Abstract
In this review, we explore different approaches for introducing bioactivity into poly(ethylene glycol) (PEG) hydrogels. Hydrogels are excellent scaffolding materials for repairing and regenerating a variety of tissues because they can provide a highly swollen three-dimensional (3D) environment similar to soft tissues. Synthetic hydrogels like PEG-based hydrogels have advantages over natural hydrogels, such as the ability for photopolymerization, adjustable mechanical properties, and easy control of scaffold architecture and chemical compositions. However, PEG hydrogels alone cannot provide an ideal environment to support cell adhesion and tissue formation due to their bio-inert nature. The natural extracellular matrix (ECM) has been an attractive model for the design and fabrication of bioactive scaffolds for tissue engineering. ECM-mimetic modification of PEG hydrogels has emerged as an important strategy to modulate specific cellular responses. To tether ECM-derived bioactive molecules (BMs) to PEG hydrogels, various strategies have been developed for the incorporation of key ECM biofunctions, such as specific cell adhesion, proteolytic degradation, and signal molecule-binding. A number of cell types have been immobilized on bioactive PEG hydrogels to provide fundamental knowledge of cell/scaffold interactions. This review addresses the recent progress in material designs and fabrication approaches leading to the development of bioactive hydrogels as tissue engineering scaffolds.
Keywords: Poly(ethylene glycol) (PEG), Hydrogel, Bioactive modification, Tissue engineering, Biomimetic scaffold, Extracellular matrix (ECM)
1. Introduction
Tissue engineering applies methods from engineering and life sciences to create artificial constructs to direct tissue regeneration [1]. Hydrogels have been studied intensively and used as tissue engineering scaffolds, because they can provide a highly swollen three-dimensional (3D) environment similar to soft tissues and allow diffusion of nutrients and cellular waste through the elastic networks [2,3]. They have been used to repair and assist regeneration of a variety of tissues, such as cartilage, bone and vasculature [4–7]. There are two major types of hydrogels, natural and synthetic hydrogels, according to their origin [3,8,9]. Natural hydrogels are made mainly from natural polymer-based materials, such as proteins (e.g., collagen, gelatin, and fibrin), and polysaccharides (e.g., alginate chitosan, hyaluronic acid, dextran). Synthetic hydrogels are made from synthetic polymers, such as poly(acrylic acid) (PAA), poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), polyacrylamide (PAAm), and polypeptides.
Natural hydrogels, such as collagen and fibrin, have been used as scaffolds because they possess many of critical biological functions like cell adhesion and biodegradation, which are lacking from synthetic polymers. However, the use of animal derived ECM proteins as scaffolds is often restricted due to concerns of potential immunogenic reactions and infection, as well as their relatively poor mechanical properties [10–12]. Synthetic hydrogels have emerged as an alternative choice for hydrogel scaffolds. Synthetic hydrogels have advantages over natural hydrogels, such as the ability for photopolymerization, adjustable mechanical properties, and convenient control of scaffold architecture and chemical compositions [8]. They can be tailored for specific applications with the incorporation of biofunctions, and their transport properties can also be customized by adjusting polymer chain lengths and density [2].
PEG has been an important type of hydrophilic polymers for biomedical applications, including surface modification, bioconjugation, drug delivery and tissue engineering because they have critical properties, such as good biocompatibility, non-immunogenity, and resistance to protein adsorption [13,14]. PEG has linear and branched (multiarm or star) structures (Fig. 1). The basic PEG structure is PEG diol with two hydroxyl end groups, which can be converted into other functional groups, such as methyloxyl, carboxyl, amine, thiol, azide, vinyl sulfone, azide, acetylene, and acrylate [15]. The two functional end groups can be the same (symmetric) or different (asymmetric), which are versatile for hydrogel formation or for conjugating with biomolecules. Three major cross-linking methods have been used to make PEG hydrogels, including radiation of linear or branched PEG polymers [15,16], free radical polymerization (FRP) of PEG acrylates [5–7,17,18], and specific chemical reactions, such as condensation [18], Michael-type addition [19,20], Click chemistry [21,22], native chemical ligation [23], and enzymatic reaction [24–26].
Fig. 1.
Structures of linear PEG and 4-arm-PEG with various functional end groups.
The most common approach to make PEG hydrogels is photopolymerization, which utilizes light to convert liquid PEG macromer solutions into solid hydrogels at physiological temperature and pH. This method is advantageous for fabricating hydrogel scaffolds in situ with spatial and temporal control and in a variety of 3D structures with encapsulation of cells and biological agents [27,28]. PEG acrylates are the major type of macromers used for photopolymerization, including PEG diacrylate (PEGDA), PEG dimethacrylate (PEGDMA), and multiarm PEG (n-PEG) acyrlate (n-PEG-Acr). PEG hydrogels are not naturally degradable, but can be altered to enhance degradation by incorporating degradable segments, such as polyester [29–31], poly(propylene fumarate) (PPF) [32,33], acetal [34] and disulfide [35]. A convenient selection of the hydrolytically degradable blocks is polyhydroxyacids, including poly(lactic acid) (PLA), and poly(glycolic acid) (PGA), and polycaprolactone (PCL). Triblock (ABA) polymers, PLA-PEG-PLA and PGA-PEG-PGA have been synthesized by ring opening polymerization, terminated with acrylates to generate PLA-PEG-PLA diacrylate and PGA-PEG-PGA diacrylate, respectively [29,36,37]. In addition, the thiol-acrylate reaction has been used to make hydrogels with enhanced degradation of the ester bonds linked to PEG chains [38–43].
PEG hydrogels are attractive scaffolds to provide 3D templates in aqueous environments for tissue regeneration; however, PEG hydrogels typically exhibit minimal or no intrinsic biological activity due to the nonadhesive nature of PEG chains [13]. It is noted that anchorage-dependent cells encapsulated in PEG hydrogels show low viability due to the bio-inert characteristic of PEG [42,43]. Inspired by nature, researchers have developed a variety of bioactively modified PEG hydrogels to mimic the natural extracellular matrix (ECM) [44–47]. Human tissues are built of different types of cells embedded within dynamic ECM hydrogels, which are composed of various proteins and glycans (polysaccharides) secreted by the cells. ECM components play a crucial instructive role in mediating cell functions, and possess critical biological functions like cell adhesion, proteolytic degradation and growth factor binding [48,49]. Thus, the natural ECM is an attractive model for design and fabrication of bioactive scaffolds for tissue engineering [45–49].
To tether ECM-derived bioactive molecules (BMs) to PEG hydrogels, various strategies have been developed to provide fundamental knowledge to understand cell/scaffold interactions [44,45]. A number of cell lines have been explored to immobilize on bioactive PEG hydrogels, including fibroblasts, chondrocytes, vascular smooth muscle cells (SMCs) and endothelial cells (ECs), osteoblasts, neural cells, and stem cells [46,47]. Much effort has been devoted to the control of ligand density and spatial distribution in PEG hydrogels to modulate specific cellular responses for tissue formation [34,37,50,53]. This review addresses the recent progress in material designs and fabrication approaches that are leading to the development of bioactive PEG hydrogels as tissue engineering scaffolds. As the fundamental biology of the cellular microenvironment is often the inspiration for material design, this review begins with a brief discussion of the structure and biofunctions of the natural ECM model for biomimetic modification, and then highlights the ECM-derived biomolecules that have been used to make various bioactive PEG hydrogels, followed by summarizing the current approaches for preparation of bioactive PEG hydrogels with the control of specific cues, such as cell adhesion, proteolytic degradation and growth factor-binding. Finally, brief conclusions are provided regarding bioactive PEG hydrogels and challenges in biomimetic scaffold modification.
2. ECM as a natural model for bioactive modification
The rapid increase in the understanding of matrix biology has provided opportunities to use the natural ECM as a model for designing biomimetic scaffolds. This section discusses the structure and biofunctions of the ECM and the general strategies for ECM-mimetic modification of PEG hydrogels.
2.1. ECM structure and components
The tissues of the human body contain significant extracellular space, into which ECM molecules are secreted by the cells to form a complex network (Fig. 2) [55,56]. The ECM provides mechanical support for tissues, organizes cells into specific tissues, and controls cell behavior. Generally, the natural ECM consists of two classes of biomacromolecules, proteins and glycans [55–57]. The ECM proteins include structural fibrous proteins (e.g., collagen, elastin and fibrin) and cell adhesive proteins (e.g., fibrolectin and laminin). Collagen, the most abundant protein in mammals, provides tensile strength to the ECM [58], while other proteins, such as elastin, give the ECM its elasticity [56]. Collagen comes in many different types [59]. Type I collagen is the most common fibrillar collagen found in skin, bone and tendons, and type II collagen possess a similar fibrillar structure which provides tensile strength to cartilage [60,61]. Type IV collagen is a network-forming collagen, which forms a meshwork, particularly in basal lamina [62]. Cells bind to the ECM mainly through adhesive proteins like laminin (LN) and fibronectin (FN). LN, which has a cross-shaped trimer structure containing α, β and γ chains [63–66], is the major adhesive protein in basal lamina with binding sites for cell membrane receptors and type IV collagen, heparan sulfate proteoglycan (HSPG) and entacin [67]. FN, evolutionarily related to fibrinogen [68–72], is another important adhesive protein in the ECM. FN is a V-shaped dimer with several binding domains for mediating the connection between the ECM and cell membrane [73], and binds a variety of proteins like collagen and fibrin, as well as cell-surface receptors like integrins and syndecan [73,74].
Fig. 2.
Model of complex 3D structure of extracellular matrix (ECM) and cell-ECM interactions.
ECM proteins are embedded in highly negatively charged, polysaccharide-rich, gelatinous ground substances (Fig. 2), called glycans, including glycosaminoglycans (GAGs) and proteoglycans (PGs) [55,56,75,76]. GAGs are linear polymers of repeated disaccharide derivative with two types, nonsulfated like hyaluronic acid (HA) and sulfated, such as chondroitin, dermatan, heparan and keratin sulfates [77]. Sulfated GAGs can assemble on serine-rich proteins to form proteoglycans (PGs), such as aggrecan and HSPG [77–79]. Both GAGs and PGs swell in the aqueous spaces between protein fibrils, taking compressive stresses, limiting tissue collapse under pressure. Glycans also allow tissues to diffuse nutrients and provide reservoir for signaling molecules [55].
2.2. Basic biofunctions of ECM
ECM components undergo self-assembly as well as cell-directed assembly to form complex 3D organized networks (Fig. 2] [80–83]. Cell receptors bind both soluble and tethered signaling cues from the ECM environment. In turn, these receptor-ligand interactions trigger complex cascades of intracellular enzymatic reactions that regulate gene and protein expression, and define the fate of a cell in a tissue [84]. Simultaneously, cells send out signals to actively construct and degrade their microenvironment. Thus, the ECM acts not only as a simple space filler and a mechanical scaffold for the cells, but also a bioactive and dynamic environment that mediates cellular functions [55,56]. Generally, the natural ECM has three basic biofunctions, including cell adhesion, proteolytic degradation and growth factor (GF)-binding.
Cell attachment to the ECM is an obvious prerequisite for a number of important cell-function processes, such as cell proliferation and cell migration [85–87]. The ECM provides cell-adhesive domains for binding cell surface receptors. There are various cell-surface receptors, such as integrins, selectins, CD44 and syndecan [88–91]. Integrins are the major family responsible for cell attachment to the ECM [92,93], which bind to specific domains present in ECM proteins such as FN, LN and collagen [94,95]. Through binding to these functional cell-binding domains, integrins play central roles in the tissue development, organization and maintenance, by providing anchorage and triggering signals that direct cell function, cell-cycle progression, and expression of differentiated phenotypes [91,96,97].
The proteolytic degradation of the natural ECM is an essential feature of a variety of biological processes, such as cell migration, tissue repair and remolding [98–100]. Most ECM proteins, including collagen, fibrin, FN and LN, have specific cleavage sites for degradation by enzymes, such as matrix metalloproteinases (MMPs), plasmin and elastase [101–105]. MMPs are zinc-dependent endopeptidases involved in the remodeling of the ECM and play important roles in morphogenesis, angiogenesis, arthritis, skin ulcer, tumor invasion and metastasis [106]. Five families of cell-secreted MMPs have been recognized, including collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs. These enzymes are composed of several domains including propeptide, catalytic, and hemopexin (except for matrilysin) domains and are involved in the degradation of collagens, proteoglycans, and various glycoproteins [107,108]. Among them, collagenases (MMP-1, MMP-8) are required by endothelial cells (ECs) for invasion and tubule formation in types I, II, and III collagen gels, and is present in the ECM at high levels during the inflammatory and early proliferative phases of wound repair. Gelatinases (MMP-2 and MMP-9) digest type IV and VII collagens, while stromelysins (MMP-3, MMP-10) degrade laminins of the basement membranes. MMPs are secreted as inactive zymogens and their activation is a prerequisite for function. Furthermore, posttranscriptional regulation of MMP activity is controlled by tissue inhibitors of metalloproteinases (TIMPs). MMPs and TIMPs play a crucial role in defining the cellular environment through regulated degradation and processing of ECM proteins [106–108].
