Abstract
Cervical cancer is the second most common cause of female cancer death worldwide. Persistent infection with `high risk' HPV genotypes is the major etiological factor in cervical cancer and thus effective vaccination against HPV provides an opportunity to reduce the morbidity and mortality associated with HPV. The FDA has approved two preventive vaccines to limit the spread of HPV. However, these are unlikely to impact upon HPV prevalence and cervical cancer rates for many years. Furthermore, preventive vaccines do not exert therapeutic effects on pre-existing HPV infections and HPV-associated lesions. In order to further impact upon the burden of HPV infections worldwide, therapeutic vaccines are being developed. These vaccines aim to generate a cell-mediated immune response to infected cells. This review discusses current preventive and therapeutic HPV vaccines and their future directions.
Keywords: HPV, therapeutic vaccines, preventive vaccines, HPV L1, HPV L2, HPV E6, HPV E7, cervical cancer
Introduction
Despite widespread implementation of cytological screening in many countries, cervical cancer represents a major cause of morbidity and mortality. Worldwide, cervical cancer is the second most common female cancer, claiming around 270,000 lives annually (1). Persistent infection with human papillomavirus (HPV) is the most important etiological factor in cervical cancer and its precursor lesions (cervical intraepithelial neoplasia, CIN), with HPV DNA being identified in more than 99% of cervical cancers (2). Although over 100 genotypes of HPV have been identified, only several are considered “high risk” due to their oncogenic potential, notably HPV-16 and 18 (3).
HPVs are non-enveloped viruses containing a circular double-stranded DNA genome of around 8,000 base pairs, which preferentially infect squamous epithelial cells. The genome encodes at least six early genes (E1, E2, E4, E5, E6, E7) and two late genes (L1, L2). The early genes regulate viral DNA replication while the late genes encode the viral capsid for packaging newly synthesized virions (Fig 1). HPV infects the basal cells of the cervical epithelium through microtrauma; however, the majority of HPV infections are self-limiting and transient (4). In persistent infection, the expression of the HPV genome is correlated to the maturation of the infected cell.
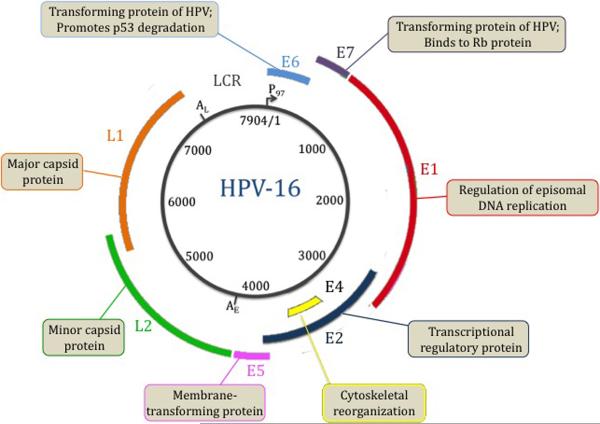
Figure 1.
HPV-16 genome and protein function. HPV-16 has a 7904 base pair, double-stranded circular DNA genome. The transcriptional promoter is designated P97. AE and AL are the early and late polyadenylation sites, respectively. The viral long control region (LCR) contains transcriptional and replication regulatory elements. The HPV-16 genome contains six early genes (E1, E2, E4, E5, E6, E7) and two late genes (L1, L2). The late genes comprise the viral capsid while the early genes are involved in viral replication. E1 regulates episomal viral DNA replication. E2 is a transcriptional regulator of E6 and E7. E4 is involved in cytoskeletal reorganization. E5 is involved in cellular transformation. E6 and E7 are responsible for the induction of malignant transformation by binding to p53 and retinoblastoma (Rb) protein, respectively.
Immature epithelial cells in the basal layer allow expression of the HPV early genes whereas in terminally differentiated cells, transcription shifts to the late genes, allowing the newly assembled virions to be released away from the submucosa, the site of immune surveillance. The HPV genome is usually found in episomal form in productive infection. However, high risk HPVs may integrate into the host genome in some persistent infections. This integration causes deletion of some of the early genes (E2, E4, E5) as well as the late genes L1 and L2. E2 is a master regulator of the viral genome and notably a transcriptional repressor of the E6 and E7 genes. Loss of E2 through integration allows upregulation of E6 and E7 transcription. E6 and E7 are oncogenes, capable of inactivating tumor suppressors p53 and retinoblastoma (Rb), leading to genomic instability and repression of apoptosis (review:(5)). HPV utilizes several mechanisms to avoid and modulate the immune system, allowing HPV to freely proliferate within cells. An understanding of these defense mechanisms, HPV virology and its role in tumorigenesis has facilitated the development of preventive and therapeutic vaccines to stimulate the immune system into responding to HPV. While preventive vaccines aim to block initial HPV entry into epithelial cells, therapeutic vaccines generate a T-cell immune response to eliminate existing HPV infection and HPV-associated neoplasms.
Preventive Vaccines
Current strategy in preventive vaccines utilizes the capsid proteins L1 and L2 as target antigens, inducing antibodies to neutralize and prevent entry of HPV into cells. Expression of recombinant L1, the major component of the capsid, in various cell types results in spontaneous assembly of virus-like particles (VLPs), which are immunologically and morphologically similar to HPV virions (6–8). Vaccination of animal models with L1 VLPs protects them against subsequent exposure to the homologous virus. The main focus of preventive vaccines has been on HPV types 16 and 18 which together account for around 70% of cervical cancers (9). Clinical trials of L1 VLP vaccines in seronegative healthy volunteers have proven their immunogenicity and safety, producing high titers of neutralizing IgG antibodies, up to 40 times those found in natural infection with HPV-16 (review:(10)).
Two preventive vaccines have recently been licensed for use: Gardasil and Cervarix (Table 1). Gardasil is a quadrivalent vaccine containing recombinant L1 VLPs for HPV genotypes 6, 11, 16 and 18 whereas the bivalent vaccine Cervarix contains L1 VLPs for HPV-16 and 18. The FDA's advisory panel has recently voted that Gardasil be approved for use in males to reduce HPV-associated cancers in males, prevent genital warts and reduce transmission to uninfected women (http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm181365.htm). The seroconversation rates for Cervarix and Gardasil is 97.5% or higher in women (11–14). The antibody response generated is unfortunately type-restricted to those HPV genotypes contained within the vaccine. However, there is some low-level cross-protection against other closely related genotypes (15). Despite this partial cross-protection, a preventive vaccine would need to contain the eight most common HPV types found in cancer to create >90% protection against cervical cancer – a costly and complex processes (16). Ongoing studies show continued protection for up to 6.4 years post vaccination with HPV-16 and 18 L1 VLPs, as well as some cross-protection to HPV 45 and HPV 31 (17). One monovalent HPV-16 L1 vaccine with an aluminium hydroxyphosphate sulfate adjuvant shows 86% of volunteers are seropositive at an average 8.5 years, where mean HPV-16 antibody titers were 71.7 mMU/mL (milli-Merk units [mMU]/mL) in contrast to 150 mMU/mL at 4 years (18, 19). It is estimated that a reduction in cervical cancer rates will not be witnessed until at least 20 years of mass vaccination due to the high prevalence in the population and slow process of carcinogenesis.
Table 1.
Comparison of Cervarix and Gardasil preventive HPV vaccines
| Cervarix | Gardasil | |
|---|---|---|
| Manufacturer | Merck & Co | GlaxoSmithKline |
|
| ||
| HPV types | 16, 18 | 16, 18, 6, 11 |
|
| ||
| Antigen (per dose) | 20 μg HPV 16 L1 20 μg HPV 18 L1 |
40 μg HPV 16 L1 20 μg HPV 18 L1 20 μg HPV 6 L1 20 μg HPV 11 L1 |
|
| ||
| Antigen source | Baculovirus | Yeast |
|
| ||
| Adjuvant | AS04 composed of: 500 μg aluminium hydroxide 50 μg MPL (3-O-desacyl-4'- monophosphoryl lipid A) |
225 μg aluminum hydroxyphosphate sulfate |
|
| ||
| Recommended administration | 0.5 mL dose at 0, 1, 6 months intramuscular dose |
0.5 mL dose at 0, 2, 6 months intramuscular dose |
|
| ||
| Approx price (USD) | $100 per dose | $120 per dose |
|
| ||
| Approved for ages | 10–25 | 9–26 |
|
| ||
| Antibody titers 1 month after completed vaccination course compared to natural infection | HPV16: 107 times HPV 18: 82 times (151) |
HPV 6: 11 times HPV 11: 7 times HPV 16: 105 times HPV 18: 19 times (152) |
|
| ||
| Geometric mean antibody titers at 7 months (age 18–26) | HPV 16: 31715 HPV 18: 13732 |
HPV 16: 8682 HPV 18: 1886 |
|
| ||
Unfortunately, L1 VLP vaccines are expensive and require repeat vaccination and specific conditions for storage. For example, the vaccines must be refrigerated and delivered via intramuscular injection, introducing several hurdles to mass vaccination in developing countries, which carry the highest burden of HPV-related disease (20). Second generation vaccines are attempting to broaden HPV type coverage, be thermo-stable, inexpensive and have needle-free administration methods while maintaining long-term protection with a single dose.
