SUMMARY
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their anti-cancer effects through cyclooxygenase-2 (COX-2)-dependent and -independent mechanisms. Here we report that Sulindac, an NSAID, induces apoptosis by binding to retinoid X receptor-α (RXRα). We identified an N-terminally-truncated RXRα (tRXRα) in several cancer cell lines and primary tumors, which interacted with the p85α subunit of phosphatidylinositol-3-OH kinase (PI3K). Tumor necrosis factor-α (TNFα) promoted tRXRα interaction with the p85α, activating PI3K/AKT signaling. When combined with TNFα, Sulindac inhibited TNFα-induced tRXRα/p85α interaction, leading to activation of the death receptor-mediated apoptotic pathway. We designed and synthesized a Sulindac analog K-80003, which has increased affinity to RXRα but lacks COX inhibitory activity. K-80003 displayed enhanced efficacy in inhibiting tRXRα-dependent AKT activation and tRXRα tumor growth in animals.
SIGNIFICANCE.
Our results demonstrate that RXRα mediates NSAID Sulindac-induced apoptosis. We provide evidence that tRXRα detected in cancer cells acts nongenomically to activate the PI3K/AKT pathway and promote cancer cell growth and survival, which is inhibited by Sulindac. Our finding that Sulindac and TNFα synergistically induce apoptosis through activation of TNFα-dependent apoptotic pathway offers therapeutic strategies to sensitize cancer cells to the killing effect of this cytokine. Our characterizations of RXR-selective Sulindac-derived analog K-80003 demonstrate that Sulindac’s anti-cancer effects can be dissociated from its COX inhibition and identify a RXRα-based lead for cancer therapy. These findings should appeal to a broad audience of clinicians, cancer biologists, molecular biologists and drug designers.
Highlights.
NSAID Sulindac induces apoptosis by binding to RXRα
Truncated RXRα (tRXRα) promotes PI3K/AKT signaling and cancer cell growth
Sulindac induces TNFα-dependent apoptosis by inhibiting tRXRα/p85α interaction
Sulindac analog lacking COX-2 activity inhibits tRXRα tumor growth in animals
INTRODUCTION
Sulindac sulfide (Sulindac hereafter) (Haanen, 2001) is one of the early non-steroidal anti-inflammatory drugs (NSAIDs) known to inhibit the activities of cyclooxygenases (COXs), of which COX-1 is constitutively expressed whereas COX-2 is induced by mitogenic and inflammatory stimuli. The discovery that regular use of aspirin, an NSAID, reduce the incidence of colon cancer has provided the impetus to develop NSAIDs for cancer prevention and treatment (Grosch et al., 2006; Thun et al., 2002). Sulindac has received extensive attention because of its potent induction of apoptosis and inhibition of cancer cell growth (Haanen, 2001; Yamamoto et al., 1999; Zhang et al., 2000). NSAIDs are believed to exert their anti-cancer effects through inhibition of COX-2, which is often overexpressed in human premalignant and malignant tissues and plays a role in carcinogenesis. Compelling evidence however also indicates that NSAIDs can function through COX-2-independent mechanisms (Grosch et al., 2006; Yamamoto et al., 1999; Zhang et al., 2000). For example, cells lacking COX-1, COX-2, or both show comparable sensitivity to NSAID-induced apoptosis, whereas NSAIDs that do not inhibit COX-2 also induce apoptosis and inhibit carcinogenesis. Recent evidence that COX-2 inhibition is associated with increased cardiovascular risk (Fitzgerald, 2004) underscores the importance in the identification of non-COX-2 targets, which may lead to strategies for developing improved anti-cancer drugs. Although several non-COX-2 targets for NSAIDs have been reported (Grosch et al., 2006), more efforts to identify additional targets and characterize their mechanism of action are needed in order to develop improved target-based drugs for cancer therapy.
Retinoid X receptor-α (RXRα), a member of the nuclear receptor superfamily, plays a role in many biological processes including carcinogenesis (Altucci and Gronemeyer, 2001; Dawson and Zhang, 2002). 9-cis-retinoic acid (9-cis-RA), several polyunsaturated fatty acids, and the NSAID Etodolac (Kolluri et al., 2005) can bind to RXRα to regulate different biological functions. Targretin, a synthetic RXR ligand, is currently used for treating cutaneous T-cell lymphoma (Dawson and Zhang, 2002), demonstrating the suitability of targeting RXRα for cancer therapy. Consistently, the oncogenic potential of RXRα has been demonstrated. Genetic disruption of RXRα enhances tumorigenesis (Huang et al., 2002; Li et al., 2001), and RXR binding to PML/RAR is essential for the development of acute promeylocytic leukemia (Zeisig et al., 2007; Zhu et al., 2007). In addition, the RXRα protein level is often reduced in cancer cells and tumor tissues (Picard et al., 1999; Takiyama et al., 2004; Zhong et al., 2003), which is in part due to limited proteolytic processing of RXRα by calpain or cathepsin (Matsushima-Nishiwaki et al., 1996; Nagaya et al., 1998; Nomura et al., 1999; Takiyama et al., 2004; Zhong et al., 2003). However, the biological function of the resulting truncated RXRα (tRXRα) proteins remains unknown.
The mechanisms by which RXRα regulates diverse biological functions remain to be fully determined and are expected to be complex (Kastner et al., 1995; Mangelsdorf and Evans, 1995). Like other nuclear receptors, RXRα is known to regulate the transcription of target genes by binding to DNA response elements. Accumulating evidence however indicates that RXRα may also have extranuclear actions. Thus, RXRα resides in the cytoplasm in certain cell types and at different stages during development (Fukunaka et al., 2001). It migrates from the nucleus to the cytoplasm in response to differentiation (Katagiri et al., 2000), apoptosis (Cao et al., 2004), and inflammation (Ghose et al., 2004; Zimmerman et al., 2006). Interestingly, tRXRα resulted from limited proteolytic cleavage in tumor cells is also cytoplasmic (Zhong et al., 2003). Whether and how it acts in the cytoplasm to modulate carcinogenesis is currently unknown.
In this study, we examined whether tRXRα serves as an intracellular target mediating the apoptotic effect of Sulindac. In addition, we investigated the mechanism by which cytoplasmic tRXRα acts to promote tumor growth. Furthermore, we explored the possibility to dissociate Sulindac’s anti-cancer effects from its COX inhibition activity.
RESULTS
Sulindac Binds to RXRα
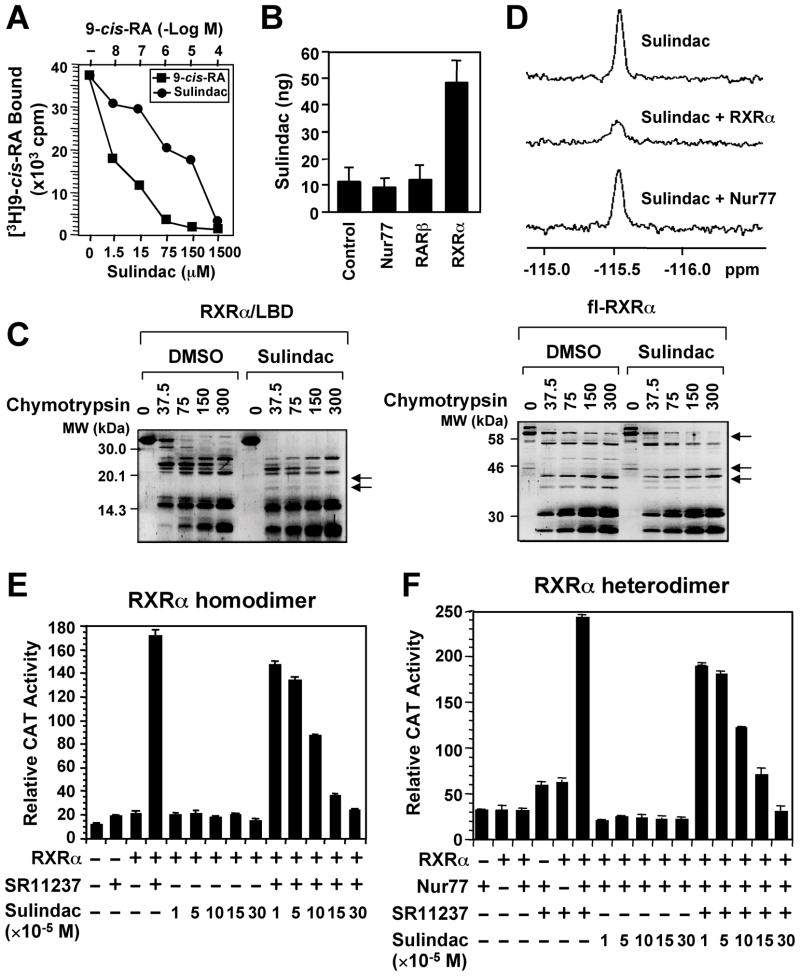
We previously reported that R-Etodolac binds RXRα and induces a RXRα-dependent apoptosis of cancer cells in vitro and in animals (Kolluri et al., 2005). During the course of identifying other NSAIDs as potential RXRα ligands, we found that Sulindac bound to RXRα, but not RAR (Figure S1A), with an IC50 of 80 μM (Figure 1A), which is in its concentration range that induces apoptosis (Yamamoto et al., 1999; Zhang et al., 2000). HPLC analysis showed a direct binding of Sulindac to RXRα protein but not other nuclear receptors such as RAR and Nur77 in cells (Figure 1B and Figure S1C). The binding was also illustrated by altered sensitivity of RXRα ligand-binding domain (LBD) or full-length (fl)-RXRα protein to chymotrypsin digestion by Sulindac in vitro (Figure 1C). Furthermore, we took advantage of the presence of fluorine atom in Sulindac and examined 19F nuclear magnetic resonance (NMR) spectra. Figure 1D shows that the signal intensity of the fluorine spectrum of Sulindac was strongly suppressed by RXRα LBD but not by Nur77 protein, demonstrating a direct and specific binding. Sulindac binding inhibited transactivation of RXRα homodimers (Figure 1E) and certain heterodimers (Figure 1F and Figure S1B) in the reporter assays, demonstrating that Sulindac is a RXRα transactivation antagonist.
