Abstract
Insulin resistance is a central feature of the polycystic ovary syndrome (PCOS) and may increase cardiovascular risk. Due to insulin resistance, the metabolic syndrome is more prevalent in PCOS women compared to normal women. Metformin improves the metabolic profile in PCOS in short-term studies. In this study, we evaluated the long-term effect of metformin on metabolic parameters in PCOS women during routine care without a controlled diet. We performed a retrospective medical chart review of 70 women with PCOS receiving metformin from an academic endocrine clinic. Metabolic risk factors were compared before and after metformin treatment. Time trends of these metabolic parameters were also analyzed. After a mean follow-up of 36.1 months with metformin treatment, improvements were observed for BMI (−1.09±3.48 kg/m2, p=0.0117), diastolic blood pressure (−2.69±10.35 mmHg, p=0.0378), and HDL cholesterol (+5.82±11.02 mg/dL, p<0.0001). The prevalence of metabolic syndrome decreased from 34.3% at baseline to 21.4% (p=0.0495). The course of BMI reduction during metformin treatment was significantly more pronounced in PCOS women with metabolic syndrome at baseline, compared with those without the metabolic syndrome (p=0.0369 for interaction). In conclusion, metformin improved the metabolic profile of PCOS women over 36.1 months, particularly in HDL cholesterol, diastolic blood pressure and BMI.
Keywords: Polycystic ovary syndrome, metformin, metabolic syndrome, cardiovascular risk factors
Introduction
PCOS is a prevalent disorder that affects approximately 6–10% of women of childbearing age,1,2 and is the major cause of female anovulatory infertility in the United States. Current evidence suggests that insulin resistance, and its compensatory hyperinsulinemia, is a central feature of PCOS.3 Hyperinsulinemia appears to play an important pathogenic role in the hyperandrogenism of both obese and lean women with PCOS.4–6 Presumably due to the insulin resistance associated with the disorder, women with PCOS are at increased risk for type 2 diabetes, dyslipidemia, hypertension, and atherosclerosis.3,7–9
Insulin resistance is also thought to play a critical role in the development of the metabolic syndrome.10,11 The National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) defined the metabolic syndrome in women as meeting at least 3 out of the 5 following criteria: a waist circumference over 88 cm; blood pressure at or above 130/85 mmHg; fasting serum glucose at least 100 mg/dL; triglycerides at least 150 mg/dL; and HDL cholesterol less than 50 mg/dL.12 The metabolic syndrome has been associated with an increased risk of incident diabetes, cardiovascular disease, and cardiovascular mortality.13–15 The prevalence of the NCEP-ATP III-defined metabolic syndrome in women with PCOS has been reported to be between 33% to 46%.16–18 In contrast, among women of reproductive age from the general population, derived from the Third National Health and Nutrition Examination Survey, the NCEP-ATP III-defined metabolic syndrome was present in 6% of women aged 20–29 years and 15% of women aged 30–39 years.19 Hence, the prevalence of the metabolic syndrome is much higher in women with PCOS compared with normal women.
The insulin sensitizing drug metformin has been shown to ameliorate insulin resistance, decrease serum free testosterone, increase serum sex-hormone binding globulin and improve ovulatory frequency in women with PCOS.20 Currently, metformin is one of the main pharmacotherapeutic options in the treatment of PCOS. In the Diabetes Prevention Program, metformin reduced the risk of incident diabetes21 and metabolic syndrome22 in individuals with impaired fasting glucose and impaired glucose tolerance. In patients with the metabolic syndrome but normal glucose tolerance, metformin has been shown to improve endothelial function.23,24 In women with PCOS, multiple short-term studies of 1 year or less have reported significant improvements in the metabolic profile with administration of metformin.16,25–30
However, to date, only one study has evaluated the long-term effects of metformin on the metabolic syndrome in women with PCOS. Glueck et al evaluated 74 women with PCOS treated by a combination of metformin and a controlled diet for four years and found a sustained improvement of the metabolic profile.31 Although lifestyle modification is the first-line therapy for all obese women with PCOS, a controlled diet over a long period of time may be hard to achieve in many women. The long-term effect of metformin, administered without a concomitant controlled diet, on metabolic parameters in PCOS has not been evaluated.
The objective of our study was to evaluate the long-term effects of metformin on metabolic syndrome parameters in women with PCOS during routine care without a controlled diet. We hypothesized that metformin would improve the metabolic profile of women with PCOS over the course of chronic follow-up.
Materials and Methods
Subjects
We reviewed the medical charts of all women with PCOS referred to a specialized endocrinology clinic at Virginia Commonwealth University from July 1, 2000 through July 1, 2005. This study was approved by the VCU Institutional Review Board. The diagnosis of PCOS was made using the criteria established at the 1990 National Institute of Child Health and Human Development conference on PCOS: ≤ 8 menses per year and biochemical or clinical evidence of hyperandrogenism, after exclusion of other causes such as thyroid dysfunction, hyperprolactinemia, Cushing’s syndrome, androgen-secreting tumors, and nonclassic congenital adrenal hyperplasia.32 Other inclusion criteria for the study included: (1) age ≥ 18 years; and (2) treatment with metformin and a follow-up period of at least 6 months.
