Abstract
Deep brain stimulation (DBS) of the thalamus is widely used in humans to treat essential tremor and tremor dominant Parkinson’s disease. After DBS lead implantation, tremor is often reduced even without electrical stimulation. Often called “microthalamotomy” effect, the exact mechanism is unknown, although it is presumed to be due to micro lesioning. Here, we tested whether microthalamotomy effect may, in fact, be mediated via release of neurotransmitters adenosine and glutamate, using fast scan cyclic voltammetry (FSCV) and amperometry, respectively. Implantation of microelectrodes into the ventrolateral (VL) thalamus of the rat resulted in transient rise in adenosine and glutamate level from mechanical stimulation. Similarly, high frequency stimulation (100 – 130 Hz) of the VL thalamus also resulted in adenosine and glutamate release. These results suggest that glutamate and adenosine release may be an important and unappreciated mechanism whereby mechanical stimulation via electrode implantation procedure may achieve the microthalamotomy effect.
I. INTRODUCTION
Thalamotomy, first introduced in the 1950s, is a surgical procedure to lesion a selected portion of the thalamus. This invasive treatment is primarily effective for tremors such as those associated with essential tremor and Parkinson’s disease. As an alternative, Deep Brain Stimulation (DBS) of the ventral intermediate nucleus (VIM) thalamus, where high frequency electrical stimulation is delivered to the identical site for thalamotomy, is becoming increasingly popular to treat tremor. Curiously, during DBS electrode implantation, tremor is often reduced simply after insertion of the DBS electrode even without stimulation. This phenomenon has been termed ‘microthalamotomy’ effect, which may be observed in as high as 53% of patients (Tasker, 1998). However, precise mechanism by which this occurs is yet unknown, although inserting the relatively large microdialysis probe in the brain has been shown to raise adenosine levels immediately after implantation (Grabb et al., 1998).
Thalamic DBS is associated with a marked increase in the local efflux of adenosine triphosphate (ATP) and extracellular accumulation of the neuromodulator catabolic product, adenosine. It was further demonstrated that intrathalamic infusions of A1 adenosine receptor agonists directly reduced tremor, implicating adenosinergic mechanisms in tremor control. Together, these findings suggested that DBS-mediated stimulation of endogenous adenosine efflux represents an important therapeutic mechanism of action of DBS in the treatment of thalamic-mediated tremor (Bekar et al., 2007).
We and others have previously reported that high frequency stimulation of the subthalamic nucleus (Lee et al., 2004) or the thalamus (Anderson et al., 2004, Lee et al., 2005) results in glutamate release in both sites, and the depolarizing effects of high frequency stimulation are eliminated in brain slice studies by glutamate receptor antagonist drugs (Anderson et al., 2004, Lee et al., 2004). These findings also suggest that glutamate release may be important for the effects of DBS. Here, we tested the hypothesis that adenosine and glutamate could be released via either mechanical or electrical stimulation.
II. METHODS
Adenosine electrochemistry
To detect the change of adenosine concentrations, FSCV was performed. Figure 1A demonstrates in vitro flow cell experiment where 10 μM adenosine was injected. The potential was scanned from −0.4 V to +1.5 V and then returned to −0.4 V, at the rate of 400 V/sec (Cehova and Venton, 2008; Swamy and Venton, 2007). Chloride coated silver wire was used as a reference electrode (World Precision Ins., FL, USA). Adenosine measurements were made using Wireless Instantaneous Neurotransmitter Concentration System (WINCS) which utilizes FSCV as previously reported (Bledsoe et al., 2009, Agnesi et al., 2009). FSCV was employed at the carbon fiber microelectrode (CFM), which was constructed by aspirating a single polyacrylonitrile-based carbon fiber (T650, Amoco Inc, SC; diam. 5 μm) into a borosilicate glass capillary and pulling to a microscopic tip using a pipette puller (P-2000, Sutter Instruments, CA, USA). Then, the exposed carbon fiber was cut to a length of 50–70 μm, under a dissecting microscope.
Fig 1.
