldt–Jakob disease (nvCJD) among young individuals in Great Britain who consumed infected beef.1 Second, much like Alzheimer's, Parkinson's, and Huntington's diseases, prion diseases involve deposits of a misfolded peptide or protein and resulting deterioration of nearby neurons.3 Consequently, understanding prion diseases is sure to help us gain a deeper understanding of more prevalent neurodegenerative disorders. The third and perhaps most fascinating feature of prion diseases is the infectious nature of the prion particle–unlike viruses or bacteria, this particle is totally devoid of any nucleic acid yet propagates a lethal neurological disorder. Thus, prion diseases represent a fundamentally new biological mechanism for transmitting infection.
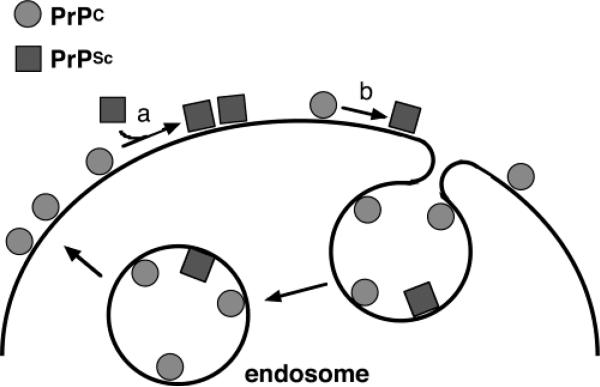
Prion diseases collectively are referred to as the transmissible spongiform encephalopathies (TSEs). The prion protein, responsible for the TSEs, is a normal component of many body tissues and is found at high levels in the central nervous system (CNS). As shown schematically in Figure 1, the protein is tethered to the outside surface of cells through a glycosylphosphatidylinositol (GPI) anchor.4 Endocytosis, in which a portion of the membrane pinches off creating an intracellular vesicle, cycles the protein from the cell surface to the endosome and back.4 The normal cellular form of the prion protein is designated PrPC. Disease results from a refolding of PrPC to the scrapie form termed PrPSc. This form contains no nucleic acid or alterations of the PrPC polypeptide backbone–its amino acid sequence is equivalent to that of PrPC. Once PrPSc forms, it acts as a template for converting additional PrPC to PrPSc thus facilitating a buildup of misfolded protein and subsequent neurodegeneration. PrPSc is therefore the infectious prion agent.
FIGURE 1.
Localization and conformations of the prion protein. PrPC represents the normal form of the prion protein found in healthy tissue; PrPSc is the conformer responsible for the TSEs. PrP is tethered to cell membranes through a GPI anchor. Conversion from PrPC to PrPSc takes place either through a templating interaction with PrPSc (a) or by a spontaneous event (b). PrPC is cycled through endocytosis.
TSEs may be sporadic, in which PrPSc forms spontaneously, inherited, where certainly family lines carry PrP sequences predisposed to forming PrPSc, or infectious, in which PrPSc is introduced from an exogenous source. Sporadic cases are by far the most common, accounting for approximately 85% of all CJD cases in the USA.
As noted above, deposition of a misfolded protein resulting in deterioration of nearby nerve tissue is common among a wide class of neurodegenerative diseases. This raises three essential questions that must be addressed for a complete understanding and hopeful treatment of these disorders:
What is the function of the precursor protein, in this case PrPC, and consequently, what function is potentially lost from misfolding?
What is the mechanism by which proteins misfold, and what endogenous or environmental factors contribute to this process?
Why are deposits of the misfolded species cytotoxic?
Remarkably, with regard to Alzheimer's disease, Parkinson's disease, Huntington's disease, as well as the TSEs, there are no complete answers to any of these questions.
Toward a Function for PrPC–The Copper Connection
The mechanism by which PrPSc is generated from PrPC, either spontaneously or via a template mechanism (Figure 1), suggests that the host animal or individual must naturally produce the prion protein to be susceptible to disease. As an essential experiment toward demonstrating the prion hypothesis, genetic techniques were used to produce knockout animals–mice in this case–devoid of PrP.5,6 These PrP-knockout mice do not contract disease even if they are inoculated with PrPSc directly into the brain. The results of these seminal experiments remain among the most convincing evidence so far that conversion of host PrPC to PrPSc is the critical event leading to prion disease. Of course, with PrP knockout mice at hand, one may get a sense of PrPC's normal function by probing for differences between these transgenic animals and wild-type. Remarkably, the knockouts exhibit reasonably normal behavior and examination of their brain tissue reveals no significant differences compared to wild-type.7 If PrPC has a function, it must be subtle.