Patients were excluded from the study if they had any of the following: (1) evidence of diabetes mellitus at baseline, according to the American Diabetes Association 2003 criteria of a fasting plasma glucose ≥ 126 mg/dL or a 2-hour plasma glucose ≥ 200 mg/dL during a 75 gram dextrose oral glucose tolerance test (OGTT);33 (2) if baseline (before metformin therapy) or follow-up assessment of metabolic parameters was not available; (3) use of other medications affecting insulin sensitivity or metabolic parameters; (4) medically-assisted weight loss with medications or surgical procedures; and (5) documented self-discontinuation from metformin. Women taking oral contraceptives and spironolactone were not excluded from the study as a considerable number of women with PCOS receive these medications as part of their routine medical care.
Metformin Therapy
In this clinic, metformin treatment is initiated in >95% of women diagnosed with PCOS unless contraindicated. Thus, possible selection bias was minimized. Metformin was initiated at 500 mg once daily, and then gradually titrated to the maximal tolerated dose, usually 1500 to 2000 mg daily. All patients with BMI ≥ 25 kg/m2 were provided general counseling on lifestyle changes by the same physician during their initial physician encounter. The identical advice was given to all patients, and patients were referred to the same written source for further information. However, no specialized diet or exercise regimen was recommended.
Data Collection
We recorded patients’ ages at presentation, race, and presence of a family history of diabetes in first-degree relatives. In addition, blood pressure, height and weight, fasting lipid profile (total cholesterol, LDL, HDL and triglycerides), fasting plasma glucose, total and free testosterone levels, were recorded at baseline, and at each subsequent clinic visit where available. Waist and hip circumference measurements were not included in our data collection because it was not part of the routine clinic visits for the majority of the study period. Laboratory analyses were performed by the Clinical Chemistry Laboratory at the Virginia Commonwealth University Health System hospital. Total and free testosterone were measured by Laboratory Corporation of America Holdings (LabCorp, Burlington, NC) through an automated immunoassay.
Definition of the Metabolic Syndrome
The primary outcome of the study was the change in metabolic syndrome parameters before and after metformin treatment. A modified NCEP-ATP III criteria were used to assess metabolic syndrome.12 As previously noted, waist circumference was not available for the majority of subjects. We have previously documented the correlation between body mass index (BMI) and waist circumference of our local women with PCOS who entered into clinical studies, who were derived from the same pool of clinic patients presented in this report.17 We determined that the BMI cutoff value of 32 kg/m2 corresponded to a waist circumference of 88 cm in this PCOS population.17 Therefore, we substituted the metabolic syndrome waist circumference criteria with a BMI of 32 kg/m2 in this study.
Statistical Analysis
The follow-up period was defined as the duration from the date metformin was initiated until the most recent assessment of metabolic syndrome parameters. All continuous variables were presented as mean values and standard deviations if they were normally distributed. Continuous variables not in normal distributions were log-transformed forstatistical analyses, and after back-transformation, were reported in their original units with 95% confidence intervals (95% CIs).
The main outcome of interest was the mean change in each of the metabolic syndrome parameters (BMI, systolic and diastolic blood pressure, triglycerides, HDL and fasting glucose) with metformin treatment compared to baseline. Because some patients began lipid-lowering or antihypertensive therapy during the follow-up period, we used the last observation of blood pressure and lipid parameters (right before initiation of lipid and blood pressure therapy) as their follow-up values.
We first evaluated the mean change in each of the metabolic parameters with metformin treatment in all women using a paired t test. To evaluate metabolic parameters across the follow-up period, we also used random coefficient models with repeated-measures analyses to model the metabolic parameters. This method tested the significance of the time-trend for each metabolic parameter, while accounting for the independent subject response to metformin, which may deviate from the overall response. We performed analyses in all women, and women with and without the metabolic syndrome at baseline as separate groups. Because we were interested in whether PCOS women with and without the metabolic syndrome at baseline had different trends of each metabolic parameter after metformin treatment, we also tested for the interaction between time-trends and baseline metabolic status. In addition, because some women used spironolactone or oral contraceptives, we evaluated whether use of these agents affected the significance of the time-trends for each metabolic parameter using the random coefficient models with repeated-measures analyses.
The secondary outcome of interest was change in the prevalence of the metabolic syndrome, as defined by the modified NCEP definition, from baseline to the last observation. Because some patients began lipid-lowering or antihypertensive therapy during the follow-up period, we used the last observation of blood pressure and lipid parameters (before initiation of lipid and blood pressure therapy) to evaluate the presence of the metabolic syndrome at follow-up. In addition, we also conducted sensitivity analyses using the following assumptions: (1) patients who began antihypertensive treatment would meet the blood pressure criteria of the metabolic syndrome; (2) patients who began lipid-lowering therapy would meet one lipid criterion of the metabolic syndrome; and (3) patients who began lipid-lowering therapy would meet two lipid criteria of the metabolic syndrome. We recalculated the prevalence of the metabolic syndrome at follow-up with these assumptions. Prevalence rates of the metabolic syndrome at baseline and at the last follow-up were compared by the McNemar’s test. For all analyses, p-values <0.05 were considered significant. Analyses were performed using JMP 7.0 and SAS 9.1.3 software (SAS Institute, Cary, NC).