Figure 1. Sulindac Binds to RXRα.
(A) Sulindac binding to RXRα in vitro. RXRα LBD protein was incubated with [3H]9-cis-RA in the presence or absence of Sulindac or unlabeled 9-cis-RA. Bound [3H]9-cis-RA was quantitated by liquid scintillation counting.
(B) Sulindac binding to RXRα in cells. HEK293 cells stably expressing RXRα, Nur77 or RARβ were treated with 100 μM sulindac for 3 hr. The same amount of purified RXRα, Nur77, or RARβ protein was subjected to HPLC analysis for the presence of Sulindac.
(C) Altered sensitivity of RXRα LBD or GST-RXRα to chymotrypsin (μg/ml) by Sulindac (100 μM).
(D) Comparison of 19F NMR spectra of Sulindac (100 μM) in the absence and presence of 10 μM RXRα LBD or Nur77 protein.
(E, F) Sulindac inhibits transactivation of RXRα homodimers and heterodimers. (TREpal)2-tk-CAT (Zhang et al., 1992a) (E) or βRARE-tk-CAT (Zhang et al., 1992b) (F), Nur77 and/or RXRα were transiently transfected into CV-1 cells. Cells were treated with or without SR11237 (10−6 M) in the presence or absence of Sulindac. CAT activity was determined.
One of three to five similar experiments is shown. Error bars represent SEM.
See also Figure S1.
Sulindac Induces RXRα-dependent Apoptosis
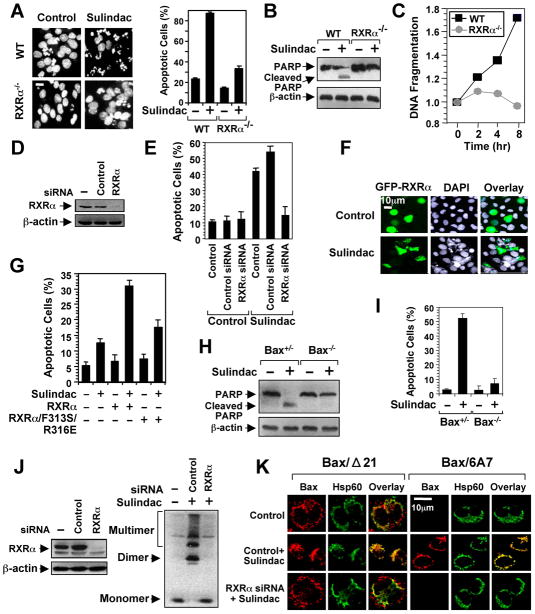
To determine the role of RXRα in Sulindac-induced apoptosis, we examined its death effect in F9 cells and F9 cells lacking RXRα (F9-RXRα−/−). Sulindac induced extensive apoptosis in F9 cells, but had little effect in F9-RXRα−/− cells (Figure 2A–2C). Moreover, the apoptotic effect of Sulindac was reduced in cells with diminished RXRα level (Figures 2D, E), whereas it was enhanced in cells with ectopically expressed RXRα in RXRα-negative CV-1 cells (Figure 2F). To address the role of Sulindac binding to RXRα, we constructed the RXRα/F313S/R316E mutant in which Phe313 and Arg316 essential for maintaining the functional integrity of RXRα ligand-binding pocket (LBP) (Bourguet et al., 2000) were substituted with Ser and Glu, respectively. The mutant failed to respond to ligand-induced homodimer or heterodimer transactivation (Figure S2) and showed reduced apoptotic responses to Sulindac (Figure 2G). Thus, RXRα is involved in Sulindac-induced apoptosis.
Figure 2. Sulindac Induces RXRα-dependent Apoptosis and Bax Activation.
(A–C) The apoptotic effects of Sulindac in F9 or F9 cells lacking RXRα (F9 RXRα−/−). Cells treated with Sulindac (75 μM) for 24 hr were analyzed by DAPI staining (A), PARP cleavage (B), and DNA fragmentation (C). Scale bar: 10 μm.
(D,E) RXRα siRNA inhibits apoptosis induction by Sulindac. H460 lung cancer cells transfected with control or RXRα siRNA were treated with Sulindac (75 μM) for 24 hr and analyzed by DAPI staining for apoptosis.
(F) Transfection of RXRα enhances the apoptotic effect of Sulindac. CV-1 cells transfected with GFP-RXRα were treated with Sulindac (75 μM) for 24 hr and analyzed by DAPI staining. GFP-RXRα-transfected cells underwent extensive nuclear fragmentation and condensation.
(G) Disruption of the RXRα LBP impairs the apoptotic effect of Sulindac. CV-1 cells transfected with GFP-RXRα or GFP-RXRα/F313S/R316E were treated with Sulindac (75 μM) for 24 hr and analyzed by DAPI staining. Apoptosis scored in receptor-transfected cells. See also Figure S2.
(H,I) Role of Bax in apoptosis induction by Sulindac. HCT116 cells or HCT116 cells lacking Bax (Bax−/−) were treated with or without sulindac (75 μM) for 24 hr. Apoptosis determined by PARP cleavage (H) and DAPI staining (I).
(J,K) RXRα siRNA inhibits sulindac-induced Bax activation. Knocking down RXRα in HCT116 cells by RXRα siRNA revealed by immunoblotting. HCT116 cells transfected with or without RXRα siRNA or control siRNA for 48 hr were treated with Sulindac for 6 hr, and analyzed for Bax oligomerization (J) and Bax conformational change and mitochondrial targeting by immunostaining/confocal microscopy using Bax/Δ21, Bax/6A7, or anti-Hsp60 antibody (K). About 60% of cells showed Bax conformational change presented.
One of three to five similar experiments is shown. Error bars represent SEM.
Bax, a proapoptotic Bcl-2 family member, is required for the apoptotic effect of Sulindac (Zhang et al., 2000). We therefore determined if RXRα was involved in activation of Bax by Sulindac. Sulindac induced cleavage of PARP (Figure 2H) and apoptosis (Figure 2I) in HCT116 colon cancer cells, but not HCT116 cells lacking Bax (Bax−/−). The fact that HCT116 cells are deficient of COX-2 (Sheng et al., 1997) demonstrates that Sulindac-induced apoptosis can be COX-2-independent. Immunoblotting assays showed that Bax underwent extensive oligomerization on mitochondria in response to Sulindac, which was abrogated by RXRα siRNA (Figure 2J). In addition, immunostaining using anti-Bax antibody (Bax/Δ21) and a Bax conformation-sensitive antibody Bax/6A7 (Nechushtan et al., 1999) demonstrated that Sulindac-induced Bax conformational change and mitochondrial targeting were impaired by RXRα siRNA (Figure 2K). Together, these results demonstrate that RXRα can act as an intracellular target mediating the apoptotic effect of Sulindac.