Future Prospects of Preventive Vaccines
The future generations of preventive vaccines must address two main issues: (1) lowering the cost in order to increase availability of the vaccine to developing countries and (2) to increase the number of HPV types covered in order to maximize protection against HPV-associated malignancies. An attractive approach to substantially reduce the cost of producing L1 vaccines is the employment of L1 capsomers. The current L1 vaccines, Cervarix and Gardasil, are produced in insect cells and yeast respectively. Production of the vaccine in Escherichia coli may be a cheaper option. Use of recombinant E. coli to produce these L1 capsomers has demonstrated success in inducing protective antibodies in animal models (21–23). Additionally, L1 capsomer vaccines are stable at room temperature, negating the need for refrigeration. Trials with VLP vaccines have investigated needle-free administration routes such as transdermal application (24) and nasal inhalation (25), which could be of practical use in future capsomer vaccines.
To overcome the genotype restriction of L1 vaccines, the highly conserved and thus cross-reactive L2 can be employed. L2-based vaccines can also be produced using E. coli to reduce costs and increase availability to the developing world. However, L2 vaccines are less immunogenic than their L1 counterparts, creating comparatively lower titers of neutralizing antibodies. This may be overcome through the use of strong adjuvants, such as TLR 2 agonists, providing a promising future vaccine (26). Another method of creating broader protection is through polyvalent L1 vaccines containing VLPs for several HPV types, for example Merck is currently recruiting for Phase II clinical trials of a nine-valent vaccine, V503 (http://clinicaltrials.gov/ct2/show/NCT00943 722).
Several factors highlight the need for a therapeutic, rather than preventive, vaccine. The most pressing of these factors is the high prevalence of existing HPV infection worldwide, on which preventive vaccines make little impact. Since over 80% of cervical cancer cases occur in the developing world, preventive vaccines would need to be in widespread use for many years to reduce this figure, which is currently improbable in the near future due to logistics and cost. Furthermore, in HPV-associated malignancies where genomic integration has occurred, infected cells may no longer express L1 or L2. To exert a therapeutic effect, a different vaccine target antigen is needed which is expressed constitutively in HPV-associated tumor cells. Such a vaccine may exert an immediate effect on the mortality and morbidity of HPV-associated lesions.
Therapeutic Vaccines
The HPV E6 and E7 antigens represent ideal targets for therapeutic vaccines since these are constitutively expressed in HPV-infected cells and not healthy cells. E6 and E7 are essential to the induction and maintenance of cellular transformation, and thus are unlikely to be lost in an attempt to evade the immune system (for review, (see (5, 27)).
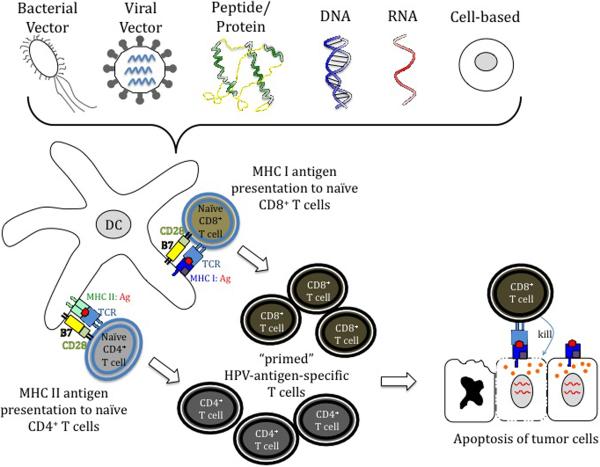
A number of therapeutic vaccines have been developed targeting E6 and E7 including live vector vaccines, peptide/protein-based vaccines, cell-based vaccines and nucleic acid-based vaccines, each with advantages and disadvantages (Table 2). These vaccines likely control HPV infection through cell-mediated immunity (Fig 2) and many have shown promise in both preclinical and clinical trials.
Table 2.
Therapeutic HPV vaccine advantages and disadvantages and future strategies
| Advantages | Disadvantages | Future Prospects | |
|---|---|---|---|
| Live Vector-based | Numerous vectors available. Highly immunogenic. Can be engineered to express cytokines and co-stimulatory molecules. |
Pre-existing immunity. Possible dominance of immune response to viral vector rather than HPV antigen. Neutralizing antibodies restrict repeated administration. Risk of disease. |
Enhancement of immunogenicity through adjuvant and fusion proteins. Circumvention of neutralizing antibodies to allow repeat dosage. |
| Peptide- based | Safe. Stable. Easy to produce. Can combine multiple epitopes in long chain peptides. |
Epitopes must be determined. HLA-restriction. Low immunogenicity. |
Enhancement of immunogenicity through. Epitope enhancement. Lipopeptide delivery. |
| Protein-based | Safe. Stable. No MHC restriction. |
Low immunogenicity; limited CTL response. | Enhancement of immunogenicity through adjuvant and fusion proteins. |
| DC-based | Highly immunogenic. Multiple methods available to load antigen. |
Expensive. Labor-intensive as individualized. Lack of agreed standards for preparation. DCs do not necessarily home to lymph nodes. |
Increase survival of DCs. More efficient loading of antigen. Identification of the most effective delivery route. |
| Tumor-cell based | Likely to express tumor antigens. | Safety concerns. Labor-intensive as individualized. Weak antigen presentation by tumor cells. |
Address safety issues. Immunogenicity enhanced by cytokines. Consistency in potency and purity established. |
| DNA-based | Safe. Stable. Easy to produce. Can administer multiple times. Several delivery methods possible. More sustained expression of antigen. |
Low immunogenicity. No intercellular spreading. Risk of genomic integration. |
Increase number and lifespan of antigen-expressing DCs. Enhanced DC antigen processing and presentation. Improve DC interaction with T cells. |
| RNA-based | Safe. Transient, non-infectious. Can administer multiple times. No risk of genomic integration. |
Difficult to produce and store - unstable. Labor intensive to produce. No intercellular spreading. |
Improved DNA-launched RNA replicons. Prevention of early apoptosis. |
Figure 2.
Therapeutic HPV vaccines. A number of therapeutic vaccines have been developed targeting HPV E6 and/or E7 antigen(s), including live vector-based vaccines, peptide/protein-based vaccines, nucleic acid-based vaccines and cell-based vaccines. These vaccines likely control HPV infection through cell-mediated immunity. Dendritic cells (DCs) prime naïve T cells through MHC:Antigen (Ag) complex with the help of costimulatory molecules (B7 on the DC and CD28 on the T cell). Antigens are processed and presented to CD4+ T cells via MHC class II pathway and presented to CD8+ T cells via MHC class I pathway. The primed effector T cells are subsequently HPV-antigen-specific T cells. Activated CD8+ T cells kill tumor cells by inducing apoptosis in the target cells. Induction of CD4+ T cell help can augment the CD8+ T cell immune response, supplementing tumor killing.
1. Live Vector-Based Vaccines
Live vector-based vaccines encompass both bacterial and viral vectors, many of which are available depending on the desired effect. These vectors are highly immunogenic as they replicate within host cells and facilitate spread of antigen. Vector-based vaccines can deliver the antigens E6 and E7 to the dendritic cells (DCs), stimulating antigen expression through MHC class I (to CD8+ cytotoxic T cells) and MHC class II (to CD4+ helper T cells). However, live vectors inherently pose a potential safety risk, especially to immunocompromised individuals. Another disadvantage is the generation of neutralizing antibodies, limiting the efficacy of repeat immunization, as well as the possibility of pre-existing immunity to the vector being employed.
Bacterial Vectors
Attenuated bacteria can deliver genes or proteins of interest, such as E6 and E7, to antigen-presenting cells. Various bacterial vectors have been explored in HPV therapeutic vaccines including Listeria monocytogenes (28, 29), Lactobacillus lactis (30, 31), and Lactobacillus plantarum (32). Listeria is a promising vector due to its ability to infect monocytes and macrophages and secrete listeriolysin O, allowing evasion from phagosomes. Literiolysin O allows Listeria to be present in both the cell cytoplasm and endosomal compartments, resulting in antigen presentation through both MHC class I and II pathways, inducing CD4+ and CD8+ immune responses (33). In preclinical trials, Listeria-based E7 vaccines were shown to cause regression of implanted solid tumors in HPV-16 E6/E7 transgenic mice (29). Currently, a clinical trial is ongoing with a Listeria-based vaccine for HPV-16 E7 (Lovaxin C) in women with advanced cervical cancer (34). Listeria–based vaccine potency can be further enhanced by the means of encoding recombinant proteins composed of HPV E6/E7 antigen fused to immunostimulatory molecules, for example, through fusion of lysteriolysin O with E7 (35). Recently, Maciag et al. utilized this method and reported the first clinical use of a live-attenuated Listeria monocytogenes vaccine that secretes HPV-16 E7 antigen fused to a fragment of listeriolysin O (Lm-LLO-E7) (36). In this Phase I safety clinical trial, Lm-LLO-E7 infusion was found to be safe and well-tolerated in end stage cervical cancer patients who had failed prior chemotherapy, radiotherapy and/or surgery. Hence, there is potential for bacterial vectors to not only serve as vaccine vectors but possibly as cancer immunotherapeutics as well.
