Abstract
Objectives/Hypothesis
Human papillomavirus (HPV) types 6 and 11 are associated with recurrent respiratory papillomatosis (RRP). Although a prophylactic vaccine has been developed that protects against HPV infection, a therapeutic vaccine is still needed for those patients infected with and/or suffering from persistent disease. Therefore, we developed a novel, therapeutic DNA vaccine targeting HPV-11 and characterized the in vivo immunologic responses generated against HPV-11 E6 and E7 after DNA vaccination in a preclinical model.
Methods
We generated a DNA vaccine that encodes the HPV-11 E6 and E7 genes in a pcDNA3 backbone plasmid. We then vaccinated C57BL/6 mice with the pcDNA3-HPV11-E6E7 DNA plasmid. Splenocytes were harvested from these vaccinated animals and were incubated with overlapping peptides spanning either the HPV-11 E6 or E7 protein. The frequency of interferon-γ–releasing CD8+ T cell responses was then analyzed by flow cytometry.
Results
Vaccinated mice with the HPV11-E6E7 DNA generated strong CD8+ T cell responses against the E6aa44-51 peptide. We determined that the epitope is presented by the MHC class I H2-Kb molecule. No significant E7 peptide-specific T cell responses were observed.
Conclusions
We developed a novel DNA vaccine that targets the E6 gene of HPV-11. Characterization of the immunologic responses elicited by this DNA vaccine reveals that the E6aa44-51 peptide contains the most immunogenic region for the HPV-11 viral type. Knowledge of this specific T cell epitope and generation of a RRP preclinical model will allow for the development and evaluation of novel vaccine strategies targeting the RRP patient population.
Keywords: HPV-11, DNA vaccine, epitope
INTRODUCTION
Recurrent respiratory papillomatosis (RRP) is a viral infection of the aerodigestive tract that causes a debilitating, lifelong disease in the pediatric and adult populations. RRP is caused by infection with the human papilloma virus (HPV) types 6 and 11. The virus induces the proliferation of benign squamous epithelium, most commonly in the larynx, trachea, or other subsites, such as the palate, pharynx, or lung. Although its overall incidence is low in the general population, the disease burden can be high for affected individuals, with profound functional consequences for airway patency and speech.1
Currently, the treatment for RRP involves serial surgical debulking and/or local infiltration of antiviral agents. The goal of these therapies is aimed at reducing the burden of the disease, but no curative therapies have yet been developed. Recently, prophylactic vaccines have been developed to protect against infection with the HPV types 6, 11, 16, and 18, but these vaccines do not treat those individuals already infected with the virus and suffering from its clinical sequelae.2
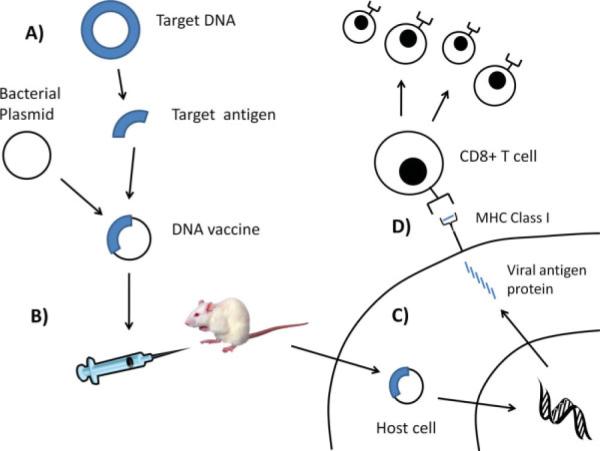
Unlike prophylactic vaccines, therapeutic DNA vaccines encode a disease-specific antigen and a promoter region that facilitates transcription of the target antigen and presentation to the immune system (see Fig. 1). There are several advantages to DNA plasmids. They are relatively safe, cost efficient, and able to sustain reasonable levels of antigen expression within cells.3,4 In addition, in contrast to viral vectors, DNA vaccines do not elicit immune responses against the viral vector in the vaccinated patient, and are well suited for indications likely to require multiple administrations to achieve and maintain antigen expression and thus targeted immune responses. Furthermore, because DNA is relatively stable in cells, long-term expression of the encoded antigen and maintenance of immunologic memory can be achieved.5–7
Fig. 1.