Results
Baseline Characteristics
During the specified time period, there were 242 women with a new diagnosis of PCOS for whom available medical charts were identified. Of these, 70 met the criteria for the study. The majority of patients were excluded due to a short or incomplete follow up (n=95). Women were also excluded for the concurrent use of medications affecting insulin sensitivity or metabolic parameters (n=31), diabetes at baseline (n=7), lack of assessment of metabolic parameters (n=22), self-discontinuation of metformin (n=7), not being treated with metformin (n=8), Cushing’s disorder (n=1), and age < 18 years (n=1). The 70 women suitable for analyses did not differ from the excluded 172 women with regard to any clinical or biochemical characteristic.
At baseline, the prevalence of NCEP ATP-III metabolic syndrome was 34.3% (24/70 women). Women with the metabolic syndrome at baseline were demographically similar to those without (Table 1). Both groups were predominantly Caucasian. The mean ages were similar: 32.6±11.5 years for women with the metabolic syndrome and 29.1±10.5 years for women without the syndrome. Five out of 24 (20.8%) women with and 6 of 46 (13.0%) women without the metabolic syndrome concurrently used oral contraceptives (p=0.4031). The number of concurrent users of spironolactone was also not different between the women with and without the metabolic syndrome (3/24 [12.5%] vs. 11/46 [23.9%], respectively, p=0.2428). Baseline clinical and biochemical characteristics in the metabolic syndrome group differed from the non-metabolic group, as expected. BMI, blood pressure, triglycerides, fasting glucose and total testosterone were all significantly higher in the group with metabolic syndrome, and HDL cholesterol was significantly lower. The mean BMI for women with the metabolic syndrome was 38.5±7.0 kg/m2, which is considered obese. The BMI for women without the metabolic syndrome was 29.2±6.1 kg/m2, which is considered overweight but not obese. Both the metabolic and non-metabolic groups achieved an adequate daily dose of metformin: 1958 ± 204 mg for women with the metabolic syndrome and 1933 ±222 mg for women without the metabolic syndrome (p=0.6381).
Table 1.
Baseline demographic, clinical and biochemical data for 70 women with the polycystic ovary syndrome, stratified by the presence or absence of the metabolic syndrome.
| No MBS (n=46) | MBS (n=24) | P-value | All | |
|---|---|---|---|---|
| Age (years) | 29.1 ± 10.5 | 32.6 ± 11.5 | 0.2052 | 30.3 ± 10.9 |
| Race | 0.4822 | |||
| Caucasian | 43 (93.5%) | 21 (87.5%) | 64 (91.4%) | |
| AA | 2 (2.8%) | 1 (4.2%) | 3 (4.3%) | |
| Other | 1 (1.4%) | 2 (8.3%) | 3 (4.3%) | |
| Length of follow up (months) | 34.8 ± 21.5 | 38.4 ± 25.7 | 0.6966 | 36.1 ± 22.9 |
| Final metformin dose (mg) | 1932.6±222.2 | 1958.3 ± 204.1 | 0.6381 | 1941.4±215 |
| Positive Family History of Diabetes | 17 (37.0%) | 10 (41.7%) | 0.3902 | 27 (38.6%) |
| Weight (kg) | 80.5 ± 17.7 | 105.9 ± 21.0 | <.0001 | 89.2 ± 22.4 |
| BMI (kg/m2) | 29.2 ± 6.1 | 38.5 ± 7.0 | <.0001 | 32.4 ± 7.8 |
| Systolic Blood Pressure (mmHg) | 114.6 ± 11.6 | 125.8 ± 12.2 | 0.0005 | 118.5 ± 12.9 |
| Diastolic Blood Pressure (mmHg) | 72.5 ± 7.9 | 80.8 ± 11.0 | 0.0004 | 75.4 ± 9.8 |
| Total Testosterone (ng/dL) | 51.6 ± 18.1 | 66.0 ± 20.3 | 0.0039 | 56.5 ± 19.9 |
| Free Testosterone (ng/dL) | 1.01 ± 0.51 | 1.31 ± 0.60 | 0.0399 | 1.11 ± 0.56 |
| Total Cholesterol (mg/dL) | 185.3 ± 38.0 | 206.8 ± 39.2 | 0.0292 | 192.7 ± 39.5 |
| LDL-C (mg/dL) | 111.2 ± 31.3 | 122.4 ± 28.6 | 0.0308 | 115.1 ± 30.7 |
| HDL-C (mg/dL) | 52.0 ± 11.9 | 44.2 ± 9.2 | 0.0067 | 49.3 ± 11.6 |
| Triglycerides (mg/dL) | 108.5 ± 88.9 | 203.2 ± 86.4 | <0.0001 | 141.0 ± 98.5 |
| Fasting Glucose (mg/dL) | 82.5 ± 7.5 | 88.1 ± 10.0 | 0.0249 | 84.4 ± 8.8 |
| Sex Hormone Binding Globulin‡(μg/mL) | 3.38 [2.63–4.34] | 4.78 [3.35–6.80] | 0.1863 | 3.80 [3.08–4.68] |
| Concurrent Oral Contraceptives | 6 (13.0%) | 5 (20.8%) | 0.4031 | 11 (15.71%) |
| Concurrent Spironolactone | 11 (23.9%) | 3 (12.5%) | 0.2428 | 14 (20.0%) |
MBS=Metabolic syndrome
p-value is for comparison between women with and without MBS at baseline
Values are presented as mean ± SD, or number (%) except where noted
Geometric mean with 95% confidence intervals
The distribution of metabolic abnormalities in patients with and without the metabolic syndrome is shown in Table 2. Decreased HDL cholesterol was the most common metabolic abnormality in women with PCOS, either with or without the metabolic syndrome, while impaired fasting glucose was the least common. As shown in Table 2, women without the metabolic syndrome still presented with suboptimal metabolic status.