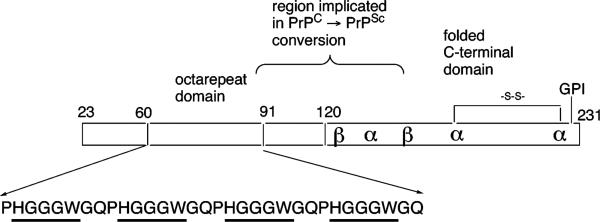
In 1995, two papers by Hornshaw et al. suggested that PrPC binds Cu2+.8,9 The region of the protein first implicated in this activity is the so-called octarepeat domain composed of multiple tandem copies of the eight-residue sequence PHGGGWGQ. PrP sequences from most species carry four or five copies of this segment. As shown in Figure 2, the octarepeat domain is in the N-terminal portion of the protein approximately from position 60 to position 90 with variation arising from different species and number of octapeptide repeats. Interestingly, across species, the octarepeat domain is among the most highly conserved portions of the PrP sequence, suggesting it plays a role in PrPC function.10
FIGURE 2.
Organization of PrP and the octarepeat domain. This schematic of PrPC locates the globular C-terminal domain, the glycosylphosphatidylinositol (GPI) membrane anchor, and the octarepeat domain. Also shown is a flexible region implicated in multimerization that accompanies PrPC → PrPSc conversion. Cu2+ binding within the octarepeats involves the specific residues HGGGW (underlined).
In 1997, Brown et al. published a landmark paper demonstrating that brain extracts from PrP-knockout mice have substantially lower copper content relative to wild-type.11 Thus, the ability of PrP to bind Cu2+ is not just incidental but indeed influences the tissue copper content. Moreover, the activity of copper-dependent enzymes was reduced suggesting that either PrP possesses metalloprotein function or helps supply other enzymes with needed copper ions. Copper binding was also found to be highly cooperative.11 Mass spectrometry studies by Whittal et al. showed that PrP N-terminal domain takes up approximately five copper ions, each with a progressively lower dissociation constant varying from the micromolar to nanomolar range at pH 7.4.12 The octarepeat domain is remarkably selective for Cu2+ with affinity for Cu+ or other metal ion species weak or nonexistent.9,12,13 Finally, Cu2+ binding is quite pH-dependent with only two copper ions found at pH 6.12
Studies of neurons in cell culture reveal additional clues regarding function. Harris and co-workers showed that both copper and zinc dramatically increase the rate of PrPC endocytosis thus demonstrating that metal ions influence PrPC trafficking in the cell.14 Elimination of the octarepeat domain abolishes this activity.15 Moreover, several studies suggest that PrP acts to protect cells from deleterious redox activity of uncomplexed copper.16,17 In mice, PrP knockouts relative to wild-type exhibit significant increases in lipid and protein oxidation, as well as loss of superoxide dismutase and catalase function.18
Despite evidence linking the normal activity of PrPC to copper binding and perhaps antioxidant activity, there has been little agreement as to specific function. Among the current theories are that PrPC is (i) a superoxide dismutase (SOD)19 participating in the scavenging and detoxification of O2-, (ii) a transmembrane copper transporter that operates through endocytosis,12,14,20 and (iii) a copper buffer that sequesters excess metal ion at the plasma membrane.17
Toward a Structure of the Prion Protein with Bound Copper Ions
Recognizing that structural information is certain to play an essential role in fleshing out specific function, a number of laboratories launched efforts aimed at determining the molecular features of the Cu2+ binding sites.13,21–28 Copper tends to rapidly precipitate recombinant PrP, so many early experimental studies used peptides corresponding to regions of the octarepeat domain. The imidazole ring of histidine, present in the PHGGGWGQ repeat, is an avid metal ion binder and is certain to participate in Cu2+ coordination. Using Raman spectroscopy, Miura et al. identified absorption bands consistent with His–Cu bonds and found that each octapeptide repeat bound a single Cu ion.21 Interestingly, they also identified bands consistent with N–Cu bonds where the nitrogen was assigned to a deprotonated backbone amide. Modeling studies identified a relaxed conformation in which the second and third glycines following the His participated in the coordination sphere. Studies from other laboratories using electron paramagnetic resonance (EPR) and companion spectroscopic approaches on peptides,22 as well as full-length protein,28 also supported a coordination environment involving deprotonated amides, as well as the possibility of additional copper sites in the globular C-terminal domain.23