During the natural tissue development, the binding of growth factors (GFs) to the ECM is a major mechanism regulating cellular activity [109]. GFs are a class of proteins that have either specific pro- or antiproliferative effects under different circumstances, and act to modulate cell functions (e.g. differentiation, migration and proliferation) and gene expression [110,111]. Naturally, the morphogenetic activities of endogenous GFs are exerted at the action site close to the site of cellular production within a given tissue compartment. Once sequestered into the surrounding ECM, GFs associated with proteoglycans, such as HSPG, syndecan and perlecan. An increasing number of GFs, including fibroblast growth factor (FGF), transforming growth factor (TGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), have been found to associate with ECM proteoglycans. These associations are important to stabilize the GF’s active conformation and protect it from immediate clearance. Liberation of factors by ECM-degrading enzymes (e.g., MMPs and plasmin) as well as heparanases, which remove the heparan sulfate, modulates the bioavailability of the GFs [112]. Once liberated, the turnover of GFs may occur rapidly within the range of minutes to a few hours [113]. Thus, the ECM plays a highly functionalized role in modulating the stability activity, release and spatial localization of GFs.
2.3. ECM-mimetic bioactive modification
The requirements of scaffolds for tissue engineering include biocompatibility, biodegradability, high porosity and no immunogenic reactions. To promote cellular functions, it is highly desirable that the scaffolds have cell specific adhesion and the ability to carry signaling biomolecules. Owing to their design flexibility, PEG hydrogels have been the primary choice of hydrogel materials for making porous scaffolds with similar characteristics to certain soft tissues such as cartilage. However, they are limited in providing an ideal environment for cells, because PEG is neither cell adhesive nor biodegradable.
Although hydrolytically labile segments like PLA and PGA have been incorporated in PEG hydrogels to enhance their biodegradability, this kind of degradation is not cell-mediated. To meet the diverse needs in tissue engineering, PEG hydrogels have been modified with the incorporation of bioactive molecules, such as cell adhesive peptides (CAPs), enzyme-sensitive peptides (ESPs) and growth factors, to mimic one or more ECM biofunctions, such as specific cell adhesion, enzyme-sensitive degradation and GF-binding (Fig. 3A).
Fig. 3.
Bioactive modification of PEG hydrogels (A) with bioactive molecules (BMs), such as cell-adhesive peptide (CAP), enzyme-sensitive peptide (ESP), and growth factor (GF), and major types of bioactive monomers from mono-, di- and multi-functionalization of BMs with various groups (B).
The functional cues presented by the ECM can be chemical in nature. Most ECM components such as collagen, elastin, FN, LN and PGs have bioactive domains for cell binding, proteolytic degradation and/or GF-binding. Usually, from these domains, short peptide sequences can be identified as motifs that are responsible for these biofunctions [54,114–123]. ECM-derived short peptides [50] as well as ECM-derived proteins [124–127] and proteoglycans [128–131] have been used to modify PEG hydrogels. Unlike the entire protein structure, which is subject to denaturation and degradation, short peptide sequences have the advantage of being relatively stable for modification, tunable for cell binding, and easy to be synthesized in a large scale [132]. The incorporation of specific peptide sequences has emerged as the major strategy for the bioactive modification of PEG hydrogels [133]. To date numerous bioactive peptide sequences derived from ECM proteins, such as FN, LN and collagen, have been incorporated into PEG hydrogels. To covalently tether ECM-derived biomolecules to the PEG hydrogel networks, mono-, di- or multivalent reactive groups, such as acrylate, amine, thiol, azide, maleimide and biotin/strepavidin, have been used to functionalize them for hydrogel formation (Fig. 3B).
3. Bioactive molecules for modification of PEG hydrogels
Biomimetic scaffolds usually mimic one or more biofunctions of the natural ECM by incorporation of different types of ECM-derived bioactive molecules (BMs) in the materials. This section classifies bioactive PEG hydrogels into four major groups, including cell-adhesive, enzyme-sensitive, growth factor-bearing (or binding) and specially biofunctionalized hydrogels. Based on this classification, biomolecules that have been used for bioactive modification of PEG hydrogels are summarized in this section.
3.1. Cell-adhesive PEG hydrogels
The major limitation of PEG hydrogels as scaffolds for tissue engineering is lack of cell specific adhesion. To overcome this limitation, a variety of ECM protein-derived cell adhesive peptides (CAPs) have been important targets for cell-adhesive modification of PEG hydrogels (Table 1). They are mainly derived from four ECM proteins, including FN (e.g., RGD, KQAGDV, REDV and PHSRN), LN (e.g., YIGSR, LGTIPG, IKVAV, PDGSR LRE, LRGDN and IKLLI), collagen (e.g., DGEA and GFOGER) and elastin (e.g., VAPG).The most commonly used CAP for cell-adhesive modification is RGD [133], the cell binding domain derived from FN, LN and collagen [121]. There are two forms of RGD peptides, including linear RGD and cyclic RGD (cRGD). The RGD sequence in the cell-binding domain of FN is exposed at the tip of a loop with a spatial constraint that results in increased affinity for cell binding [122]. Research has demonstrated that cRGD peptides have the advantage of increasing the affinity to integrin αvβ3 and enhance biological activity up to 240 times, in comparison with linear RGD analogues [157–160]. The incorporation of cRGD peptides into the PEGDA hydrogels can better mimic the native RGD loop structure and benefits the cell specific adhesion [148].
Table 1.
Cell-adhesive peptides (CAPs) that have been used for cell-adhesive modification of PEG hydrogels.
| CAP | Origin a | Cell receptor | Monomer type | Ref. |
|---|---|---|---|---|
| RGD | FN, LN, Collagen | Integrins | Monoacrylate | 7,134–146 |
| Diacrylate | 147,148 | |||
| Monothiol | 21,149–154 | |||
| Monoazide | 155 | |||
| Diazide | 155 | |||
| Monomaleimide | 23 | |||
| Mono-QAP b | 26 | |||
| KQAGDV | FN | Integrin | Monoacrylate | 138–141,145 |
| REDV | FN | Integrin α4β1 | Monoacrylate | 118 |
| PHSRN | FN | Integrin α5β1 | Monoacrylate | 142,143 |
| Monothiol | 150 | |||
| IKVAV | LN α1 | 110 kDa protein | Monoacrylate | 144,146 |
| Monothiol | 151 | |||
| Monobiotin | 124 | |||
| YIGSR | LN | 67 kDa protein | Monoacrylate | 144–146 |
| Monothiol | 150 | |||
| PDGSR | LN | Integrin | Monoacrylate | 144,146 |
| LRGDN | LN | Integrin | Monoacrylate | 146 |
| LRE | LN | Integrin | Monoacrylate | 144 |
| IKLLI | LN | Heparin | Monoacrylate | 144,146 |
| GFOGER | Collagen-I | Integrin α2β1 | Monoacrylate | 141 |
| VAPG | Elastin | 67 kDa protein | Monoacrylate | 139,140,145,156 |
FN, fibronectin; LN, laminin.
QAP, Gln acceptor peptide (NQEQVSPL).
3.2. Enzyme-sensitive PEG hydrogels
The degradation rate of scaffolds is one of most important considerations and it is highly desirable to ensure that the degradation rate matches with new tissue regeneration at the defect site. If the degradation is more rapid than the tissue regeneration, the scaffolds will lose their carrier function for cell growth; on the other hand, if the degradation is too slow compared with tissue regeneration, the scaffolds will impede tissue regeneration. PEG hydrogels from photopolymerization of PEGDA have ester bonds for potential hydrolysis; however, the hydrolytic degradation is relatively slow both in vitro and in vivo. Although the incorporation of polyester segments (e.g. PLA and PGA) has been used to enhance the hydrolytic degradation of PEG hydrogels, this hydrolytic degradation process is not responsive to cellular signals or cell-secreted enzymes. The best way to impart biodegradability is to exploit the proteolytic degradation mechanisms presented in the ECM with the incorporation of enzyme-sensitive peptide (ESP) sequences. Various ESPs have been used for enzyme-sensitive degradation of PEG hydrogels (Table 2). Peptides like collagen-derived GPQG↓IAGQ and peptide library-derived GPQG↓IWGQ, APG↓L and L↓GPA (↓ indicating the cleavage site) have been used to make MMP-sensitive PEG hydrogels, while fibrin-derived YK↓NRD and VR↓N have been used to make plasmin-sensitive PEG hydrogels. The elastase-sensitive peptides, such as AAPV↓RGGG and AAAAAAA also have been used for proteolytic modification of PEG hydrogels [138,172].
Table 2.
Enzyme-sensitive peptides (ESPs) that have been used for preparation of proteolytically degradable PEG hydrogels.
| ESPa | Origin | Sensitive enzyme | Monomer type | Ref. |
|---|---|---|---|---|
| GPQG↓IAGQ | Collagen-I | MMP-1 | Dithiol | 161 |
| GPQG↓IWGQ | Peptide library | MMP-1 | Diacrylate | 162 |
| Dithiol | 19,149,163–167 | |||
| GPQG↓ILGQ | Collagen-I | MMP-1 | Dialkyne | 152 |
| L↓GPA | Peptide library | MMP-1 | Diacrylate | 7,138,168–170 |
| APG↓L | Peptide library | MMP-1 | Diacrylate | 171 |
| YK↓NRD | Fibrinogen | Plasmin | Dithiol | 164,165 |
| VR↓N | Fibrinogen | Plasmin | Diacrylate | 171 |
| AAAAAAAAA | Peptide library | Elastase | Diacrylate | 138 |
| AAPV↓RGGG | Peptide library | Elastase | Monoacrylate | 172 |
| PEN↓FF | Aggrecan | MMP-13 | Monothiol | 153 |
↓ indicates the cleavage site.
The enzyme-sensitive designs also have been used to modulate cell adhesion to PEG hydrogels. The incorporation of enzyme-cleavable CAPs is expected to mimic the natural ECM that provides temporary cues for regulating of cellular responses and tissue development. PE↓NFF is one of the major peptide sequence at the MMP-13 cleavage site of aggrecan, a cartilage ECM component. Anseth et al, incorporated a cysteine-containing bifunctional peptide, CPENFFRGD into PEG hydrogels by thiol– acrylate photopolymerization. This peptide has the RGD motif for cell adhesion and the sequence of PENFF for MMP-13-sensitive cleavage [153]. The resulting hydrogels provide a platform that mimics the native upregulation and downregulation of cell adhesive proteins by the cell-secreted enzymes in the ECM for differentiating human mesenchymal stem cells (hMSCs).
3.3. Growth factor-bearing or binding PEG hydrogels
Growth factors (GFs) are polypeptides that transmit signals to modulate cellular activities. GFs initiate their action by binding to specific receptors on the surface of target cells, and need to bind to matrix molecules for activity and stabilization due to their short half-lives in free forms or in the circulation. Most GFs are involved in binding to proteoglycans, which also modulate their release from sulfated GAG chains, to guide cell functions and tissue formation [109,173–175]. Purified GFs are highly sensitive to proteolytic degradation in soluble forms. The easiest way to incorporate GFs into PEG hydrogels is to load them in hydrogels directly during hydrogel formation; however, this direct loading method typically shows a rapid burst release during the initial swelling phase [176–178]. Since the rate of protein release is generally diffusion-controlled through aqueous channels within the hydrogels, it is a great challenge for the direct loading method to control the growth factor release over a long time without burse release [180–182].
The dosage response of GFs like VEGF is highly sensitive for tissue formation. For example, low doses of VEGF result in increased vascular permeability in therapeutic vascularization and overdoses result in hemangioma formation and fatal vascular leakage. Most research demonstrates that sustained stimulation with an optimal level of VEGF is required for the formation of stable vasculature in vivo [183]. Thus, a variety of delivery systems (e.g., microparticles and nanoparticles) for GFs have been designed and fabricated from diverse types of synthetic and natural materials [184–189]. GFs have been incorporated into hydrogels during or after the fabrication by covalent and non-covalent means; the latter includes simple adsorption, electrostatic interaction, or complexation [188,189]. ECM components like proteins and glycans have functional domains for binding GFs and for modulating their release. To mimic the GF-binding function of the ECM, researchers have developed two major strategies to modify PEG hydrogels with binding of GFs, including covalent attachment and specific interaction (Table 3).
Table 3.
Bioactive molecules (BMs) that have been used for preparation of GF-bearing or binding PEG hydrogels.
| BM a | Monomer type | Method | Ref. |
|---|---|---|---|
| VEGF | Monothiol | Covalently attaching VEGF to hydrogels by Michael- type addition of VEGF-SH with n-PEG-VS. b |
190 |
| Mono-QAP | Covalently attaching VEGF to hydrogels using Factor XIIIa through enzymatic reaction of VEGF-QAP with n- PEG-KDP. c |
25 | |
| bFGF | Multiacrylate | Covalently attaching bFGF to hydrogels by copolymerization of acrylated bFGF with PEGDA. |
191 |
| EGF | Multiacrylate | Covalently attaching EGF to hydrogels by copolymerization of acrylated EGF with PEGDA. |
192 |
| TGF-β | Multiacrylate | Covalently attaching TGF-β to hydrogels by copolymerization of acrylated TGF-β with PEGDA. |
193 |
| BMP peptide a | Monoazide | Covalently incorporating KIPKASSVPTELSAISTLYL (corresponding to residues 73–92 of BMP2) into hydrogels by Click chemistry for induction of osteogenesis. |
194 |
| Heparin | Multicarboxyl | Forming hydrogels by carboxyl/amine conjugation | 195–197 |
| Multiacrylate | Forming hydrogels by copolymerization. | 198,199 | |
| Multimaleimide | Conjugating with PEG or star PEG and forming hydrogels by Michael addition, or by specific binding between heparin and GFs or HBPs. d |
200–203 | |
| Multithiol | Forming hydrogels by Michael addition All the above hydrogels were studied for binding bFGF |
204,205 | |
| CS | Multiacrylate | Forming hydrogels by copolymerization. | 206,207 |
| Multithiol | Forming PEG hydrogels with incorporation of HA by Michael addition for binding bFGF. |
205 | |
| HA | Multithiol | Forming PEG hydrogels with incorporation of heparin by Michael addition for binding bFGF. |
205 |
| Biotin | Monothiol | Incorporating thiol-modified biotion into PEG hydroges by thiol-acrylate photopolymerization, followed by specific binding streptavidin-modified bFGF. |
208 |
| KRTGQYKL | Monothiol | Incorporating thiol-containing peptide, CKRGGAYKL into PEG hydroges by thiol-acrylate photopolymerization for specific binding of bFGF. |
208 |
BMP, bone morphogenetic protein; CS, chondroitin sulfate; HA, hyaluronic acid.