Sulindac Inhibits RXRα-dependent AKT Activation by TNFα
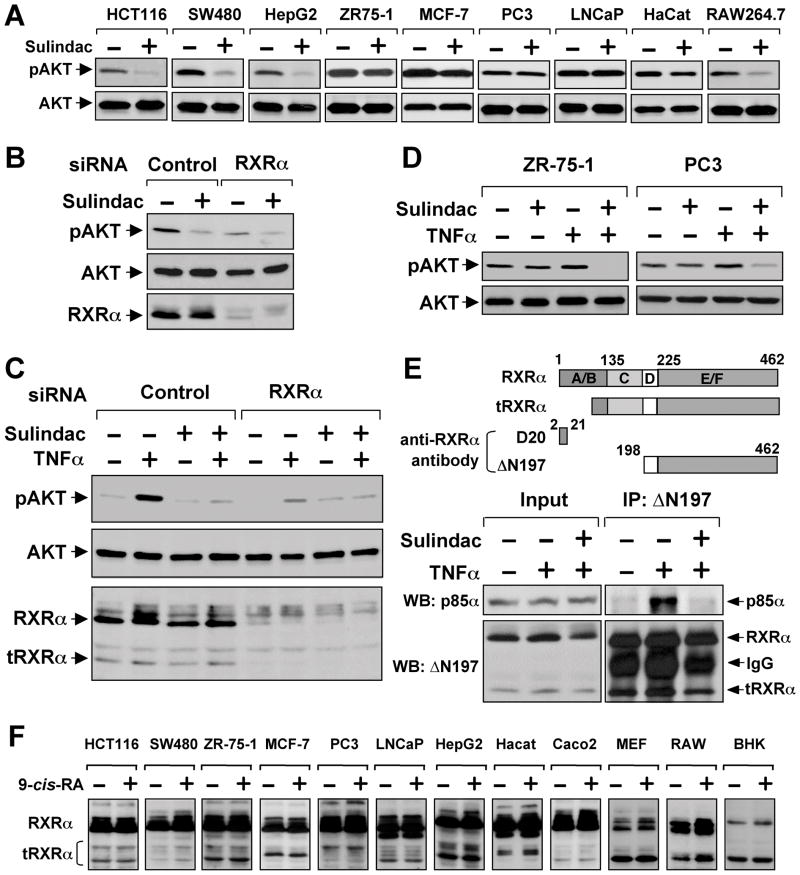
Activation of phosphatidylinositol-3-OH kinase (PI3K) and its downstream effector, AKT, regulates the biological function of substrates such as Bax (Cantley, 2002). We therefore investigated whether Sulindac activated Bax through inhibition of AKT activation and found that Sulindac potently suppressed AKT activation in HCT116 and other cancer cell lines (Figure 3A). Transfection of RXRα siRNA significantly reduced AKT activation (Figure 3B), similar to the effect of Sulindac, raising the possibility that Sulindac might inhibit RXRα-mediated AKT activation. Although Sulindac failed to inhibit AKT activation induced by epidermal growth factor (Figure S3A), it potently inhibited AKT activation induced by retinoic acid in a RXRα-dependent manner (Figures S3B–C).
Figure 3. Sulindac Inhibits TNFα-induced AKT Activation and tRXRα-p85α Interaction.
(A) Inhibition of AKT activation by Sulindac. The indicated cells starved overnight and treated with Sulindac (100 μM) for 1 hr were analyzed for AKT activation by immunoblotting.
(B) Inhibition of basal AKT activation by RXRα siRNA. HepG2 cells transfected with RXRα siRNA for 48 hr were treated with Sulindac (100 μM) for 1 hr. AKT activation and RXRα expression were analyzed by immunoblotting.
(C) Inhibition of TNFα-induced AKT activation by Sulindac and RXRα siRNA. A549 lung cancer cells transfected with RXRα or control siRNA for 48 hr were pretreated with Sulindac (100 μM) for 1 hr before exposed to TNFα (10 ng/ml) for 30 min.
(D) Synergistic inhibition of AKT activation by TNFα and Sulindac. ZR-75-1 and PC3 cells were pretreated with Sulindac for 1 hr before exposed to TNFα (10 ng/ml) for 30 min.
(E) Induction of RXRα-p85α interaction by TNFα. A549 cells treated with TNFα (10 ng/ml) and/or Sulindac (100 μM) for 30 min were analyzed for RXRα-p85α interaction by co-immunoprecipitation using ΔN197 antibody. Above, schematic representation of anti-RXRα antibodies used in co-immunoprecipitation and immunoblotting assays. D20 antibody recognizes amino acids 2–21 in the N-terminal A/B domain, while ΔN197 antibody recognizes the C-terminal E/F domain. A RXRα truncated protein with about 44 kDa is also shown.
(F) Expression of tRXRα in various cancer cell lines. The indicated cell lines treated with or without 9-cis-RA (10−7 M) for 30 min were analyzed by immunoblotting using the ΔN197 RXRα antibody.
One of three to six similar experiments is shown.
See also Figure S3.
TNFα could also activate PI3K/AKT signaling (Ozes et al., 1999; Pincheira et al., 2008). We thus examined whether RXRα played a role in AKT activation by TNFα. Treatment of A549 lung cancer cells with TNFα led to strong AKT activation, which was potently inhibited by Sulindac (Figure 3C). Transfection of RXRα siRNA, which inhibited not only the expression of the 54-kDa fl-RXRα but also a 44-kDa tRXRα, significantly impaired the ability of TNFα to activate AKT (Figure 3C), demonstrating that RXRα was critical for AKT activation by TNFα. Although Sulindac showed little inhibitory effect on AKT activation in cancer cells with high basal AKT activation, such as ZR-75-1 breast cancer and PC3 prostate cancer cells, it completely inhibited AKT activation when used together with TNFα (Figure 3D), raising an intriguing possibility that TNFα can sensitize cancer cells to Sulindac by converting AKT activation from a RXRα-independent to a RXRα-dependent manner.
TNFα-induced Interaction of tRXRα with p85α and Its Inhibition by Sulindac
Our observations that RXRα was required for AKT activation by TNFα and retinoic acid prompted us to examine whether RXRα interacted with p85α. Our initial intensive attempts by co-immunoprecipitation assays using anti-RXRα antibody (D20) against sequences in the N-terminus of RXRα (Figure 3E) failed to detect a clear interaction, although the antibody effectively immunoprecipitated the RXRα protein (Figure S3E). As tRXRα proteins produced through limited proteolytic cleavage in cancer cells were cytoplasmic (Nagaya et al., 1998; Zhong et al., 2003), we asked whether the cytoplasmic tRXRα was responsible for binding to p85α. For this purpose, we used another anti-RXRα antibody (ΔN197) that recognizes the RXRα LBD (Figure 3F). Indeed, p85α was readily co-immunoprecipitated by the ΔN197 antibody in a TNFα (Figure 3E) or RA (Figures S3D–E) dependent manner. Co-immunoprecipitation of p85α was accompanied with immunoprecipitation of tRXRα, which was not detected by the D20 RXRα antibody (Figure S3E), indicating its lack of N-terminal sequences. Using the ΔN197 antibody, we also observed that interaction of p85α with tRXRα in the presence of TNFα (Figure 3E) or 9-cis-RA (Figure S3D) was inhibited by Sulindac. These results suggested that tRXRα might bind to p85α, leading to AKT activation.
Regulation of tRXRα Production and Its Activation of AKT
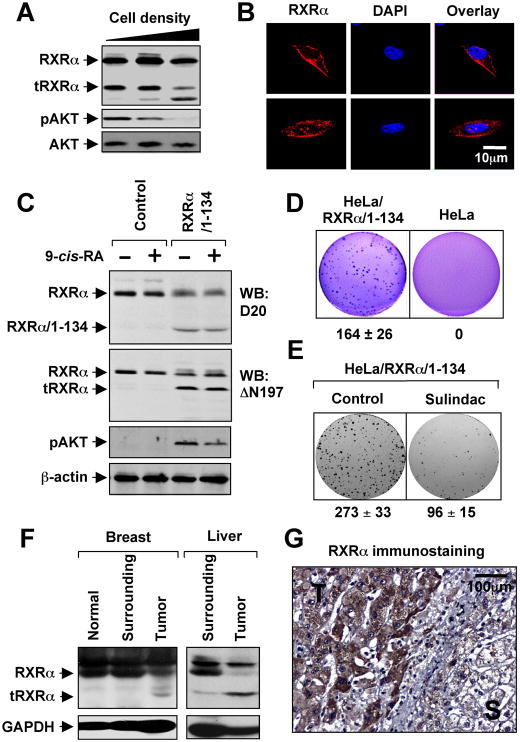
We reported previously that cell density plays a critical role in determining the cytoplasmic localization of RARγ (Han et al., 2009). We similarly observed that the level of the 44-kDa tRXRα reduced as the density of cells increased, which was accompanied with appearance of a smaller RXRα fragment. Interestingly, the levels of the 44-kDa tRXRα protein correlated with AKT activation (Figure 4A), suggesting that cell density-dependent proteolytic cleavage of RXRα might be an important mechanism regulating AKT activation. Consistent with cytoplasmic localization of tRXRα (Figure S4A), immunostaining of MEFs with the ΔN197 antibody revealed RXRα staining predominantly in the cytoplasm and occasionally on the plasma membrane (Figure 4B), likely due to the high levels of tRXRα in MEFs. Thus, deletion of the N-terminal sequences of RXRα could alter its subcellular localization, conferring its ability to interact with p85α.
Figure 4. Role of tRXRα in AKT Activation and Anchorage-Independent Cell Growth.
(A) Cell density dependent production of tRXRα and AKT activation. MEFs seeded at different cell density were analyzed for RXRα expression using ΔN197 antibody and for AKT activation by immunoblotting.
(B) Subcellular localization of endogenous RXRα in MEFs was visualized by confocal microscopy after immunostaining using anti-RXRα (ΔN197). Cells were also stained with DAPI to visualize the nucleus. More than 60% of cells showed the images presented.
(C) Stable expression of RXRα/1–134 induces RXRα cleavage and AKT activation. HeLa or HeLa cells stably expressing RXRα/1–134 were treated with 9-cis-RA for 30 min and analyzed for AKT activation and expression of RXRα.