Table 2.
Distribution of metabolic abnormalities at baseline.
| No MBS (n=46) | MBS (n=24) | All (n=70) | |
|---|---|---|---|
| BMI >32 kg/m2 | 10 (21.7%) | 19 (79.2%) | 29 (41.4%) |
| Systolic Blood Pressure ≥130 mmHg | 7 (15.2%) | 12 (50.0%) | 19 (27.1%) |
| Diastolic Blood Pressure ≥85 mmHg | 2 (4.4%) | 10 (41.7%) | 12 (17.1%) |
| HDL-C < 50 mg/dL | 22 (47.8%) | 20 (83.3%) | 42 (60.0%) |
| Triglycerides ≥ 150 mg/dL | 5 (10.9%) | 19 (79.2%) | 24 (34.3%) |
| Fasting Plasma Glucose ≥ 100 mg/dL | 0 (0%) | 5 (20.8%) | 5 (7.1%) |
MBS= metabolic syndrome
Effects of Metformin on Individual Metabolic Syndrome Parameters
The women with PCOS were followed via routine clinic visits and the length of follow-up ranged from 6 months to 102 months. The mean was 36.1±22.9 months (median 32.0 months). Comparing metabolic parameters at the last follow-up to baseline (Table 3), significant improvements were observed for BMI (−1.09 ±3.48 kg/m2, p=0.0117), diastolic blood pressure (−2.69±10.35 mmHg, p=0.0378), and HDL cholesterol (+5.82±11.02 mg/dL, p<0.0001). In the random coefficient models with repeated-measures analyses, these same 3 parameters showed a significant improvement across the duration of metformin treatment (p=0.0185 for BMI, p=0.0420 for diastolic blood pressure, and p=0.0031 for HDL cholesterol, Table 4). Even after adjusting for concurrent spironolactone and oral contraceptive use, the time-trends for these three metabolic parameters remained materially unchanged (p=0.0569 for BMI, p=0.0365 for diastolic blood pressure, and p=0.0026 for HDL cholesterol after adjusting for spironolactone use; p=0.0571 for BMI, p=0.0417 for diastolic blood pressure, and p=0.0025 for HDL cholesterol after adjusting for oral contraceptive use).
Table 3.
Changes in clinical and metabolic parameters from baseline after metformin therapy in all women, and in women with and without the metabolic syndrome.
| All (n=70) | p | |
|---|---|---|
| BMI (kg/m2) | −1.09 ± 3.48 | 0.0117 |
| Systolic Blood Pressure (mmHg) | −1.23 ± 18.06 | 0.5764 |
| Diastolic Blood Pressure (mmHg) | −2.69 ± 10.35 | 0.0378 |
| Total Cholesterol (mg/dL) | −5.46 ± 35.29 | 0.2096 |
| LDL-C (mg/dL) | −5.43 ± 33.33 | 0.1868 |
| HDL-C (mg/dL) | +5.82 ± 11.02 | <0.0001 |
| Triglycerides (mg/dL) | −10.31 ± 88.01 | 0.3410 |
| Fasting Glucose (mg/dL) | −1.00 ± 10.62 | 0.5328 |
| Testosterone (ng/dL) | −19.28 ± 22.90 | <0.0001 |
| Free Testosterone (ng/dL) | 0.30 ± 1.36 | 0.0792 |
MBS=Metabolic syndrome
p-value is for comparison between last observation and baseline
Values are mean ± SD
Table 4.
Effect of baseline metabolic syndrome status and time on metformin on trend of metabolic parameters during follow-up period.