Lead by Drs. Colin Burns and Eliah Aronoff-Spencer, our laboratory approached this problem using a suite of EPR techniques and peptide design. Cu2+ is a d9 species with a single unpaired electron. EPR spectra are very sensitive to the metal ion's environment and can be used to estimate the geometry of the center and the number and type of equatorially coordinating atoms.29 At the outset, we tried to determine whether each octarepeat bound an individual Cu2+ as an independent unit or whether there was a more complex organization of the fully copper bound octarepeat domain. Working with Professor Haydn Ball, now at the University of Texas Southwestern Medical Center in Dallas, we prepared a library of peptides ranging from the full octarepeat domain–WGQ(PHGGGWGQ)4–down to a small HGG tripeptide derived from the octarepeat sequence. Remarkably, we found that the minimal sequence HGGGW bound a single Cu2+ and captured all the EPR spectral features of the full four repeat domain with four bound copper ions.30 Using direct EPR-detected titrations, we further showed that the number of HGGGW units determined the number of copper ions taken up in a 1:1 fashion. These findings demonstrated that HGGGW constitutes the fundamental copper-binding unit in the PrP octarepeat domain.30
Next, in collaboration with Professors Gary Gerfen and Jack Peisach at Albert Einstein College of Medicine, we applied electron spin–echo envelope modulation (ESEEM) and showed that indeed each Cu2+ was coordinated by a single imidazole ring.30 Analysis of the conventional EPR spectra suggested that approximately three nitrogens were equatorially coordinated the Cu2+; the next step was to determine which amino acids were contributing these nitrogens. Here, in collaboration with Professor William Antholine of the Medical College of Wisconsin, we employed low-frequency S-band EPR, which is remarkably sensitive to Cu–N hyperfine coupling.20 A library of octarepeat peptides was produced where each was 15N labeled at a distinct position. Analysis of the hyperfine patterns immediately pointed to the amide nitrogens of the two glycines following the histidine thus directly implicating deprotonated amides in copper coordination.20,30
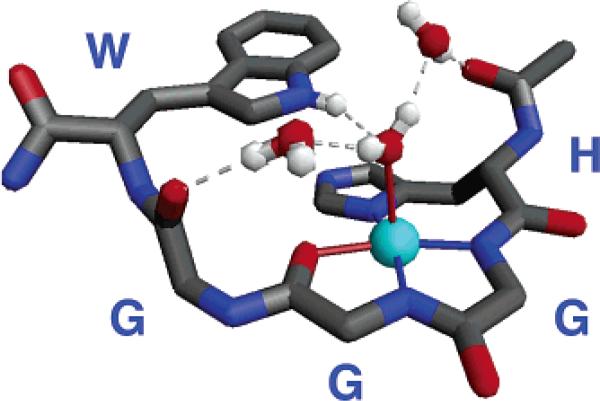
The N-terminal domain of PrP encompassing the octarepeat region is rich with glycine. Correspondingly, NMR structural studies find this domain to be largely disordered.31 Our work showing a specific binding mode for HGGGW in complex with copper, however, suggested that we might be able to grow crystals of this fundamental segment. Collaborating with Professors William Scott and Alice Vrielink and Dr. Christine Dunham of UCSC and Professor Marilyn Olmstead of UC Davis, we found that crystals formed in aqueous solution at pH 7.4 and diffracted to better than 0.70 Å.20 The structure of the complex is shown in Figure 3. As first identified by EPR, three nitrogens contribute to the equatorial plane and arise from the imidazole and the two deprotonated glycines immediately following the histidine. In addition, the second glycine contributes its amide carbonyl and the indole NH of the tryptophan hydrogen bonds to an axial water thus creating a highly organized copper binding unit.
FIGURE 3.
Crystal structure of the Cu–HGGGW complex. Equatorial Cu2+ coordination is from the histidine imidazole, the deprotonated amides from the next two glycines, and the amide carbonyl of the second glycine. In addition, the NH of the indole is within hydrogen bonding distance to the oxygen of the axial water. Two additional intramolecular ordered water molecules are also shown.
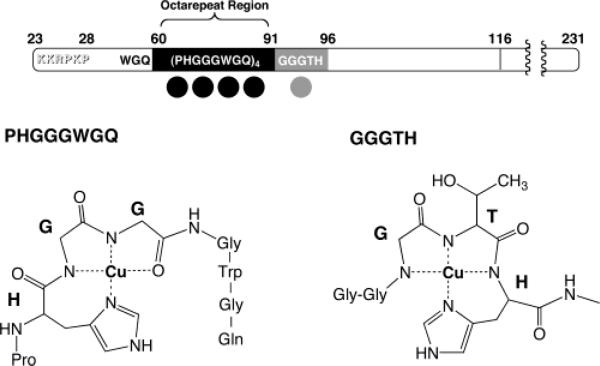
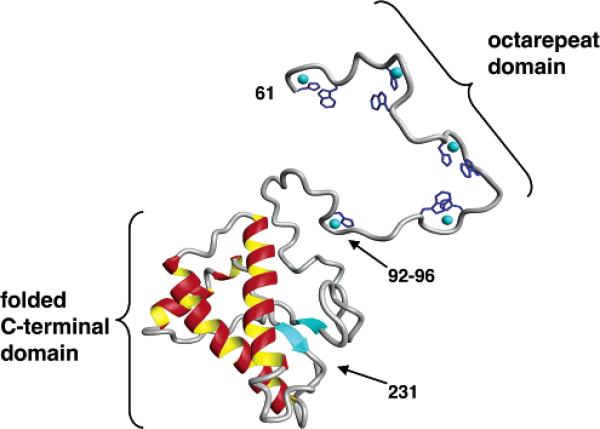
To establish physiological relevance, it is essential to demonstrate that findings garnered from N-terminal peptides apply to the full-length recombinant protein. Working in collaboration with Professors Giuseppe Legname and Stanley Prusiner of UCSF, we obtained EPR spectra of Cu2+-loaded Syrian hamster PrP(29-231) that were quite consistent with that obtained from octarepeat peptide constructs.32 However, stoichiometric measurements identified an additional copper binding site outside of the octarepeat domain. By systematic analysis using PrP-derived peptides, we showed that the GGGTH segment involving His96 constituted a newly identified copper site adjacent to the octarepeat domain. This histidine, as well as those in the octarepeat domain, were previously identified as Cu2+ coordination sites by Qin et al. using an elegant mass spectrometry protocol.33 A summary of the copper sites characterized by our studies is shown in Figure 4. In addition, a structural model of PrP(60–231) with all coppers present is shown in Figure 5. Here we used NMR structural coordinates for PrP(97–231) provided by Professor Thomas James of UCSF34 and coordinates for the copper sites obtained from our crystallographic and EPR studies. Relaxed conformations were assumed for polypeptide linker segments between the copper sites.32 Figure 5 constitutes the first structural model of PrP with its full complement of bound copper. Quite likely there is organization among the copper sites of which we are currently unaware. Further experiments are in progress to investigate this.