Overview of DNA vaccines. (A) A DNA vaccine is a recombinant gene product consisting of a target antigen placed into a bacterial DNA backbone plasmid with a mammalian promoter. (B) The recombinant DNA vaccine is injected into a mouse or mammalian host. (C) The DNA vaccine is taken up by the host cells and the recombinant DNA is transcribed and translated to express the target antigen proteins within the cells. (D) These proteins are processed and presented to the immune system via the MHC class I pathway. The target antigenic peptide is presented to the immune system in complex with the MHC class I molecule, and this complex can be recognized and activated by antigen-specific CD8+ cytotoxic T cells. These T cells then proliferate and circulate systemically to eradicate any cell that expresses the target antigen. [Color figure can be viewed in the online issue, which is available at www.interscience. wiley.com.]
We report herein the generation of a DNA vaccine targeting HPV-11, the characterization of the in vivo immunologic responses, and generation of a HPV-11 E6-specific T cell line, which can serve as a positive control in immunoassays that evaluate various vaccine strategies. These are the first steps toward the development and evaluation of targeted immunotherapeutic strategies in a preclinical model prior to its application in humans.
MATERIALS AND METHODS
Mice
C57BL/6 female mice, 6 to 8 weeks old, were purchased from the National Cancer Institute–Frederick Animal Production Area (Frederick, MD) and kept in the oncology animal facility of the Johns Hopkins Hospital (Baltimore, MD). All animal procedures were performed according to approved animal protocols and in accordance with the recommendations for the proper ethical use and care of laboratory animals.
DNA Vaccine Plasmid
A fusion product consisting of the HPV-11 E6 and E7 genes was cloned and codon optimized (GenScript USA Inc., Piscataway, NJ). The resulting 756 base pair gene product was subsequently cloned into a pcDNA3 backbone vector to generate the plasmid, pcDNA3.1-HPV11-E6E7. The plasmid was confirmed by direct sequencing.
Gene Gun Mediated Intradermal DNA Vaccination
DNA-coated gold particles were delivered to the shaved abdominal region of mice using a helium-driven gene gun (Bio-Rad, Hercules, CA) with a discharge pressure of 400 psi.8 DNA bullets were created so that the vaccination dose of 2 μg of DNA vaccine was divided between two bullets for each set of vaccinations. Subsequent boosts were administered to areas of fresh epidermis to avoid the scar created from the previous sites of vaccinations.
Intracellular Cytokine Staining With Flow Cytometric Analysis to Detect Interferon-γ–Secreting HPV-11 E6- or E7-Specific CD8+ T Cells
Cell surface marker staining for CD8 and intracellular cytokine staining for interferon (IFN)-γ was performed followed by flow cytometry as described previously.9 Five mice in each group were vaccinated with 2 μg of DNA plasmid and received a booster vaccination 1 week later. Overlapping peptides spanning the full length of the HPV-11 E6 and E7 proteins were generated. One week after the last vaccination, splenocytes were harvested and incubated for 20 hours either with or without pools of the overlapping peptides. The number of IFN-γ–secreting CD8+ T cells was then analyzed using flow cytometry. Analysis was performed on a Becton-Dickinson FACScan with CELLQuest software (Becton-Dickinson Immunocytometry System, Mountain View, CA). The advantage to using overlapping peptides in this assay is that it does not require prior identification of immunologic epitopes and is broadly applicable to multiple major histocompatibility complex (MHC) class I haplotypes.
Generation of a HPV-11 E6aa41-70-Specific CD8+ T Cell Line
We generated a HPV-11 E6-specific T cell line that could serve as a positive control for our immunologic assays. Briefly, splenocytes from pcDNA3- HPV11-E6E7 immunized C57BL/6 mice were harvested after DNA vaccination, and then stimulated with HPV-11 E6aa41-70 peptides for the first week, and then weekly in the presence of murine IL-2 (20 U/mL), thereafter. The specificity of the CD8+ cytotoxic T cell line was tested by intracellular IFN-γ staining followed by flow cytometric analysis. We demonstrated that the E6-specific T cell line can be activated to secrete IFN-γ only by the E6aa41-70 peptide (data not shown).