Viral Vectors
Highly efficient infection rates and expression of encoded antigen make viral vectors a feasible option in therapeutic HPV vaccines. Viruses that have been used include adenovirus (37), adeno-associated virus (38), vaccinia virus (39) and alphaviruses, such as the Venezuelan equine encephalitis (VEE) virus (40, 41).
Vaccinia's large genome and highly infectious nature make it a promising vector. Vaccinia-based vaccines include vaccinia encoding fusion of E7 and calreticulin (CRT) to enhance MHC I processing in DCs (39, 42), and vaccinia encoding E7 and listeriolysin O (42), to facilitate MHC I and II presentation. Phase I/II clinical trials have shown that a recombinant vaccinia virus expressing the HPV-16 and 18 E6 and E7 fusion protein, TA-HPV, induces potent antigen-directed antibody and cytotoxic responses in patients with CIN III and cervical cancer (43–45).
Adenoviruses have also shown promising results. A recent study demonstrated that vaccination with replication-deficient adenoviruses encoding CRT/E7 fusion protein conferred immunity to an E7-expressing tumor challenge, and eradicated established tumors in mice (37). Creation of an adenovirus vaccine encoding a fusion protein of Hepatitis B surface antigen (HBsAg) and HPV-16 E7 has been described. Vaccination with adenovirus vector encoding HBsAg/E7 fusion protein stimulated E7-specific antibody and CD8+ T cell immune responses in vaccinated mice (46). The adeno-associated viruses can also be engineered to express E7 linked to Mycobacterium tuberculosis heat shock protein 70 (hsp70) enhancing MHC class I antigen processing in a number of ways, such as through translocation of E7 to sub-cellular compartments, inducing CD8+ T cell immune responses (47, 48).
RNA viruses, such as the Semliki Forest virus (SFV) can also be utilized as live vectors. SFV expressing HPV-16 E7 can induce E7-specific cytotoxic T cells in HPV-transgenic mice (49). This response can be enhanced through the co-administration of interleukin-12, inhibiting tumor angiogenesis (50, 51).
The future of therapeutic live vector-based vaccines requires identification of efficient bacterial and viral vectors and enhancement of the immunogenicity of these vector-based vaccines through expression of cytokines or co-stimulatory molecules. A further hurdle to overcome is the generation of neutralizing antibodies upon first exposure to the vaccine; a means to prevent this is required to allow effective repeated administration. A recent study showed that COX-2 inhibitors, such as Celecoxib, can prevent the generation of neutralizing antibodies to vaccinia, allowing repeated administration without losing infectivity, a promising advance (52).
2. Peptide/Protein-based Vaccines
Administered peptides and proteins derived from HPV antigens are taken up by DCs, processed and expressed via MHC II and/or I to the appropriate CD4+/CD8+ T cell.
Peptide-based Vaccines
Peptide vaccines are safe, stable and easy to produce. However, widespread use is restricted by the necessity to identify immunogenic epitopes corresponding to the polymorphic MHC molecules within the population. This can be partially overcome through the use of overlapping long peptides that contain several epitopes of E6/E7. Preclinical studies in mice and rabbits have shown that long E6/E7 peptides are able to induce antigen-specific T cells in all animal subjects (53, 54).
Due to the poor immunogenicity of peptide vaccines, adjuvants such as chemokines, cytokines and Toll-like receptor ligands, must be simultaneously administered. Examples of these include GM-CSF to activate DCs (54), the co-stimulatory 4-IBB ligand (55), mutant cholera toxin (56) and CpG oligodeoxynucleotides (CpG ODN), which mimic bacterial danger signals (57, 58).
Despite these limitations, several peptide vaccines have advanced to clinical trials. Phase I trials in end-stage cervical cancer patients employing HPV-16 E6 and/or E7 long peptides with the adjuvant Montanide ISA-51 show a significant E6-specific T cell response (59). A similar study using HPV-16 E6 and E7 long peptides with Montanide ISA-51 in women with HPV-16+ cervical cancer induced E6-specific CD4+ and CD8+ immune responses in all 6 patients, and E7-specific CD4+ and CD8+ immune responses in 5 out of 6 patients, an encouraging result (60).
The future of peptide-based vaccine relies on augmentation of their immunogenicity and epitope enhancement to prevent degradation and thus prolong antigen presentation. Furthermore, targeting delivery specifically to DCs will additionally increase antigen presentation, for example, using liposomal vehicles, which also act as a potent adjuvant. A recent study utilizing this method created an E7 lipopeptide vaccine, which greatly enhanced the E7-specific CD8+ activity in TC-1 tumor-bearing mice compared to E7 peptide alone (61).
Protein-based Vaccines
Protein-based vaccines contain all antigenic epitopes, circumventing the MHC-restriction limitation associated with peptide vaccines. However, while protein-based vaccines are safe, they suffer low immunogenicity. Strategies to improve their potency are similar to those employed in peptide-based vaccines. Unfortunately, due to their exogenous nature, protein-based vaccines are presented via MHC class II pathway and thus predominantly generate an antibody response, rather than a T cell response, necessitating strategies to create a predisposition for the MHC I presentation pathway.
Adjuvants trialled include liposome-polycationic-DNA (LPD) carrier particles (62) and the saponin-based ISCOMATRIX (63), both which enhance endogenous processing and thus MHC I expression of antigen. Creation of fusion proteins to target the antigen to DCs can increase MHC I presentation and thus CD8+ responses. Examples of these include HPV-16 E7 linked to Bordetella pertussis adenylyl cyclase (CyaA), which interacts with the DC's integrin receptors (64), and fusion of the translocation domain of Pseudomonas aeruginosa exotoxin A with HPV-16 E7 to target E7 to the MHC class I presentation pathway (65). A similar strategy includes fusion of antigen to heat shock proteins (hsp) that act as molecular chaperones to target antigen to DCs for cross-priming, stimulate DC maturation and induce cytokines (66). Such examples include a fusion of Mycobacterium hsp65 with HPV-16 E7 (HspE7) (67) and a fusion of hsp 70, CRT and HPV-16 E7 (68).
A clinical trial of HspE7 in 58 women with CIN III generated a complete pathologic response (defined as a LEEP specimen being negative for CIN) in 13 and a partial clinical response (defined as a colposcopic lesion regression of >50% based on measurements made on a grid form) in 32 women. However, it is difficult to determine if this observed regression was contributed by spontaneous regression (69).
Another protein-based vaccine that has progressed to clinical trials is TA-CIN, containing a fusion protein composed of HPV-16 L2, E6 and E7. Injection of the vaccine into 40 healthy volunteers induced antibody response to L2 in all, and T cell-mediated responses to HPV-16 E6 and E7 in 8 of the 11 patients receiving the highest dose (70). The inclusion of L2 within this vaccine offers a new step in the evolution of HPV vaccines by combining both preventive and therapeutic vaccines. In general, the future of protein-based vaccines relies upon enhancement of immunogenicity and CD8+ T cell response through adjuvant and fusion protein strategies.
3. Whole Cell Vaccines
Dendritic Cell-based Vaccines
Circumvention of HPV-induced immunosuppression can be achieved by delivering antigenic peptides directly to DCs in those with HPV-associated lesions. In such a setting, DCs act as natural adjuvants (review:(71)). Unfortunately the preparation involved is costly and time-consuming and consequently widespread use is currently impractical. A lack of agreed standards and culturing techniques for the generation of such vaccines adds a further challenge. Various methods employed in preparing DCs ex vivo include the usage of viral vectors (72, 73), transfection with DNA or RNA encoding antigen (74, 75) and pulsation of DCs with antigenic protein, peptide or tumor cell lysates (76–79).
T-cell mediated apoptosis limits the lifespan of DCs and their ability to prime T cells. Therefore, methods to prolong DC survival enhance antigen-specific responses. One way to achieve this is through transfection of DCs with siRNAs intended to interfere with the expression of pro-apoptotic molecules. DCs loaded with E7 and transfected with siRNA targeting the pro-apoptotic Bak and Bax proteins generate enhanced E7-specific CD8+ activation and antitumor effects in mice (77). Similarly, E7-presenting DCs transfected with siRNA to Bim, Bid, Bak, Bax and caspase 8 found that siRNA to Bim generated the strongest E7-specific CTL response in mice (78).