(D) Growth of HeLa/RXRα/1–134 and HeLa cells in soft agar.
(E) Sulindac inhibits clonogenic survival of HeLa/RXRα/1–134 cells. Cells grown in 6-well plates for 5 days were treated with Sulindac (25 μM) for 3 days.
(F) Production of tRXRα in human tumor tissues of breast (5 out of 6) or liver (4 out of 6) compared to tumor surrounding and normal tissues.
(G) Cytoplasmic localization of RXRα in liver tumor specimens immunostained by ΔN197 antibody. T, tumor tissue; S, tumor surrounding tissue.
One of three to five similar experiments is shown.
See also Figure S4.
In an effort to study the regulation of tRXRα production, we found that expression of the N-terminal region of RXRα, RXRα/1–134, enhanced the tRXRα level (Figure S4B). To study the biological function of the endogenous tRXRα, we stably expressed RXRα/1–134 in HeLa cells, which resulted in production of a significant amount of 44-kDa tRXRα protein. Comparing to parental HeLa cells, HeLa/RXRα/1–134 stable clone had much higher AKT activation (Figure 4C) and were able to rapidly grow in soft agar (Figure 4D). Sulindac strongly reduced colonies formed by the stable clone in the colony formation assay (Figure 4E). Together, these results demonstrate that tRXRα may contribute to the growth and survival of cancer cells by activating AKT and that tRXRα-mediated activities can be negatively regulated by Sulindac.
To study the possible pathological function of tRXRα, we examined its expression in tumor tissues. Immunoblotting of tissue samples showed the presence of tRXRα in breast and liver cancer tissues but not in tumor surrounding tissues or distant normal tissues from the same patients (Figure 4F). Previous studies revealed an extensive cytoplasmic RXRα immunostaining in malignant human prostatic tumor (Zhong et al., 2003) and thyroid tumor (Takiyama et al., 2004) specimens. Immunohistochemical analysis using the ΔN197 antibody also revealed a strong cytoplasmic RXRα staining in liver tumor tissue but not the surrounding tissue (Figure 4G), confirming that tRXRα produced in tumor tissues is cytoplasmic. Together, these results suggest that tRXRα may play a role in the development of cancer through its ability to activate AKT.
N-terminally Truncated RXRα Mediates TNFα Activation of the PI3K/AKT Pathway and Promotes Cancer Cell Growth and Survival
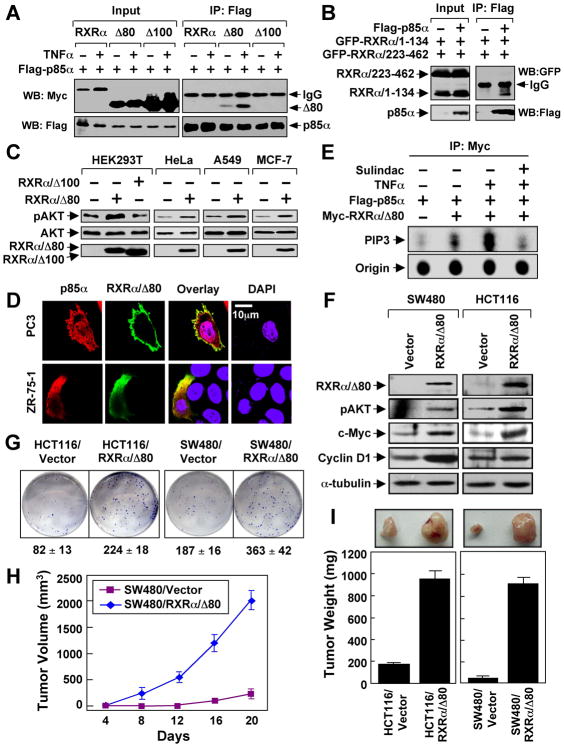
To directly address the role of N-terminally truncated RXRα, we constructed a RXRα mutant lacking its N-terminal 80 amino acids (RXRα/Δ80) with a molecular weight similar to the endogenous tRXRα. Also similar to tRXRα, RXRα/Δ80 interacted with p85α, which was strongly enhanced by TNFα (Figure 5A). In contrast, the full-length RXRα did not interact with p85α either in the absence or presence of TNFα, suggesting that the N-terminal sequences of RXRα prevented its binding to p85α. Interestingly, RXRα mutant lacking the N-terminal 100 amino acids (RXRα/Δ100) was unable to interact with p85α (Figure 5A). This was consistent with the fact that RXRα/1–134 but not RXRα/223–462 could interact with p85α (Figure 5B). The role of RXRα/Δ80 in AKT activation was demonstrated by that expression of RXRα/Δ80 but not RXRα/Δ100 strongly activated AKT in different cell types (Figure 5C). Consistent with cytoplasmic localization of tRXRα (Figures 4B and S4A), RXRα/Δ80 predominantly resided in the cytoplasm, with occasional punctate plasma membrane localization (Figure 5D). Thus, deletion of the N-terminal sequences of RXRα alters its subcellular localization and confers its ability to interact with p85α.
Figure 5. Role of N-terminally Truncated RXRα in PI3K/AKT Activation by TNFα and Cancer Cell Growth.
(A) HEK293T cells were transfected with Flag-p85α and RXRα, RXRα/Δ80, or RXRα/Δ100 tagged with the Myc epitope, treated with TNFα (10 ng/ml) for 30 min, and analyzed by co-immunoprecipitation using anti-Flag antibody.
(B) N-terminal A/B domain of RXRα interacts with p85α. Flag-p85α was cotransfected with GFP-RXRα/1–134 and GFP-RXRα/223–462 into HEK293T cells, and analyzed for their interaction by co-immunoprecipitation using anti-Flag antibody.
(C) RXRα/Δ80 is a potent AKT activator. AKT activation of the indicated cells transfected with RXRα/Δ80 or RXRα/Δ100 was determined by immunoblotting.
(D) Cytoplasmic co-localization of RXRα/Δ80 and p85α. Myc-RXRα/Δ80 and p85α were cotransfected into the indicated cell lines, immunostained with anti-Myc and anti-p85α antibody, and their subcellular localization revealed by confocal microscopy. RXRα/Δ80 predominantly resided in the cytoplasm of about 80% of cells. About 15% of cells showed the images presented.
(E) Activation of PI3K by RXRα/Δ80 immunoprecipitates. A549 cells transfected with Flag-p85α and Myc-RXRα/80 were treated with TNFα and/or Sulindac, immunoprecipitated with anti-Myc antibody, and subjected to in vitro PI3K assay.
(F) AKT activation by stable expression of RXRα/Δ80. Cells stably transfected with GFP-RXRα/Δ80 or control GFP vector were analyzed by immunoblotting.
(G) RXRα/Δ80 promotes clonogenic survival of cancer cells grown in 6-well plates for 8 days.
(H,I) RXRα/Δ80 promotes cancer cell growth in nude mice (n=6) for three weeks. One of three to five similar experiments is shown. Error bars represent SEM.
See also Figure S5.
To determine how tRXRα/p85α interaction induced AKT activation, we examined whether RXRα/Δ80 immunocomplex possessed PI3K activity in vitro. The PI3K activity exhibited by the Myc-RXRα/Δ80 immunocomplex was dramatically enhanced by TNFα treatment (Figures 5E and S5A–B), which correlated well with its ability to interact with p85α (Figure 5A) and activation of AKT (Figure 5C). Thus, TNFα-induced tRXRα/p85α interaction can activate the PI3K/AKT signaling.
To further study the role of tRXRα, we stably expressed RXRα/Δ80 in SW480 and HCT116 colon cancer cells. The resulting stable clones, SW480/RXRα/Δ80 and HCT116/RXRα/Δ80, showed elevated AKT activation and induction of its downstream targets c-Myc and cyclin D1 (Figure 5F) and increased clonogenic survival than do the control cells (Figure 5G). We then examined the effect of RXRα/Δ80 on the growth of cancer cells in animals by injecting the same number of RXRα/Δ80 expressing cells and the control cells into different flanks of same nude mice. Our results showed that tumors formed by SW480/RXRα/Δ80 and HCT116/RXRα/Δ80 grew much faster than those formed by the control cells (Figures 5H–I). Together, these results demonstrate that the N-terminally truncated RXRα is a potent promoter of cancer cell growth.