Determination of MHC Class I Subtype
The C1R cell line is a human plasma leukemia cell line that was transduced with either the murine MHC class I molecule H-2Db (referred to as the C1R-Db cell line) or H-2Kb (referred to as the C1R-Kb cell line). The C1R-Db and C1R-Kb cell lines were pulsed with the HPV-11 E6aa41-70 peptide (10 μg/mL) overnight. These cells were then labeled with 10 μM carboxyfluorescein succinimidyl ester (CFSE), which is a fluorescent cell-staining dye. CFSE consists of a fluorescent molecule containing a succinimidyl ester functional group and two acetate moieties. CFSE diffuses freely inside the cells, and intracellular esterases cleave the acetate groups, converting CFSE into a fluorescent membrane-impermeable dye. This dye cannot be transferred to adjacent cells. CFSE is retained by the cell in the cytoplasm and during each round of cell division; relative fluorescence intensity of the dye is decreased by one half. Thus, this method allows us to examine specific populations of proliferating cells. After extensive cell washing, the CFSE-labeled cells were then cocultured with the HPV-11 E6aa41-70 peptide-specific CD8+ T cell line at various effector:target (E:T) ratios at 37° C for 3 hours. The specific cell killing was detected by staining for active caspase-3 and the cells analyzed by flow cytometry.
Characterization of the Immunologic HPV-11 E6 Epitope
Once the E6aa41-70 region was determined to contain the immunologically relevant epitope that could be presented by the H2-Kb molecule, HLA binding predictive software was used to identify the 8, 9, or 10 amino acid-long candidate epitopes (Table I). C1R H2-Kb transduced cells were then pulsed with the indicated HPV-11 E6 candidate epitope peptides at serially diluted concentrations for 2 hours at 37° C. These cells were then labeled with 10 μM CFSE. After extensive cell washing, the C1R H2-Kb epitope-pulsed cells were cocultured with HPV-11 E6aa41-70 peptide-specific CD8+ T cell line (E:T ratio of 2:1) at 37° C for 3 hours. The specific cell killing was detected by staining for active caspase-3 and analyzed by flow cytometry.
TABLE 1.
Candidate H-2b-Restricted CTL Epitopes in HPV-11 E6aa41-70.
| BIMAS |
SYFPEITHI |
||||||
|---|---|---|---|---|---|---|---|
| Restriction Element | Peptide Length | Peptide Sequence | Position | Score | Peptide Sequence | Position | Score |
| Db | 8 | N/A | N/A | ||||
| 9 | YAYKNLKVV | 46–54 | 30 | YAYKNLKVV | 46–54 | 22 | |
| 10 | VVWRDNFPFA | 53–62 | 25.9 | YAYKNLKVW | 46–55 | 17 | |
| Kb | 8 | YAYAYKNL | 44–51 | 120 | YAYAYKNL | 44–51 | 28 |
| 9 | IYAYAYKNL | 43–51 | 26.4 | N/A | |||
| 10 | EIYAYAYKNL | 42–51 | 144 | N/A | |||
BIMAS, BioInformatics and Molecular Analysis Section, Center for Information Technology, NIH; SYFPEITHI, EU Biomed.
CTL = cytotoxic T cell line.
RESULTS
HPV-11 E7 Is Not an Immunogenic Antigen
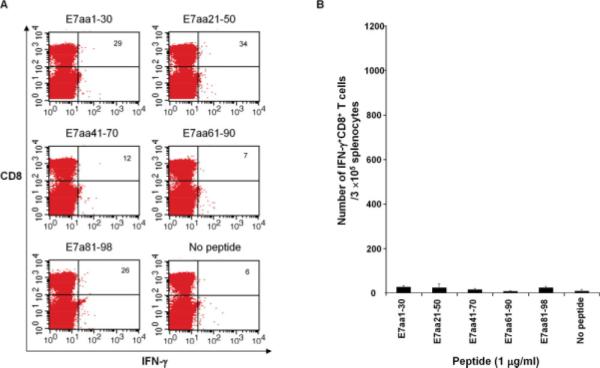
We generated a pcDNA3-HPV11-E6E7 DNA vaccine and sought to characterize the CD8+ T cell immune responses to the E6 and E7 oncogenic proteins of HPV-11. First, we evaluated the ability of the pcDNA3-HPV11-E6E7 DNA vaccine to elicit E7-specific CD8+ T cells by performing intracellular cytokine staining on splenocytes harvested from vaccinated mice. DNA vaccine was administered, and 1 week after vaccination the splenocytes from vaccinated groups of mice were harvested and incubated with pools of overlapping HPV-11 E7 peptides. Five mice were used in each group, and as shown in Figure 2. HPV-11 E7 did not elicit strong activation of CD8+ T cell responses (P > .05). Our data suggests that HPV-11 E7 is not an immunogenic antigen in our preclinical model.