In clinical trials, autologous DCs loaded with HPV-16 or HPV-18 E7 antigen were administered to women with HPV-16+ or HPV-18+ late-stage cervical cancer respectively. E7-specific T cell responses were present in 4 out of 11 patients (80). A similar study of DCs loaded with HPV-16 or HPV-18 E7 co-administered with IL-2 in HPV-16/18+ refractory cervical cancer patients showed E7-specific CD4+ responses in 2 of 4 patients and E7-specific CD8+ responses in all 4 patients (81). An ongoing clinical study using DC-based vaccines with HPV-16 E7 is currently underway in patients with HPV-16+ recurrent cervical cancer at the National Taiwan University Hospital (http://clinicaltrials.gov/ct2/show/NCT00155766?term=HPV%2C+DC&rank=1).
Antigen-loaded DCs must travel to lymphoid organs in order to prime T cells and as a result, the route of administration of DC-based vaccines is an important issue. Methods previously used include intramuscular, subcutaneous, intravenous and intranodal delivery. Improvement strategies for future generations of DC-based vaccines include elucidating the most effective delivery route and developing methods to enhance antigen loading and prolong DC survival.
Tumor Cell-based Vaccines
Isolating and manipulating tumor cells ex vivo to express immunomodulatory proteins can enhance their immunogenicity in vivo. The cytokines IL-2 (82), IL-12 (83, 84) and GM-CSF (84, 85) have been trialled in mice with HPV-16 induced tumors. Clinical studies have not yet begun for HPV-associated tumor-based vaccines, although tumor-based vaccines have undergone clinical trials in melanoma, pancreatic cancer and renal cell carcinoma (review: (86)). These vaccines are advantageous in that tumour antigens do not have to be identified. However, we already hold this knowledge for cervical cancers, limiting the usefulness of this approach for the development of cervical cancer vaccines.
Some reluctance surrounds tumor-based vaccination due to the risk of seeding new cancers in patients, preventing clinical trials in healthy individuals or those with mild CIN. Due to the nature of these vaccines, their potency and purity may be inconsistent and must be individualized, creating additional problems for clinical studies.
4. Nucleic Acid-Based Vaccines
DNA-based Vaccines
Naked DNA is safe, stable, relatively easy to manufacture on a large scale at high purity and capable of sustaining antigen expression in cells longer than RNA or protein vaccines. DNA vaccines can be repeatedly administered, as they do not generate neutralizing antibodies. Although no supportive evidence currently exists, there is a risk that DNA vaccines could integrate into the host genome. Furthermore, administering HPV E6 and E7 DNA may cause cellular transformation as they are oncogenes. However, this is addressed through modification of E6 and E7 DNA into proteins incapable of oncogenic transformation.
An important limitation of DNA vaccines is their intrinsic low immunogenicity due to an inability to amplify or spread from transfected cells into surrounding cells in vivo. To overcome this, strategies to enhance DNA vaccine potency have been developed, taking into consideration the central role that DCs play in vaccine-mediated immunity. Strategies include i) increasing numbers of DCs expressing antigen, ii) enhancing antigen processing and presentation in DCs and iii) improving the interaction between DCs and T cells (for review, see (87, 88)).
i) Increasing the antigen-expressing/antigen-loaded DC population
Delivery methods targeting DNA directly to areas rich in DCs increase the population of DCs presenting the antigen. Intradermal administration via gene gun ballistically delivers gold particles coated in DNA directly to the immature DCs of the skin, the Langerhan cells. This route of administration is convenient and a potent method of DNA delivery. Head-to-head comparison shows that the gene gun requires the lowest dose to generate a comparable antigen-specific CD8+ T cell immune response, compared to the biojector or intramuscular injection (89). Unfortunately, the DNA dose that can be delivered with each shot of the gene gun is limited (90), which may necessitate multiple administration sites, risking local side effects. Another effective administration method is the combination of intramuscular injection with electroporation. Electroporation enhances DNA uptake through the application of a small electric current, creating large numbers of muscle cells expressing the desired antigen and increasing release of antigen, which local DCs can then process and present through the MHC class I pathway. Electroporation can also induce cytokine release, creating a favourable environment for the DCs. A Phase I trial is currently underway delivering VGX-3100, a DNA vaccine targeting HPV-16 and 18 E6 and E7, via intramuscular injection and electroporation in patients with a diagnosis of CINII/III (http://clinicaltrials.gov/ct2/show/NCT00685412?term=VGX-3100&rank=1). A recent study comparing several methods of DNA administration found the highest numbers of E7-specific CD8+ were produced through intramuscular injection with electroporation (91). Other novel methods to enhance DNA delivery include laser (92) and microencapsulation of DNA (93).
Since DNA vaccines are unable to spread between cells, the linkage of HPV antigen with proteins capable of intercellular transport in the context of DNA vaccination allows this spread of antigen in cells transfected with DNA. One example is the use of DNA encoding both HPV-16 E7 and herpes simple virus type 1 VP22 (HSV-1 VP22), which has been shown to have intercellular trafficking properties. Although questions have been raised as to whether VP22 transports DNA between cells, or if this is a fixation artifact, vaccinated mice unequivocally generate around a 50-fold increase in the number of E7-specific CD8+ compared to vaccination with wild-type E7 DNA (94). Further strategies to enhance DNA-encoded antigen uptake by DCs include linkage of HPV antigen to molecules that target the antigen to the DC surface, such as FMS-like tyrosine kinase 3 (flt3) ligands (95) and heat shock protein (hsp), which binds with scavenger receptors on DCs, such as CD91 (89, 96).
ii) Improving antigen expression, processing and presentation in DCs
Antigen expression can be increased through codon optimization, which replaces codons infrequently used by the host cells with more commonly used codons to enhance translation of the encoded antigens in cells transfected with DNA. In preclinical mouse models, CD8+ T cell immune responses and antitumor effects are enhanced through codon optimization of HPV DNA vaccines (97–100).
A second strategy to enhance antigen expression is the application of demethylation agents. There is reduced expression of DNA when methylated, and thus demethylating agents upregulate gene expression. A DNA vaccine encoding calreticulin (CRT) plus E7 combined with the demethylation agent 5-aza-2'- deoxycytidine (DAC) upregulates CRT/E7 expression in mice, enhancing anti-tumor effects against an E7-expressing tumor (101).
Antigen-specific CD8+ T cell responses rely upon presentation of antigen via MHC class I on DCs. To increase MHC class I processing, HPV E7 DNA can be linked with molecules that localise antigen to the endoplasmic reticulum (102) or that facilitate proteasome degradation (103). Other MHC I targeting proteins trialled in HPV DNA vaccines to enhance cross-priming include M. tuberculosis hsp70 (104), calreticulin (105–107), the heat shock protein Gp96 (108), the translocation domain of Pseudomonas aeruginosa exotoxin A (109) and γ-tubulin which targets HPV antigen to the centrosomal compartment, rich in proteasomes (110). All of these show improvement in MHC I presentation of the HPV E6/E7 antigen, inducing potent CD8+ T cell immune responses to the DNA vaccines.
MHC I single-chain trimer (SCT) technology can be utilized to circumvent antigen processing and presentation altogether. DNA vaccines encoding antigenic peptide is linked to β2-microglobulin and MHC class I heavy chain genes. The gene encoding the SCT is transcribed and expressed on the DC surface as MHC I molecules already loaded with the desired peptide. HPV-16 E6 SCT vaccines greatly increase E6-specific CD8+ T cell immune response in vaccinated mice, protecting them from a lethal challenge of E6-specific TC-1 tumor cells (111).
MHC class II processing can also be enhanced, resulting in greater CD4+ T cell responses to augment CD8+ T cell responses. DNA vaccine encoding E7 antigen linked to the sorting signal peptide of lysosomal-associated membrane protein 1 (LAMP-1) has been shown to generate greater numbers of E7-specific CD4+ and CD8+ cells and antitumor effects in vaccinated mice than wild type E7 (112). A second way to target antigen through the MHC class II processing pathway is through the use of the invariant chain (Ii). Substitution of the CLIP region of Ii, which normally occupies the antigen peptide-binding groove of MHC II to prevent premature binding of MHC class II molecules to antigenic peptides, with a T helper epitope such as the pan-DR helper T lymphocyte epitope (PADRE), allows presentation of PADRE via MHC II (113). A DNA vaccine encoding this (Ii-PADRE) generates significant PADRE-specific CD4+ responses in vaccinated mice and co-administration of Il-PADRE DNA with HPV E7 DNA elicits potent E7-specific CD8+ responses compared to E7 DNA co-administered with unmodified Ii (113). Recent advances show that both MHC I and II expression are regulated by CIITA, and thus administering DNA for CIITA with CRT/E6 DNA leads to enhanced E6-specific CD8+ T cell immune responses in vaccinated mice, which can be further improved with co-administration of Ii-PADRE DNA (114).
iii) Enhancing DC and T cell interaction
Strategies to enhance DC and T cell interaction may rely upon prolonging DC survival, increasing DC expression of cytokines and blocking negative regulation of DC activation.
