Abstract
Vaccines are attractive as consolidation therapy after autologous stem cell transplantation (ASCT) for multiple myeloma (MM). We report the results of a phase II trial of the immunotherapeutic, APC8020 (Mylovenge™), given after ASCT for MM. We compared the results with that of other patients with MM who underwent ASCT at Mayo Clinic during the same time period. Twenty-seven patients were enrolled on the trial between July, 1998 and June, 2001, and the outcomes were compared to that of 124 consecutive patients transplanted during the same period, but not enrolled on the trial. The median (range) follow-up for patients still alive from the vaccine trial is 6.5 (2.9–8 years), and 7.1 (6–8 years) in the control group. The median age was 57.4 range (36.1–71.3) in the DB group and 56.4 (range, 30–69) in the trial group. Known prognostic factors including PCLI, B2M, and CRP were comparable between the groups. The median overall survival for the trial patients was 5.3 years (95% CI: 4.0 years—N/A) compared to 3.4 years (95% CI: 2.7–4.6 years) for the DB group (P = 0.02). The median time to progression and progression-free survival for the trial group was similar to the DB group. Although not a controlled trial, the vaccines given after ASCT appear to be associated with improved overall survival compared to historical controls. This approach warrants further investigation to confirm this and define the role of vaccine therapy in myeloma.
Introduction
Multiple myeloma is characterized by malignant transformation of mature, immunoglobulin secreting plasma cells. New therapeutic options have improved survival in patients with multiple myeloma [1], but the disease remains incurable despite these recent advances. This fact has motivated the search for alternative therapeutic approaches. One such alternative is to utilize the immune system to specifically target and eliminate neoplastic cells on the basis of their expression of immunogenic markers. We hypothesized immunotherapy is most likely to work when the tumor burden is low and the immediate interval following autologous stem cell transplantation is an opportune time for patients to benefit from such therapy.
B cell malignancies, including malignant lymphoma and multiple myeloma, are unique in their expression of immunoglobulin (Ig). The Ig on malignant cells can be distinguished from normal B cells or plasma cells by virtue of specific idiotypic determinants [2]. Because these malignancies are monoclonal, all the cells of a given tumor express or secrete identical Ig. Despite the identification of tumor associated antigens, such as immunoglobulin idiotype, these antigens are ordinarily not recognized as foreign by the body’s immune system.
We conducted a trial using idiotype loaded antigen presenting cells, APC8020 (Mylovenge™), as consolidation therapy following autologous stem cell transplant (ASCT) for patients with MM. APC8020 is prepared from autologous antigen presenting cells (APC), including dendritic cells, partially purified from an unmobilized leukapheresis product by gradient density isolation and then incubated for 2 days with autologous serum containing M protein obtained pretransplant. We now present data on durability of response, time to progression (TTP), progression free survival (PFS) and overall survival with the use of APC8020 vaccine therapy as consolidation following ASCT for myeloma. However, because patients were enrolled when in remission post transplant, it was difficult to ascertain the effects of the vaccines from delayed responses to the transplant. We report long term results of the vaccine trial compared retrospectively to all other patients with MM who underwent autologous stem cell transplant at Mayo Clinic during the same time period.
Patients and Methods
Clinical trial patients
Patient enrolled in the vaccine trial had to have a diagnosis of multiple myeloma and have completed an autologous stem cell transplant either at relapse or as part of the initial planned induction therapy. They had to be at least 60 days status post the autologous stem cell transplant. Patients had to have a quantifiable M-protein in the serum or have had serum with a quantifiable M-protein frozen and stored prior to ASCT for vaccine production. Patients also needed to have platelet count greater than 50 × 109/L, absolute neutrophil count greater than 1.5 × 109/L, total bilirubin less than five times the upper limits of normal and creatinine less than 5 mg/dL. Pregnant or nursing women were required to have a negative pregnancy test and all sexually active patients were required to practice a medically acceptable form of birth control. Patients were required to be at least 18 years of age. Patients were ineligible if they had progressed after ASCT or if they had non-secretory or light chain only myeloma. Responses were assessed according to the criteria used by the Eastern Cooperative Oncology Group (ECOG) [3]. The study was approved by the Mayo Clinic Institutional Review Board in accordance with federal regulations and the Declaration of Helsinki.