Fig. 2.
HPV-11 E7 is not an immunogenic antigen. C57BL/6 mice were vaccinated with 2 μg of pcDNA3-HPV11-E6E7 DNA via intradermal delivery and were boosted 7 days later with the same regimen. One week after the last vaccination, splenocytes were harvested and stimulated with the indicated HPV-11 E7 overlapping peptides (1 μg/mL). The HPV-11 E7 peptide-specific CD8+ T cells were then analyzed by cell surface staining of CD8 and intracellular interferon (IFN)-γ staining. (A) Representative flow cytometry data. (B) Summary of the flow cytometry data. There were no significant differences from the negative control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
HPV-11 E6aa41-70 Contains Immunogenic Epitope(s) Recognized by CD8+ T Cells
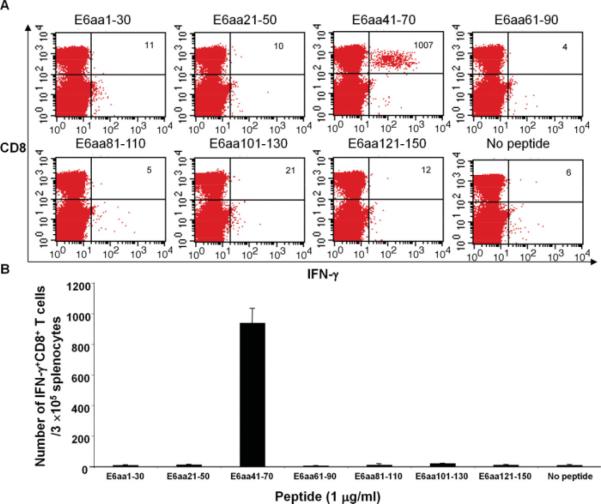
Figure 3 demonstrates that E6-specific CD8+ T cells can be elicited through DNA vaccination with pcDNA3-HPV11-E6E7. Splenocytes from vaccinated groups of mice were harvested and incubated with pools of overlapping peptides that spanned the HPV-11 E6 protein. Robust IFN-γ release by activated CD8+ T cells was observed in the peptide pool containing the E6aa41-70 peptide, indicating that an immunogenic epitope to HPV-11 E6 is present in this region. The number of activated CD8+ T cells found in this peptide sequence was statistically significant as compared to all other E6 peptide sequences (P < .05).
Fig. 3.
HPV-11 E6aa41-70 contains an immunogenic epitope(s) recognized by CD8+ T cells. C57BL/6 mice were vaccinated with 2 μg of pcDNA3-HPV11-E6E7 DNA via intradermal delivery, and were boosted 7 days later with the same regimen. One week after last vaccination, splenocytes were prepared and stimulated with the indicated HPV11 E6 overlapping peptides (1 μg/mL). The HPV-11 E6 peptide-specific CD8+ T cells were then analyzed by cell surface staining of CD8 and intracellular interferon (IFN)-γ staining. (A) Representative flow cytometry data. (B) Summary of the flow cytometry data. Only E6aa41-70 was significantly different from the negative control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
HPV-11 E6aa41-70-Specific T Cell Response is H2-Kb–Restricted
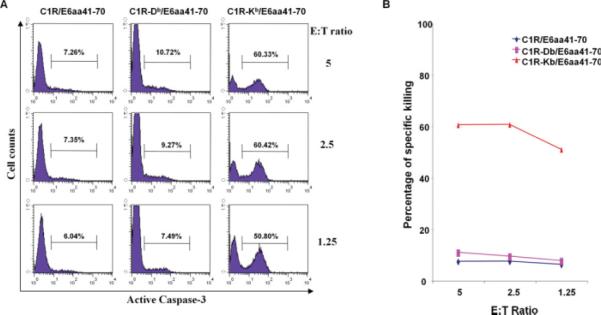
C57BL/6 mice express two major isoforms of MHC class I proteins for presentation of peptides to CD8+ cytotoxic T cells. To determine which relevant MHC I molecule was presenting the E6aa41-70 peptide, the human C1R cell line was transduced with either the murine MHC class I molecule H2-Db or H2-Kb to generate the cell lines C1R-Db or C1R-Kb. Each of these cell lines was then incubated with the E6aa41-70 peptide. Figure 4 demonstrates cell-specific killing as measured by caspase-3 expression after incubation with a HPV-11 E6aa41-70 peptide-specific CD8+ T cell line at the indicated E:T ratios. Incubation of the E6aa41-70 peptide with the C1RKb cell line revealed significant cell killing by the CD8+ T cell line, indicating that E6aa41-70 is being presented by the H2-Kb MHC I molecule, and this molecular complex is being recognized by the cytotoxic CD8+ T cells.