Sulindac Activates TNFα-induced Extrinsic Apoptotic Pathway
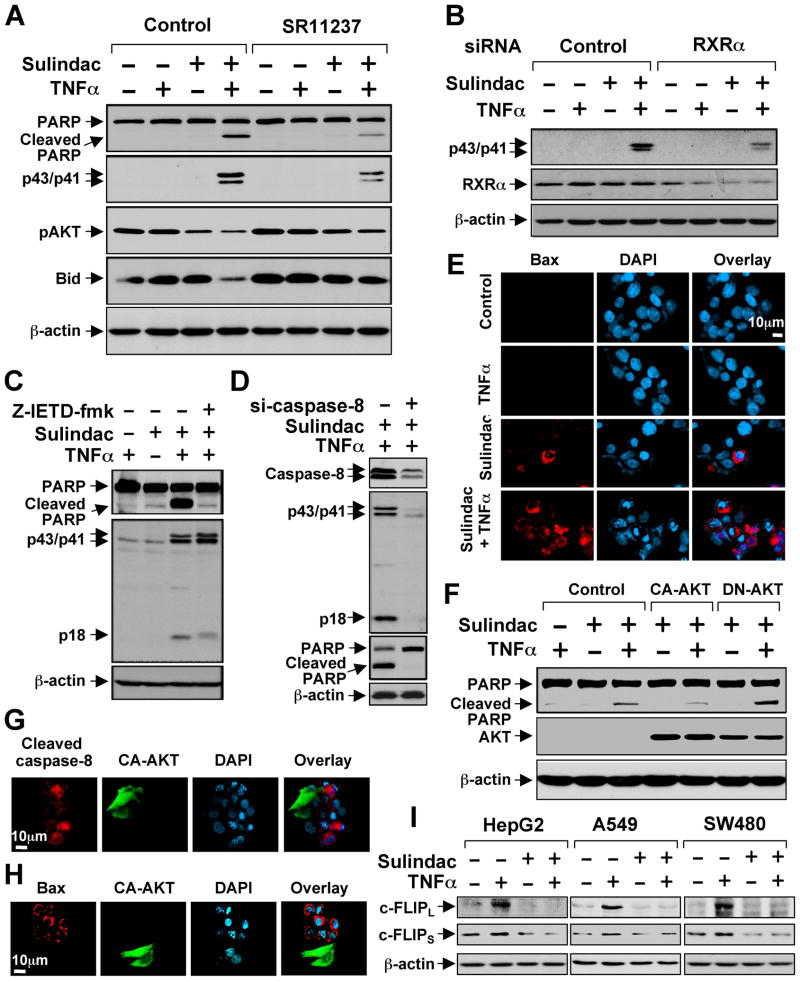
We next determined whether and how synergistic inhibition of AKT activation by Sulindac and TNFα induced apoptosis. Treatment of various cancer cell lines with Sulindac and TNFα effectively induced PARP cleavage and caspase-8 activation (indicated by cleaved caspase-8 products, p43/p41), while treatment of these cells with either Sulindac or TNFα alone had little effect (Figures 6A and S6). The apoptotic effect of Sulindac/TNFα combination was partially suppressed by RXRα-selective ligand SR11237 (Figure 6A) or transfection of RXRα siRNA (Figure 6B).
Figure 6. Activation of TNFα-induced Extrinsic Apoptotic Pathway by Sulindac.
(A) Synergistic induction of apoptosis by Sulindac/TNFα combination and its inhibition by RXRα ligand. HepG2 cells cultured in medium with 1% FBS were treated with SR11237 (1 μM) for 1 hr, then TNFα (10 ng/ml) and/or Sulindac (75 μM) for 4 hr, and analyzed by immunoblotting.
(B) Inhibition of Sulindac/TNFα-induced caspases-8 cleavage by RXRα siRNA. HepG2 cells transfected with control or RXRα siRNA were treated with TNFα and/or Sulindac and analyzed by immunoblotting.
(C,D) Inhibition of Sulindac/TNFα-induced PARP cleavage by caspase-8 inhibitor and siRNA. HepG2 cells transfected with control or caspase-8 siRNA or pretreated with Z-IETD-fmk (40 μM) for 1 hr were treated with TNFα and Sulindac and analyzed by immunoblotting.
(E) Activation of Bax by Sulindac and TNFα. HepG2 cells treated with TNFα and/or Sulindac were immunostained with Bax/6A7 antibody. About 15% Sulindac-treated cells while about 60% Sulindac/TNFα-treated cells showed Bax staining.
(F) Regulation of Sulindac/TNFα-induced PARP cleavage by AKT. PC3 cells transfected with CA-AKT or DN-AKT were treated with TNFα and/or Sulindac, and analyzed by immunoblotting.
(G,H) CA-AKT inhibits activation of caspase-8 (G) and Bax (H) by Sulindac and TNFα. HepG2 cells transfected with CA-AKT were treated with TNFα and Sulindac, and immunostained with anti-cleaved caspase-8 or Bax/6A7 antibody. About 80% nontransfected and 15% CA-AKT-transfected cells showed caspase-8 staining. About 60% nontransfected and about 13% CA-AKT-transfected cells exhibited Bax staining.
(I) Regulation of c-FLIP expression by TNFα and Sulindac. Cells treated with TNFα and/or Sulindac for 6 hr were analyzed by immunoblotting.
One of three to five similar experiments is shown.
See also Figure S6.
Our observation that Sulindac/TNFα activated caspase-8 suggested that apoptosis induction might be due to the activation of TNFα-mediated extrinsic apoptotic pathway. To address this, we treated cells with the caspase-8 inhibitor Z-IETD-fmk or with Caspase-8 siRNA and observed suppression of Sulindac/TNFα-induced PARP cleavage (Figures 6C, 6D and S6B–D). Thus, Sulindac/TNFα-induced apoptosis is mediated by the extrinsic apoptotic pathway.
We also examined whether Sulindac/TNFα activation of the extrinsic apoptotic pathway resulted in Bax activation by immunostaining cells using conformation-sensitive Bax/6A7 antibody. Significant Bax staining was observed only when cells were treated with both TNFα and Sulindac (Figure 6E). Cross-talk between extrinsic and intrinsic apoptotic pathways can be linked through Bid cleavage and activation (Li et al., 1998). Indeed, we observed that Bid was significantly degraded in cells treated with TNFα and Sulindac (Figure 6A), suggesting that Sulindac/TNFα-induced Bax activation might be mediated through Bid activation.
Our observation that Sulindac/TNFα combination synergistically induced apoptosis and inhibited AKT activation suggested that AKT activity might be critical for their induction of apoptosis. Indeed, Sulindac/TNFα-induced PARP cleavage was inhibited by the expression of a constitutive-active AKT (CA-AKT) and enhanced by the expression of a dominant-negative AKT (DN-AKT) (Figure 6F). Consistently, induction of apoptosis and activation of caspase-8 and Bax by Sulindac/TNFα combination was inhibited by CA-AKT (Figures 6G, 6H, and S6E). To study how Sulindac promoted apoptosis through its inhibition of AKT, we examined the expression of c-FLIP, a downstream target gene of AKT signaling (Panka et al., 2001), which acts as a potent inhibitor of the extrinsic apoptotic pathway by inhibiting caspase-8 activation (Irmler et al., 1997). Treatment of cells with TNFα resulted in strong induction of both short form (c-FLIPS) and long form (c-FLIPL) of c-FLIP, which was inhibited by Sulindac (Figure 6I). Thus, Sulindac might induce apoptosis by suppressing the inducing effect of TNFα on c-FLIP expression.
Design and Synthesis of RXRα-selective Sulindac Analogs
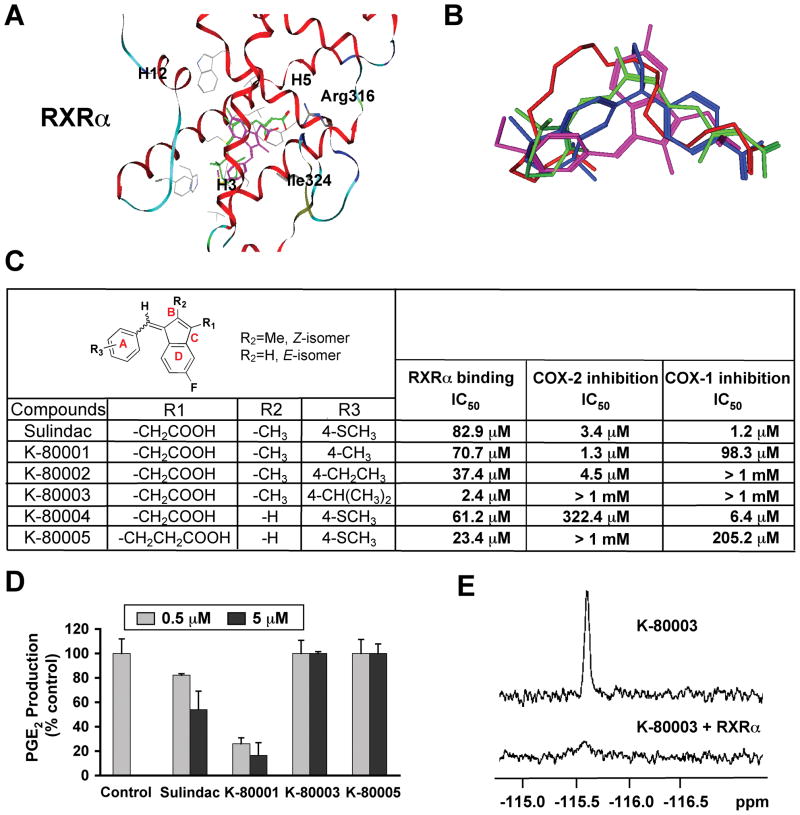
Our finding that RXRα served as an intracellular target of Sulindac action provided an opportunity to design RXRα-selective Sulindac derivatives for cancer therapy. Thus, we conducted docking of Sulindac to three-dimensional structures of the RXRα LBD to identify strategies for structural modifications of Sulindac in order to dissociate its COX inhibition from RXRα-binding activity. Docking of Sulindac to RXRα (Figure 7A) showed that Sulindac bound in a mode where its carboxylate group was aligned with the carboxylate group found in all RXRα ligands examined (Figure 7B), interacting with Arg316 in the RXRα LBP. The benzyl methyl sulfide portion of Sulindac bound to the hydrophobic region of the RXRα LBP, overlapping with the a-ionone ring of 9-cis-RA. In this binding mode, Van der Waals interaction of the –SCH3 group at position 4 (Figure 7C) with the RXRα protein was not optimal and there was room around it for modification to improve the binding to RXRα. The idea of making use of position 4 to design RXRα-selective analogs was fully supported by the fact that sulindac prodrug, sulindac sulfoxide and the metabolite sulindac sulfone show no COX-inhibiting activity, whereas the metabolite sulindac sulfide (used in this study) is a potent COX inhibitor (Haanen, 2001). As shown in Figure 7A, the carboxylate group of Sulindac was positioned away from Arg316 compared to the equivalent ones in RXRα ligands DHA, BMS649, and 9-cis-RA. Replacing –CH2COOH at position D with a bulkier group such as –CH2CH2COOH would help place the carboxylate group closer to Arg316 to achieve good charge-charge interaction with RXRα as observed in 9-cis-RA. Our candidate compounds were also examined by docking to the crystal structure of COX-2 (Kurumbail et al., 1996) (Figure S7) to identify non-COX binders. Based on these considerations, five analogs were designed and synthesized (Figures 7C and S7). Their evaluation showed that all analogs retained RXRα-binding activity, with K-80003 being the most potent, likely due to its iso-propyl (i-Pr) group at position 4, which has improved interaction with the hydrophobic residues on Helix7 of RXRα. Significantly, K-80003 and K-80005 had no detectable inhibition of COX activities and failed to inhibit constitutive and TNFα or IL-1β-induced prostaglandin E2 (PGE2) production (Figures 7C, D, and data not shown). The binding of K-80003 to RXRα was also confirmed by 19F NMR binding assays (Figure 7E). Thus, Sulindac’s RXRα-binding can be dissociated from its COX-binding.