| MBS Status | Time on metformin | |||
|---|---|---|---|---|
| F-statistic | p-value | F-statistic | p-value | |
| BMI | 20.68 | <0.0001 | 6.05 | 0.0185 |
| Systolic BP | 18.97 | <0.0001 | 0.65 | 0.4321 |
| Diastolic BP | 26.61 | <0.0001 | 4.60 | 0.0420 |
| HDL-C | 9.02 | 0.0037 | 12.14 | 0.0031 |
| Triglycerides | 33.27 | <0.0001 | 0.31 | 0.5820 |
| Fasting glucose | 8.72 | 0.0045 | 0.00 | 0.9526 |
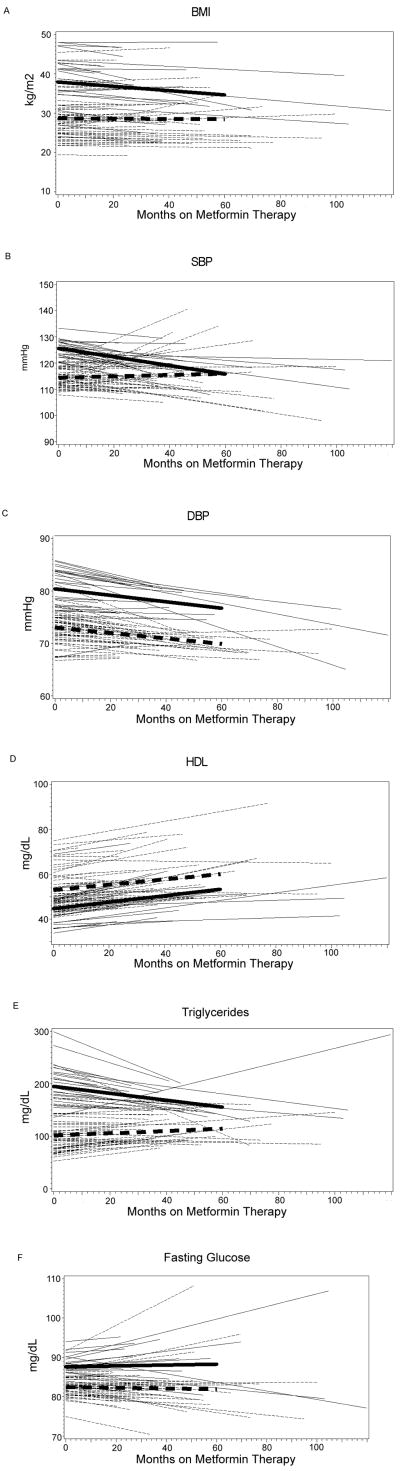
We also observed that both metabolic syndrome status at baseline and metformin treatment were important predictors for the change in the metabolic parameters during follow-up (Table 4). Hence, we evaluated the change in metabolic parameters with metformin treatment, stratified by patients with and without metabolic syndrome at baseline (Table 5). Figures 1A–F show the summary (thicker lines) and individual (thinner lines) time-trends during metformin treatment for each metabolic parameter in women with and without the metabolic syndrome.
Table 5.
Change in metabolic parameters from baseline to median follow-up time (32 months) stratified by baseline metabolic syndrome status.
| No MBS at Baseline | MBS at Baseline | |||||
|---|---|---|---|---|---|---|
| Baseline Mean (SD) | 32 months Mean (SD) | p-value | Baseline Mean (SD) | 32 months Mean (SD) | p-value | |
| BMI (kg/m2) | 28.7 (0.98) | 28.6 (1.00) | 0.8076 | 37.9 (1.31) | 36.2 (1.34) | 0.0060 |
| Systolic BP (mmHg) | 114.4 (1.48) | 115.2 (1.74) | 0.7002 | 125.7 (1.91) | 120.4 (2.40) | 0.0785 |
| Diastolic BP (mmHg) | 73.0 (1.09) | 71.4 (0.87) | 0.1287 | 80.4 (1.41) | 78.5 (1.19) | 0.1772 |
| Triglycerides (mg/dL) | 101.4 (9.82) | 108.2 (7.98) | 0.4728 | 194.8 (12.80) | 173.7 (10.81) | 0.0947 |
| HDL (mg/dL) | 53.0 (1.67) | 56.8 (1.82) | 0.0181 | 44.6 (2.23) | 49.3 (2.52) | 0.0400 |
| Fasting Glucose (mg/dL) | 82.4 (1.13) | 82.2 (1.47) | 0.8558 | 87.6 (1.56) | 87.9 (2.08) | 0.8680 |
Figure 1. Time-Trends of Metabolic Parameters During Metformin Therapy.
—Time trend for women with metabolic syndrome at baseline
---Time trend for women without metabolic syndrome at baseline
Thick lines are summary time-trends; thin lines are individuals’ time-trends
Interaction between time-trend and baseline metabolic syndrome: p=0.0369 for BMI, p=0.0989 for SBP, p=0.8595 for DBP, p=0.7305 for HDL, p=0.0785 for triglycerides, p=0.8093 for fasting glucose.
Among the metabolic parameters, the interaction between baseline metabolic syndrome status and time on metformin was significant for BMI (p=0.0369). In women with the metabolic syndrome, metformin significantly reduced BMI (p=0.0060, Table 5), an effect not seen in women without the metabolic syndrome (p=0.8076). The course of BMI reduction during metformin treatment was more pronounced in women with the metabolic syndrome: the summary time-trend of BMI in PCOS women with metabolic syndrome decreased by 0.5 kg/m2 for each 10-month period, and the trend of BMI in women without the metabolic syndrome only decreased by 0.03 kg/m2 for each 10-month period (Figure 1A). HDL increased in both groups (p=0.0400 in those with metabolic syndrome and p=0.0181 in those without metabolic syndrome, Figure 1D and Table 5), and its time-trend was not different according to baseline metabolic status (p=0.7305 for interaction, figure 1D). There was a trend towards differential change in triglycerides (p=0.0785 for interaction) between the metabolic and non-metabolic groups, but the trend in the metabolic group was of marginal significance (p=0.0947, Table 5). The time-trends for fasting glucose, systolic and diastolic blood pressure were not different depending upon the women’s metabolic syndrome status at baseline (Figures 1B, C, F and Table 5), and the time-trends for either groups separately were not significant, except for systolic blood pressure, which was borderline significant (p=0.0785) in women with baseline metabolic syndrome. Hence, while all PCOS women derived a beneficial increase in HDL with metformin, women who also had the metabolic syndrome at baseline appeared to decrease their BMI with metformin more than those without metabolic syndrome. There was also a trend towards differential benefit for systolic blood pressure and triglycerides in women with the metabolic syndrome at baseline.