Control group
The group of patients used for comparison consisted of consecutive patients with multiple myeloma who underwent autologous stem cell transplant at Mayo Clinic during the same interval as those who were enrolled in the trial, July of 1998 and May of 2001. Data pertaining to the transplant patients were captured prospectively into a database which is continuously updated. Complete follow-up was available for all the patients. All patients had provided written informed consent for use of their medical records.
Preparation and administration of APC8020
Patients underwent a series of four treatments. Treatment was given intravenously in weeks 0, 2, 4, and 16. APC8020 was prepared fresh for each treatment course. Dendritic-cell precursors and antigen presenting cells were harvested from the peripheral blood by a standard 1.5–2.0 blood volume mononuclear cell leukapheresis. Mobilization with a colony-stimulating factor was not required. Dendritic-cell precursors were collected by two sequential buoyant density centrifugation steps by a modification of the method of Hsu et al. [4,5]. Briefly, the leukapheresis product is layered over a buoyant density solution (specific gravity = 1.077 g/mL) and centrifuged at 1,000 g for 20 min to deplete erythrocytes and granulocytes. The interface cells are collected, washed, layered over a second buoyant density solution (specific gravity = 1.065 g/mL), and centrifuged at 805g for 30 min to deplete platelets and low-density monocytes and lymphocytes. The cell pellet, which contains dendritic-cell precursors, is washed and incubated in AIM media with 10 μg/mL of patient serum as a source of M-protein. The culture media did not contain serum or exogenous cytokines. After incubation for 40 hr at 37°C in 5% CO2 atmosphere, the cells were washed and formulated at the desired clinical dose in 250 mL of lactated Ringers’ solution. Criteria for releasing the products for infusion included the following: (1) in-process sterility tests with no growth at 40 hr; (2) endotoxin less than 1.4 EU/ mL; (3) CD54 expression greater than 3 SD above T = 0 value; (4) cell viability greater than 72%; and (5) phenotype consistent with the values listed in Table I. Phenotype was determined by flow cytometry using monoclonal antibodies to CD4, CD8, CD54, CD56, CD66b, and CD86 (Becton Dickenson, San Jose, CA; Coulter, Miami, FL).
TABLE I.
Phenotype of Antigen Presenting Cells
| Final cell count (106) | Viability (%) | CD14 (%) | CD19 (%) | CD66b (%) | CD56 (%) | HLA-DR (%) | CD3 (%) | CD54 (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Min | 141 | 74 | 0 | 0 | 0 | 0 | 6 | 27 | 2 |
| Max | 7,406 | 98 | 62 | 21 | 9 | 28 | 62 | 89 | 60 |
| Average | 3,139 | 89 | 21 | 8 | 0 | 9 | 21 | 65 | 24 |
| Median | 3243.5 | 89 | 19 | 7 | 0 | 8 | 18 | 66 | 21 |
| N | 104 | 104 | 104 | 104 | 95 | 95 | 95 | 103 | 104 |
The cell product was stored at 4°C until infusion into the patient. The APC8020 product was infused into the patient within 6 hr after it was released by Mayo Cell Processing Center. APC8020 was infused over 30 min. Patients were not routinely premedicated before the infusion. They were observed for 30 min after infusion and then discharged to home.
Statistical analysis
Time to progression (TTP) was defined as the time from transplant, as recorded in the database, to the first documentation of disease progression. Patients who died without progression were censored at the date of death for progression. Progression free survival (PFS) was defined as the time from the date of transplant to the earlier of the dates of death from any cause or disease progression. Overall survival (OS) was defined as the time from the date of transplant until the date of death. Fisher’s exact tests and Wilcoxon rank-sum tests were used to compare the baseline characteristics between the database and vaccine trial patients. The distributions of time to event data were estimated using the Kaplan-Meier method, and compared between the vaccine trial and the database using the stratified log rank test statistic, with disease group (relapsed versus newly diagnosed) as a stratification factor.