VEGF-SH, recombinant VEGF with incorporation of a cysteine residue; n-PEG-VS, multiarm PEG vinyl sulfone.
QAP, Gln acceptor peptide (NQEQVSPL); KDP, Lys donor peptide (FKGG); VEGF-QAP, QAP-modified VEGF; n-PEG-KDP, KDP-capped multiarm PEG.
HBP, heparin-binding peptide, CGGRMKQLEDKVKKLLKKNYHLENEVARLKKLVG derived from the heparin-binding domain of human platelet factor 4.
Various functional groups have been used to modify GFs for covalent attachment, including thiol, Gln, acrylate, and azide. Hubbell et al., engineered recombinant VEGF with cysteine (VEGF-SH) for tethering to PEG networks by Michael-type addition with multiarm PEG vinyl sulfone (n-PEG-VS) [190]. They also developed recombinant VEGF with attachment of a native Gln acceptor peptide (QAP), NQEQVSPL (derived from the N-terminus of α2-plasmin inhibitor), which can be incorporated into hydrogels with the Lys donor peptide (KDP) (e.g., FKGG)-functionalized multiarm PEG by the enzymatic reaction using Factor XIIIa [25]. West et al., developed a copolymerization method for covalently tethering acrylated GFs to PEG networks [191–193]. GFs, including bFGF, EGF and TGFβ, were acrylated by conjugating with Acr-PEG-NHS, followed by photo-copolymerization with PEG macromers to form hydrogels. Their results show the covalently tethered GFs maintaining mitogenic activity and enhancing fibroblast proliferation and migration. In addition to attach the intact GFs on hydrogels, Jabbari and coworkers reported on the incorporation of bone morphogenetic protein (BMP)-derived peptide, KIPKASSVPTELSAISTLYL (corresponding to residues 73–92 of BMP-2) into PEG hydrogels by Click chemistry [194], in order to enhance the osteogenic differentiation of bone morrow stromal cells.
Apart from the covalent tethering of GFs and GF-derived peptides, the development of GF-binding hydrogels has emerged another important strategy for delivering GFs. This method has the advantage to maintain the biological bioactivity of GFs upon release, and overcome the potential damage to GFs that may be resulted from the method of covalent tethering. To mimic the GF binding mechanism of GAGs in the natural ECM, much efforts have been devoted to chemical modification of heparin [195–205], chondroitin sulfate (CS) [205–207] and hyaluronic acid (HA) [205] with various reactive groups, such as acrylate , thiol, or maleimide, followed by reacting with the functionalized derivatives of PEG or multiarm PEG to form GAG-bearing PEG hydrogels by carboxyl/amine conjugation [195–197], copolymerization [198,199,206,207], Michael addition [200,201,204,205], and specific interaction between heparin and GFs [202] or heparin-binding peptides (HBPs) [203](Table 3). Another method to make GF-binding PEG hydrogels is to develop affinity hydrogels. Anseth et al., used thiol-acrylate photopolymerization to incorporate thiol-containing biotin into PEG hydrogels for specific interaction with strepavidin-modified GFs like bFGF [208]. Also they used similar chemistry to make hydrogels with incorporated GF-binding peptide, KRTGQYK for binding of bFGF [208].
3.4. Specially biofunctionalized PEG hydrogels
In addition to the above three major bioactive PEG hydrogels, some special biofunctions have been incorporated in PEG hydrogels for specific biomedical applications. Currently, there are three types of specially biofunctionalized PEG hydrogels, including matrix-protein-binding, immune-isolating and NO-bearing hydrogels (Table 4).
Table 4.
Functionalized PEG hydrogels with specific bioactivities.
| Bioactive PEG hydrogel | BM a | Origin | Monomer type | Ref. |
|---|---|---|---|---|
| Matrix protein-binding | (POG)7Y | Collagen | Monoacrylate | 209 |
| KLER | Decrorin | Monothiol | 210 | |
| Immuno-isolating | MnTMPyP | N/A b | Tetra-acrylate | 211 |
| Anti-Fas | N/A | Monoacrylate | 212 | |
| YC*WSQYLC*Y | TNF receptor 1 | 213 | ||
| NO-bearing | K5/NO | N/A | Monoacrylate | 214,215 |
| Cys/NO | N/A | Monoacrylate | 214 | |
| DETA/NO | N/A | Monoacrylate | 214 |
POG, proline-hydroxyproline-glycine; MnTMPyP, Mn(III) tetrakis(1-Methyl-4-pyridyl)porphyrin pentachloride; * indicates disulfide form ; DETA, diethylenetriamine.
N/A, not applicable.
3.4.1. Matrix protein-binding PEG hydrogels
Cells can respond to extracellular signals not only through direct interaction between cell surface receptors and ECM components, but also through matrix protein deposition and organization. To enhance the matrix collagen binding ability of PEG hydrogels, Lee et al., incorporated collagen-mimetic proline-hydroxyproline-glycine (POG) peptide, (POG)7Y into PEGDA hydrogel to retain cell-secreted collagens and promote cell matrix production [209]. The peptide sequence of KLER is one of the major sites found in decrorin that plays a key role in matrix deposition by aiding in the fibril growth, extension, and ultimately organization of type II collagen in the cartilage matrix. Salinas and Anseth incorporated KLER into PEGDA hydrogels to promote cartilage-specific matrix deposition and organization [210]. KLER was found to bind type II collagen and stabilize its triple-helical structure, which leads to inhibiting collagenase degradation and regulating the influence of TGFβ on chondrocytes.
3.4.2. Immuno-isolating PEG hydrogels
Although the survival and function of cells encapsulated in PEG hydrogels can be controlled in most in vitro studies, these cells encounter additional challenges when they are transplanted in vivo. The host responds rapidly to the injury caused by the implantation procedure and triggers normal wound healing cascades with pro-inflammatory cytokines secreted by inflammatory cells. Semi-permeable PEG hydrogels can prevent direct contact between immune and inflammatory cells and the encapsulated cells; however, they cannot prevent the diffusion of small cytotoxic molecules, such as reactive oxygen species (ROS) and pro-inflammatory cytokines, e.g., tumor necrosis factor-α (TNFα, 17.4 KDa), and interleukin-1β (IL-1β, 17 KDa). Once these cytotoxic molecules penetrate into the hydrogel, they can trigger apoptotic pathways that lead to apoptosis or impaired cell function. To overcome this challenge, Anseth and co-workers synthesized a polymerizable superoxide dismutase (SOD)-mimetic macromer, tetraacrylate of Mn(III) tetrakis(1-Methyl-4-pyridyl)porphyrin pentachloride (MnTMPyP), and coplymerized it with PEGDA to form hydrogel networks that provide SOD-mimetic activity assigned to protect encapsulated cells from ROS-mediated damage [211]. They also immobilized cytokine-antagonizing antibody, anti-Fas MAb (binding to the Fas antigen of Jurkat T cells) in PEG hydrogels [212]; however, there are concerns in conjugating modified antibodies in PEG hydrogels, including the large size for conjugation, poor stability and immunogenicity. As an alternative, a short peptide of YC*WSQYLC*Y (* indicating disulfide form) derived from a critical TNFα recognition loop on TNF receptor 1, has been incorporated into PEG hydrogels to inhibit TNFα-mediated cell apoptosis by binding TNFα [213].
3.4.3. Nitric oxide-bearing PEG hydrogels
Nitric oxide (NO), a molecule produced by uninjured ECs, reduces platelet adhesion and SMC proliferation, while stimulating EC proliferation. West et al., reported on the synthesis of nucleophile-containing PEG hydrogels to complex and delivery NO [214,215]. Three kind nucleophiles, including K5 with 5 lysine residues, diethylenetriamine (DETA), cysteine (Cys), were chosen to conjugate with Acr-PEG-NHS and complex with NO, followed by copolymerizing with PEGDA (Mw 3400) to form NO-containing PEG hydrogels. NO was released from these hydrogels over periods ranging from hours to months, depending on the hydrogel formulation. The NO-releasing hydrogels inhibit SMC growth and platelet adhesion, which could provide localized and sustained production of NO to reduce thrombosis and restenosis following procedures such as balloon angioplasty [214].
4. Approaches for bioactive modification of PEG hydrogels
ECM-derived bioactive molecules (BMs), especially short peptides are major targets for bioactive modification of PEG hydrogels. To specifically direct cell adhesion and tissue formation, it is essential to develop suitable strategies to tether the BMs to the PEG hydrogel networks and tailor the hydrogel chemistry and composition with controlled biofunctions. This section reviews a variety of key approaches that have been developed for bioactive modification of PEG hydrogels.
4.1. Post-grafting
The approach of post-grafting is to make PEG hydrogels first, followed by grafting peptides (or proteins) on the hydrogel surface. The hydrogels prepared by photopolymerization of PEG macromers, like PEGDA, have no functional groups for further modification with peptides. To provide reactive sites on the hydrogel surface, acrylic acid has been copolymerized with PEGDA to make hydrogels with carboxyl groups, followed by conjugation with the amine groups of peptides like RGD [216] or proteins like collagen [217], as shown in Fig. 4A. The amount of acrylic acid in the PEG hydrogel network directly control the amount of incorporated RGD peptides. This post-grafting method also was used to attach other peptides, such as YIGSR and REDV on PEG hydrogel surfaces [203]. Furthermore, it was developed to modify PEG hydrogels with attachment of maleimide or thiol groups on the hydrogel surface, followed by attachment of peptides through Michael-type addition with the cysteine- or maleimide-containing peptides [23,151].
Fig. 4.
Fabrication of cell-adhesive PEG hydrogels (A) by copolymerization of PEGDA and acrylic acid, followed by post-grafting of cell-adhesive peptides (CAPs) on the hydrogel surface through the reaction between the N-terminal amino groups of CAPs and the carboxyl groups provided by acrylic acid from the hydrogel. Microfabrication of patterned cell-adhesive hydrogel surfaces (B) by microcontact printing (µCP) of biotinated CAPs (Biotin-CAPs) on streptavidin-bearing PEG hydrogels.
An important application of the post-grafting approach is to prepare patterned medical devices with biological components by microcontact printing (µCP) [218–221]. The surface grafting of biomolecules is crucial for soft lithography to tailor the chemical and structural properties of the desired surfaces to mediate cell attachment, proliferation and differentiation [222–226]. Hynd and coworkers applied streptavidin-biotin chemistry to make patterned PEG hydrogel surfaces to direct cell attachment and growth [124]. Streptavidin-functionalized PEG hydrogel surfaces were first made by photo-copolymerization of acrylated streptavidin with PEGDA. Subsequently, biotin-labeled LN, FN, or biotin-IKVAV were transferred by polydimethylsiloxane (PDMS) stamps to react with streptavidin on the hydrogel surface, which resulted in the formation of a patterned cell-adhesive hydrogel surface (Fig. 4B). LRM55 astroglioma cells selectively adhered to LN, FN or IKVAV-stamped regions of the hydrogels, and exhibited significant neurite extension after 72 h in vitro [124].
4.2. Free radical polymerization
Free radical polymerization (FRP), especially photopolymerization has been widely used to make PEG hydrogels from PEGDA macromers in the presence of photoinitiators. To incorporate bioactive molecules into PEG hydrogel networks, copolymerization of acrylated biomolecules has been an important strategy to make bioactive PEG hydrogels.