FIGURE 4.
Location and molecular features of the five main copper binding sites in PrP. Bond-line models of the equatorial Cu2+ coordination spheres for the two different types of binding are shown below. The structure for the single HGGGW–Cu2+ unit was determined from crystallographic and spectroscopic data. This structure is maintained for each HGGGW unit in the full octarepeat region. The coordination sphere depicted for GGGTH–Cu2+ is a model based on spectroscopic data.
FIGURE 5.
Three-dimensional rendering of PrP(61–231) with coppers included. Crystal structure coordinates were used for the octarepeat binding units HGGGW. The PrP(92–96) segment was based on the model shown in Figure 4. Intervening regions were built in a relaxed conformation.
Form, Affinity, and Function
PrP is remarkably selective for Cu2+. Screens against other divalent ions, such as Ca2+, Co2+, Mg2+, Mn2+, and Ni2+, failed to find high-affinity interactions. The structure in Figure 3 now explains this selectivity. As noted above, copper coordination is achieved through interaction with two deprotonated amides from the glycines following the histidine. This is a somewhat unusual copper binding site but by no means unprecedented. In most copper binding proteins, side chain functionalities such as histidine imidazoles or cysteine thiols make contact to the metal ion.35 However, elegant studies dating back to the 1960s showed that unstructured peptides containing histidine coordinate in a fashion similar to that now identified for PrP.36,37 The pKa of amide protons is typically 13–15, and consequently, the amide nitrogen is not ionized at pH 7. However, nitrogen and Cu2+ are well matched on the hard–soft scale of Lewis acid–base interactions. Thus, with the histidine imidazole anchoring the metal ion close to the polypeptide backbone, Cu2+ is uniquely able to displace a nearby amide proton at physiological pH.38 A similar copper binding motif is well-known in serum albumin, which carries the ion in an NH2-Xaa-Xaa-His sequence at its N-terminus.39
Copper binding affinity, as reflected by the dissociation constant Kd, has been a controversial issue. At the outset, several studies demonstrated cooperative binding with Kd's in the low micromolar to nanomolar range.12,40 Extracellular Cu2+ concentrations in the CNS are estimated at greater than 10 μM thus suggesting that the affinity of PrP for copper is tuned for this milieu. Moreover, neuronal depolarization often results in localized Cu2+ concentrations in excess of 100 μM.41 Nevertheless, other copper metalloproteins, such as copper-dependent SODs, typically exhibit much higher affinities with Kd's of approximately 10–14 M leading some to question the micromolar affinities found for PrP. For example, using a fluorescence assay in the presence of a competitive glycine buffer that binds Cu2+, Jackson et al. identified two copper sites with Kd values of 1 × 10–14 and 4 × 10–14 M.26 These results have been challenged by Garnett and Viles who used circular dichroism to show that low concentrations of glycine and l-histidine readily compete Cu2+ from PrP, again placing the affinity of PrP in the micromolar range.42
Regarding function, several lines of research suggest that PrP functions to protect neurons from reactive oxygen species (ROS) such as superoxide, peroxide, and redox-active metal ions. Thus, one possibility already noted above is that PrP is itself a superoxide dismutase.19 Despite the attractiveness of this proposal, the structures in Figures 3, 4, and 5 suggest this is unlikely. The redox cycle of SOD requires that the copper ion have two accessible oxidation states, typically Cu+ and Cu2+. Although Cu2+ readily coordinates to deprotonated amide bonds, this is not the case for Cu+.43 Voltammetric data obtained from the octarepeat bound with copper suggest dissociation of the complex as a consequence of one-electron reduction.22 Consequently, the binding sites identified in our studies will likely not coordinate Cu+ as required for SOD function. These findings do not rule out an SOD function for PrPC but do suggest that the copper binding domain would have to significantly restructure to support this activity.