Effect of Metformin on the Prevalence of the Metabolic Syndrome
At the last follow up, the prevalence of metabolic syndrome had decreased from 34.3% (24/70 women) at baseline to 21.4% (15/70 women, χ2= 3.857, p=0.0495).
During the follow-up period, two women began antihypertensive therapy (1 woman on diuretic, and another on a calcium channel blocker), and 6 women began lipid-lowering therapies (5 women started a statin and 1 woman on niacin). For these individuals, in addition to using the last observations of lipid and blood pressure parameters before initiation of therapies, we also conducted a sensitivity analyses by assuming that (1) patients who began antihypertensive treatment would have met the blood pressure criteria of the metabolic syndrome; (2) patients who began lipid-lowering therapy would have met one lipid criterion of the metabolic syndrome; and (3) patients who began lipid-lowering therapy would have met two lipid criteria of the metabolic syndrome. The prevalence of metabolic syndrome at follow-up did not change with assumptions 1 and 2, and remained at 21.4% (15/70 women). If lipid-lowering therapy were counted as two lipid criteria of the metabolic syndrome, the prevalence of the metabolic syndrome would have been 22.9% (16/70 women) at follow up, which was not materially different from the prevalence rate of 21.4% as determined in our main analysis.
Of the 24 women with metabolic syndrome at baseline, 15 of them no longer met the metabolic syndrome criteria at the last follow-up after metformin treatment, while 9 of them continued to have the metabolic syndrome. Of the 46 women without metabolic syndrome at baseline, only 6 developed the metabolic syndrome during follow-up. These 6 women were significantly older (39.2±11.0 vs. 27.6±9.7 years, p=0.0103), and more obese (BMI 37.3±6.6 vs. 28.0±5.0 kg/m2, p=0.0002) as compared with the other 40 women without metabolic syndrome at baseline and who did not develop the syndrome during the observation period.
Discussion
The aim of this study was to evaluate the long-term effects of metformin on metabolic parameters in women with PCOS during routine care without a controlled diet. We hypothesized that metformin would improve overall metabolic profiles in women with PCOS.
In this study, the initial prevalence of metabolic syndrome was 34.3%, which is consistent with previously reported prevalence rates.16–18 This is considerably higher than the prevalence rate for normal women in their 30’s, which is only 15%.19 However, at the end of a mean follow-up of 36.1 months of treatment with metformin, the prevalence rate of the metabolic syndrome had decreased significantly to 21.4%. Metformin appears to have decreased the prevalence of metabolic syndrome in a PCOS population to a prevalence rate closer to that of a normal, non-PCOS population. In all women, without stratification based on metabolic syndrome status at baseline, there were significant improvements in three metabolic parameters: BMI, HDL cholesterol, and diastolic blood pressure.
There is a wide body of evidence suggesting that the presence of the metabolic syndrome greatly increases an individual’s risk for cardiovascular events.14,34,35 Although still debated, women with PCOS may be at a greater risk of cardiovascular disease.7,36 Therefore, the prevention and amelioration of metabolic syndrome may be an important factor in the long-term care of PCOS patients. In this study, metformin appears to be effective in ameliorating the metabolic syndrome in PCOS women over 3 years of follow-up.
Besides ameliorating the metabolic syndrome already present, metformin appears to be also effective in preventing the onset of the metabolic syndrome. At baseline, even women without the metabolic syndrome commonly had metabolic abnormalities (Table 2). However, only 6 out of 46 women (13%) developed metabolic syndrome over the course of their treatment. Although there currently is no published figure on the annual conversion rate from a non-metabolic status to incident metabolic syndrome in untreated PCOS women, one might expect it to be higher than 13% given the high prevalence of the metabolic syndrome in PCOS16–18 and the greater risk of developing metabolic syndrome with increasing age or time.19
Our findings are consistent with multiple short term studies of 1 year or less on the effects of metformin in women with PCOS.16,25–28,37,38 In addition our results are consonant with those reported by Glueck et al, in which women with PCOS treated with a combination of metformin and controlled diet had significant and sustained improvements in all parameters of the metabolic syndrome over 4 years.31 Previous research studies have suggested that lifestyle changes in PCOS are difficult to maintain long-term,39 and may be particularly so in a non-research environment. Our study shows that beneficial effects of metformin on the metabolic syndrome, without a specific lifestyle modification regimen, can be sustained over 3 years of routine clinic follow-up in women with PCOS.
Baseline metabolic syndrome status also affected the time-trend of metabolic parameters in women with PCOS treated with metformin. Specifically, BMI reduction was significantly more pronounced in women with PCOS who also had the metabolic syndrome at baseline compared with those without the metabolic syndrome. When women were stratified into those with and without metabolic syndrome at baseline, despite a smaller number of women in each group, metformin treatment was associated with a significant improvement in BMI and HDL, and a trend toward improvement in systolic blood pressure in the women with the metabolic syndrome (Table 5). In contrast, in women without the metabolic syndrome at baseline, metformin treatment significantly improved only HDL cholesterol, with other parameters remained essentially unchanged (Table 5).