4.2.1. Copolymerization with peptide monoacrylates
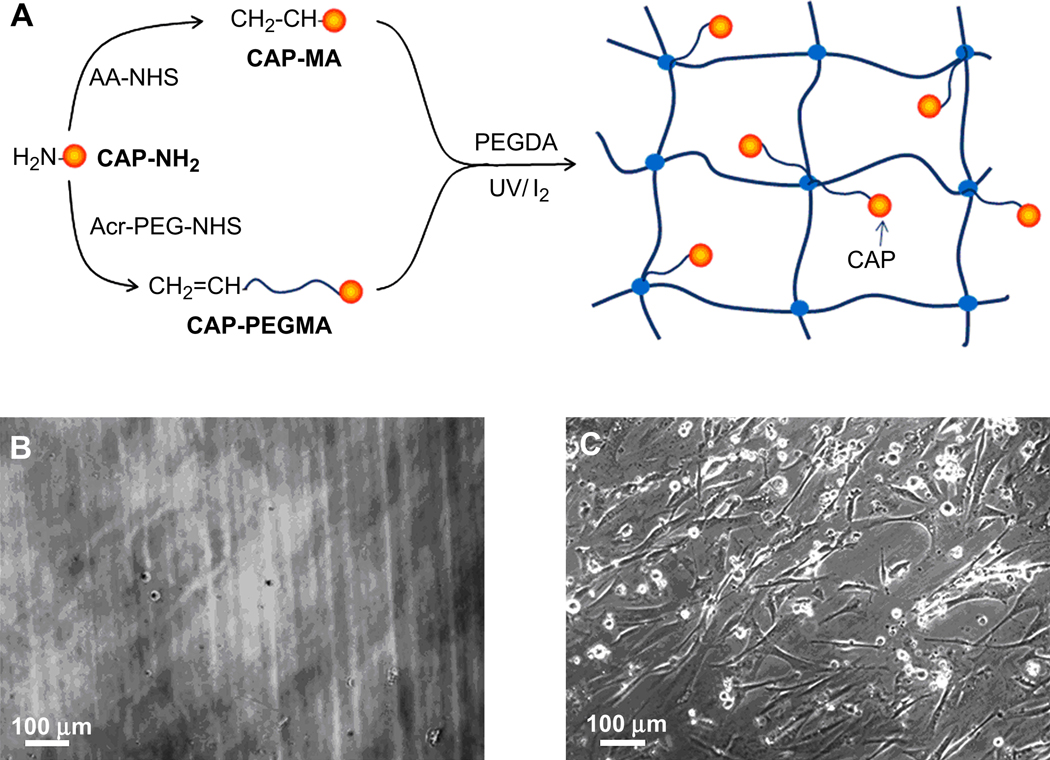
The post-grafting approach is limited to attaching peptides on the hydrogel surface. However, cell culturing in a 3D hydrogel network needs to incorporate bioactive peptides throughout the bulk hydrogel. Copolymerization of PEGDA with monoacrylates of cell-adhesive peptide (CAPs) has emerged as the major approach to make bulk cell-adhesive hydrogels (Fig. 5A). Hern and Hubbell reported on the synthesis of monoarcylated RGD with/without PEG spacers by functionalizing the N-terminal amines of RGD peptides with N-hydroxyl succinimide (NHS) ester of acrylic acid (AA-NHS) and acryloyl-PEG-NHS (Acr-PEG-NHS, Mw 3400) to produce mono-acrylamidoyl RGD (RGD-MA) and RGD-PEG monoacrylate (RGD-PEGMA), respectively [134]. Subsequently, RGD-MA or RGD-PEGMA monomers were copolymerized with PEGDA upon photopolymerization to create cell-adhesive hydrogels (Fig. 5A). The modified PEG hydrogels with various RGD densities were studied in vitro for their ability to promote the spreading of human foreskin fibroblasts over 24 h. The PEG spacer (Mw 3400) can enable the immobilized peptide to move flexibly in the biological environment, which is required to permit cell spreading to be mediated specifically, while PEGDA hydrogels with RGD-MA without a PEG spacer mediated cell spreading nonspecifically.
Fig. 5.
Synthesis of cell adhesive peptide (CAP) monoacrylamide (CAP-MA) and CAP-containing PEG monoacrylate (CAP-PEGMA) for preparation of cell-adhesive PEG hydrogels (A). Phase contrast images of human artery SMCs 6 h after seeding on 10% (w/v) PEGDA (Mw 6000) hydrogels (B) and on 10% (w/v) PEGDA (Mw 6000) hydrogels with incorporation of 0.5% (w/v) RGD-PEGMA (PEG Mw 3400; RGD sequence, GRGDSP, density ∼ 1 mM) (C).
The copolymerization method with peptide monoacrylates presents a versatile means for cell-adhesive modification of PEG hydrogels. For example, 10% (w/v) PEGDA (Mw 6000) hydrogels showed minimum cell adhesion and spreading 6 h after seeding human artery SMCs seeding on the hydrogel surface (Fig. 5B), while 10% (w/v) PEGDA hydrogels with incorporation of 0.5% (w/v) RGD-PEGDA (PEG Mw 3400; RGD sequence, GRGDSP, density ∼ 1.0 mM) exhibited significantly higher cell adhesion and spreading at the same time point (Fig. 5C). This method for incorporating cell adhesion into PEG hydrogels has been studied extensively with various other CAPs, such as KQAGDV, YIGSR, REDV, VAPG and IKVAV (Table 1) [100–111,115, 227]. Various cell lines have been explored to immobilize on bioactive PEG hydrogels, including fibroblasts, chondrocytes, vascular endothelial cells (ECs), osteoblasts, neural cells, and stem cells [43,44].
4.2.2. Copolymerization with peptide diacrylates
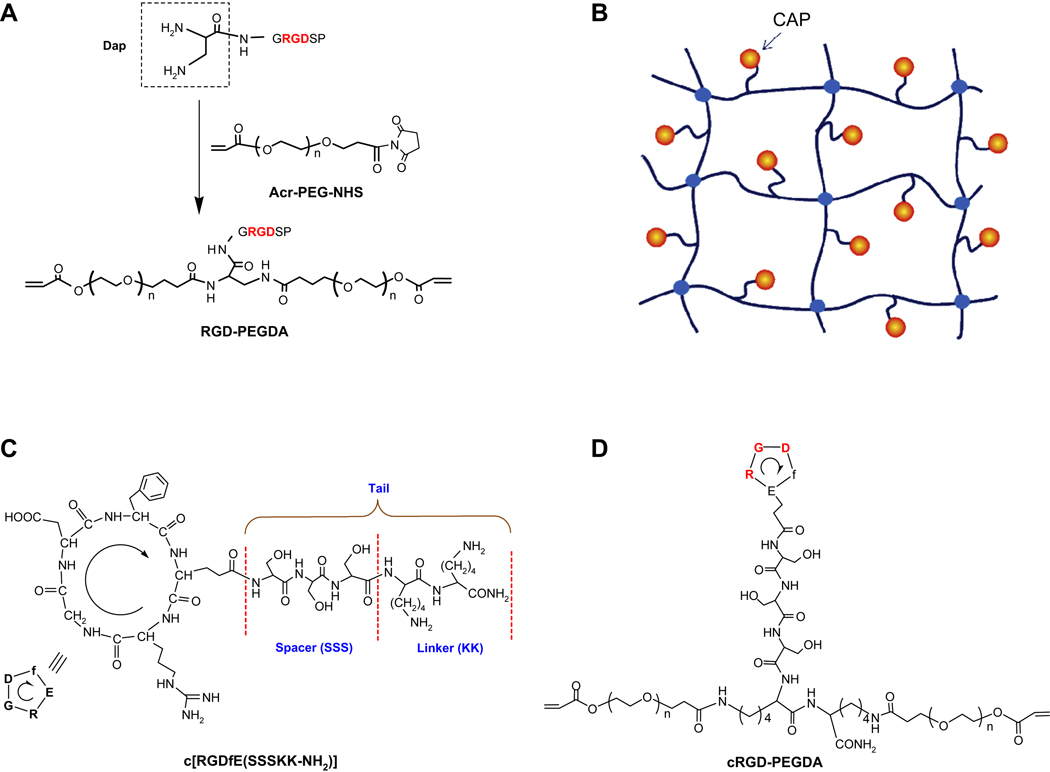
Peptide monoacrylates like RGD-PEGMA can copolymerize with PEGDA to create cell-adhesive PEG hydrogels, but the distribution of RGD peptides in hydrogels is random (Fig. 5A), and the extent of peptide incorporation in hydrogels is limited due to the monoacrylation of peptide affecting hydrogel formation and its mechanical properties. Since ligand presentation in scaffolds plays an important role in controlling cell behavior, Zhu et al., developed a strategy to create a peptide-containing PEGDA macromer like RGD-PEGDA with RGD attached with two PEG monoacrylates, which has a similar structure to PEGDA with two C=C double bonds for polymerization (Fig. 6A) [147]. This approach has the advantage of controlling the spatial organization of peptide ligands in hydrogels (Fig. 6B) and eliminating the effort of peptide incorporation on hydrogel mechanical properties. RGD-PEGDA was synthesized by conjugation of diaminopropionic acid (Dap)-capped GRGDSP peptides with Acr-PEG-NHS (Mw 3400). To mimic the RGD loop structure, Zhu et al., synthesized cyclic RGDfE peptide (Fig. 6C), c[RGDfE(SSSKK-NH2)] with a hydrophilic tail consisting of a spacer of three serine residues (SSS) and a linker of two lysine residues (KK) [148], which can be conjugated with Acr-PEG-NHS to form cRGD-PEGDA (Fig. 6D) [148,228]. Both RGD-PEGDA and cRGD-PEGDA can be photopolymerized to form cell-adhesive hydrogels with well-controlled spatial organization of peptide ligands. The results show that cRGD-PEGDA hydrogels facilitate EC adhesion and spreading on the hydrogel surfaces, and exhibit significantly higher EC population in comparison with linear RGD-modified hydrogels at low peptide incorporation [148].
Fig. 6.
Synthesis of RGD-containing PEGDA (RGD-PEGDA) (A) by conjugating diaminopropionic acid (Dap)-capped GRGDSP with Acr-PEG-NHS. Model of cell-adhesive PEG hydrogels (B) from photopolymerization of cell adhesive peptide (CAP)-containing PEGDA (CAP-PEGDA) with controlled spatial organization of CAPs. Structures of cyclic RGD (cRGD), c[(RGDfE)SSSKK(NH2)] (C) with a hydrophilic tail, consisting a spacer of three serine residues (SSS) and a linker of two lysine residues (KK), and cRGD-containing PEGDA (cRGD-PEGDA) (D).
Another important type of peptide-modified PEG diacrylates is enzyme-sensitive peptide (ESP)-containing PEGDA (ESP-PEGDA) (Fig. 7). The structure of ESP-PEGDA is different from that of CAP-PEGDA. ESP usually have two reactive groups on both ends for conjugation with Acr-PEG-NHS to create ESP-PEGDA with ESP inserted between two PEG monoacrylate (PEGMA) chains, while CAP-PEGDA was synthesized by a CAP with two amines on one end for attachment as a pendant on the PEGDA chain. ESP-PEGDA has the ability for photopolymerization to form hydrogels, which mimic the proteolytic degradation of the natural ECM by specific enzymes, e.g., plasimin, elastase and MMPs. A variety of enzyme-sensitive peptide (ESP) sequences (Table 2), such as GPQG↓IWGQ (↓ indicating the cleavage site) [162], YK↓NRD [164,165], L↓GPA [7,138,168–170], APG↓L [171], VR↓N [171] and AAAAAAAAA [138], have been used for enzyme-sensitive modification of PEG hydrogels. Also, ESP-PEGDA can be used to copolymerize cell-adhesive PEG macromers like RGD-PEGMA to make bioactive PEG hydrogels with dual biofucntions, i.e., enzyme-sensitive degradation and specific cell adhesion [168–170].
Fig. 7.
Synthesis of enzyme-sensitive peptide (ESP)-containing PEGDA (ESP-PEGDA) by conjugating Acr-PEG-NHS with ESP diamine (ESP-2NH2), and preparation of proteolytically degradable PEG hydrogels from photopolymerization of ESP-PEGDA.
4.2.3. Thiol-acrylate photopolymerization
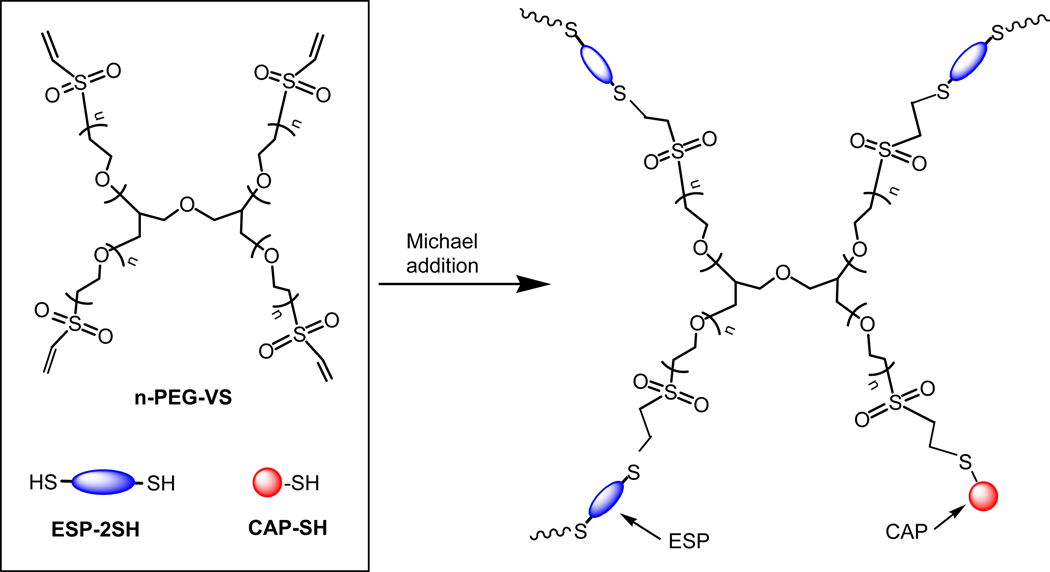
Copolymerization of acrylated bioactive peptides has been a major method to incorporate bioactive peptides in PEG hydrogels, but this method needs one more step to synthesize acrylated peptides off resin using Acr-PEG-NHS. It will be beneficial to develop a method to tether peptides into hydrogels directly. Since thiol-containing compounds have been used as chain transfer reagents to control the molecular weight of polymers by free radical polymerization (FRP), it is expected that cysteine-containing peptides can play the same role as chain transfer reagents in the FRP of PEGDA, which leads to the incorporation of cysteine-containing peptides into the PEG hydrogel network [229–232]. Based on this principle, an alternative approach, called thiol-acrylate photopolymerization has been developed to bioactively modify PEG hydrogels (Fig. 8) [21,152,153,233]. Anseth and co-workers synthesized thiol-bearing RGD peptide in the form of CGRGDSG, which was photopolymerized with PEGDA. The results show that approximate 95% of the CGRGDSG peptide was incorporated after 10 min reaction with incorporation of cysteine-containing peptides with appropriately functionalized PEG or multiarm PEG macromers (e.g., acrylate, maleimide and vinyl sulfone) [234–238]. The reaction proceeds via a stepwise growth mechanism [239,240]. Hubbell and coworkers synthesized multiarm PEG vinyl sulfone (n-PEG-VS) for Michael-type addition of SH-containing RGD peptide (CRGDSP), and dithiol-bearing ESP (ESP-2SH), CRD-GPQG↓IWGQ-DRC (↓ indicating the cleavage site) (Fig. 9) [154,161,163,241]. The PEG-based network can be readily formed under physiological conditions in direct contact with tissues, cells and biological molecules. The resultant hydrogel mimics the cell-adhesive and biodegradable properties of the natural ECM, and has utility as tissue engineering scaffolds and as delivery matrixes for sensitive biomolecules, such as recombinant human bone morphogenetic protein-2 (rhBMP-2). Primary human fibroblasts migrated within the bioactive hydrogels by integrin- and MMP-dependent mechanisms [241].