Yet another possibility, considered by several labs, is that PrP endocytosis transports Cu2+ from the extracellular space to the cell interior.12,14,20 Here, the pH sensitivity of copper binding and copper-stimulated endocytosis are key. As noted above, the affinity of PrP for Cu2+ is well matched to exchangeable, extracellular concentrations of the metal ion. Consequently, PrP loads up with Cu2+ which, in turn, stimulates PrP endocytosis. Once in the low pH environment of the sealed endosome, PrP releases part of its copper complement. Such a mechanism would explain the results of studies demonstrating that PrP protects cells from copper toxicity. However, several labs have now examined potential copper transport function. In general these studies have failed to find direct evidence that copper transport depends on the level of PrP expression.17,44
Perhaps PrP protects against deleterious redox activity by sequestering excess Cu2+.17 Wild-type cells are more resistant to oxidative stress, relative to PrP knockouts, and concentrate copper at the plasma membrane.17 This suggests that PrPC functions as a copper buffer or part of a “cuprostat” that helps maintain neuron integrity in the copper-rich environment of the central nervous system. With regard to such function, interesting new results demonstrate that cultured cells infected with PrPSc are more susceptible to oxidative stress and exhibit a significant reduction in the ability to bind copper despite maintaining normal PrP levels.45 A buffering or regulatory function is fully consistent with the multiple copper site structure identified by our studies. Moreover, a loss of this function would certainly result in neuronal disturbances due to unregulated redox activity, perhaps contributing to neurodegeneration in the TSEs.
Vassallo and Herms have expanded on this scenario arguing that, beyond copper buffering, PrP may also contribute to synaptic integrity and neurophysiological function.41 PrPC is primarily localized at presynaptic membranes. They argue that this location, along with the protein's ability to modulate redox activity of copper or ROS, suggests that PrPC may function as a redox sensor in turn coupling copper levels to neuronal calcium flux and signaling. As yet, no mechanism by which PrP communicates with proteins involved with calcium signaling has been identified.
Copper and the TSEs
Treatment of the infectious prion with proteinase K cuts PrPSc at approximately residue 90 but without loss of infectivity, suggesting to some that the octarepeat domain, and hence copper, does not play a role in the TSEs. While copper may not be essential for PrP conversion and disease, it may well play a role in modulating kinetics and pathology. Indeed, the octarepeat domain and copper have been directly implicated in neurological disease. Humans with PrP genes coding for extra octarepeats are predisposed to CJD.46 In addition, PrP knockout mice possessing a transgene that produces PrP devoid of residues 32-93 retain their susceptibility to prion infection although with longer incubation times and altered pathology.47 Finally, recent studies show that addition of copper to PrPC converts the protein to a partially protease-resistant state, and this conversion requires only a single copper binding site.48 Our findings, as well as those of Qin et al.,33 demonstrate that copper binding takes place at His96, which is outside of the octarepeat domain. Several lines of evidence suggest that this site may possess higher affinity for Cu2+ than the octarepeat sites.32 This site is beyond the proteinase K cleavage site and thus suggests that copper may be found in the PrPSc particle, perhaps with a stabilizing role.
Metals, Neurological Disease, and Concluding Remarks
The connection to copper takes prion biology in a new direction with a focus on the normal function of PrPC. Perhaps by uncovering specific function, we will not only advance our knowledge of the molecular workings of the CNS but may also gain insight into loss of function associated with disease. Such insights may be critical for a thorough understanding of neurodegeneration in the TSEs. Moreover, the lessons learned with PrP may contribute to the understanding and treatment of other neurodegenerative disorders, especially if metal participation is ubiquitous. In fact, the connection between PrP and metal ions is not unique among neurodegenerative diseases. Brain lesions associated with Alzheimers disease are rich with metal ions,49,50 and metals such as iron, copper, zinc, and manganese facilitate amyloid fibril formation both in vivo and in vitro.51,52 Bush and colleagues have proposed that the Aβ peptide, one of the plaque components in Alzheimer's disease, may participate in metal ion transport at membranes.51 If, however, the peptide becomes hypermetalated, a cascade ensues in which redox activity stabilizes toxic peptide–metal aggregates ultimately leading to an increase in localized peroxide production, degradation of proteins and nucleic acids, and mounting cell stress. Brain copper and iron content increase with age, and this perhaps explains, in part, why neurodegenerative diseases are so much more prevalent among the elderly.53 Metal chelators are now under investigation for treatment of Alzheimer's disease. Tests with transgenic mice that overexpress the amyloid precursor protein (which releases Aβ upon proteolytic cleavage) show that clioquinol significantly reduces Aβ deposition.51 Human trials are currently underway.54
With regard to the prion protein, there are fundamental issues yet to be addressed. At the molecular level, we still do not understand the basis of cooperative Cu2+ binding in PrP, nor is it clear at this point whether other metal ions bind, although likely with weaker interactions. There is no sense as to how the octarepeat domain organizes in the fully Cu2+-loaded protein or how this organization changes when part of the copper complement is lost. Regarding PrPC function in the healthy CNS, sophisticated ideas are just now emerging. Participation in copper buffering and consequent regulation of ROS are certainly consistent with our structural findings. Finally, in stabilizing PrPSc or enhancing its cytotoxicity, the role of copper or other metals is not clear, but at least this author believes their involvement is likely to be very important.