Our report of a differential effect of metformin on metabolic syndrome parameters in women with PCOS with and without the metabolic syndrome awaits confirmation by other studies. Although previous studies did not evaluate metabolic syndrome parameters, these studies yielded conflicting data regarding whether obese or lean women may derive more clinical and biochemical benefits from metformin. Administration of metformin for 12 weeks in women with PCOS and a mean BMI of 39 kg/m2 did not improve hyperinsulinemia or androgen excess.40 Other evidence suggests that lean PCOS women may not benefit from metformin in regards to metabolic risk factors. In a 6-month study, metformin improved menstrual cyclicity, but not glucose and insulin responses to OGTT, or insulin resistance as assessed by the homeostatic model (HOMA-IR) in lean women with PCOS.41 In another 6-month evaluation in which metformin and placebo was administered in a cross-over design, fasting glucose and insulin concentrations, blood pressure and HOMA did not improve in non-obese women with PCOS.42 In yet another study, treatment with metformin for 6 months in lean (mean BMI 22.0 kg/m2), overweight (mean BMI 26.8 kg/m2), and obese (mean BMI 38.1 kg/m2) women with PCOS improved HOMA-IR, fasting glucose and insulin, BMI, testosterone and ovulatory frequency.43 The beneficial effects of metformin in this study were irrespective of pre-treatment BMI and pre-treatment insulin resistance.
Our study has several limitations. This is not a randomized controlled study and we do not have a control group of women treated with placebo for 3 years. Leaving such a group of women with signs and symptoms of hyperandrogenemia untreated for several years was not an option and might be considered unethical. While the improvements seen in the metabolic parameters with metformin in this study could simply be due to the passage of time or attendance at a PCOS clinic, this is unlikely. From data derived from other specialized PCOS centers, the conversion rate from normal glucose tolerance to impaired glucose tolerance is high in women with PCOS (up to 16% per year).44,45 This suggests that despite specialized care, the metabolic profile of PCOS women generally worsens over time. While it is possible that other confounding factors, such as changes in diet or weight loss, could have led to an improvement in the metabolic profile, we have excluded PCOS women who received any medically-assisted drug or surgical weight loss treatment during the observation period. It is also possible that obese women may have had received more encouragement to lose weight and hence had more improvement in metabolic parameters. However, overweight or obese PCOS patients received only general counseling on lifestyle changes during their initial physician encounter. The same lifestyle counseling was provided to these patients by the same physician, and no specialized diet or exercise regimen was recommended. It is not expected that such general lifestyle counseling would have significant beneficial effects on weight reduction. In fact, in the Diabetes Prevention Project (DPP), patients receiving the standard lifestyle recommendations, which included written information and an annual 20-to-30 minute individual session, did not lose weight at any time point during the study.46 The DPP standard lifestyle recommendations were more intensive than the general counseling provided in this study. Hence, we did not expect intentional weight loss to be an important confounding factor in the interpretation of our results.
Because data were collected retrospectively in this study, some information was not available. Waist circumference was not widely recorded in the clinic during the period of the study as it was not routine clinical practice at the time; however, we were able to substitute waist circumference with BMI, based on their correlation derived from a similar local PCOS population.17 This modified NCEP-III criteria was also used in one of the first studies that defined the prevalence rate of metabolic syndrome in PCOS.17 Other investigators have also reported a similar prevalence of the metabolic syndrome when using either waist circumference or BMI to assess the syndrome.47
In conclusion, metformin improved the metabolic profile of women with PCOS over a mean follow-up of 36.1 months, particularly in HDL cholesterol, diastolic blood pressure and BMI. In addition, women with PCOS who also had the metabolic syndrome at baseline seemed to derive more metabolic benefits from metformin. Whether metformin’s amelioration of the metabolic syndrome would also decrease the risk of cardiovascular events and diabetes remains to be tested.
Acknowledgments
This study was supported in part by National Institutes of HealthGrants K23HD049454 (to K.I.C), K24HD40237 (to J.E.N.), and Virginia Commonwealth University Honor’s Summer Research Fellowship (to J.M.H.).
Footnotes
Conflict of interest statement: All authors have nothing to declare.