Fig. 4.
HPV-11 E6aa41-70-specific T cell response is H2-Kb-restricted. C1R cells that express H-2Db or Kb were pulsed with HPV-11 E6aa41-70 peptide (10 μg/mL) overnight and then labeled with 10 μM carboxyfluorescein succinimidyl ester. The cells were cocultured with a HPV-11 E6aa41-70 peptide-specific CD8+ T cell line at the indicated effector:target (E:T) ratios for 3 hours. The specific cell killing was detected by staining for active caspase-3 and analyzed with flow cytometry. (A) Representative flow cytometry data. (B) Summary of the flow cytometry data. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
E6aa44-51 is the Optimal Sequence for H2-Kb–Restricted HPV-11 E6 CD8+ T Cell Responses
Once the relevant MHC class I molecule was determined, predictive software was used to identify punitive H2-Kb epitopes. Table I shows the results of two different predictive schemes. BIMAS uses known binding motifs and predicated peptide binding of MHC molecules based on computational predictions alone. SYFPEITHI is a database of published and verified peptides that are known to bind MHC proteins. The consensus from the predictive software was a region within the E6aa41-70 peptide that consisted of eight amino acids.
Using dilutions of the various candidate epitopes, the percentage of activated T cells was studied by detecting active caspase-3 expression after coculture with a HPV-11 E6aa41-70 peptide-specific CD8+ T cell line at an E:T ratio of 2:1. These results are demonstrated in Figure 5. At a concentration of 10−9 M, the percentage of T cells activated by the E6aa44-51 epitope is statistically higher than the other peptides (P < .05), confirming this peptide sequence to be the H2-Kb binding epitope.
Fig. 5.
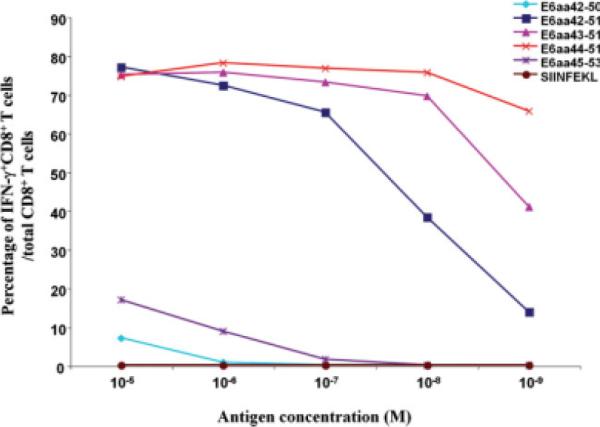
E6aa44-51 is the immunogenic epitope presented by the H2-Kb-MHC class I molecule and recognized by CD8+ T cells. C1R H2-Kb transduced cells were pulsed with the indicated HPV-11 E6 peptides, or OVA SIINFEKL peptide at serially diluted concentrations. These cells were then labeled with 10 μM carboxyfluorescein succinimidyl ester, washed, and cocultured with the HPV-11 E6aa41-70 peptide-specific CD8+ T cell line (effector:target ratio of 2:1). Cell specific killing was detected by staining for active caspase-3, analyzed with flow cytometry, and graphed as the percentage of activated CD8+ T cells at each antigen dilution. Our results demonstrate that at a concentration of 10−9 M, the percentage of Tcells activated by epitope E6aa44-51 is statistically significant from the other peptides (P <.05). IFN = interferon. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We report the development of a DNA vaccine targeting HPV-11 and characterization of the HPV-11 E6-specific immune responses generated after DNA vaccination. In addition, we have generated a HPV-11 E6-specific T cell line, which can be used to evaluate immunologic responses in the development of novel vaccine strategies against RRP in a preclinical model.