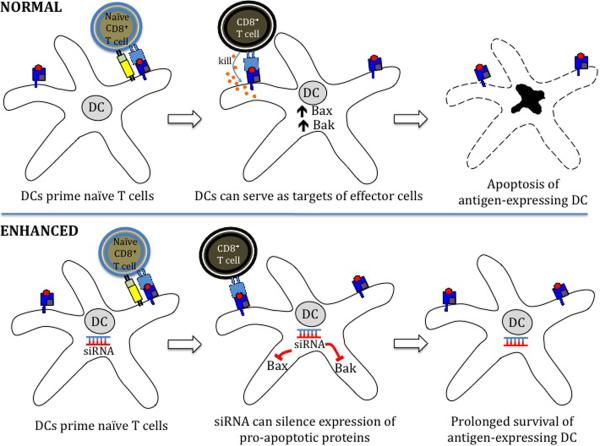
Once DCs have primed naive T cells, they become the targets of these effectors cells and undergo apoptosis. Inhibiting this T cell-mediated apoptosis allows DCs to prime a greater number of T cells and can be achieved through the use of anti-apoptotic proteins (Fig 3A). For example, DNA encoding E7 co-administered with DNA for inhibitors of apoptosis, such as BCL-xL, BCL-2, X-linked inhibitor of apoptosis protein (XIAP) and dominant-negative capsases has been shown to enhance E7-specific CD8+ responses in mice (115). However, introduction of DNA encoding anti-apoptotic proteins raises concerns of oncogenicity. This may be alleviated through the use of short interfering RNA (siRNA) to instead transiently silence the expression of pro-apoptotic proteins. SiRNA targeting the key proapoptotic proteins Bak and Bax with the E7 DNA vaccine improves DNA-transfected DC resistance to apoptosis and enhances CD8+ antitumor effects in mice (116). A recent study established that DNA encoding connective tissue growth factor (CTGF) linked to E7 can prolong survival of DCs, generating potent E7-specific antitumor responses without any oncogenic risk (117).
Figure 3.
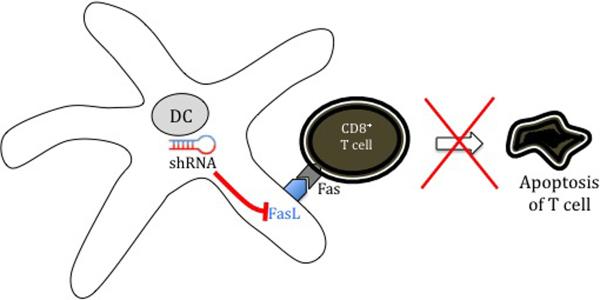
Strategies to improve DC-T cell interaction in enhancing therapeutic DNA vaccine potency. (A) Prolonging DC survival. Once DCs have primed naïve T cells, they can become the target of these effector T cells. The use of short interfering RNA (siRNA) targeting key pro-apoptotic proteins such as Bak and Bax can transiently silence expression of Bak and Bax, improving DNA-transfected DC resistance to apoptosis and improving DC-T cell interaction. (B) Prevention of activated CD8+ T cell apoptosis. The Fas ligand (FasL) found on the surface of DCs is a pro-apoptotic signalling protein that binds to the Fas receptor on T cells, causing them to undergo apoptosis. Creation of DNA encoding small hairpin RNA (shRNA) to block FasL can prevent apoptosis of activated T cells and improve DC-T cell interaction.
Preventing activated T cell apoptosis is another way to enhance overall CD8+ responses (Fig 3B). The Fas ligand (FasL) found on the surface of DCs is a pro-apoptotic signalling protein that binds to the Fas receptor on T cells causing them to undergo apoptosis. Creation of DNA encoding small hairpin RNA (shRNA) to block FasL allows co-administered E7 DNA to generate significant E7-specific CD8+ responses and antitumor effects in vaccinated mice (118).
Enhancing stimulatory cytokine release from DCs further improves DC and T cell interaction. DNA-encoded cytokines can be included within the E6/E7 DNA vaccine, for example GM-CSF (119), IL-2 (120) and IL-12 (121). Combining HPV E7 DNA vaccines with DNA encoding sequence-optimized (as opposed to wild type) IL-2 and IL-12 as adjuvants caused tumor regression in mice through E7-specific CD8+ responses (100).
Several DNA vaccines have translated to clinical trials. A microencapsulated DNA vaccine encoding several HLA-A2-restricted HPV-16 E7 epitopes (ZYC-101) has been tested in patients with HPV-16+ CIN II/III, causing complete histological response in 5 of the 15 women and E7-specific T cell responses in 11 of the 15 patients (122). An updated version of this, ZYC-101a, contains HPV-16 and HPV-18 E6 and E7-derived epitopes and was studied in a Phase II trial in patients with CIN II/III lesions providing resolution in 70% of those patients younger than 25, although this may be attributable to spontaneous resolution (123). One DNA vaccine encoding HPV-16 E7, modified through the abolition of the Rb-binding site, was linked to M tuberculosis hsp 70 and administered to women with CIN II/III. Results revealed that those receiving the maximum dose had detectable E7-specific CD8+ T cell responses and complete histological regression was observed in 3 of the 9 women receiving the highest dose (124). Plans are underway to initiate a Phase I trial with a DNA vaccine encoding this modified E7 linked with CRT (CRT/E7 detox) in patients with high grade CIN through use of a clinical-grade gene gun (Huh and Trimble, personal communication).
Naked RNA replicon vaccines
Naked RNA replicon vaccines provide a new and interesting approach to HPV vaccination. RNA replicons can be derived from alphaviruses, such as the Sindbis virus (125, 126), Semliki Forest virus (127) and Venezuelan Equine Encephalitis virus (41, 128). Self-replication of the RNA replicon allows a sustained level of antigen expression, enhancing immunogenicity and making them superior to DNA vaccines in this manner. The replicon vectors are modified to exclude viral structural genes, preventing production of viral particles and ensuring safe administration. This also allows repeat administration without the generation of neutralizing antibodies.
Unfortunately RNA is less stable than DNA. Attempts to overcome this have used more stable `suicidal DNA', which is translated into RNA replicons within the transfected cell. Transfected cells eventually undergo apoptosis, alleviating concerns of possible genomic integration and cellular transformation, an anxiety associated with DNA vaccines. Despite this advantage, the apoptosis leads to poor immunogenicity in DCs directly transfected with RNA replicons. Apoptosis can be delayed by suicidal DNA encoding the E7 antigen linked to anti-apoptotic proteins, such as BCL-xL, which in mice produces significantly higher E7-specific CD8+ T cell immune responses than wild type E7 alone, due to prolonged survival of DCs (129). Another strategy to overcome the problem of apoptosis is to exploit the flavivirus Kunjin (KUN) vector to deliver replicons. The advantage of KUN is that it does not induce apoptosis in transfected cells, prolonging antigen presentation by DCs. DNA-launched KUN replicons encoding HPV-16 E7 have been shown to generate E7-specific T cell responses and protect mice against a challenge of E7-expressing tumor (130).
RNA replicon-based vaccines can be enhanced through employment of intercellular spreading and intracellular targeting strategies as utilised in DNA based vaccines (126, 131, 132). Despite the relative success of RNA replicon vaccines in preclinical models, there has not yet been progression to clinical trails.
5. Combinational Approach
Prime-Boost Regimen
The variety of therapeutic vaccines available creates opportunities to enhance overall potency through prime-boost regimens. For example, an initial priming HPV-16 E6/E7 DNA vaccine can be followed by a boost with recombinant vaccinia (133), adenovirus (134) or with HPV-16 E6/E7 expressing tumor cell-based vaccine (135) eliciting greater HPV-specific CD8+ T cell responses than the vaccines delivered alone. Several prime-boost studies in mice have shown significantly increased E7-specific CTL responses, for example through priming with a Sindbis virus RNA replicon containing HPV-16 E7 linked with hsp70 (E7/hsp70) and boosting with a recombinant vaccinia virus encoding E7/hsp70 (136).
Prime-boost regimens have been evaluated in therapeutic clinical trials. TA-CIN (HPV-16 L2/E6/E7 fusion protein vaccine) has been boosted with a recombinant vaccinia virus encoding HPV-16/18 E6/E7 fusion protein (TA-HPV) in patients with anogenital intraepithelial neoplasia. Increases in HPV-16 antigen-specific T cell mediated immune responses were shown in 5 out of 29 patients (137, 138). However, this is not a significant advantage over TA-HPV alone as no additional efficacy is observed (137). A second study using TA-HPV followed by TA-CIN in 10 women with HPV-16+ high grade vulvar intraepithelial neoplasia reduced lesion size in 3 patients and created HPV-16 antigen-specific T cell responses in 9 of the 10 vaccinated patients. Unfortunately, there is no correlation between immunological and clinical responses (139). A clinical trial using a DNA vaccine encoding a signalling peptide (Sig), the mutated E7 antigen (E7(detox)) and hsp70 (i.e. pNGVL4a/Sig/E7(detox)/Hsp70), boosted with TA-HPV is currently in progress at Johns Hopkins University in women with CIN II/III lesions (http://clinicaltrials.gov/ct2/show/NCT00788164?term=pNHVL4a-Sig%2FE7%28detox%29%2FHSP70%rank=2).