Fig. 8.
Preparation of cell-adhesive PEG hydrogels by thiol-acrylate photopolymerization of PEGDA with monothiol-containing cell adhesive peptide (CAP-SH).
Fig. 9.
Preparation of cell-adhesive PEG hydrogels by Michael addition of 4-arm-PEG vinyl sulfone (4-PEG-VS) with monothiol-containing cell-adhesive peptide (CAP-SH) and dithiol-containing enzyme-sensitive peptide (ESP-2SH).
4.4. Click chemistry
Recently, Click chemistry has been employed to fabricate PEG hydrogels, which exhibit enhanced swelling capacities and improved mechanical properties [21,22,152]. Yang et al., reported on synthesis of cell-adhesive PEG hydrogels by Click chemistry between 4-arm PEG acetylene (4-PEG-Ace) and RGD diazide (RGD-2N3) (Fig. 10) [145]. RGD-2N3 was prepared by solid phase peptide synthesis (SPPS), while 4-PEG-Ace was synthesized by acetylenation of tetrahydroxy terminated 4-arm PEG. PEG networks were formed by the copper (I)-catalyzed formation of 1,2,3-triazoles between RGD-2N3 and 4-PEG-Ace, which has demonstrated complete specificity under physiological conditions. The gelation time ranged from 2 to 30 min, depending on temperature, catalyst and precursor concentration. Cell studies using primary human dermal fibroblasts show that the PEG hydrogels with the incorporation of RGD peptides achieved significantly improved cell attachment and greater cell proliferation, compared with the control hydrogels without RGD peptides.
Fig. 10.
Preparation of cell-adhesive PEG hydrogels by Click chemistry between 4-arm-PEG acetylene (4-PEG-Ace) and cell adhesive peptide diazide (CAP-2N3) in the presence of copper (II) sulfate and sodium ascorbate.
4.5. Enzymatic reaction
Enzymatic reactions have become increasingly attractive targets for the fabrication of advanced materials. Recent development efforts in this area have produced a number of remarkable examples for such smart enzyme responsive materials [24,242–244]. Molecular building blocks have been designed to form hydrogels by the catalytic action of crosslinking enzymes. Most enzymes catalyze chemical reactions under mild conditions, such as at low temperature and neutral pH, and in buffered aqueous solutions. Enzymes also can be exceptionally selective for their substrates, allowing for sophisticated, biologically inspired hydrogel designs without the complication of side reactions and cellular toxicity. The enzymatic formation of hydrogels is versatile to functionalize hydrogels with the incorporation of necessary biomolecular signals to elicit a desired cellular response.
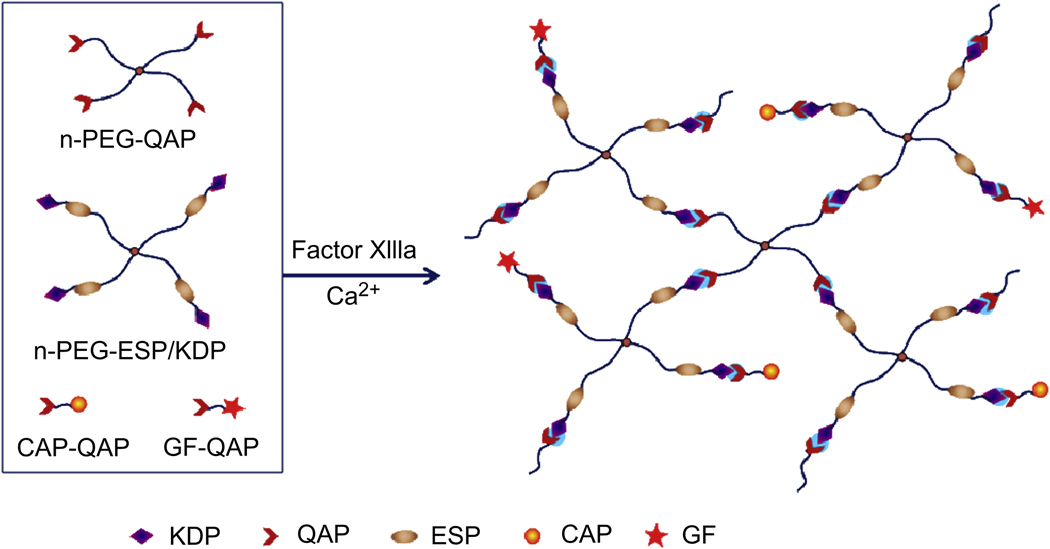
Hubbell et al., developed a method using coagulation transglutaminase Factor XIIIa to form bioactive hydrogels by the enzymatic reaction between Gln- and Lys-containing substrates [25,26]. A α2-plasmin inhibitor-derived peptide, NQEQVSPL was used as the Gln acceptor peptide (QAP), while FKGG was used as the Lys donor peptide (KDP) conjugated with an ESP, GPQG↓IWGQ. Both QAP and KDP-ESP were attached with a cysteine residue for coupling with multiarm PEG vinyl sulfone (n-PEG-VS) by Michael-type addition, producing n-PEG-QAP and n-PEG-ESP/KDP, respectively (Fig. 11). Factor XIIIa was used for enzymatic crosslinking n-PEG-QAP and n-PEG-ESP/KDP to form MMP-sensitive hydrogels. To incorporate CAPs and GFs, RGD and VEGF were attached with QAP to generate RGD-QAP and VEGF-QAP, respectively. Both RGD-QAP and VEGF-QAP can be incorporated into the enzyme-sensitive PEG networks by the same enzymatic reaction using Factor XIIIa. VEGF was quantitatively incorporated and released upon cell-derived proteolytic degradation of the gels, and primary stromal cells invaded and proteolytically remodeled these networks both in vitro and in vivo [25].
Fig. 11.
Preparation of bioactive PEG hydrogels by enzymatic reaction between Lys donor peptide (KDP)-capped multiarm PEG (n-PEG-KDP) and Gln acceptor peptide (QAP)-capped enzyme-sensitive peptide (ESP)-containing multiarm PEG (n-PEG-ESP/QAP) with incorporation of QAP-functionalized cell adhesive peptide (CAP-QAP) and/or QAP-functionalized growth factor (GF-QAP) in the presence of Factor XIIIa and calcium ion.
4.6. Photoregulation of hydrogel bioactivity
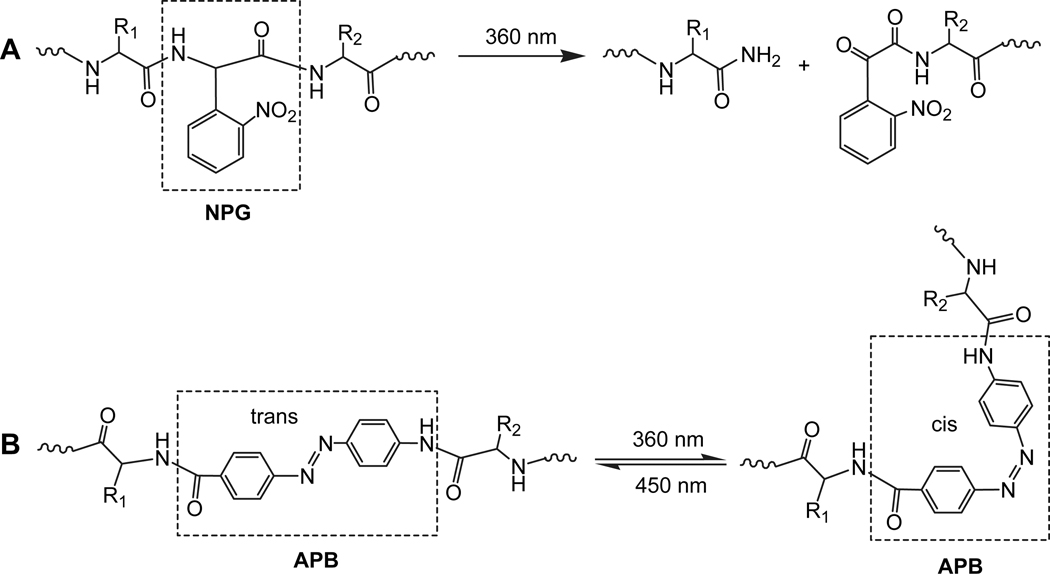
Natural proteolytic activities are involved in a wide array of biological processes, including zymogen activation, protein degradation, and the removal of signal peptides [245]. Photochemical regulation of such a cleavable process is used increasingly to probe cellular events. The ability to initiate protein or peptide cleavage photochemically would allow spatial and temporal control over the activity, lifetime and localization of bioactive motifs both in vitro and in living cells [246,247]. Photoactive molecules have attracted much attention since they can be used with high space and time selectivity. For example, photocleavable molecules like 2-(nitrophenyl)glycine (NPG) have been introduced into proteins to study the folding mechanism [248–251], whereby the native protein structure is produced instantly by irradiation of the modified protein with light (Fig. 12A). A photoisomerizable intramolecular crosslinker, 4-[(4-amino)phenylazo]benzoic acid (APB) has been used to control optically not only the peptide conformation, but also its complex formation with biomolecules (Fig. 12B) [252–256]. The desired gel property for altering cell function or fabricating a device is externally triggered and directed with irradiation by photolytic cleavage and removal of the macromolecules that compose the gel.
Fig. 12.
Photodegradable peptide backbone (A) containing the residue of 2-(nitrophenyl)glycine (NPG), and photoisomerizable peptide backbone (B) containing the residue of 4-[(4-amino)phenylazo]benzoic acid (APB).
The ability to manipulate the hydrogel bioactivity is an essential feature in the development of novel scaffolds for tissue engineering. To date there are few examples of bioactive PEG hydrogels with photoresponsive bioactivities. Anseth and coworkers developed photodegradable PEG hydrogels for the dynamic tuning of gel physical and chemical properties and for the control of cell adhesion in real time [257]. Photodegradable acrylate (PDA) with a carboxyl group was synthesized by acrylation of the photodegradable functionality, a nitrobenzyl ether-derived moiety. Photodegradable PEG diacrylate (PD-PEGDA) was synthesized by conjugation of PEG diamine with the carboxyl group of PDA, while photodegradable RGD monoacrylate (PD-RGDMA) was synthesized by the same chemistry by the reaction of N-terminal amine of RGD with the carboxyl group of PDA [257]. PD-RGDMA and PD-PEGDA are capable of redox-initiated polymerizing to produce photolytically degradable hydrogels (Fig. 13).
Fig. 13.
Preparation of photocleavable cell-adhesive PEG hydrogels by copolymerization of PEGDA with photocleavable cell-adhesive peptide monoacrylate (PD-CAPMA), and photodegradable PEG hydrogels by copolymerization of photocleaveable PEGDA (PD-PEGDA) with PEG monoacrylate (PEGMA).
This type of hydrogels is capable of polymerizing in the presence of cells like hMSCs, and the gel physical or chemical properties and cell morphology can be manipulated by light irradiation and degradation of these PD-PEGDA hydrogels at any time in culture [257]. To tune the cell adhesion of hydrogels temporally and spatially with light, PD-RGDMA was copolymerized with PEGDA to make hydrogels with photocleavable cell adhesion. Upon photolytic removal of RGD peptides from the photolabile tether gels on day 10, hMSC viability was unaffected; however, by day 21, a four-fold statistical increase in the production of GAGs occurred relative to the persistently presented RGD or PEG-only hydrogels. Cells persistently presented with RGD expressed integrins, whereas most cells with photocleavable RGD ceased integrin expression by day 21, indicating that the cells have locally sensed and responded to the chemical change in their environment [257].