DNA vaccines are a powerful tool to direct immune responses against targeted antigens and are already in clinical development for multiple human diseases.10–13 Delivery and optimization of DNA vaccines is an area of intensive study, and multiple methods of delivery—including gene gun, electroporation, and intramuscular injection—are being studied in clinical trials to determine optimal dosing and timing of vaccinations in humans.14–16 Linkage of the disease-specific antigen with immunostimulatory molecules, such as calreticulin, can significantly enhance immune responses to the targeted antigen.17
Relevant preclinical models provide the basis for many new pharmacologic and technological innovations, and their development can play an important role in facilitating the transition of novel therapies to human clinical trials. DNA vaccines against HPV-16 for the treatment of cervical cancer18 or HPV-induced head and neck cancer19 are currently being evaluated in the clinical arena, after rigorous testing in the preclinical mouse model. Using a similar methodical approach, we plan to evaluate the efficacy of a disease-specific therapeutic DNA vaccine targeting HPV-11—the causative infectious agent of a subset of recurrent respiratory papillomatosis—with a goal of translating our findings into the clinical setting.
Recurrent respiratory papillomatosis is an ideal disease to target with therapeutic vaccination. Several lines of evidence demonstrate that immune modulation can play an important role in the development of RRP, and current treatment modalities cannot address the etiologic viral infection. Infection with either HPV-6 or HPV-11 can result in local proliferation of epithelial cells, which can progress to airway obstruction and/or dysphonia with reports of rare progression to carcinoma.20,21 Currently, serial surgical debulking and/or focal infiltration of antiviral agents (cidofovir)22 are the current treatment options available to patients. Recent analysis suggests that a clinically significant portion of the United States population is infected with HPV-11, specifically 3.6% of males and 5.7% of females.23 Despite this high prevalence, RRP occurs in only 4.3 per 100,000 children,24 and it is estimated that only one in approximately 300 children born to mothers with active HPV condylomas will develop RRP.25 Although the factors that lead to disease susceptibility have not been elucidated, host immune function likely plays a role.
Studies on patients with severe RRP disease have identified HLA haplotypes, which appear to be risk factors for development of more aggressive disease.26,27 Analysis of peripheral T cell from patients with RRP suggests that these T cells mount an ineffective T helper type 2 response when challenged with papilloma.28 A prospective study of children with RRP identified abnormal cell-mediated immune responses in those children with frequent recurrence of their disease.29 If those who develop RRP do so because of a deficiency in their immune response to chronic viral infection, targeted immune therapy is a logical therapeutic strategy.
The identification of immunogenic antigens and/or epitopes for HPV-11 is an important first step in further evaluating the efficacy of novel vaccine strategies in vivo. We found that vaccination with HPV-11 E6 and E7 DNA resulted in poor E7-specific immunologic responses in a preclinical model. It is unclear why HPV-11 E7-specific responses were poor. However, several explanations may include the inability of the E7 peptide to bind murine MHC class I molecules, the inability of the CD8+ T cells to efficiently bind the E7 peptide/MHC class I complex to become activated, or that the HPV-11 E7 protein is inherently nonimmunogenic in a murine model. In contrast, we found strong immunologic responses against the HPV-11 E6 protein and identified an immunogenic epitope spanning E6aa41-70. Interestingly, this E6 epitope was also found to be immunogenic in a HPV-6 preclinical model by our group (data not shown). These findings suggest that future vaccine strategies may want to focus on the HPV-6 and HPV-11 E6 gene because it appears more immunogenic than E7.
Further studies will now be required to evaluate the CD8+ cytotoxic T cell responses against HPV-11 E6/E7 expressing tumors in vivo to demonstrate the antitumor efficacy of the immune responses generated by the therapeutic DNA vaccine. Demonstration of disease-controlling, or ultimately disease-eliminating, therapeutic immunologic responses induced by DNA vaccination could provide a strong basis for transitioning this therapy to human clinical trials.
CONCLUSION
Currently, there is no curative therapy for RRP. We present herein the promising first steps toward achieving our goal of developing a novel therapeutic DNA vaccine against the causative human papillomavirus. Proof-of-concept experiments in preclinical models and evidence for the efficacy of the HPV-11 DNA vaccine will hopefully lead to the translation of this novel therapy to human clinical trials.
Acknowledgments
This work was supported by The Milton J. Dance, Jr. Head and Neck Center at the Greater Baltimore Medical Center and the William F. Reinhoff Jr. MD Scholars Program at the Johns Hopkins University.
Footnotes
The authors have no conflicting financial interests.