Figure 7. Design, Synthesis and Evaluation of RXRα-selective Sulindac Analogs.
(A) Docking of sulindac sulfide (magenta) to the LBP of RXRα in reference to 9-cis-RA (green). Side chains within 4Å of the ligands are displayed in grey.
(B) Comparison of orientation and position of docked sulindac sulfide (magenta) to the crystal structures of 9-cis-RA (green), DHA (red) and BMS649 (blue).
(C) RXRα binding and inhibition of COX-1 and COX-2 activities by Sulindac analogs. RXRα binding was measured by competition ligand-binding assays. COX inhibition assays used Cayman’s COX Fluorescent Activity Assay Kits.
(D) Inhibition of PGE2 production by Sulindac and analogs. A549 cells stimulated with TNFα (10 ng/ml) for 24 hr were treated with Sulindac or analogs for 30 min. PGE2 production was measured and expressed as the ratio of PGE2 produced in the presence of compound to that with vehicle. Error bars represent SEM.
(E) Comparison of 19F NMR spectra of K-80003 (100 μM) in the absence and presence of 10 μM RXRα LBD.
One of three to five similar experiments is shown.
See also Figure S7.
RXRα-selective Analog K-80003 is a Potent Inhibitor of AKT Activation and Cancer Cell Growth
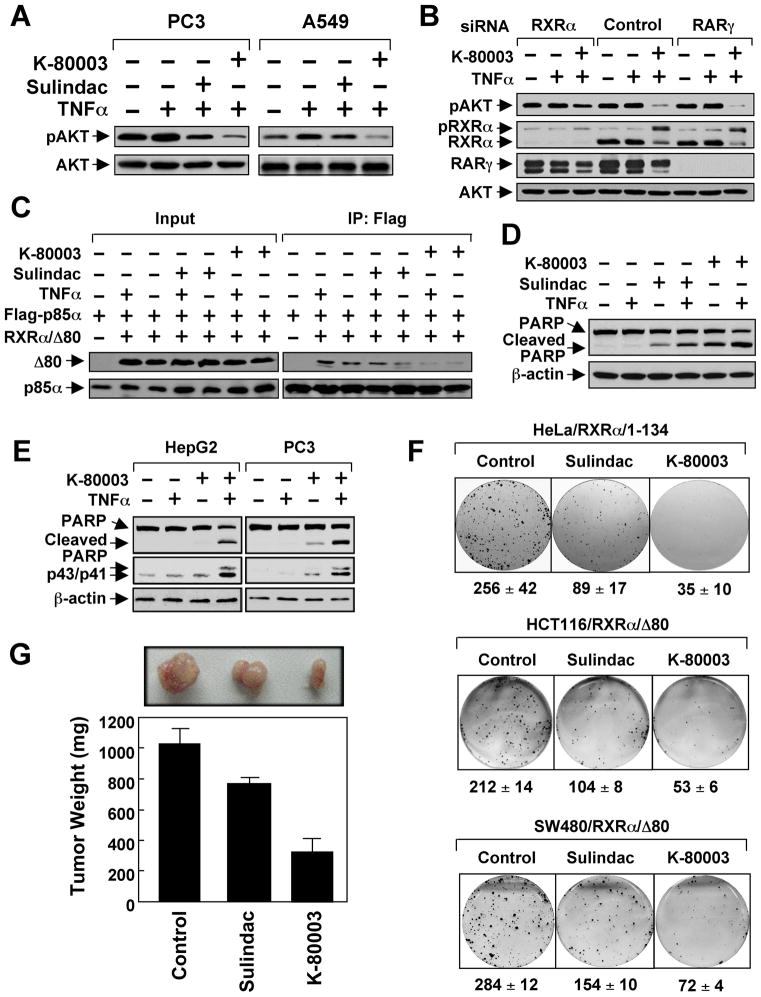
Because of its much-improved affinity to RXRα and lack of COX inhibitory effect, K-80003 was chosen for further evaluation. Immunoblotting showed that K-80003 was much more effective than Sulindac in inhibiting RA- and TNFα-induced AKT activation (Figures 8A and S8). Figure 8B shows that the inhibitory effect of K-80003 on AKT activation in PC3 cells is largely impaired by reducing RXRα, but not RARγ, expression by siRNA. Thus, inhibition of AKT activation by K-80003 was also dependent on RXRα expression. The interaction of RXRα/Δ80 with p85α either in the absence or presence of TNFα was more potently inhibited by K-80003 than by Sulindac (Figure 8C). K-80003 was also more effective than Sulindac in inducing PARP cleavage when used together with TNFα in ZR-75-1 cells (Figure 8D). Similar to Sulindac, K-80003 combination with TNFα synergistically induced PARP cleavage and caspase-8 activation (Figure 8E). In clonogenic survival assays, colony formation of HeLa/RXRα/1–134 and RXRα/Δ80 cells was almost completely suppressed by K-80003 (Figure 8F). Significantly, K-80003 exhibited much more potent inhibitory effect than Sulindac on the growth of RXRα/Δ80 tumor in animals (Figure 8G). Together, the RXRα-selective Sulindac analog K-80003 is a potent inhibitor of RXRα-mediated PI3K/AKT signaling and cancer cell growth.
Figure 8. K-80003 is a Potent Inhibitor of RXRα Dependent AKT Activation.
(A) Inhibition of AKT activation by Sulindac (50 μM) or K-80003 (50 μM) in the presence of TNFα.
(B) RXRα-dependent inhibition of AKT activation by K-80003. PC3 cells transfected with RXRα or RARγ siRNA were pre-treated with K-80003 (50 μM) for 1 hr before exposed to TNFα (10 ng/ml) for 30 min. pRXRα: phosphorylated RXRα.
(C) Inhibition of RXRα/Δ80 interaction with p85α by Sulindac and K-80003. A549 cells were transfected with Flag-p85α and Myc-RXRα/Δ80, treated with Sulindac (50 μM) or K-80003 (50 μM) for 1 hr before exposed to TNFα for 30 min, and analyzed by co-immunoprecipitation using anti-Flag antibody.
(D) Induction of PARP cleavage by Sulindac or K-80003 in the presence of TNFα. ZR-75-1 cells treated with TNFα and/or Sulindac (75 μM) or K-80003 (50 μM) for 6 hr were analyzed by immunoblotting.
(E) Activation of caspase-8 by K-80003 in the presence of TNFα. Cells treated with TNFα and/or K-80003 (50 μM) were analyzed by immunoblotting.
(F) Inhibition of clonogenic survival of RXRα/1–134 cells and RXRα/Δ80 stable clones by Sulindac (25 μM) and K-80003 (25 μM).
(G) Inhibition of RXRα/Δ80 tumor growth in animals by Sulindac and K-80003. Mice (n=6) were treated intraperitoneally with corn oil, Sulindac (60 mg/kg), or K-80003 (60 mg/kg) for two weeks. Tumors were removed and measured. Error bars represent SEM.