HPV Therapeutic Vaccines with other Therapies
Combinational approaches employ HPV therapeutic vaccines in addition to other therapeutic modalities such as chemotherapy, radiotherapy or biotherapeutic agents have been described. For example, agipenin, a chemotherapeutic agent that induces apoptotic tumor cell death in vitro in a dose-dependent manner has been tested in conjunction with HPV DNA vaccines. Mice bearing E7-expressing tumors treated with apigenin combined with HPV E7 DNA vaccines show enhanced E7-specific CD8+ responses and potent antitumor effects as apigenin increases tumor cell susceptibility to the CD8+ cells (140). Low-dose radiotherapy has also been combined with therapeutic HPV DNA vaccines (CRT/E7(detox)) to control E7-expressing tumors in TC-1 tumor-bearing mice (141)
Tumor Microenvironment
Effective immunotherapy for HPV-associated lesions must consider modulation of the tumor microenvironment, which may be hindering the success of therapeutic vaccines. For example, T regulatory cells release immunosuppressive cytokines, such as IL-10 (142) and TGF-β (143) in the microenvironment, which can paralyze T cell function, preventing clearance of HPV-associated lesions. Depletion of T regulatory cells from the tumor microenvironment significantly enhances the potency of therapeutic HPV DNA vaccines (144). The tumor also induces a state of immunosuppression through B7 homolog-1 (B7-H1) (145), signal transducer and activator of transcription 3 (STAT-3) (review:(146), the enzyme indoleamine 2,3-dioxygenase (IDO) (147), galectin-1 (148) and MHC class I polypeptide-related sequence A and B (MICA/MICB) (149). Each of these are potential targets of immune modulation which may enhance therapeutic effects of HPV vaccines in the future (for review, see (150)).
Summary
While the approval of Gardasil and Cervarix preventive HPV vaccines represents a breakthrough in the development of HPV immunotherapy, the much-needed therapeutic vaccines require further development before full-scale implementation. The high prevalence of HPV malignancies and HPV-associated lesions worldwide represents a pressing need for effective therapeutic HPV vaccines. Further study into the tumor microenvironment and molecular mechanisms impeding immune attack against HPV will lead to novel targets for therapeutic intervention in the future. Discovery of such targets, development of new adjuvants, and improved understanding of tumor biology will allow HPV vaccines to be used in combinational therapies in a synergistic manner in the future.
Acknowledgments
This review is not intended to be an encyclopedic one, and the authors apologize to those not cited. The authors would like to thank Barbara Ma for critical review of the manuscript. This work was supported by the NCI SPORE in Cervical Cancer P50 CA098252, NCI 1RO1 CA114425-01 and 1RO1 CA118790.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Einstein MH, Schiller JT, Viscidi RP, et al. Clinician's guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis. 2009;9:347–56. doi: 10.1016/S1473-3099(09)70108-2. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 6.Rose RC, Reichman RC, Bonnez W. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J Gen Virol. 1994;75(Pt 8):2075–9. doi: 10.1099/0022-1317-75-8-2075. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185:251–7. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- 8.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–4. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roden RB, Ling M, Wu TC. Vaccination to prevent and treat cervical cancer. Hum Pathol. 2004;35:971–82. doi: 10.1016/j.humpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–45. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 12.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 13.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 14.Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26:201–9. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 15.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 16.Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 17.David MP, Van Herck K, Hardt K, et al. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: Modeling of sustained antibody responses. Gynecol Oncol. 2009 doi: 10.1016/j.ygyno.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27:5612–9. doi: 10.1016/j.vaccine.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 20.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 21.Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–67. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 22.Rose RC, White WI, Li M, Suzich JA, Lane C, Garcea RL. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J Virol. 1998;72:6151–4. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Cripe TP, Estes PA, Lyon MK, Rose RC, Garcea RL. Expression of the human papillomavirus type 11 L1 capsid protein in Escherichia coli: characterization of protein domains involved in DNA binding and capsid assembly. J Virol. 1997;71:2988–95. doi: 10.1128/jvi.71.4.2988-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP. Cutting edge: priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol. 2005;174:2476–80. doi: 10.4049/jimmunol.174.5.2476. [DOI] [PubMed] [Google Scholar]
- 25.Nardelli-Haefliger D, Lurati F, Wirthner D, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23:3634–41. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Alphs HH, Gambhira R, Karanam B, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A. 2008;105:5850–5. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roden RB, Monie A, Wu TC. Opportunities to improve the prevention and treatment of cervical cancer. Curr Mol Med. 2007;7:490–503. doi: 10.2174/156652407781387127. [DOI] [PubMed] [Google Scholar]
- 28.Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souders NC, Sewell DA, Pan ZK, et al. Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 2007;7:2. [PMC free article] [PubMed] [Google Scholar]
- 30.Bermudez-Humaran LG, Langella P, Miyoshi A, et al. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl Environ Microbiol. 2002;68:917–22. doi: 10.1128/AEM.68.2.917-922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bermudez-Humaran LG, Cortes-Perez NG, Le Loir Y, et al. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol. 2004;53:427–33. doi: 10.1099/jmm.0.05472-0. [DOI] [PubMed] [Google Scholar]
- 32.Cortes-Perez NG, Azevedo V, Alcocer-Gonzalez JM, et al. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. J Drug Target. 2005;13:89–98. doi: 10.1080/10611860400024219. [DOI] [PubMed] [Google Scholar]
- 33.Peters C, Paterson Y. Enhancing the immunogenicity of bioengineered Listeria monocytogenes by passaging through live animal hosts. Vaccine. 2003;21:1187–94. doi: 10.1016/s0264-410x(02)00554-6. [DOI] [PubMed] [Google Scholar]
- 34.Lowry F. Live Listeria Vaccine Proves Safe Against End-Stage Cervical Cancer in Human Trial. Ob Gyn News. 2008 Sect. 2. [Google Scholar]
- 35.Sewell DA, Shahabi V, Gunn GR, 3rd, Pan ZK, Dominiecki ME, Paterson Y. Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res. 2004;64:8821–5. doi: 10.1158/0008-5472.CAN-04-1958. [DOI] [PubMed] [Google Scholar]
- 36.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–83. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, Shirwan H, Sam Zhou H, McMasters KM. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer Immunol Immunother. 2007;56:997–1007. doi: 10.1007/s00262-006-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin HS, Park EK, Lee JM, et al. Immunization with adenoviral vectors carrying recombinant IL-12 and E7 enhanced the antitumor immunity to human papillomavirus 16-associated tumor. Gynecol Oncol. 2005;97:559–67. doi: 10.1016/j.ygyno.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CJ, Kim TW, Hung CF, et al. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine. 2004;22:3993–4001. doi: 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 40.Velders MP, McElhiney S, Cassetti MC, et al. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001;61:7861–7. [PubMed] [Google Scholar]
- 41.Cassetti MC, McElhiney SP, Shahabi V, et al. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine. 2004;22:520–7. doi: 10.1016/j.vaccine.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Lamikanra A, Pan ZK, Isaacs SN, Wu TC, Paterson Y. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. J Virol. 2001;75:9654–64. doi: 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borysiewicz LK, Fiander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–7. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 44.Adams M, Borysiewicz L, Fiander A, et al. Clinical studies of human papilloma vaccines in cervical cancer. Adv Exp Med Biol. 2001;495:419–27. doi: 10.1007/978-1-4615-0685-0_61. [DOI] [PubMed] [Google Scholar]
- 45.Kaufmann AM, Stern PL, Rankin EM, et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res. 2002;8:3676–85. [PubMed] [Google Scholar]
- 46.Baez-Astua A, Herraez-Hernandez E, Garbi N, et al. Low-dose adenovirus vaccine encoding chimeric hepatitis B virus surface antigen-human papillomavirus type 16 E7 proteins induces enhanced E7-specific antibody and cytotoxic T-cell responses. J Virol. 2005;79:12807–17. doi: 10.1128/JVI.79.20.12807-12817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brodsky JL. Post-translational protein translocation: not all hsc70s are created equal. Trends Biochem Sci. 1996;21:122–6. [PubMed] [Google Scholar]
- 48.Cyr DM, Neupert W. Roles for hsp70 in protein translocation across membranes of organelles. In: Feige RIM U, Yahara I, Polla BS, editors. Stress-inducible cellular responses. Birkhauser; Switzerland: 1996. pp. 25–40. [DOI] [PubMed] [Google Scholar]