Reference List
- 1.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–38. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 2.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 3.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–69. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 4.Nestler JE. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol. 1997;15:111–22. doi: 10.1055/s-2007-1016294. [DOI] [PubMed] [Google Scholar]
- 5.Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. J Clin Endocrinol Metab. 1997;82:4075–79. doi: 10.1210/jcem.82.12.4431. [DOI] [PubMed] [Google Scholar]
- 6.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–23. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 7.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1357–63. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 8.Dunaif A. Hyperandrogenic anovulation (PCOS): a unique disorder of insulin action associated with an increased risk of non-insulin-dependent diabetes mellitus. Am J Med. 1995;98:33S–9S. doi: 10.1016/s0002-9343(99)80057-6. [DOI] [PubMed] [Google Scholar]
- 9.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–46. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, D’Agostino R, Jr, Festa A, Bergman RN, Mykkanen L, Karter A, et al. Low insulin sensitivity (S(i) = 0) in diabetic and nondiabetic subjects in the insulin resistance atherosclerosis study: is it associated with components of the metabolic syndrome and nontraditional risk factors? Diabetes Care. 2003;26:2796–803. doi: 10.2337/diacare.26.10.2796. [DOI] [PubMed] [Google Scholar]
- 11.Hanley AJ, D’Agostino R, Jr, Wagenknecht LE, Saad MF, Savage PJ, Bergman R, et al. Increased proinsulin levels and decreased acute insulin response independently predict the incidence of type 2 diabetes in the insulin resistance atherosclerosis study. Diabetes. 2002;51:1263–70. doi: 10.2337/diabetes.51.4.1263. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–22. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 14.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–89. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 15.Trevisan M, Liu J, Bahsas FB, Menotti A. Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol. 1998;148:958–66. doi: 10.1093/oxfordjournals.aje.a009572. [DOI] [PubMed] [Google Scholar]
- 16.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 17.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 18.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–59. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 20.Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951–53. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–19. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Aguiar LG, Bahia LR, Villela N, Laflor C, Sicuro F, Wiernsperger N, et al. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006;29:1083–89. doi: 10.2337/diacare.2951083. [DOI] [PubMed] [Google Scholar]
- 24.Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GM. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258:250–56. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg N, Glueck CJ, Loftspring M, Sherman A, Wang P. Metformin-diet benefits in women with polycystic ovary syndrome in the bottom and top quintiles for insulin resistance. Metabolism. 2005;54:113–21. doi: 10.1016/j.metabol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Lord J, Thomas R, Fox B, Acharya U, Wilkin T. The effect of metformin on fat distribution and the metabolic syndrome in women with polycystic ovary syndrome--a randomised, double-blind, placebo-controlled trial. BJOG. 2006;113:817–24. doi: 10.1111/j.1471-0528.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C, McGrath BP, Teede HJ. Effects of medical therapy on insulin resistance and the cardiovascular system in polycystic ovary syndrome. Diabetes Care. 2007;30:471–78. doi: 10.2337/dc06-0618. [DOI] [PubMed] [Google Scholar]
- 28.Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:4649–54. doi: 10.1210/jc.2002-021688. [DOI] [PubMed] [Google Scholar]
- 29.Orio F, Manguso F, Di Biase S, Falbo A, Giallauria F, Labella D, et al. Metformin administration improves leukocyte count in women with polycystic ovary syndrome: a 6-month prospective study. Eur J Endocrinol. 2007;157:69–73. doi: 10.1530/EJE-07-0133. [DOI] [PubMed] [Google Scholar]
- 30.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21:80–89. doi: 10.1093/humrep/dei311. [DOI] [PubMed] [Google Scholar]
- 31.Glueck CJ, Aregawi D, Agloria M, Winiarska M, Sieve L, Wang P. Sustainability of 8% weight loss, reduction of insulin resistance, and amelioration of atherogenic-metabolic risk factors over 4 years by metformin-diet in women with polycystic ovary syndrome. Metabolism. 2006;55:1582–89. doi: 10.1016/j.metabol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Boston: Blackwell Scientific; 1992. pp. 377–84. [Google Scholar]
- 33.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–67. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–97. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 35.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- 36.Cobin RH, Futterweit W, Nestler JE, Reaven GM, Jellinger PS, Handelsman Y, et al. American Association of Clinical Endocrinologists Position Statement on Metabolic and Cardiovascular Consequences of Polycystic Ovary Syndrome. Endocr Pract. 2005;11:125–34. doi: 10.4158/EP.11.2.125. [DOI] [PubMed] [Google Scholar]
- 37.Krstevska B, Dimitrovski C, Pemovska G, Misevska S, Dimova Z, Simeonova S, et al. Metformin improves menstrual patterns, endocrine and metabolic profile in obese hyperinsulinemic women with a polycystic ovary syndrome. Prilozi. 2006;27:57–66. [PubMed] [Google Scholar]
- 38.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–46. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 39.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13:251–57. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- 40.Ehrmann DA, Cavaghan MK, Imperial J, Sturis J, Rosenfield RL, Polonsky KS. Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:524–30. doi: 10.1210/jcem.82.2.3722. [DOI] [PubMed] [Google Scholar]
- 41.Sahin Y, Unluhizarci K, Yilmazsoy A, Yikilmaz A, Aygen E, Kelestimur F. The effects of metformin on metabolic and cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007 doi: 10.1111/j.1365-2265.2007.02985.x. [DOI] [PubMed] [Google Scholar]
- 42.Trolle B, Flyvbjerg A, Kesmodel U, Lauszus FF. Efficacy of metformin in obese and non-obese women with polycystic ovary syndrome: a randomized, double-blinded, placebo-controlled cross-over trial. Hum Reprod. 2007;22:2967–73. doi: 10.1093/humrep/dem271. [DOI] [PubMed] [Google Scholar]
- 43.Tan S, Hahn S, Benson S, Dietz T, Lahner H, Moeller LC, et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur J Endocrinol. 2007;157:669–76. doi: 10.1530/EJE-07-0294. [DOI] [PubMed] [Google Scholar]
- 44.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–42. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 45.Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16:1995–98. doi: 10.1093/humrep/16.9.1995. [DOI] [PubMed] [Google Scholar]
- 46.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meigs JB, Wilson PW, Nathan DM, D’Agostino RB, Sr, Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160–67. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]