BIBLIOGRAPHY
- 1.Gallagher TQ, Derkay CS. Recurrent respiratory papillomatosis: update 2008. Curr Opin Otolaryngol Head Neck Surg. 2008;16:536–542. doi: 10.1097/MOO.0b013e328316930e. [DOI] [PubMed] [Google Scholar]
- 2.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 3.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 4.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll DM. Cancer vaccines. Nat Med. 1998;4(5 suppl):525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 6.Robinson HL. Nucleic acid vaccines: an overview. Vaccine. 1997;15:785–787. doi: 10.1016/s0264-410x(96)00249-6. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 8.Trimble C, Lin CT, Hung CF, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21:4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Wang TL, Hung CF, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 10.MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 11.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;13:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.LeBlanc R, Vasquez Y, Hannaman D, Kumar N. Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation. Vaccine. 2008;26:185–192. doi: 10.1016/j.vaccine.2007.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luxembourg A, Hannaman D, Ellefsen B, Nakamura G, Bernard R. Enhancement of immune responses to an HBV DNA vaccine by electroporation. Vaccine. 2006;24:4490–4493. doi: 10.1016/j.vaccine.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Phase I of human papillomavirus (HPV) DNA plasmid (VGX-3100) + electroporation for CIN 2 or 3. [Accessed October 7, 2009];ClinicalTrials Web site. NCT00685412. Available at: http://www.clinicaltrials.gov/ct2/results?term=NCT00685412.
- 15.Vaccine therapy in treating patients with stage IIB, stage IIC, stage III, or stage IV melanoma. [Accessed October 7, 2009];ClinicalTrials Web site. NCT00398073. Available at: http://www.clinicaltrials.gov/ct2/results?term=NCT00398073.
- 16.Study of a potential preventive vaccine against HIV in healthy volunteers. [Accessed October 7, 2009];ClinicalTrials Web site. NCT00545987. Available at: http://www.clinicaltrials.gov/ct2/results? term=NCT00545987.
- 17.Cheng WF, Hung CF, Chai CY, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trimble CL, Peng S, Kos F, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia ⅔. Clin Cancer Res. 2009;15:361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 20.Rady PL, Schnadig VJ, Weiss RL, Hughes TK, Tyring SK. Malignant transformation of recurrent respiratory papillomatosis associated with integrated human papillomavirus type 11 DNA and mutation of p53. Laryngoscope. 1998;108:735–740. doi: 10.1097/00005537-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Schraff S, Derkay CS, Burke B, Lawson L. American Society of Pediatric Otolaryngology members' experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg. 2004;130:1039–1042. doi: 10.1001/archotol.130.9.1039. [DOI] [PubMed] [Google Scholar]
- 22.Snoeck R, Wellens W, Desloovere C, et al. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] J Med Virol. 1998;54:219–225. doi: 10.1002/(sici)1096-9071(199803)54:3<219::aid-jmv13>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Hariri S, Dunne EF, Sternberg M, et al. Seroepidemiology of human papillomavirus type 11 in the United States: results from the third National Health And Nutrition Examination Survey, 1991–1994. Sex Transm Dis. 2008;35:298–303. doi: 10.1097/OLQ.0b013e31815abaef. [DOI] [PubMed] [Google Scholar]
- 24.Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg. 1995;121:1386–1391. doi: 10.1001/archotol.1995.01890120044008. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg MJ, Thorsen P, Lindeberg H, Grant LA, Shah KV. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet Gynecol. 2003;101:645–652. doi: 10.1016/s0029-7844(02)03081-8. [DOI] [PubMed] [Google Scholar]
- 26.Gelder CM, Williams OM, Hart KW, et al. HLA class II polymorphisms and susceptibility to recurrent respiratory papillomatosis. J Virol. 2003;77:1927–1939. doi: 10.1128/JVI.77.3.1927-1939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonagura VR, Vambutas A, DeVoti JA, et al. HLA alleles, IFN-gamma responses to HPV-11 E6, and disease severity in patients with recurrent respiratory papillomatosis. Hum Immunol. 2004;65:773–782. doi: 10.1016/j.humimm.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Bonagura VR, Hatam L, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol. 1999;93:302–311. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 29.Stern Y, Felipovich A, Cotton RT, Segal K. Immunocompetency in children with recurrent respiratory papillomatosis: prospective study. Ann Otol Rhinol Laryngol. 2007;116(3):169–171. doi: 10.1177/000348940711600302. [DOI] [PubMed] [Google Scholar]