Acknowledgments
Colin Burns, Eliah Aronoff-Spencer, and Gary Gerfen have been partners in this project since its inception. At every stage of the project, their energy, insights, and vision have been remarkable. I thank our wonderful collaborators, many of whom are mentioned above. Finally, I thank Professor Pradip Mascharak and Ms. Madhuri Chattopadhyay for helpful comments on the manuscript. The author's prion work is supported by NIH Grant GM 65790 and NSF Instrumentation Grant DBI 0217922.
Footnotes
On December 23, 2003, shortly after the acceptance of this Account, the U.S. confirmed its first case of mad cow disease. The fallout has been immediate–quarantine of suspected herds, international embargos against export of U.S. beef, and calls for new federal regulations requiring both widespread testing for the prion agent and elimination of nerve tissue and downer cattle from the human food chain.
References
- 1.Prusiner SB. Prion diseases and the BSE crisis. Science. 1997;278:245–251. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem. Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 4.Caughey B, Chesebro B. Prion Protein and the Transmissible Spongiform Encephalopathies. Trends Cell Biol. 1997;7:56–62. doi: 10.1016/S0962-8924(96)10054-4. [DOI] [PubMed] [Google Scholar]
- 5.Sailer A, Bueler H, Fischer M, Aguzzi A, Weissmann C. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 6.Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 7.Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 8.Hornshaw MP, McDermott JR, Candy JM. Copper Binding to the N-Terminal Tandem Repeat Regions of Mammalian and Avian Prion Protein. Biochem. Biophys. Res. Commun. 1995;207:621–629. doi: 10.1006/bbrc.1995.1233. [DOI] [PubMed] [Google Scholar]
- 9.Hornshaw MP, McDermott JR, Candy JM, Lakey JH. Copper binding to the N-terminal tandem repeat region of mammalian and avian prion protein: structural studies using synthetic peptides. Biochem. Biophys. Res. Commun. 1995;214:993–999. doi: 10.1006/bbrc.1995.2384. [DOI] [PubMed] [Google Scholar]
- 10.Wopfner F, Weidenhofer G, Schneider R, von Brunn A, Gilch S, Schwarz TF, Werner T, Schatzl HM. Analysis of 27 Mammalian and 9 Avian Prps Reveals High Conservation of Flexible Regions of the Prion Protein. J. Mol. Biol. 1999;289:1163–1178. doi: 10.1006/jmbi.1999.2831. [DOI] [PubMed] [Google Scholar]
- 11.Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, von Bohlen A, Schulz-Schaeffer W, Giese A, Westway D, Kretzschmar H. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 12.Whittal RM, Ball HL, Cohen FE, Burlingame AL, Prusiner SB, Baldwin MA. Copper binding to octarepeat peptides of the prion protein monitored by mass spectrometry. Protein Sci. 2000;9:332–343. doi: 10.1110/ps.9.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stöckel J, Safar J, Wallace AC, Cohen FE, Prusiner SB. Prion Protein Selectively Binds Copper(II) Ions. Biochemistry. 1998;37:7185–7193. doi: 10.1021/bi972827k. [DOI] [PubMed] [Google Scholar]
- 14.Pauly PC, Harris DA. Copper Stimulates Endocytosis of the Prion Protein. J. Biol. Chem. 1998;273:33107–33119. doi: 10.1074/jbc.273.50.33107. [DOI] [PubMed] [Google Scholar]
- 15.Sumudhu W, Perera WS, Hooper NM. Ablation of the metal ion-induced endocytosis of the prion protein by disease-associated mutation of the octarepeat region. Curr. Biol. 2001;11:519–523. doi: 10.1016/s0960-9822(01)00147-6. [DOI] [PubMed] [Google Scholar]
- 16.Brown DR, Schmidt B, Kretzschmar HA. Effects of copper on survival of prion protein knockout neurons and glia. J. Neurochem. 1998;70:1686–1693. doi: 10.1046/j.1471-4159.1998.70041686.x. [DOI] [PubMed] [Google Scholar]
- 17.Rachidi W, Vilette D, Guiraud P, Arlotto M, Riondel J, Laude H, Lehmann S, Favier A. Expression of Prion Protein Increases Cellular Copper Binding and Antioxidant Enzyme Activities but Not Copper Delivery. J. Biol. Chem. 2003;278:9064–9072. doi: 10.1074/jbc.M211830200. [DOI] [PubMed] [Google Scholar]
- 18.Klamt F, Dal-Pizzol F, Conte DA Frota ML, Jr., Walz R, Andrades ME, Gomes DA Silva E, Brentani RR, Izquierdo I, Moreira JCF. Imbalance of antioxidant defense in mice lacking cellular prion protein. Free Radical Biol. Med. 2001;30:1137–1144. doi: 10.1016/s0891-5849(01)00512-3. [DOI] [PubMed] [Google Scholar]