- 49.Riezebos-Brilman A, Regts J, Freyschmidt EJ, Dontje B, Wilschut J, Daemen T. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene Ther. 2005;12:1410–4. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 50.Riezebos-Brilman A, Regts J, Chen M, Wilschut J, Daemen T. Augmentation of alphavirus vector-induced human papilloma virus-specific immune and anti-tumour responses by co-expression of interleukin-12. Vaccine. 2009;27:701–7. doi: 10.1016/j.vaccine.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 51.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–6. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 52.Chang CL, Ma B, Pang X, Wu TC, Hung CF. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther. 2009;17:1365–72. doi: 10.1038/mt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vambutas A, DeVoti J, Nouri M, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005;23:5271–80. doi: 10.1016/j.vaccine.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 54.Zwaveling S, Ferreira Mota SC, Nouta J, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:350–8. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 55.Sharma RK, Elpek KG, Yolcu ES, et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res. 2009;69:4319–26. doi: 10.1158/0008-5472.CAN-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manuri PR, Nehete B, Nehete PN, et al. Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection. Vaccine. 2007;25:3302–10. doi: 10.1016/j.vaccine.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen YF, Lin CW, Tsao YP, Chen SL. Cytotoxic-T-lymphocyte human papillomavirus type 16 E5 peptide with CpG-oligodeoxynucleotide can eliminate tumor growth in C57BL/6 mice. J Virol. 2004;78:1333–43. doi: 10.1128/JVI.78.3.1333-1343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daftarian P, Mansour M, Benoit AC, et al. Eradication of established HPV 16-expressing tumors by a single administration of a vaccine composed of a liposome-encapsulated CTL-T helper fusion peptide in a water-in-oil emulsion. Vaccine. 2006;24:5235–44. doi: 10.1016/j.vaccine.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 59.Kenter GG, Welters MJ, Valentijn AR, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–77. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 60.Welters MJ, Kenter GG, Piersma SJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14:178–87. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 61.Chen W, Huang L. Induction of cytotoxic T-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine. Mol Pharm. 2008;5:464–71. doi: 10.1021/mp700126c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui Z, Huang L. Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: therapeutic effect against cervical cancer. Cancer Immunol Immunother. 2005;54:1180–90. doi: 10.1007/s00262-005-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart TJ, Drane D, Malliaros J, et al. ISCOMATRIX adjuvant: an adjuvant suitable for use in anticancer vaccines. Vaccine. 2004;22:3738–43. doi: 10.1016/j.vaccine.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 64.Preville X, Ladant D, Timmerman B, Leclerc C. Eradication of established tumors by vaccination with recombinant Bordetella pertussis adenylate cyclase carrying the human papillomavirus 16 E7 oncoprotein. Cancer Res. 2005;65:641–9. [PubMed] [Google Scholar]
- 65.Liao CW, Chen CA, Lee CN, et al. Fusion protein vaccine by domains of bacterial exotoxin linked with a tumor antigen generates potent immunologic responses and antitumor effects. Cancer Res. 2005;65:9089–98. doi: 10.1158/0008-5472.CAN-05-0958. [DOI] [PubMed] [Google Scholar]
- 66.Walker KB, Keeble J, Colaco C. Mycobacterial heat shock proteins as vaccines - a model of facilitated antigen presentation. Curr Mol Med. 2007;7:339–50. doi: 10.2174/156652407780831575. [DOI] [PubMed] [Google Scholar]
- 67.Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16 E7. Clin Exp Immunol. 2000;121:216–25. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu B, Ye D, Song X, et al. A novel therapeutic fusion protein vaccine by two different families of heat shock proteins linked with HPV16 E7 generates potent antitumor immunity and antiangiogenesis. Vaccine. 2008;26:1387–96. doi: 10.1016/j.vaccine.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 69.Einstein MH, Kadish AS, Burk RD, et al. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol Oncol. 2007;106:453–60. doi: 10.1016/j.ygyno.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Jong A, O'Neill T, Khan AY, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20:3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 71.Santin AD, Bellone S, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharm Des. 2005;11:3485–500. doi: 10.2174/138161205774414565. [DOI] [PubMed] [Google Scholar]
- 72.Tillman BW, Hayes TL, DeGruijl TD, Douglas JT, Curiel DT. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res. 2000;60:5456–63. [PubMed] [Google Scholar]
- 73.Mackova J, Kutinova L, Hainz P, et al. Adjuvant effect of dendritic cells transduced with recombinant vaccinia virus expressing HPV16-E7 is inhibited by co-expression of IL12. Int J Oncol. 2004;24:1581–8. [PubMed] [Google Scholar]
- 74.Wang TL, Ling M, Shih IM, et al. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gene Ther. 2000;7:726–33. doi: 10.1038/sj.gt.3301160. [DOI] [PubMed] [Google Scholar]
- 75.Benencia F, Courreges MC, Coukos G. Whole tumor antigen vaccination using dendritic cells: comparison of RNA electroporation and pulsing with UV-irradiated tumor cells. J Transl Med. 2008;6:21. doi: 10.1186/1479-5876-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murakami M, Gurski KJ, Marincola FM, Ackland J, Steller MA. Induction of specific CD8+ T-lymphocyte responses using a human papillomavirus-16 E6/E7 fusion protein and autologous dendritic cells. Cancer Res. 1999;59:1184–7. [PubMed] [Google Scholar]
- 77.Peng S, Kim TW, Lee JH, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther. 2005;16:584–93. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim JH, Kang TH, Noh KH, et al. Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death. Immunol Lett. 2009;122:58–67. doi: 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Adams M, Navabi H, Jasani B, et al. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R) Vaccine. 2003;21:787–90. doi: 10.1016/s0264-410x(02)00599-6. [DOI] [PubMed] [Google Scholar]
- 80.Ferrara A, Nonn M, Sehr P, et al. Dendritic cell-based tumor vaccine for cervical cancer II: results of a clinical pilot study in 15 individual patients. J Cancer Res Clin Oncol. 2003;129:521–30. doi: 10.1007/s00432-003-0463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santin AD, Bellone S, Palmieri M, et al. HPV16/18 E7-pulsed dendritic cell vaccination in cervical cancer patients with recurrent disease refractory to standard treatment modalities. Gynecol Oncol. 2006;100:469–78. doi: 10.1016/j.ygyno.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 82.Bubenik J, Simova J, Hajkova R, et al. Interleukin 2 gene therapy of residual disease in mice carrying tumours induced by HPV 16. Int J Oncol. 1999;14:593–7. doi: 10.3892/ijo.14.3.593. [DOI] [PubMed] [Google Scholar]
- 83.Hallez S, Detremmerie O, Giannouli C, et al. Interleukin-12-secreting human papillomavirus type 16-transformed cells provide a potent cancer vaccine that generates E7-directed immunity. Int J Cancer. 1999;81:428–37. doi: 10.1002/(sici)1097-0215(19990505)81:3<428::aid-ijc17>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 84.Mikyskova R, Indrova M, Simova J, et al. Treatment of minimal residual disease after surgery or chemotherapy in mice carrying HPV16-associated tumours: Cytokine and gene therapy with IL-2 and GM-CSF. Int J Oncol. 2004;24:161–7. [PubMed] [Google Scholar]
- 85.Chang EY, Chen CH, Ji H, et al. Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine. Int J Cancer. 2000;86:725–30. doi: 10.1002/(sici)1097-0215(20000601)86:5<725::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 86.Thompson PL, Dessureault S. Tumor cell vaccines. Adv Exp Med Biol. 2007;601:345–55. doi: 10.1007/978-0-387-72005-0_37. [DOI] [PubMed] [Google Scholar]
- 87.Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5:20–4. [PubMed] [Google Scholar]
- 88.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev Vaccines. 2007;6:227–39. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Trimble C, Lin CT, Hung CF, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–42. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 90.Babiuk LA, van Drunen Littel-van den H, Babiuk SL. Immunization of animals: from DNA to the dinner plate. Vet Immunol Immunopathol. 1999;72:189–202. doi: 10.1016/s0165-2427(99)00132-4. [DOI] [PubMed] [Google Scholar]
- 91.Best SR, Peng S, Juang CM, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsen SW, Wu CY, Meneshian A, Pai SI, Hung CF, Wu TC. Femtosecond laser treatment enhances DNA transfection efficiency in vivo. J Biomed Sci. 2009;16:36. doi: 10.1186/1423-0127-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klencke B, Matijevic M, Urban RG, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clin Cancer Res. 2002;8:1028–37. [PubMed] [Google Scholar]
- 94.Hung CF, Cheng WF, Chai CY, et al. Improving vaccine potency through intercellular spreading and enhanced MHC class I presentation of antigen. J Immunol. 2001;166:5733–40. doi: 10.4049/jimmunol.166.9.5733. [DOI] [PubMed] [Google Scholar]
- 95.Hung CF, Hsu KF, Cheng WF, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Fms-like tyrosine kinase 3-ligand. Cancer Res. 2001;61:1080–8. [PubMed] [Google Scholar]
- 96.Hauser H, Chen SY. Augmentation of DNA vaccine potency through secretory heat shock protein-mediated antigen targeting. Methods. 2003;31:225–31. doi: 10.1016/s1046-2023(03)00136-1. [DOI] [PubMed] [Google Scholar]