5. Conclusions
The natural ECM is an attractive model for bioactive modification of PEG hydrogels. Short peptide sequences derived from ECM proteins, such as fibronectin, laminin and collagen, have been the major targets for fabricating biomimetic hydrogels. To tether ECM-derived bioactive molecules to PEG hydrogels, various strategies have been developed to provide fundamental knowledge to understand cell/scaffold interactions through specific cell adhesion, proteolytic degradation, and signal molecule conjugation. Much effort has been devoted to the control of ligand density and spatial distribution in PEG hydrogels to modulate specific cellular responses. Despite the recent advances toward the development of bioactive PEG hydrogels, several challenges still remain including precisely spatial and temporal control of scaffold architecture and biofunctions, cell-mediated delivery of GFs without burst release, and design of a dynamic cellular microenvironment for tissue engineering.
Acknowledgements
This work was supported in part by the National Institutes of Health (Grants 1RC1EB010795, 1R01HL087843 and 5R01EB002067). The author gratefully thanks Dr. Roger Marchant for valuable revisions and Lin Lin for some constructive discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliver Rev. 2002;43:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 4.Temenoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2002;21:2405–2412. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 5.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci U S A. 1999;96:3104–3107. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton AN, Zhu J, Marchant RE, West JL, Yoo JU, Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13:2549–2560. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 7.Hahn MS, McHale MK, Wang E, Schmedlen RH, West JL. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann Biomed Eng. 2007;35:190–200. doi: 10.1007/s10439-006-9099-3. [DOI] [PubMed] [Google Scholar]
- 8.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 9.Traphagen S, Yelick PC. Reclaiming a natural beauty: whole-organ engineering with natural extracelluar materials. Regen Med. 2009;4:747–758. doi: 10.2217/rme.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nerem RM, Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225–243. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 11.Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM. Crosslinking of collagen gels by, transglutaminase. J Biomed Mater Res A. 2004;68:756–762. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Hunt JA. Biomimetic materials processing for tissue-engineering processes. J Mater Chem. 2007;17:3974–3979. [Google Scholar]
- 13.Lee JH, Lee HB, Andrade JD. Blood compatibility of polyethylene oxide surfaces. Prog Polym Sci. 1995;20:1043–1079. [Google Scholar]
- 14.Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res. 2000;51:343–351. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62:81–87. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 16.Keys KB, Andreopoulos FM, Peppas NA. Poly(ethylene glycol) star polymer hydrogels. Macromolecules. 1998;31:8149–8156. [Google Scholar]
- 17.Beamish JA, Zhu J, Kottke-Marchant K, Marchant RE. The effects of monoacrylate poly(ethylene glycol) on the properties of poly(ethylene glycol) diacrylate hydrogels used for tissue engineering. J Biomed Mater Res A. 2010;92:441–450. doi: 10.1002/jbm.a.32353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbell JA. Synthetic biodegradable polymers for tissue engineering and drug delivery. Curr Opin Solid ST M. 1998;3:246–251. [Google Scholar]
- 19.Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6:290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 20.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 21.Polizzotti BD, Fairbanks BD, Anseth KS. Three-dimensional biochemical patterning of Click-based composite hydrogels via thiol-ene photopolymerization. Biomacromolecules. 2008;9:1084–1087. doi: 10.1021/bm7012636. [DOI] [PubMed] [Google Scholar]
- 22.Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason A, et al. Synthesis of well-defined hydrogel networks using Click chemistry. Chem Commun. 2006:2774–2776. doi: 10.1039/b603438a. [DOI] [PubMed] [Google Scholar]
- 23.Hu BH, Su J, Messersmith PB. Hydrogels cross-linked by native chemical ligation. Biomacromolecules. 2009;10:2194–2200. doi: 10.1021/bm900366e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanborn TJ, Messersmith PB, Barron AE. In situ crosslinking of a biomimetic peptide-PEG hydrogel via thermally triggered activation of factor XIII. Biomaterials. 2002;23:2703–2710. doi: 10.1016/s0142-9612(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 25.Ehrbar M, Rizzi SC, Hlushchuk R, Dionov V, Zisch AH, Hubbell JA, et al. Enzymatic formation of modular cell-instructive fibrin analogs for tissue engineering. Biomaterials. 2007;28:3856–3866. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Ehrbar M, Rizzi SC, Schoenmakers RG, Miguel BS, Hubbell JA, Weber FE, et al. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules. 2007;8:3000–3007. doi: 10.1021/bm070228f. [DOI] [PubMed] [Google Scholar]
- 27.Truong K, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 28.Fisher JP, Dean D, Engel PS, Mikos AG. Photoinitiated polymerization of biomaterials. Annu Rev Mater Res. 2001;31:171–181. [Google Scholar]
- 29.Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(α-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581–587. [Google Scholar]
- 30.Clapper JD, Skeie JM, Mullins RF, Guymon CA. Development and characterization of photopolymerizable biodegradable materials from PEG-PLA-PEG block macromonomers. Polymer. 2007;48:6554. [Google Scholar]
- 31.Jiang Z, Hao J, You Y, Liu Y, Wang Z, Deng X. Biodegradable and thermosensitive hydrogels of poly(ethylene glycol)-poly(ε-caprolactone-co-glycolide)-poly(ethylene glycol) aqueous solutions. J Biomed Mater Res A. 2008;87:45–51. doi: 10.1002/jbm.a.31699. [DOI] [PubMed] [Google Scholar]
- 32.Jo S’ Engel PS, Mikos AG. Synthesis of poly(ethylene glycol)-tethered poly(propylene fumarate) and its modification with GRGD peptide. Polymer. 2000;41:7595–7604. [Google Scholar]
- 33.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliver Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Ksihara S, Matsumura S, Fisher JP. Synthesis and characterization of cyclic acetal based degradable hydrogels. Eur J Pharm Biopharm. 2008;68:67–73. doi: 10.1016/j.ejpb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Skardal A, Prestwich GD. Engineered extracellular matrices with cleavable crosslinkers for cell expansion and easy cell recovery. Biomaterials. 2008;29:4521–4531. doi: 10.1016/j.biomaterials.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J Control Release. 2002;83:53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 37.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 38.Jo YS, Gantz J, Hubbell JA, Lutolf MP. Tailoring hydrogel degradation and drug release via neighboring amino acid controlled ester hydrolysis. Soft Matter. 2009;5:440–446. [Google Scholar]
- 39.Hudalla GA, Eng TS, Murphy WL. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules. 2008;9:842–849. doi: 10.1021/bm701179s. [DOI] [PubMed] [Google Scholar]
- 40.Rydholm AE, Anseth KS, Bowman CN. Effects of neighboring sulfides and pH on ester hydrolysis in thiol-acrylate photopolymers. Acta Biomater. 2007;3:449–455. doi: 10.1016/j.actbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rydholm AE, Bowman CN, Anseth KS. Degradable thiol-acrylate photopolymers: polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials. 2005;26:4495–4506. doi: 10.1016/j.biomaterials.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 42.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog Polym Sci. 2008;33:167–170. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lutolf MP. Spotlight on hydrogels. Nat Mater. 2009;8:451–453. doi: 10.1038/nmat2458. [DOI] [PubMed] [Google Scholar]
- 45.Cushing MC, Anseth KS. Hydrogel cell culture. Science. 2007;316:1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 46.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 47.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Devp Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 49.Badylak SF. The extracellular matrix as a biological scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 50.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 51.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliver Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao S, Chan CK, Ramakrishna S. Stem cells and biomimetic materials strategies for tissue engineering. Mater Sci Eng C. 2008;28:1189–1202. [Google Scholar]
- 53.Sands RW, Mooney DJ. Polymers to direct cell fate by controlling the microenvironment. Curr Opin Biotech. 2007;18:448–453. doi: 10.1016/j.copbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Mel A, Jell G, Stevens MM, Sefalian AM. Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review. Biomacromolecules. 2008;9:2969–2979. doi: 10.1021/bm800681k. [DOI] [PubMed] [Google Scholar]
- 55.Scott JE. Extracellular matrix, supramolecular organization and shape. J Anat. 1995;187:259–269. [PMC free article] [PubMed] [Google Scholar]
- 56.Rhodes JM, Simons M. The extracellular matrix and blood vessel formation; not just a scaffold. J Cell Mol Med. 2007;11:176–205. doi: 10.1111/j.1582-4934.2007.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gailit J, Clark RA. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994;6:717–725. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 58.Ottani V, Raspanti M, Ruggeri A. Collagen structure and functional implications. Micro. 2001;32:251–260. doi: 10.1016/s0968-4328(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 59.Celse K, Poschl E, Aigner T. Collagens-structure, function, and biosynthesis. Adv Drug Deliver Rev. 2003;55:1513–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Kuhn K. Structure and biochemistry of collagen. Aesthet Plast Surg. 1985;9:141–144. doi: 10.1007/BF01570346. [DOI] [PubMed] [Google Scholar]
- 61.Brodsky B, Ramshaw JAM. The collagen triple-helix structure. Matrix Biol. 1997;15:545–554. doi: 10.1016/s0945-053x(97)90030-5. [DOI] [PubMed] [Google Scholar]
- 62.Kuhn K. Basement membrane (type IV) collagen. Matrix Biol. 1994;14:439–445. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 63.Aumailley M, Smyth N. The role of laminins in basement membrane function. J Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- 65.Gole T, Pohl U. Laminin binding conveys mechanosensing in endothelial cells. News Physiol Sci. 2002;17:166–169. doi: 10.1152/nips.01381.2001. [DOI] [PubMed] [Google Scholar]
- 66.Kumagai C, Okano M, Kitagawa Y. Three heterotrimeric laminins produced by human keratinocytes. Cytotechnology. 2000;33:167–174. doi: 10.1023/A:1008186912975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yurchenco P, O’Rear J. Basal lamina assembly. Curr Opin Cell Biol. 1994;6:674–681. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 68.Herrick S, Blanc-Brude O, Gray A, Laurent G. Fibrinogen. Int J Biochem Cell Biol. 1999;31:741–746. doi: 10.1016/s1357-2725(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 69.Fuss C, Palmaz JC, Sprague EA. Fibrinogen: structure, function, and surface interactions. J Vasc Interv Radiol. 2001;12:677–682. doi: 10.1016/s1051-0443(07)61437-7. [DOI] [PubMed] [Google Scholar]
- 70.Spraggeon G, Everse SJ, Doolittle RF. Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin. Nature. 1997;389:455–462. doi: 10.1038/38947. [DOI] [PubMed] [Google Scholar]
- 71.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 72.Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscl Throm Vas. 1998;18:1363–1370. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]
- 73.Potts JR, Campbell ID. Fibronectin structure and assembly. Curr Opin Cell Biol. 1994;6:648–655. doi: 10.1016/0955-0674(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 74.Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin-integrin interactions. Front Biosci. 1997;2:d126–d146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 75.Papagiannopoulos A, Waign TA, Hardingham TE. The viscoelasticity of self-assembled proteoglycan combs. Faraday Discuss. 2008;139:337–357. doi: 10.1039/b714864j. [DOI] [PubMed] [Google Scholar]
- 76.Horkay F, Basser PJ, Hecht AM, Geissler E. Gel-like behavior in aggrecan assemblies. J Chem Phys. 2008;128:135103. doi: 10.1063/1.2884350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dudhia J. Aggrecan, aging and assembly in articular cartilage. Cell Mol Life Sci. 2005;62:2241–2256. doi: 10.1007/s00018-005-5217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo W, Guo C, Zheng J, Chen TL, Wang PY, Vertel BM, et al. Aggrecan from start to finish. J Bone Miner Metab. 2000;18:51–56. doi: 10.1007/s007740050011. [DOI] [PubMed] [Google Scholar]
- 79.Vertel BM. The ins and outs of aggrecan. Trends Cell Biol. 1995;5:458–464. doi: 10.1016/s0962-8924(00)89115-1. [DOI] [PubMed] [Google Scholar]
- 80.Traub W. Molecular assembly in collagen. FEBS Lett. 1978;92:114–120. [Google Scholar]
- 81.Obrink B, Laurent TC, Carlsson B. The binding of chondroitin sulphate to collagen. FEBS Lett. 1975;56:166–169. doi: 10.1016/0014-5793(75)80133-5. [DOI] [PubMed] [Google Scholar]
- 82.Guidetti GF, Bartolini B, Bernardi B, Tira ME, Berndt MC, Balduini C, et al. Binding of von Willebrand factor to the small proteoglycan decorin. FEBS Lett. 2004;574:95–100. doi: 10.1016/j.febslet.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Zhu W, Latridis JC, Hlibczuk V, Ratcliffe A, Mow VC. Determination of collagen-proteoglycan interactions in vitro. J Biomech. 1996;29:773–783. doi: 10.1016/0021-9290(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 84.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–639. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 85.Jensen LT, Host NB. Collagen: scaffold for repair or execution. Cardiovasc Res. 1997;33:535–539. doi: 10.1016/s0008-6363(96)00247-7. [DOI] [PubMed] [Google Scholar]
- 86.Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 87.Humphries MJ, Newham P. The structure of cell-adhesion molecules. Trends Cell Biol. 1998;8:78–83. [PubMed] [Google Scholar]