Results
Data was frozen in January 2008 for this analysis. A total of 151 patients were included; 124 patients from the database, and 27 patients from the vaccine trial. The median (range) of follow-up for alive patients in the vaccine trial and the database is 6.5 (range, 2.9–8), and 7.1 (range, 6–8) years respectively. All follow-up was censored at 8 years for OS, and 5 years for TTP and PFS analysis.
Patient characteristics
The baseline characteristics are outlined in Table II. Among the database patients, 50 were transplanted in response/plateau, 15 after failing induction therapy (primary refractory), 45 relapsed off therapy and 14 relapse on therapy (refractory relapse). Among the trial patients, the status prior to ASCT consisted of: relapse off treatment 11 patients; in response/plateau 10 patients; primary refractory to initial chemotherapy 6 patients. One hundred and seven (88%) database patients had a response to a prior therapy. Twenty-six trial patients (96%) had a response to a prior therapy. There are no significant differences in the baseline factors between the trial and the database patients. The median time from diagnosis to transplant was 7.7 and 7.9 months, respectively for the database and the vaccine trial patients (P = 0.44).
TABLE II.
Patient Characteristics (All Patients)
| Database patients (N = 124) | Vaccine trial patients (N = 27) | P value | |
|---|---|---|---|
| Age | 0.99 | ||
| N | 124 | 27 | |
| Mean (SD) | 57.1 (7.25) | 56.4 (9.34) | |
| Median | 57.4 | 56.0 | |
| Q1, Q3 | 52.5, 62.5 | 52.0, 64.0 | |
| Range | (36.1–71.3) | (30.0–69.0) | |
| Gender | 1.00 | ||
| f | 44 (35.5%) | 9 (33.3%) | |
| m | 80 (64.5%) | 18 (66.7%) | |
| B2M | 0.91 | ||
| N | 122 | 27 | |
| Mean (SD) | 2.7 (1.93) | 2.4 (1.15) | |
| Median | 2.1 | 2.3 | |
| Q1, Q3 | 1.6, 3.0 | 1.4,3.3 | |
| Range | (1.0–10.1) | (1.0–5.3) | |
| CRP | 0.66 | ||
| N | 123 | 27 | |
| Mean (SD) | 1.1 (2.69) | 0.6 (0.67) | |
| Median | 0.4 | 0.4 | |
| Q1, Q3 | 0.4, 0.5 | 0.4, 0.5 | |
| Range | (0.0–25.7) | (0.1–3.1) | |
| PCLI | 0.08 | ||
| N | 123 | 26 | |
| Mean (SD) | 1.0 (1.78) | 0.4 (0.62) | |
| Median | 0.4 | 0.1 | |
| Q1, Q3 | 0.0, 1.2 | 0.0, 0.6 | |
| Range | (0.0–11.2) | (0.0–2.4) | |
| Salmon-Durie Stage | |||
| II | 33 | 8 | |
| III | 91 | 19 | |
| Time from diagnosis to transplant | |||
| 7.73 months (range: 3.71–84.40 months) | 7.92 months (range: 5.09–57.95) | ||
| Status prior to Transplant | |||
| Response/plateau | 48 | 10 | |
| Primary refractory | 15 | 6 | |
| Relapsed off therapy | 46 | 11 | |
| Relapse on therapy | 15 |
All P values for categorical variables are Fisher’s exact. All P values for continuous variables are Rank Sum.
Objective responses
Following ASCT, 26 (96%) patients in the vaccine trial and 109 (88%) database patients had achieved an objective response. Among the trial patients further improvement of response following the vaccines consisted of CR in 6 patients and PR in 2 patients. The remaining 19 patients had continued stable disease (SD). The vaccines were well tolerated. Toxicity is outlined in Table III.
TABLE III.