- 97.Cheung YK, Cheng SC, Sin FW, Xie Y. Plasmid encoding papillomavirus Type 16 (HPV16) DNA constructed with codon optimization improved the immunogenicity against HPV infection. Vaccine. 2004;23:629–38. doi: 10.1016/j.vaccine.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 98.Liu WJ, Gao F, Zhao KN, et al. Codon modified human papillomavirus type 16 E7 DNA vaccine enhances cytotoxic T-lymphocyte induction and anti-tumour activity. Virology. 2002;301:43–52. doi: 10.1006/viro.2002.1584. [DOI] [PubMed] [Google Scholar]
- 99.Lin CT, Tsai YC, He L, et al. A DNA vaccine encoding a codon-optimized human papillomavirus type 16 E6 gene enhances CTL response and anti-tumor activity. J Biomed Sci. 2006;13:481–8. doi: 10.1007/s11373-006-9086-6. [DOI] [PubMed] [Google Scholar]
- 100.Ohlschlager P, Quetting M, Alvarez G, Durst M, Gissmann L, Kaufmann AM. Enhancement of immunogenicity of a therapeutic cervical cancer DNA-based vaccine by co-application of sequence-optimized genetic adjuvants. Int J Cancer. 2009;125:189–98. doi: 10.1002/ijc.24333. [DOI] [PubMed] [Google Scholar]
- 101.Lu D, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Treatment with demethylating agent, 5-aza-2'-deoxycytidine enhances therapeutic HPV DNA vaccine potency. Vaccine. 2009;27:4363–9. doi: 10.1016/j.vaccine.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smahel M, Polakova I, Pokorna D, Ludvikova V, Duskova M, Vlasak J. Enhancement of T cell-mediated and humoral immunity of beta-glucuronidase-based DNA vaccines against HPV16 E7 oncoprotein. Int J Oncol. 2008;33:93–101. [PubMed] [Google Scholar]
- 103.Massa S, Simeone P, Muller A, Benvenuto E, Venuti A, Franconi R. Antitumor activity of DNA vaccines based on the human papillomavirus-16 E7 protein genetically fused to a plant virus coat protein. Hum Gene Ther. 2008;19:354–64. doi: 10.1089/hum.2007.122. [DOI] [PubMed] [Google Scholar]
- 104.Chen CH, Wang TL, Hung CF, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–42. [PubMed] [Google Scholar]
- 105.Cheng WF, Hung CF, Chai CY, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim JW, Hung CF, Juang J, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004;11:1011–8. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 107.Peng S, Ji H, Trimble C, et al. Development of a DNA vaccine targeting human papillomavirus type 16 oncoprotein E6. J Virol. 2004;78:8468–76. doi: 10.1128/JVI.78.16.8468-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bolhassani A, Zahedifard F, Taghikhani M, Rafati S. Enhanced immunogenicity of HPV16E7 accompanied by Gp96 as an adjuvant in two vaccination strategies. Vaccine. 2008;26:3362–70. doi: 10.1016/j.vaccine.2008.03.082. [DOI] [PubMed] [Google Scholar]
- 109.Hung CF, Cheng WF, Hsu KF, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61:3698–703. [PubMed] [Google Scholar]
- 110.Hung CF, Cheng WF, He L, et al. Enhancing major histocompatibility complex class I antigen presentation by targeting antigen to centrosomes. Cancer Res. 2003;63:2393–8. [PubMed] [Google Scholar]
- 111.Huang CH, Peng S, He L, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–6. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ji H, Wang TL, Chen CH, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. 1999;10:2727–40. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 113.Hung CF, Tsai YC, He L, Wu TC. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther. 2007;15:1211–9. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim D, Hoory T, Monie A, Ting JP, Hung CF, Wu TC. Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun. J Immunol. 2008;180:7019–27. doi: 10.4049/jimmunol.180.10.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim TW, Hung CF, Boyd D, et al. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies. J Immunol. 2003;171:2970–6. doi: 10.4049/jimmunol.171.6.2970. [DOI] [PubMed] [Google Scholar]
- 116.Kim TW, Lee JH, He L, et al. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–16. [PubMed] [Google Scholar]
- 117.Cheng WF, Chang MC, Sun WZ, et al. Connective tissue growth factor linked to the E7 tumor antigen generates potent antitumor immune responses mediated by an antiapoptotic mechanism. Gene Ther. 2008;15:1007–16. doi: 10.1038/gt.2008.25. [DOI] [PubMed] [Google Scholar]
- 118.Huang B, Mao CP, Peng S, Hung CF, Wu TC. RNA interference-mediated in vivo silencing of fas ligand as a strategy for the enhancement of DNA vaccine potency. Hum Gene Ther. 2008;19:763–73. doi: 10.1089/hum.2007.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leachman SA, Tigelaar RE, Shlyankevich M, et al. Granulocyte-macrophage colony-stimulating factor priming plus papillomavirus E6 DNA vaccination: effects on papilloma formation and regression in the cottontail rabbit papillomavirus--rabbit model. J Virol. 2000;74:8700–8. doi: 10.1128/jvi.74.18.8700-8708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231–52. doi: 10.1007/BF02255855. [DOI] [PubMed] [Google Scholar]
- 121.Kim MS, Sin JI. Both antigen optimization and lysosomal targeting are required for enhanced anti-tumour protective immunity in a human papillomavirus E7-expressing animal tumour model. Immunology. 2005;116:255–66. doi: 10.1111/j.1365-2567.2005.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sheets EE, Urban RG, Crum CP, et al. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am J Obstet Gynecol. 2003;188:916–26. doi: 10.1067/mob.2003.256. [DOI] [PubMed] [Google Scholar]
- 123.Garcia F, Petry KU, Muderspach L, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2004;103:317–26. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
- 124.Trimble CL, Peng S, Kos F, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15:361–7. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hariharan MJ, Driver DA, Townsend K, et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72:950–8. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng WF, Hung CF, Hsu KF, et al. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Hum Gene Ther. 2002;13:553–68. doi: 10.1089/10430340252809847. [DOI] [PubMed] [Google Scholar]
- 127.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retroviruses. 1997;13:1487–95. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 128.Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology. 1997;239:389–401. doi: 10.1006/viro.1997.8878. [DOI] [PubMed] [Google Scholar]
- 129.Kim TW, Hung CF, Juang J, He L, Hardwick JM, Wu TC. Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death. Gene Ther. 2004;11:336–42. doi: 10.1038/sj.gt.3302164. [DOI] [PubMed] [Google Scholar]
- 130.Herd KA, Harvey T, Khromykh AA, Tindle RW. Recombinant Kunjin virus replicon vaccines induce protective T-cell immunity against human papillomavirus 16 E7-expressing tumour. Virology. 2004;319:237–48. doi: 10.1016/j.virol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 131.Cheng WF, Hung CH, Chai CY, et al. Enhancement of sindbis virus self-replicating RNA vaccine potency by linkage of herpes simplex virus type 1 VP22 protein to antigen. J Virol. 2001;75:2368–76. doi: 10.1128/JVI.75.5.2368-2376.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng WF, Hung CF, Hsu KF, et al. Enhancement of sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments. Hum Gene Ther. 2001;12:235–52. doi: 10.1089/10430340150218387. [DOI] [PubMed] [Google Scholar]
- 133.Chen CH, Wang TL, Hung CF, Pardoll DM, Wu TC. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18:2015–22. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- 134.Wlazlo AP, Deng H, Giles-Davis W, Ertl HC. DNA vaccines against the human papillomavirus type 16 E6 or E7 oncoproteins. Cancer Gene Ther. 2004;11:457–64. doi: 10.1038/sj.cgt.7700723. [DOI] [PubMed] [Google Scholar]
- 135.Rittich S, Duskova M, Mackova J, Pokorna D, Jinoch P, Smahel M. Combined immunization with DNA and transduced tumor cells expressing mouse GM-CSF or IL-2. Oncol Rep. 2005;13:311–7. [PubMed] [Google Scholar]
- 136.Lin CT, Hung CF, Juang J, et al. Boosting with recombinant vaccinia increases HPV-16 E7-Specific T cell precursor frequencies and antitumor effects of HPV-16 E7-expressing Sindbis virus replicon particles. Mol Ther. 2003;8:559–66. doi: 10.1016/s1525-0016(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 137.Fiander AN, Tristram AJ, Davidson EJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16:1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 138.Smyth LJ, Van Poelgeest MI, Davidson EJ, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004;10:2954–61. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 139.Davidson EJ, Faulkner RL, Sehr P, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004;22:2722–9. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 140.Chuang CM, Monie A, Wu A, Hung CF. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J Biomed Sci. 2009;16:49. doi: 10.1186/1423-0127-16-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tseng CW, Trimble C, Zeng Q, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58:737–48. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yue FY, Dummer R, Geertsen R, et al. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer. 1997;71:630–7. doi: 10.1002/(sici)1097-0215(19970516)71:4<630::aid-ijc20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 143.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 144.Chuang CM, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+CD25+ T regulatory cells. Vaccine. 2009;27:684–9. doi: 10.1016/j.vaccine.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–92. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 147.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–8. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 148.Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–51. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 149.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 150.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–36. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 151.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 152.Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–83. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]