One of three to five similar experiments is shown.
See also Figure S8.
DISCUSSION
RXRα is an attractive molecular target for drug development. Here we report that Sulindac could bind to RXRα in the range of concentrations commonly used to study the anti-cancer effects of Sulindac. Conventional administration of Sulindac could result in about 10–15 μM Sulindac in the serum of patients and up to approximately 50 μM of Sulindac could be detected in the plasma of humans (Yamamoto et al., 1999). Sulindac could be also concentrated in epithelial cells at concentrations that are at least 20-fold higher than those in the serum (Duggan et al., 1980). Thus, the binding affinity of Sulindac to RXRα is relevant to in vivo cancer prevention by this drug. The facts that Sulindac can bind to RXRα and that the apoptotic effect of Sulindac largely depends on RXRα expression and its intact LBP strongly suggest that RXRα is an intracellular target of Sulindac.
An important finding of this study is that the N-terminally truncated RXRα protein acts differently from the full-length RXRα protein. Cytoplasmic tRXRα interacted with p85α to activate the PI3K/AKT survival pathway and induce anchorage-independent cell growth in vitro and tumor growth in animals, implying that tRXRα may serve as an important tumor promoter. Our mutational analysis suggested that amino acids from 80 to 100 in RXRα are critical for tRXRα binding to p85α. The region is enriched with proline resides, which can presumably form several polyproline helices (PPII helix) known to bind to the SH3 domain (Kaneko et al., 2008) that is present in p85α. The p85α-binding motif(s) in RXRα are likely masked by the N-terminal end sequences and regulated by phosphorylation. This is consistent with the regulation of tRXRα production and AKT activation by cell density. Regulated proteolysis is a key step in a number of different signaling pathways. Caspase-mediated cleavage of the BH3-only protein Bid into a truncated protein (tBid) and subsequent translocation of tBid to mitochondria are implicated in death receptor signaling (Li et al., 1998), whereas proteolytic processing of Notch and nuclear translocation of truncated product are crucial steps in transduction of the Notch signaling (Ye et al., 1999). STAT signaling is also regulated by proteolytic processing (Hendry and John, 2004). Thus, cleavage of RXRα may represent a mechanism that triggers nongenomic tRXRα signaling by removing the inhibitory N-terminal domain, allowing tRXRα to expose its p85α-binding motif and activate the PI3K/AKT signaling.
Our finding that tRXRα is often produced in tumor tissues but not in normal tissues is consistent with previous findings that RXRα is cleaved in tumor but not in premalignant or normal tissues from patients with prostate or thyroid cancer (Takiyama et al., 2004; Zhong et al., 2003). Thus, agents targeting tRXRα-mediated pathway can be effective and tumor specific. To this end, we showed that Sulindac could inhibit the tRXRα-mediated PI3K/AKT activation, suggesting that Sulindac represents a lead for a class of anti-cancer agents targeting this pathway.
Our observation that Sulindac and TNFα synergistically inhibit tRXRα-dependent AKT activation provides insight into the crosstalk between retinoid receptor and TNFα signaling pathways. Retinoids in combination with cytokines, such as TNFα and TNF-related apoptosis inducing ligand (TRAIL), can synergistically induce differentiation or apoptosis of human transformed cells (Altucci et al., 2005) whereas combination of retinoids and TNFα can overcome RA resistance (Witcher et al., 2004). The fact that Sulindac and TNFα synergistically inhibit AKT activation in cancer cells implies that TNFα and probably other cytokines can prime cancer cells for their responsiveness to RXRα ligands such as Sulindac by converting AKT activation from a RXRα-independent to a RXRα-dependent manner.
TNFα plays important roles in diverse cellular events such as cell survival and death. However, it often fails to induce apoptosis in cancer cells due to its simultaneous activation of the NF-κB and/or the PI3K/AKT pathway (Aggarwal, 2003; Balkwill, 2009). Our observation that tRXRα mediates AKT activation by TNFα suggests a possibility of using Sulindac or analogs to suppress TNFα-induced AKT-mediated survival function, thereby shifting its function from survival to death. Consistently, we have provided evidence that Sulindac in combination with TNFα potently induce tRXRα-dependent caspase-8 activation and apoptosis, demonstrating that Sulindac was able to sensitize cancer cells to TNFα-induced death receptor-mediated extrinsic apoptotic pathway. The fact that TNFα-induced c-FLIP expression is completely prevented by Sulindac places c-FLIP in a central position for integrating TNFα-induced AKT activation and its inhibition by Sulindac and induction of apoptosis by Sulindac and TNFα combination.
Our finding that RXRα serves as an intracellular target of Sulindac action provides a rationale to design RXRα-selective Sulindac derivatives for suppressing AKT activity. Our identification of a Sulindac analog, K-80003, with improved affinity to RXRα but lacking COX inhibitory effects offers an example to this approach. It is expected that K-80003 will lack or have much reduced COX-2-associated side effects. The fact that K-80003 could effectively inhibit the tRXRα pathway and the growth of cancer cells in vitro and in animals warrants its further development for cancer therapy.
EXPERIMENTAL PROCEDURES
Plasmids
Flag-p85α, RXRα/F313S/R316E, RXRα/Δ80, and RXRα/Δ100 were constructed using standard methods.
Antibodies and Regents
Antibodies for pAkt (Ser473, D9E), Bid, and cleaved caspase-8 (p43/p41)(Cell Signaling); Flag (M2), and Bax(6A7) (Sigma-Aldrich); p85α (Millipore); AKT1(C-20), GFP(B-2), Hsp60(N-20), c-Myc(9E10), RARγ(C-19), RXRα(D20), RXRα(N197), Bax(Δ21), c-FLIP, PARP(H-250) (Santa Cruz Biotechnology) were used. Sulindac and analogs were dissolved in dimethyl sulfoxide.
Ligand-Binding Assay
RXR LBD was incubated with [3H]-9-cis-RA in the presence or absence of unlabeled 9-cis-RA or Sulindac. Bound [3H]-9-cis-RA was determined.
HPLC Analysis of Sulindac Binding
EK293 cells stably transfected with vector containing receptor fused to C-terminal TAP fusion were treated with or without 100 μM Sulindac for 3 hr. Receptor proteins purified using InterPlay Mammalian TAP System (Stratagene) were analyzed by HPLC using microsorb-mv 100-3 C18 100×4.6 column (Varian). Sulindac was detected using a photoarray detector (Waters model 2996). A standard solution of Sulindac was used to obtain the calibration curve.
Proteolytic Protection Assay
In vitro translated [35S]-labeled RXRα-LBD or GST-RXRα was incubated with solvent or Sulindac (100 μM) for 30 min and then digested with chymotrypsin.
NMR Binding Assays
F-19 NMR Spectra on Bruker Avance500 NMR Spectrometer with a F-H-D-O probe were recorded with 1024 transients using a sweep width of 18797 Hz. The repetition time is 2 sec.
Apoptosis Assays
Apoptosis assays followed those described (Kolluri et al., 2008; Li et al., 2000; Lin, 2004).
Bax Oligomerization Assay
Lysates were incubated with BMH (Pierce) to cross-link the oligomerized Bax protein. To study Bax oligomerization on mitochondria, heavy membrane fractions were lysed for immunoblotting using anti-Bax antibody (Bax 2D2).
RXRα and RARγ siRNA
RXRα siRNA siGENOME SMARTpool (M-003443-02), RARγ siRNA siGENOME SMARTpool (M-003439-01), and siRNA Non-specific Control IX (D-001206-09-05) were from DHARMACON.
Co-immunoprecipitation and Immunoblotting Assays
The assays followed those described (Li et al., 2000; Lin, 2004).
Confocal Microscopy
Cells seeded on chamber slides were fixed, permeabilized, and stained with appropriate antibodies (Li et al., 2000).
PI3K Kinase Assay
Cells transfected with Flag-p85α and Myc-RXRα/80 were serum-starved for 4 hr, pretreated with Sulindac for 0.5 hr, and then TNFα for 15 min. Cell lysates immunoprecipitated with anti-Myc antibody were incubated with phosphatidylinositol and [γ-32P] ATP and subjected to thin layer chromatography.
Stable Transfection and Soft Agar Assay
RXRα/1–134 cloned into pNTAP vector was stably transfected into HeLa cells, while GFP-RXRα/Δ80 was stably transfected into SW480 and HCT116 cells. Cells seeded at 5 × 103 cells/well (6-well plate) in DMEM supplemented with 10% FBS and 0.35% agarose with 0.5% bed agar for 12 days were stained with 0.005% crystal violet.
Colony Formation Assay
Cells were seeded in 6-well plate for 5 days, treated with compound in 0.5% serum medium for 3 days, and fixed with 4% paraformaldehyde. Colonies were stained with 0.1% crystal violet.