Toxicity of Vaccines
| Grade |
||||
|---|---|---|---|---|
| 1 % | 2 % | 3 % | ||
| Body System | Toxicity | |||
| Hematology | Anemia | 7 | ||
| Hemorrhage | Hematuria | 4 | ||
| Hepatic | SGOT (AST) | 7 | 4 | |
| SGPT (ALT) | 4 | 7 | ||
| ALK PHOS | 4 | |||
| Infection | Infection | 4 | 26 | |
| Musculoskeletal | Arthritis | 4 | ||
| Musculoskeletal | 4 | |||
| Neurology | Insomnia | 4 | ||
| Depression | 4 | |||
| Pain | Headache | 4 | ||
| Myalgia | 7 | 4 | 4 | |
| Arthralgia | 4 | 4 | ||
| Pain-bone | 11 | 4 | ||
| Pain-abdominal | 4 | |||
| Pain-chest | 4 | |||
| Neuralgia | 4 | |||
| Pulmonary | Cough | 4 | ||
| Pneumonitis | 4 | 4 | ||
| Renal/Genitourinary | Creatinine | 4 | 4 | |
| Cardiovascular | Hypotension | 4 | ||
| Constitutional Symptoms | Fatigue | 11 | 4 | |
| Fever | 4 | |||
| Weight loss | 4 | |||
| Dermatology/Skin | Rash | 4 | ||
| Dermatology | 4 | |||
| Gastrointestinal | Nausea | 4 | ||
| Colitis | 4 | |||
| Vomiting | 4 | |||
| Diarrhea | 4 | |||
Time to event outcomes
The Kaplan-Meier survival curves for TTP, PFS, and OS distributions for the vaccine trial and the database patients are given in Figures 1–3, respectively. The median TTP for the vaccine and database patients was respectively 1.5 years (95% CI: 1.3–2.4 years), and 1.6 years (95% CI: 1.3–1.8 years; stratified log rank P value = 0.46). The median PFS for the vaccine patients was 1.5 years (95% CI: 1.3–2.4 years). The median PFS for database patients was 1.5 years (1.1–1.8 years) There was no statistically significant difference in the PFS distributions between the two groups (stratified log rank P value = 0.30). There was a statistically significant difference in OS between the vaccine trial and the database patients, with the vaccine patients having a better survival outcome (median OS: vaccine-5.3 years (95% CI: 4.0 years—N/A); database patients 3.4 years (95% CI: 2.7–4.6 years); stratified log rank P value of 0.02).
Figure 1.
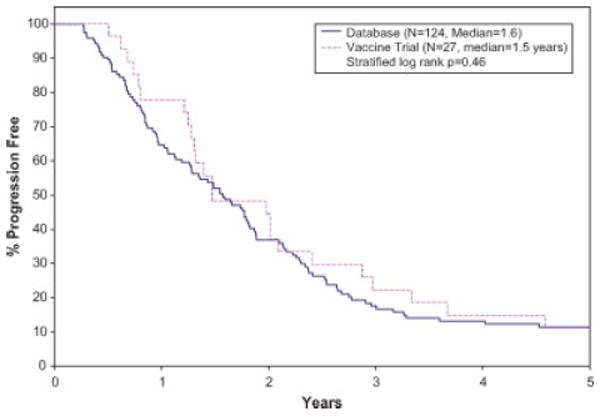
Kaplan-Meier curves for time to progression. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3.
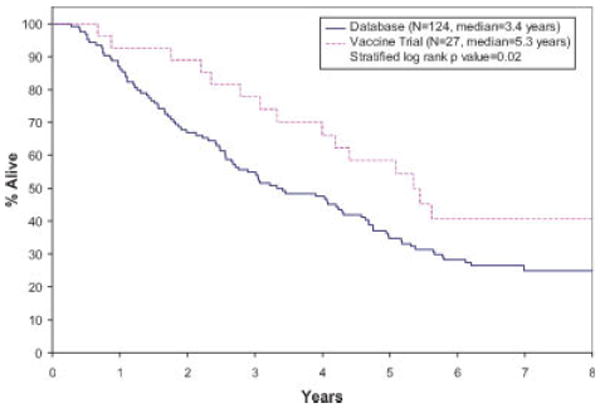
Kaplan-Meier curves for overall survival. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
With advent of novel therapies including proteosome inhibitors and immunomodulatory drugs (IMiDs) interest in myeloma vaccine trials has waned. The novel agents produce significant and rapid objective responses [6–11]. Dendritic cell (antigen presenting cell) vaccine trials are expensive and cumbersome to conduct with uncertain clinical benefits, although the relative costs are less when compared those of the more recent therapeutic agents. Introduction of novel agents into clinical use has been associated with improvements in median survival [1]. However, still there is no known cure for myeloma and our study suggests there may be a role for dendritic cell vaccine-based immunotherapy. There is a growing body of literature suggesting the degree to which the immune system is intact predicts prognosis in myeloma [12–17], thus hinting at the therapeutic benefits of immunotherapies.