- 88.Cohen M, Joester D, Geiger B, Addadi L. Spatial and temporal sequence of events in cell adhesion: from molecular recognition to focal adhesion assembly. ChemBioChem. 2004;5:1393–1399. doi: 10.1002/cbic.200400162. [DOI] [PubMed] [Google Scholar]
- 89.Henricks PAJ, Nijkamp FP. Pharmacological modulation of cell adhesion molecules. Eur J Pharmacol. 1998;344:1–13. doi: 10.1016/s0014-2999(98)00036-3. [DOI] [PubMed] [Google Scholar]
- 90.Woods A, Couchman JR. Syndecans: synergistic activators of cell adhesion. Trends Cell Biol. 1998;8:189–191. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- 91.Woods A, Oh ES, Couchman JR. Syndecan proteoglycans and cell adhesion. Matrix Biol. 1998;17:477–483. doi: 10.1016/s0945-053x(98)90095-6. [DOI] [PubMed] [Google Scholar]
- 92.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 93.Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 94.Chen W, Chang C, Gilson MK. Concepts in receptor optimization: targeting the RGD peptide. J Am Chem Soc. 2006;128:4675–4684. doi: 10.1021/ja056600l. [DOI] [PubMed] [Google Scholar]
- 95.Woods A, Couchman JR. Integrin modulation by lateral association. J Biol Chem. 2000;275:24233–24236. doi: 10.1074/jbc.R000001200. [DOI] [PubMed] [Google Scholar]
- 96.Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 97.Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells – over and over and over again. Nat Cell Biol. 2002;4:E97–E99. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- 98.Ellis V, Murphy G. Cellular strategies for proteolytic targeting during migration and invasion. FEBS Lett. 2001;506:1–5. doi: 10.1016/s0014-5793(01)02845-9. [DOI] [PubMed] [Google Scholar]
- 99.Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol. 1999;11:614–621. doi: 10.1016/s0955-0674(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 100.Murphy G, Knauper V, Atkinson S, Gavriliovic J, Edwards D. Cellular mechanisms for focal proteolysis and the regulation of the microenvironment. Fibrinol Proteol. 2000;14:165–174. [Google Scholar]
- 101.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 102.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 104.Ogura Y, Matsunaga Y, Nishiyama T, Amano S. Plasmin induces degradation and dysfunction of laminin 332 (laminin 5) and impaired assembly of basement membrane at the dermal-epidermal junction. Brit J Dermatol. 2008;159:49–60. doi: 10.1111/j.1365-2133.2008.08576.x. [DOI] [PubMed] [Google Scholar]
- 105.Mydel P, Shipley JM, Adair-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, et al. Neutrophil elastase cleaves laminin-332 (lamini-5) generating peptides that are chemotactic for neutrophils. J Biol Chem. 2008;15:9513–9522. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amano S, Akutsu N, Matsunaga Y, Kadoya K, Nishiyama T, Champliaud MF, et al. Importance of balance between extracellular matrix synthesis and degradation in basement membrane formation. Exp Cell Res. 2001;271:249–262. doi: 10.1006/excr.2001.5387. [DOI] [PubMed] [Google Scholar]
- 107.Nagase H, Fields GB. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers. 1996;40:399–416. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C399::AID-BIP5%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 108.Turk BE, Huang LL, Piro ET, Cantley LC. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat Biotechnol. 2001;19:661–667. doi: 10.1038/90273. [DOI] [PubMed] [Google Scholar]
- 109.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 110.Silva AKA, Richard C, Bessodes M, Scherman D, Merten OW. Growth factor delivery approaches in hydrogels. Biomacromolecules. 2009;10:9–18. doi: 10.1021/bm801103c. [DOI] [PubMed] [Google Scholar]
- 111.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 112.Whitelock JM, Murdoch AD, Lozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 113.Bikfalvi A, Sauzeau C, Moukadiri H, Maclouf J, Busso N, Bryckaert M, et al. Interaction of vasculotropin/vascular endothelial cell growth factor with human unbilical vein endothelial cells: binding, internalization, degradation, and biological effects. J Cell Physiol. 1991;149:50–59. doi: 10.1002/jcp.1041490108. [DOI] [PubMed] [Google Scholar]
- 114.Heino J. The collagen family members as cell adhesion proteins. BioEssays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- 115.Mineur P, Guignandon A, Lambert CA, Lapiere CM, Nusgens BV. RGDS and DGEA-induced [Ca2+]i signaling in human dermal fibroblast. BBA-Mol Cell Res. 2005;1746:28–37. doi: 10.1016/j.bbamcr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 116.Luzak B, Golanski J, Rozalski M, Boncler MA, Watala C. Inhibition of collagen-induced platelet reactivity by DGEA peptide. Acta Biochim Pol. 2003;50:1119–1128. [PubMed] [Google Scholar]
- 117.Pocza P, Suli-Vargham H, Darvas Z, Falus A. Locally generated VGVAPG and VAPG elastin-derived peptides amplify melanoma invasion via the galectin-3 receptor. Int J Cancer. 2008;122:1972–1980. doi: 10.1002/ijc.23296. [DOI] [PubMed] [Google Scholar]
- 118.Girotti A, Reguera J, Rodriguez-Cabello JC. Design and bioproduction of recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J Mater Sci Mater M. 2004;15:479–484. doi: 10.1023/b:jmsm.0000021124.58688.7a. [DOI] [PubMed] [Google Scholar]
- 119.Nomizu M, Weeks BS, Weston CA, Kim WH, Kleinman HK, Yamada Y. Structure-activity study of a laminin α1 chain active peptide segment Ile-Lys-Val-Ala-Val (IKVAV) FEBS Lett. 1995;365:227–231. doi: 10.1016/0014-5793(95)00475-o. [DOI] [PubMed] [Google Scholar]
- 120.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 121.Ruoslahti E. The RGD story: a personal account. Matrix Biol. 2003;22:459–465. doi: 10.1016/s0945-053x(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 122.Leahy DJ, Aukhil I, Erickson HP. 2.0Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- 123.Lin X, Takahashi K, Liu Y, Zamora PO. Enhancement of cell attachment and tissue integration by a IKVAV containing multi-domain peptide. BBA-Gen Subjects. 2006;1760:1403–1410. doi: 10.1016/j.bbagen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 124.Hynd MR, Frampton JP, Dowell-Mesfin N, Turner JN, Shain W. Directed cell growth on protein-functionalized hydrogel surfaces. J Neurosci Meth. 2007;162:255–263. doi: 10.1016/j.jneumeth.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Halstenberg S, Panitch A, Rizzi S, Hall H, Hubbell JA. Biologically engineering protein-graft-poly(ethylene glycol) hydrogels: a cell adhesive and plasmin-degradable biosynthetic material for tissue repair. Biomacromolecules. 2002;3:710–723. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 126.Nie T, Akins RE, Kiick KL. Production of heparin-containing hydrogels for modulating cell responses. Acta Biomater. 2009;5:865–875. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moon JJ, Lee SH, West JL. Synthetic biomimetic hydrogels incorporated with Ephrin-A1 for therapeutic angiogenesis. Biomacromolecules. 2007;8:42–49. doi: 10.1021/bm060452p. [DOI] [PubMed] [Google Scholar]
- 128.Bryant SJ, Arthur JA, Anseth KS. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater. 2005;1:243–252. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 129.Li Q, Williams CG, Sun DDN, Wang J, Leong K, Elisseeff JH. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68:28–33. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 130.Masters KS, Shah DN, Walker G, Leinwand LA, Anseth KS. Designing scaffolds for valvular interstitial cells: cell adhesion and function on naturally derived materials. J Biomed Mater Res A. 2004;71:172–180. doi: 10.1002/jbm.a.30149. [DOI] [PubMed] [Google Scholar]
- 131.Khetan S, Katz JS, Burdick JA. Sequential crosslinking to control cellular spreading in 3-dimensional hydrogels. Soft Mater. 2009;5:1601–1606. [Google Scholar]
- 132.Massia SP, Hubbell JA. Tissue engineering in the vascular graft. Cytotechnology. 1992;10:189–204. doi: 10.1007/BF00146670. [DOI] [PubMed] [Google Scholar]
- 133.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 134.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 135.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 136.Guarnieri D, Borzacchielo A, De Capua A, Ruvo M, Netti PA. Engineering of covalently immobilized gradients of RGD peptides on hydrogel scaffolds: effect on cell behavior. Macromol Symp. 2008;266:36–40. [Google Scholar]
- 137.DeLong SA, Gobin AS, West JL. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J Control Rel. 2005;109:139–148. doi: 10.1016/j.jconrel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 138.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesion and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 139.Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 140.Mann BK, Schmedlen RH, West JL. Tethered-TGF-β increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 141.Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, et al. Fabrication of 3D hepatic tissues by additive photopattering of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 142.Schmidt DR, Kao WJ. Monocyte activation in response to polyethylene glycol hydrogels grafted with RGD and PHSRN separated by interpositional spacers of various length. J Biomed Mater Res A. 2007;83:617–625. doi: 10.1002/jbm.a.31270. [DOI] [PubMed] [Google Scholar]
- 143.Benoit DSW, Anseth KS. The effect on osteoblast function of coloclized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209–5220. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 144.Webber LM, Haydam KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated β-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Mann BK, Tsa AT, Scott-Burden T, West JL. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20:2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 146.Webber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–673. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu J, Beamish JA, Tang C, Kottke-Marchant K, Marchant RE. Extracellular matrix-like cell-adhesive hydrogels form RGD-containing poly(ethylene glycol) diacrylate. Macromolecules. 2006;39:1305–1307. [Google Scholar]
- 148.Zhu J, Tang C, Kottke-Marchant K, Marchant RE. Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug Chem. 2009;20:333–339. doi: 10.1021/bc800441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 150.Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, et al. The selective modulating of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005;26:167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 151.Saha K, Irwin EF, Kozhukh J, Schaffer DV, Healy KE. Biomimetic interfacial interpenetrating polymer networks control neural stem cell behavior. J Biomed Mater Res A. 2007;81:240–249. doi: 10.1002/jbm.a.30986. [DOI] [PubMed] [Google Scholar]
- 152.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Herten M, Jung RE, Ferrari D, Rothamel D, Golubovic V, Molenberg A, et al. Biodegradation of different synthetic hydrogels made of polyethylene glycol hydrogel/RGD-peptide modifications: an immunohistochemical study in rats. Clin Oral Implan Res. 2009;20:116–125. doi: 10.1111/j.1600-0501.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- 155.Liu SQ, Ee PLR, Ke CY, Hedrick JL, Yang YY. Biodegradable poly(ethylene glycol)-peptide hydrogels with well-defined structure and properties for cell delivery. Biomaterials. 2009;30:1453–1461. doi: 10.1016/j.biomaterials.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 156.Gobin AS, West JL. Val-ala-pro-gly, an elastin-derived non-integrin ligand: smooth muscle cell adhesion and specificity. J Biomed Mater Res. 2003;67:255–259. doi: 10.1002/jbm.a.10110. [DOI] [PubMed] [Google Scholar]
- 157.Haubner R, Schmitt W, Holzemann G, Goodman SL, Jonczyk A, Kessler H. Cyclic RGD peptides containing β-turn mimetics. J Am Chem Soc. 1996;118:7881–7891. [Google Scholar]
- 158.Haubner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J Am Chem Soc. 1996;118:7461–7472. [Google Scholar]
- 159.Locardi E, Mullen DG, Mattern RH, Goodman M. Conformations and pharmacophores of cyclic RGD containing peptides which selectively bind integrin αvβ3. J Pept Sci. 1999;5:491–506. doi: 10.1002/(SICI)1099-1387(199911)5:11<491::AID-PSC218>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 160.Kaufmann D, Fiedler A, Junger A, Auernheimer J, Kessler H, Weberskirch R. Chemical conjugation of liner and cyclic RGD moieties to a recombinant elastin-mimetic polypeptide – a versatile approach towards bioactive protein hydrogels. Macromol Biosci. 2008;8:577–588. doi: 10.1002/mabi.200700234. [DOI] [PubMed] [Google Scholar]
- 161.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee SH, Miller JS, Moon JJ, West JL. Proteolytically degradable hydrogels with a fluorogenic substrate for studies of cellular proteolytic activity and migration. Biotechnol Progr. 2005;21:1736–1741. doi: 10.1021/bp0502429. [DOI] [PubMed] [Google Scholar]
- 163.Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA. Cell-responsive synthetic hydrogels. Adv Mater. 2003;15:888–892. [Google Scholar]
- 164.Pratt AB, Weber FE, Schmoekel HG, Muller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86:27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 165.Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, et al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 167.Raeber GP, Lutolf MP, Hubbell JA. Mechanisms of 3-D migration and matrix remodeling of fibroblasts within artificial ECSs. Acta Mater. 2007;3:615–629. doi: 10.1016/j.actbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 168.Patel PN, Gobin AS, West JL, Patrick CW. Poly(ethylene glycol) hydrogels system supports preadipocyte viability, adhesion, and proliferation. Tissue Eng. 2005;11:1498–1505. doi: 10.1089/ten.2005.11.1498. [DOI] [PubMed] [Google Scholar]
- 169.Lee SH, Moon JJ, Miller JS, West JL. Poly(ethylene glycol) hydrogels conjugated with a collagenase-sensitive fluorogenic substrate to visualized collagenase activity during three-dimensional cell migration. Biomaterials. 2007;28:3163–3170. doi: 10.1016/j.biomaterials.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 170.Gobin AS, West JL. Effects of epidermal growth factor on fibroblast migration through biomimetic hydrogels. Biotechnol Progr. 2003;19:1781–1785. doi: 10.1021/bp0341390. [DOI] [PubMed] [Google Scholar]