- 19.Brown DR, Wong B-S, Hafiz F, Clive C, Haswell SJ, Jones IM. Normal prion protein has an activity like that of superoxide dismutase. Biochem. J. 1999;344:1–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Burns CS, Aronoff-Spencer E, Dunham CM, Lario P, Avdievich NI, Antholine WE, Olmstead MM, Vrielink A, Gerfen GJ, Peisach J, Scott WG, Millhauser GL. Molecular features of the copper binding sites in the octarepeat domain of the prion protein. Biochemistry. 2002;41:3991–4001. doi: 10.1021/bi011922x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura T, Hori-i A, Mototani H, Takeuchi H. Raman Spectroscopic Study on the Copper(II) Binding Mode of Prion Octapeptide and Its pH Dependence. Biochemistry. 1999;38:11560–11569. doi: 10.1021/bi9909389. [DOI] [PubMed] [Google Scholar]
- 22.Bonomo RP, Impellizzeri G, Pappalardo G, Rizzarelli E, Tabbi G. Copper(II) binding modes in the prion octarepeat PHGGGWGQ: A spectroscopic and voltammetric study. Chem.–Eur. J. 2000;6:4195–4202. doi: 10.1002/1521-3765(20001117)6:22<4195::aid-chem4195>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Cereghetti GM, Scheweiger A, Glockshuber R, Van Doorslaer S. Electron paramagnetic resonance evidence for binding Cu2+ to the C-terminal domain of the murine prion protein. Biophys. J. 2001;81:516–525. doi: 10.1016/S0006-3495(01)75718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luczkowski M, Kozlowski H, Stawikowski M, Rolka K, Gaggelli E, Valensin D, Valensin G. Is the monomeric prion octapep-tide repeat PHGGGWGQ a specific ligand for Cu2+ ions? J. Chem. Soc., Dalton Trans. 2002:2269–2274. [Google Scholar]
- 25.Hasnain SS, Murphy LM, Strange RW, Grossmann JG, Clarke AR, Jackson GS, Collinge J. XAFS study of the high-affinity copper-binding site of human PrP91–231 and its low-resolution structure in solution. J. Mol. Biol. 2001;311:467–473. doi: 10.1006/jmbi.2001.4795. [DOI] [PubMed] [Google Scholar]
- 26.Jackson GS, Murray I, Hosszu LLP, Gibbs N, Waltho JP, Clarke AR, Collinge J. Location and properties of metal-binding sites on the human prion protein. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8531–8535. doi: 10.1073/pnas.151038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pushie MJ, Rauk A. Computational studies of Cu(II)[peptide] binding motifs: Cu[HGGG] and Cu[HG] as models for Cu(II) binding to the prion protein octarepeat region. J. Biol. Inorg. Chem. 2003;8:53–65. doi: 10.1007/s00775-002-0386-7. [DOI] [PubMed] [Google Scholar]
- 28.Van Doorslaer S, Cereghetti GM, Glockshuber R, Schweiger A. Unraveling the Cu2+ binding sites in the C-terminal domain of the murine prion protein: A pulse EPR and ENDOR study. J. Phys. Chem. B. 2001;105:1631–1639. [Google Scholar]
- 29.Peisach J, Blumberg WE. Structural Implications Derived from the Analysis of Electron Paramagnetic Resonance Spectra of Natural and Artificial Copper Proteins. Arch. Biochem. Biophys. 1974;165:691–708. doi: 10.1016/0003-9861(74)90298-7. [DOI] [PubMed] [Google Scholar]
- 30.Aronoff-Spencer E, Burns CS, Avdievich NI, Gerfen GJ, Peisach J, Antholine WE, Ball HL, Cohen FE, Prusiner SB, Millhauser GL. Identification of the Cu2+ binding sites in the N-terminal domain of the prion protein by EPR and CD spectroscopy. Biochemistry. 2000;39:13760–13771. doi: 10.1021/bi001472t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donne DG, Viles JH, Groth D, Mehlhorn I, James TL, Cohen FE, Prusiner SB, Wright PE, Dyson HJ. Structure of the recombinant full-length hamster prion protein PrP(29–231): the N-terminus is highly flexible. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13452–13456. doi: 10.1073/pnas.94.25.13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns CS, Aronoff-Spencer E, Legname G, Prusiner SB, Antholine WE, Gerfen GJ, Peisach J, Millhauser GL. Copper coordination in the full-length, recombinant prion protein. Biochemistry. 2003;42:6794–6803. doi: 10.1021/bi027138+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin K, Yang Y, Mastrangelo P, Westaway D. Mapping Cu(II) bindings sites in prion proteins by diethylpyrocarbonate modification and MALDI-TOF mass spectrometric “footprinting”. J. Biol. Chem. 2002;277:1981–1990. doi: 10.1074/jbc.M108744200. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Farr-Jones S, Ulyanov NB, Llinas M, Marqusee S, Groth D, Cohen FE, Prusiner SB, James TL. Solution structure of Syrian hamster prion protein rPrP(90–231). Biochemistry. 1999;38:5362–5377. doi: 10.1021/bi982878x. [DOI] [PubMed] [Google Scholar]