Human Tissues and Evaluation
Breast and liver tumor tissues and their surrounding tissues were obtained by surgical resection from cancer patients. Histological normal specimens, which were about at least 3~5 cm distant from the tumor nodule, were obtained from the corresponding patients. The study was approved by Xiamen University Institute for Biomedical Research Ethics Committee, and all of the patients gave informed consent. Tissues with primary hepatocellular carcinoma (HCC, n=6) or breast cancer (n=6) were collected for detecting the expression of RXRα using ΔN197 antibody. For immunohistochemistry analysis, tissue sections were incubated with the ΔN197 antibody (1:500) overnight at 4°C and detected with goat anti-rabbit-specific immunoglobulins (1:100). The slides were counterstained with hematoxylin.
COX Assays
COX Fluorescent Activity Assay, COX Fluorescent Inhibitor Screening Assay and Prostaglandin E2 Enzyme Immunoassay Kits were from Cayman Chemical. Assays were performed according to the manufacturer’s protocols.
Animal Studies
Nude mice (BALB/c, 4–5-week old) were injected subcutaneously with 100 μl of cells (2×106). For drug treatment, mice (n=6) were treated intraperitoneally after 7 days of transplantation with Corn oil, Sulindac (60 mg/kg), or K-80003 (60 mg/kg) once every other day (six injections). Body weight and tumor sizes were measured every 4 days. All manipulations involving live mice were approved by the Animal Care and Use Committee of Xiamen University.
Chemical Synthesis
Compound synthesis followed schemes in Supplemental Experimental Procedures. Optical rotations, 1H NMR and 13C NMR spectra, IR spectra, and Mass spectra were recorded on Perkin-Elmer 341 automatic polarimeter, Bruker 400 MHz spectrometer, Nicolet Avatar 360 FT-IR spectrophotometer, and Bruker Dalton Esquire 3000 plus or a Finnigan Mat-LCQ (ESI direct injection), respectively. Unless noted, 1H NMR spectra were registered in CDCl3 or DMSO-d6, and chemical shifts were expressed in parts per million (δ) relative to internal Me4Si. Elemental analyses were performed using a Vario RL analyzer. Melting points were determined on an X-4 Micro-melting point apparatus and were uncorrected.
Statistical Analyses
Data were analyzed using an analysis of variance or Student’s t-test and were presented as the mean ± SEM.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (CA109345, CA140980, GM089927, and GM062848), the Susan G. Komen Breast Cancer Foundation, the California Breast Cancer Research Program, the U.S. Army Medical Research and Material Command, the California Tobacco-Related Diseases Research Program, the 985 Project grant from Xiamen University, the 863 Program (2007aa09z404), and the Natural Science Foundation (30971445). We thank Dr. P. Chambon for providing F9-RXR−/− cells, Dr. B. Vogelstein for providing HCT116-Bax−/− cells, and Dr. D. Finlay for reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- Altucci L, Rossin A, Hirsch O, Nebbioso A, Vitoux D, Wilhelm E, Guidez F, De Simone M, Schiavone EM, Grimwade D, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65:8754–8765. doi: 10.1158/0008-5472.CAN-04-3569. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand- binding domains. Mol Cell. 2000;5:289–298. doi: 10.1016/s1097-2765(00)80424-4. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, Han YH, Dawson MI, Zhang X-k. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24:9705–9725. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Current Medicinal Chemistry. 2002;9:623–637. doi: 10.2174/0929867023370789. [DOI] [PubMed] [Google Scholar]
- Duggan DE, Hooke KF, Hwang SS. Kinetics of the tissue distributions of sulindac and metabolites. Relevance to sites and rates of bioactivation. Drug Metab Dispos. 1980;8:241–246. [PubMed] [Google Scholar]
- Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- Fukunaka K, Saito T, Wataba K, Ashihara K, Ito E, Kudo R. Changes in expression and subcellular localization of nuclear retinoic acid receptors in human endometrial epithelium during the menstrual cycle. Mol Hum Reprod. 2001;7:437–446. doi: 10.1093/molehr/7.5.437. [DOI] [PubMed] [Google Scholar]
- Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2:4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- Haanen C. Sulindac and its derivatives: a novel class of anticancer agents. Curr Opin Investig Drugs. 2001;2:677–683. [PubMed] [Google Scholar]
- Han YH, Zhou H, Kim JH, Yan TD, Lee KH, Wu H, Lin F, Lu N, Liu J, Zeng JZ, Zhang XK. A unique cytoplasmic localization of retinoic acid receptor-gamma and its regulations. J Biol Chem. 2009;284:18503–18514. doi: 10.1074/jbc.M109.007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry L, John S. Regulation of STAT signalling by proteolytic processing. Eur J Biochem. 2004;271:4613–4620. doi: 10.1111/j.1432-1033.2004.04424.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Powell WC, Khodavirdi AC, Wu J, Makita T, Cardiff RD, Cohen MB, Sucov HM, Roy-Burman P. Prostatic intraepithelial neoplasia in mice with conditional disruption of the retinoid X receptor alpha allele in the prostate epithelium. Cancer Res. 2002;62:4812–4819. [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Li L, Li SS. The SH3 domain--a family of versatile peptide- and protein-recognition module. Front Biosci. 2008;13:4938–4952. doi: 10.2741/3053. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Katagiri Y, Takeda K, Yu ZX, Ferrans VJ, Ozato K, Guroff G. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat Cell Biol. 2000;2:435–440. doi: 10.1038/35017072. [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Corr M, James SY, Bernasconi M, Lu D, Liu W, Cottam HB, Leoni LM, Carson DA, Zhang XK. The R-enantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. Proc Natl Acad Sci U S A. 2005;102:2525–2530. doi: 10.1073/pnas.0409721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, Tian X, Town J, Cao X, Lin F, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14:285–298. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3 [see comments] [comment] Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li M, Chiba H, Warot X, Messaddeq N, Gerard C, Chambon P, Metzger D. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- Lin B, Kolluri S, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from Protector to Killer by Interaction with Nuclear Orphan Receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Matsushima-Nishiwaki R, Shidoji Y, Nishiwaki S, Moriwaki H, Muto Y. Limited degradation of retinoid X receptor by calpain. Biochem Biophys Res Commun. 1996;225:946–951. doi: 10.1006/bbrc.1996.1276. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Murata Y, Yamaguchi S, Nomura Y, Ohmori S, Fujieda M, Katunuma N, Yen PM, Chin WW, Seo H. Intracellular proteolytic cleavage of 9-cis-retinoic acid receptor alpha by cathepsin L-type protease is a potential mechanism for modulating thyroid hormone action. J Biol Chem. 1998;273:33166–33173. doi: 10.1074/jbc.273.50.33166. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Nagaya T, Yamaguchi S, Katunuma N, Seo H. Cleavage of RXRalpha by a lysosomal enzyme, cathepsin L-type protease. Biochem Biophys Res Commun. 1999;254:388–394. doi: 10.1006/bbrc.1998.9941. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Panka DJ, Mano T, Suhara T, Walsh K, Mier JW. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6896. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, Martinet N, Vignaud JM. Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- Pincheira R, Castro AF, Ozes ON, Idumalla PS, Donner DB. Type 1 TNF receptor forms a complex with and uses Jak2 and c-Src to selectively engage signaling pathways that regulate transcription factor activity. J Immunol. 2008;181:1288–1298. doi: 10.4049/jimmunol.181.2.1288. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiyama Y, Miyokawa N, Sugawara A, Kato S, Ito K, Sato K, Oikawa K, Kobayashi H, Kimura S, Tateno M. Decreased expression of retinoid X receptor isoforms in human thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:5851–5861. doi: 10.1210/jc.2003-032036. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- Witcher M, Shiu HY, Guo Q, Miller WH., Jr Combination of retinoic acid and tumor necrosis factor overcomes the maturation block in a variety of retinoic acid-resistant acute promyelocytic leukemia cells. Blood. 2004;104:3335–3342. doi: 10.1182/blood-2004-01-0023. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. J Biol Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- Ye Y, Lukinova N, Fortini ME. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature. 1999;398:525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- Zeisig BB, Kwok C, Zelent A, Shankaranarayanan P, Gronemeyer H, Dong S, So CW. Recruitment of RXR by homotetrameric RARalpha fusion proteins is essential for transformation. Cancer Cell. 2007;12:36–51. doi: 10.1016/j.ccr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- Zhang XK, Lehmann J, Hoffmann B, Dawson MI, Cameron J, Graupner G, Hermann T, Tran P, Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992a;358:587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- Zhang XK, Hoffmann B, Tran PB, Graupner G, Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992b;355:441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhong C, Yang S, Huang J, Cohen MB, Roy-Burman P. Aberration in the expression of the retinoid receptor, RXRalpha, in prostate cancer. Cancer Biol Ther. 2003;2:179–184. doi: 10.4161/cbt.2.2.281. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nasr R, Peres L, Riaucoux-Lormiere F, Honore N, Berthier C, Kamashev D, Zhou J, Vitoux D, Lavau C, de The H. RXR is an essential component of the oncogenic PML/RARA complex in vivo. Cancer Cell. 2007;12:23–35. doi: 10.1016/j.ccr.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. J Biol Chem. 2006;281:15434–15440. doi: 10.1074/jbc.M508277200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.