Idiotypic vaccinations alone have met with limited success in human trials [18,19]. A decade ago technological advances facilitated the study of dendritic cell-based vaccination approaches [5]. Dendritic cell-based vaccination is a more potent way to induce antitumor immunity than peptide vaccines. Multiple reports demonstrate that dendritic cell vaccinations with idiotype are feasible and safe [20–23]. Most of the reports involved small numbers of patients with short follow-up, so information regarding the long-term clinical benefit of this approach is sparse. The data presented here represent the largest series of myeloma patients treated with dendritic cell vaccines and include lengthy follow up. We recognize the limitations of comparing a trial retrospectively to contemporaneous controls. Given the relatively small sample sizes involved these analyses are largely exploratory. Despite these limitations the 2 year difference in overall survival is striking. We believe this is the first report suggesting a benefit in overall survival for dendritic cell vaccines in myeloma. We hypothesize that the vaccination strategies used in the post transplantation setting may lead to an improved platform for use of immunomodulatory drugs such as lenalidomide at the time of relapse, which could explain the improved OS in the absence of PFS improvements.
Our results are similar for immunotherapy for other tumor systems. Clinical trials have been conducted in multiple tumor systems, but there is a scarcity of trials that provide insights into long-term clinical benefits or include information about effects on overall survival. A similar platform was used to test dendritic cell vaccines in prostate cancer [24]. Sipuleucel-T (Provenge, APC8015; Dendreon Corp, Seattle, WA) is an immunotherapy product consisting of autologous antigen presenting cells (dendritic cells) loaded ex vivo with a recombinant fusion protein consisting of prostatic acid phosphatase (PAP) linked to granulocyte-macrophage colony-stimulating factor. A double-blinded, placebo controlled phase three trial was conducted to evaluate the effect of APC8015 in men with asymptomatic, metastatic androgen independent prostate cancer demonstrated improved survival (30.7 months vs. 22.3 months) in men with Gleason scores of 7 or less but not in men with Gleason scores greater than 7. This trial also failed to demonstrate a difference in time to progression and only with prolonged follow-up was the survival advantage seen.
Our study raises several important questions. We need to identify the optimal method of generating the dendritic cells, the best protein to target with the vaccines, or the optimal timing and duration of the DC vaccines. We also need to study if there is a role for combining dendritic cell vaccination with IMiDs. However, the improvement in overall survival seen in our cohort justifies pursuing well-designed clinical trials to answer these questions.
Figure 2.
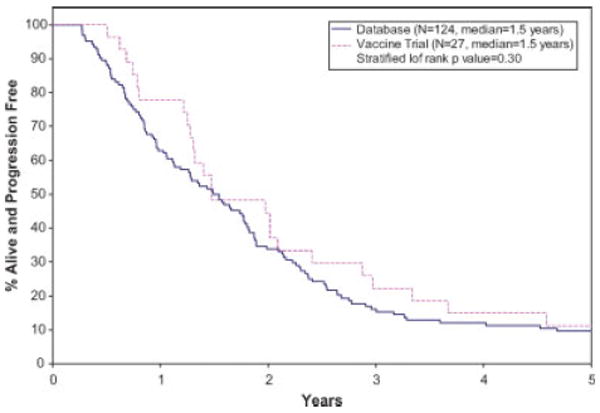
Kaplan-Meier curves for progression free survival. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Acknowledgments
We wish to thank Dendreon Corp. for providing reports and materials for manufacturing the antigen presenting cells.