- 171.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 172.Aimetti AA, Tibbitt MW, Anseth KS. Human neutrophil elastase responsive delivery from poly(ethylene glycol) hydrogels. Biomacromolecules. 2009;10:1484–1489. doi: 10.1021/bm9000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-β binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 174.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 175.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 176.Andreopoulos FM, Persaud I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials. 2006;27:2468–2476. doi: 10.1016/j.biomaterials.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 177.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J Control Release. 2002;83:53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 178.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J Control Release. 2005;102:619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 179.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliver Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 180.Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hiemstra C, Zhong Z, van Steenbergen MJ, Hennink WE, Feijen J. Release of model proteins and basic fibroblast growth factor from in situ forming degradable dextran hydrogels. J Contr Rel. 2007;122:71–78. doi: 10.1016/j.jconrel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 182.Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2001;76:11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 183.Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4:65–80. doi: 10.2217/17460751.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 185.Ferreira LS, Gerecht S, Fuller J, Shieh HF, Vunjak-Novakovic G, Langer R. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007;28:2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Elisseeeff J, McIntosh W, Fu K, Blunk T, Langer R. Controlled-release of IGF-1 and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 187.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 188.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 189.Zhang S, Uludag H. Nanoparticle systems for growth factor delivery. Pharm Res. 2009;26:1561–1579. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 190.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68:704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 191.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 192.Gobin AS, West JL. Effects of epidermal growth factor on fibroblast migration through biomimetic hydrogels. Biotechnol Progr. 2003;19:1781–1785. doi: 10.1021/bp0341390. [DOI] [PubMed] [Google Scholar]
- 193.Mann BK, Schmedlen RH, West JL. Tethered-TGF-β increase extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 194.He X, Ma J, Jabbari E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic diffierentiation of marrow stromal cells. Langmuir. 2008;24:12508–12516. doi: 10.1021/la802447v. [DOI] [PubMed] [Google Scholar]
- 195.Tae G, Scatena M, Stayton PS, Hoffman AS. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci-Polym E. 2006;17:187–197. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 196.Freudenberg U, Herman A, Welzel PB, Stirl K, Schwarz SC, Crimmer M, et al. A star-PEG-heaprin hydrogel platform to aid cell replacement therapies for neurodegeneration diseases. Biomaterials. 2009;30:5049–5060. doi: 10.1016/j.biomaterials.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 197.Tsurkan MV, Levental KR, Freudenberg U, Werner C. Enzymatically degradable heparin-polyethylene glycol gels with controlled mechanical properties. Chem Commun. 2010;46:1141–1143. doi: 10.1039/b921616b. [DOI] [PubMed] [Google Scholar]
- 198.Benoit DSW, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 199.Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 200.Yamaguchi N, Kiick KL. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6:1921–1930. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nie T, Akins RE, Kiick KL. Production of heparin-containing hydrogels for modulating cell responses. Acta Biomater. 2009;5:865–875. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL. Growth factor mediated assembly of cell receptor-responsive hydrogels. J Am Chem Soc. 2007;129:3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang L, Furst EC, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions. J Control Release. 2006;114:130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tae G, Kim YJ, Choi WI, Kim M, Stayton PS, Hoffman AS. Formation of a novel heparin-based hydroel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8:1979–1986. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 205.Cai S, Liu Y, Zheng X, Prestwich GD. Injectable glycosaminoglyosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 206.Li Q, Williams CG, Sun DDN, Wang J, Leong K, Elisseeff JH. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res. 2004;68A:28–33. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 207.Ramaswamy S, Wang DA, Fishbein KW, West JH. An analysis of the integration between articular cartilage and nondegradable hydrogel using magnetic resonance imaging. J Biomed Mater Res B. 2006;77:144–148. doi: 10.1002/jbm.b.30404. [DOI] [PubMed] [Google Scholar]
- 208.Lin CC, Anseth KS. Controlling affinity binding with peptide-functionalized poly(ethylene glycol) hydrogels. Adv Funct Mater. 2009;19:2325–2331. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseef JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 210.Salinas CN, Anseth KS. Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells. J Biomed Mater Res A. 2009;90:456–464. doi: 10.1002/jbm.a.32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cheung CY, McCartney SJ, Anseth KS. Synthesis of polymerizable superoxide dismutase mimetics to reduce reactive oxygen species damage in transplanted biomedical devices. Adv Funct Mater. 2008;18:3119–3126. [Google Scholar]
- 212.Cheung CY, Anseth KS. Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets. Bioconjug Chem. 2006;17:1036–1042. doi: 10.1021/bc060023o. [DOI] [PubMed] [Google Scholar]
- 213.Lin CC, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFα. Biomaterials. 2009;30:4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bohl KS, West JL. Nitric oxide-generating polymers reduce platelet adhesion and smooth muscle cell proliferation. Biomaterials. 2000;21:2273–2278. doi: 10.1016/s0142-9612(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 215.Lipke EA, West JL. Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta Biomater. 2005;1:597–606. doi: 10.1016/j.actbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 216.Drumbeller PD, Hubbell JA. Polymer networks with grafted cell adhesion peptides for highly biospecific cell adhesive substrates. Anal Biochem. 1994;222:380–388. doi: 10.1006/abio.1994.1506. [DOI] [PubMed] [Google Scholar]
- 217.Lee W, Lee TG, Koh WG. Grafting of poly(acrylic acid) on the poly(ethylene glycol) hydrogel using surface-initiated photopolymerization for covalent immobilization of collagen. J Ind Eng Chem. 2007;13:1195–1200. [Google Scholar]
- 218.Simms HM, Bowman CM, Anseth KS. Using living radical polymerization to enable facile incorporation of materials in microfludic cell culture devices. Biomaterials. 2008;29:2228–2236. doi: 10.1016/j.biomaterials.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Albrecht DR, Tsang VL, Sah RL, Bhatia SN. Photo- and electropatterning of hydrogel-encapsulated living cell arrays. Lab Chip. 2005;5:111–118. doi: 10.1039/b406953f. [DOI] [PubMed] [Google Scholar]
- 220.Tsang VL, Bhatia SN. Three-dimensional tissue fabrication. Adv Drug Deliver Rev. 2004;56:1635–1647. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 221.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–2524. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 222.Roger JA, Nuzzo RG. Recent progress in soft lithography. Mater Today. 2005;8:50–56. [Google Scholar]
- 223.Xia Y, Whitesides GM. Soft lithography. Angew Chem Int Ed Engl. 1998;37:550–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 224.Perl A, Reinhoudt DN, Huskens J. Microcontact printing: limitations and achievements. Adv Mater. 2009;21:2257–2268. [Google Scholar]
- 225.Quist AP, Pavlovic E, Qscarsson S. Recent advances in microcontact printing. Anal Bioanal Chem. 2005;381:591–600. doi: 10.1007/s00216-004-2847-z. [DOI] [PubMed] [Google Scholar]
- 226.Ruiz SA, Chen CS. Microcontact printing, a tool to pattern. Soft Matter. 2007;3:168–177. doi: 10.1039/b613349e. [DOI] [PubMed] [Google Scholar]
- 227.Beamish JA, Fu AY, Choi A, Haq NA, Kottke-Marchant K, Marchant RE. The influence of RGD-bearing hydrogels on the re-expression of contractile vascular smooth muscle cell phenotype. Biomaterials. 2009;30:4127–4135. doi: 10.1016/j.biomaterials.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhu J, Marchant RE. Solid-phase synthesis of tailed cyclic RGD peptides using glutamic acid: unexpected glutarimide formation. J Pept Sci. 2008;14:690–696. doi: 10.1002/psc.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Reddy SK, Cramer NB, Bowman CN. Thiol-vinyl mechanisms. 1. Termination and propagation kinetics in thiol-ene photopolymerizations. Macromolecules. 2006;39:3673–3680. [Google Scholar]
- 230.Cramer NB, Reddy SK, O’Brien AK, Bowman CN. Thiol-ene photopolymerization mechanism and rate limiting step changes for various vinyl functional group chemistries. Macromolecules. 2003;36:7964–7969. [Google Scholar]
- 231.Cramer NB, Bowman CN. Kinetics of thiol-ene and thiol-acrylate photopolymerizations with real-time Fourier transform infrared. J Polym Sci Polym Chem. 2001;39:3311–3319. [Google Scholar]
- 232.Houllier LL, Bunel BY. Photoinitiated corss-linking of a thiol-methacrylate system. Polymer. 2001;42:2727–2736. [Google Scholar]
- 233.Salinas CN, Anseth KS. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules. 2008;41:6019–6026. [Google Scholar]
- 234.Lutolf MP, Cerritelli S, Cavalli L, Hubbell JA. systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug Chem. 2001;12:101–1056. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- 235.Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6:290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 236.Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-liked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 237.Hiemstra C, Van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Rapid in situ-forming degradable hydrogels from dextran thiols through Michael addition. Biomacromolecules. 2007;8:1548–1556. doi: 10.1021/bm061191m. [DOI] [PubMed] [Google Scholar]
- 238.Hiemstra C, Van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Novel in situ forming degradable hydrogels by Michael addition chemistry: synthesis, rheology, and degradation. Macromolecules. 2007;40:1165–1173. [Google Scholar]
- 239.Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci. 2006;31:487–531. [Google Scholar]
- 240.Tirelli N, Lutolf MP, Napoli A, Hubbell JA. Poly(ethylene glycol) block copolymers. Rev Mol Biotechnol. 2002;90:3–15. doi: 10.1016/s1389-0352(01)00057-5. [DOI] [PubMed] [Google Scholar]
- 241.Park Y, Lutolf MP, Hubbell JA, Hunziker EH, Wong M. Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng. 2004;10:515–522. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 242.Jin R, Hiemstra C, Zhong Z, Feijen J. Enzyme-mediated fast in situ formation of hydrogels from dextran-tyramine conjugates. Biomaterials. 2007;28:2791–2800. doi: 10.1016/j.biomaterials.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 243.Yang Z, Gu H, Fu D, Gao P, Lam JK, Xu B. Enzymatic formation of supramolecular hydrogels. Adv Mater. 2004;16:1440–1444. [Google Scholar]
- 244.Thornton K, Smith AM, Merry CLR, Ulijn RV. Controlling stiffness in nanostructured hydrogels produced by enzymatic dephophorylation. Biochem Soc T. 2009;37:660–664. doi: 10.1042/BST0370660. [DOI] [PubMed] [Google Scholar]
- 245.Young DD, Deiters A. Photochemical control of biological processes. Org Biomol Chem. 2007;5:999–1005. doi: 10.1039/b616410m. [DOI] [PubMed] [Google Scholar]
- 246.Willner I. Photoswitchable biomaterials: en route to optobioelectronic systems. Accounts Chem Res. 1997;30:347–356. [Google Scholar]
- 247.Pelliccioli AP, Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photoch Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 248.England PM, Lester HA, Davidson N, Dougherty DA. Site-specific, photochemical proteolysis applied to ion channels in vivo. Proc Natl Acad Sci U S A. 1997;94:11025–11030. doi: 10.1073/pnas.94.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Peters FB, Brock A, Wang J, Schultz PG. Photocleavage of the polypeptide backbone by 2-nitrophenylalanine. Chem Biol. 2009;16:148–152. doi: 10.1016/j.chembiol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li H, Hah JM, Lawrence DS. Light-mediated liberation of enzymatic activity: “Small molecule” caged protein equivalents. J Am Chem Soc. 2008;130:10474–10475. doi: 10.1021/ja803395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Takahashi I, Kuroiwa S, Lindfors HE, Ndamba LA, Yoshitaka H, et al. Modulation of protein-ligand interactions by photocleavage of a cyclic peptide using phosphatidylinositol 3-kinase SH3 domain as model system. J Pept Sci. 2009;15:411–416. doi: 10.1002/psc.1132. [DOI] [PubMed] [Google Scholar]
- 252.Renner C, Moroder L. Azobenzene as conformational switch in model peptides. ChemBioChem. 2006;7:868–878. doi: 10.1002/cbic.200500531. [DOI] [PubMed] [Google Scholar]
- 253.Ulysse L, Chmielewski J. Photoregulation of cyclic peptide conformation. J Am Chem Soc. 1995;117:8466–8467. [Google Scholar]
- 254.Auernheimer J, Dahmen C, Hersel U, Bausch A, Kessler H. Photoswitched cell adhesion on surfaces with RGD peptides. J Am Chem Soc. 2005;127:16107–16110. doi: 10.1021/ja053648q. [DOI] [PubMed] [Google Scholar]
- 255.Schutt M, Krupka SS, Milbradt AG, Deindl S, Sinner EK, Oesterhelt D, et al. Photocontrol of cell adhesion processes: model studies with cyclic azobenzene-RGD peptides. Chem Biol. 2003;10:487–490. doi: 10.1016/s1074-5521(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 256.Zheng Y, Andreopoulos FM, Micic M, Huo Q, Pham SM, Leblanc RM. A novel photoscissible poly(ethylene glycol)-based hydrogel. Adv Funt Mater. 2001;11:37–40. [Google Scholar]
- 257.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]