- 35.Lippard SJ, Berg JM. Principles of bioinorganic chemistry. University Science Books; Mill Valley, CA: 1994. [Google Scholar]
- 36.Freeman HC. In: The Biochemistry of Copper. Peisach J, Aisen P, Blumberg WE, editors. Academic Press; New York: 1966. [Google Scholar]
- 37.Bryce GF, Gurd FR. Visible spectra and optical rotatory properties of cupric ion complexes of L-histidine-containing peptides. J. Biol. Chem. 1966;241:122–129. [PubMed] [Google Scholar]
- 38.Sundberg RJ, Martin RB. Interactions of Histidine and Other Imidazole Derivatives with Transition Metal Ions in Chemical and Biological Systems. Chem. Rev. 1974;74:471–517. [Google Scholar]
- 39.Harford C, Sarkar B. Amino Terminal Cu(II)- and Ni(II)-Binding (ATCUN) Motif of Proteins and Peptides – Metal Binding, DNA Cleavage, and Other Properties. Acc. Chem. Res. 1997;30:123–130. [Google Scholar]
- 40.Kramer ML, Kratzin HD, Schmidt B, Romer A, Windl O, Liemann S, Hornemann S, Kretzschmar H. Prion protein binds copper within the physiological concentration range. J. Biol. Chem. 2001;276:16711–16719. doi: 10.1074/jbc.M006554200. [DOI] [PubMed] [Google Scholar]
- 41.Vassallo N, Herms J. Cellular prion protein function in copper homeostasis and redox signaling at the synapse. J. Neurochem. 2003;86:538–544. doi: 10.1046/j.1471-4159.2003.01882.x. [DOI] [PubMed] [Google Scholar]
- 42.Garnett AP, Viles JH. Copper Binding to the Octarepeats of the Prion Protein. Afffinity, Specificity, Folding and Cooperativity: Insights from Circular Dichroism. J. Biol. Chem. 2003;278:6795–6802. doi: 10.1074/jbc.M209280200. [DOI] [PubMed] [Google Scholar]
- 43.Kroneck PM, Vortisch V, Hemmerich P. Model studies on the coordination of copper in biological systems. The deprotonated peptide nitrogen as a potential binding site for copper(II). Eur. J. Biochem. 1980;109:603–612. doi: 10.1111/j.1432-1033.1980.tb04833.x. [DOI] [PubMed] [Google Scholar]
- 44.Waggoner DJ, Drisaldi B, Bartnikas TB, Casareno RLB, Prohaska JR, Gitlin JD, Harris DA. Brain copper content and cuproenzyme activity do not vary with prion protein expression level. J. Biol. Chem. 2000;275:7455–7458. doi: 10.1074/jbc.275.11.7455. [DOI] [PubMed] [Google Scholar]
- 45.Rachidi W, Mange A, Senator A, Guiraud P, Riondel J, Benboubetra M, Favier A, Lehmann S. Prion infection impairs copper binding of cultured cells. J. Biol. Chem. 2003;278:14595–14598. doi: 10.1074/jbc.C300092200. [DOI] [PubMed] [Google Scholar]
- 46.Goldfarb LG, Brown P, McCombie WR, Goldgaber D, Swergold GD, Wills PR, Cervenakova L, Baron H, Gibbs CJ, Jr., Gajdusek DC. Transmissible familial Creutzfeldt-Jakob disease associated with five, seven, and eight extra octapeptide coding repeats in the PRNP gene. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10926–10930. doi: 10.1073/pnas.88.23.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flechsig E, Shmerling D, Hegyi I, Raeber AJ, Fischer M, Cozzio A, von Mering C, Aguzzi A, Weissmann C. Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuron. 2000;27:399–408. doi: 10.1016/s0896-6273(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 48.Quaglio E, Chiesa R, Harris DA. Copper converts the cellular prion protein into a protease-resistant species that is distinct from the scrapie isoform. J. Biol. Chem. 2001;276:11432–11438. doi: 10.1074/jbc.M009666200. [DOI] [PubMed] [Google Scholar]
- 49.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 50.Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ. Histochemically reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer's diseased brains. Brain Res. 2000;852:274–278. doi: 10.1016/s0006-8993(99)02096-x. [DOI] [PubMed] [Google Scholar]
- 51.Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 52.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J. Biol. Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 53.Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of Alzheimer's disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002;277:44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- 54.Finefrock AE, Bush AI, Doraiswamy PM. Current Status of Metals as Therapeutic Targets in Alzheimer's Disease. J. Am. Geriatr. Soc. 2003;51:1143–1148. doi: 10.1046/j.1532-5415.2003.51368.x. [DOI] [PubMed] [Google Scholar]