Footnotes
Conflict of interest: Nothing to report.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salmon S. Immunoglobulin synthesis and tumor kinetics of multiple myeloma. Semin Hematol. 1973;10:136–147. [PubMed] [Google Scholar]
- 3.Oken MM, Leong T, Lenhard RE, Jr, et al. The addition of interferon or high dose cyclophosphamide to standard chemotherapy in the treatment of patients with multiple myeloma. Cancer. 1999;86:957–968. [PubMed] [Google Scholar]
- 4.Hsu FJ, Caspar CB, Czerwinski D, et al. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma–long-term results of a clinical trial. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 5.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nature Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 6.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82:1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson PG, Barlogie B, Berenson J, et al. A phase II multicenter study of the proteasome inhibitor bortezomib (VELCADE™, Formerly PS-341) in multiple myeloma patients (pts) with relapsed/refractory disease. Blood. 2002;100:104a. A385. [Google Scholar]
- 9.Jagannath S, Richardson PG, Barlogie B, et al. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006;91:929–934. [PubMed] [Google Scholar]
- 10.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 11.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 12.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 13.Kay NE, Leong T, Kyle RA, et al. Circulating blood B cells in multiple myeloma: Analysis and relationship to circulating clonal cells and clinical parameters in a cohort of patients entered on the Eastern Cooperative Oncology Group phase III E9486 clinical trial. Blood. 1997;90:340–345. [PubMed] [Google Scholar]
- 14.Kay NE, Leong T, Bone N, et al. Blood levels of immune cells predict survival in myeloma patients: results of an Eastern Cooperative Oncology Group phase 3 trial for newly diagnosed multiple myeloma patients. Blood. 2001;98:23–28. doi: 10.1182/blood.v98.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Kay N, Leong T, Kyle RA, et al. Altered T cell repertoire usage in CD4 and CD8 subsets of multiple myeloma patients, a Study of the Eastern Cooperative Oncology Group (E9487) Leuk Lymphoma. 1999;33:127–133. doi: 10.3109/10428199909093733. [DOI] [PubMed] [Google Scholar]
- 16.Osterborg A, Nilsson B, Bjorkholm M, et al. Natural killer cell activity in monoclonal gammopathies: Relation to disease activity. Eur J Haematol. 1990;45:153–157. doi: 10.1111/j.1600-0609.1990.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 17.Dianzani U, Pileri A, Boccadoro M, et al. Activated idiotype-reactive cells in suppressor/cytotoxic subpopulations of monoclonal gammopathies: Correlation with diagnosis and disease status. Blood. 1988;72:1064–1068. [PubMed] [Google Scholar]
- 18.Kwak LW, Taub DD, Duffey PL, et al. Transfer of myeloma idiotype-specific immunity from an actively immunised marrow donor. Lancet. 1995;345:1016–1020. doi: 10.1016/s0140-6736(95)90757-2. [DOI] [PubMed] [Google Scholar]
- 19.Massaia M, Borrione P, Battaglio S, et al. Idiotype vaccination in human myeloma: Generation of tumor-specific immune responses after high-dose chemotherapy. Blood. 1999;94:673–683. [PubMed] [Google Scholar]
- 20.Titzer S, Christensen O, Manzke O, et al. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: Immunological and clinical aspects. Br J Haematol. 2000;108:805–816. doi: 10.1046/j.1365-2141.2000.01958.x. [DOI] [PubMed] [Google Scholar]
- 21.Lim SH, Bailey-Wood R. Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer. 1999;83:215–222. doi: 10.1002/(sici)1097-0215(19991008)83:2<215::aid-ijc12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Cull G, Durrant L, Stainer C, et al. Generation of anti-idiotype immune responses following vaccination with idiotype-protein pulsed dendritic cells in myeloma. Br J Haematol. 1999;107:648–655. doi: 10.1046/j.1365-2141.1999.01735.x. [DOI] [PubMed] [Google Scholar]
- 23.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—A feasibility study. Blood. 1999;93:2411–2419. [PubMed] [Google Scholar]
- 24.Schellhammer PF, Hershberg RM. Immunotherapy with autologous antigen presenting cells for the treatment of androgen independent prostate cancer. World J Urol. 2005;23:47–49. doi: 10.1007/s00345-004-0475-z. [DOI] [PubMed] [Google Scholar]