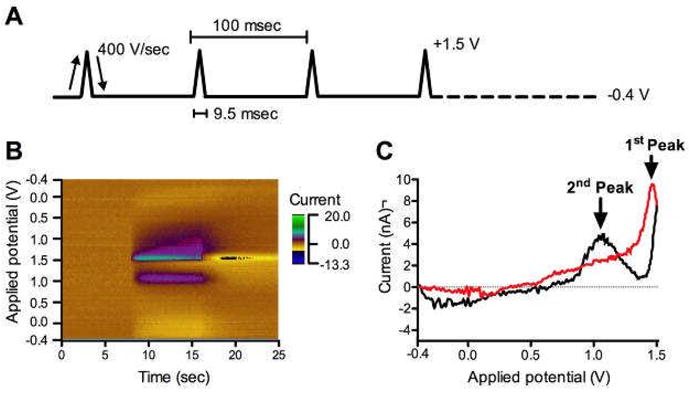
In vitro adenosine (10 μM) FSCV. A. FSCV waveform. B. The pseudocolor plot exhibits three-dimensional information; each x-, y-axis, and color gradient indicates time, potential waveform applied at the CFM, and currents detected from the CFM, respectively. C. Representative CV of adenosine. Black line is obtained from forward-going potentials and red line is from reverse-going potentials.
A pseudocolor plot (Fig. 1B) was either generated by WINCS software (WINCSware v3.2, Mayo Clinic) or recalled by LabView software (national Ins., Texas, USA). Time is plotted on the x-axis and the applied electrode potential is plotted on the y-axis. The resulting color gradient represents oxidation/reduction current detected at the CFM. A light-brown color representing background current can be seen to change to green and purple, due to the current detected at CFM. The current detected during the scanning was plotted against the applied potential, called cyclic voltammogram (CV) (Fig. 1C). The CV of a substance is generally unique in its potential of oxidation and reduction, and its shape. Therefore, FSCV is useful to identify the analyte of interest.
Glutamate electrochemistry
Glutamate is non-electroactive. Therefore, fixed potential amperometry was performed, using an enzyme coated glutamate electrode (Pinnacle Technology Inc., Lawrence, KS). The glutamate electrode is coated with glutamate oxydase (GluOX), which can selectively catalyze the conversion of glutamate to α-ketoglutarate and then to H2O2 as a final co-product, which can be oxidized at the surface of electrode (Fig. 2). Theoretically, the rate of H2O2 production is directly related to glutamate concentration. To perform this experiment, potential was fixed at + 0.6 V, using a Picostat (e-DAQ, Sydney, NSW, Australia), and current was recorded through eCorder.
Figure 2.
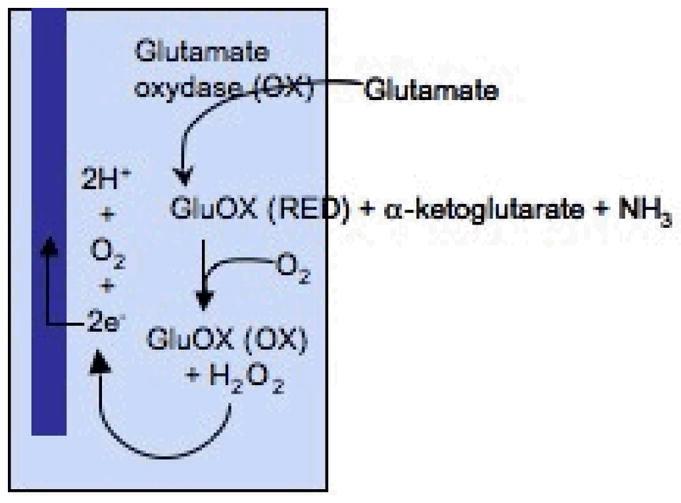
Schematic diagram of the glutamate sensing mechanism
Animal
Sprague-Dawley rats (male, 250–350 g) were deeply anesthetized with urethane (1.6 g/kg i.p.) and placed in a stereotaxic frame (David Kopf Inst., Tujunga, CA, USA). Multiple craniectomies were performed for implanting the reference, stimulating, and recording electrodes. All coordinates, anteroposterior (AP), mediolateral (ML), and dorsoventral (DV), are given in mm and utilized bregma, midline, and dura as reference points. Glutamate electrode was implanted in the VL thalamus at the coordinates: AP 2.4, ML 2.2, and DV 6.0. A twisted bipolar electrode (Plastic One, Roanoke, VA) was implanted 0.2 mm lateral from the glutamate electrode and lowered at 1 mm increments. MCF for adenosine measurement was implanted in the VL thalamus at the coordinates: AP 2.3, ML 2.0, and DV 5.6 – 6.5. All animal cares were provided by the following the Mayo Clinic Animal Care and Use Committees in accordance with National Institute of Health guidelines for use of animals in teaching and research.
Slice preparation
After deep anesthesia achieved, brain was rapidly removed and transferred into ice-cold slicing solution, in which NaCl was replaced with sucrose while maintaining an osmolarity of 307 mOsm to increase tissue viability (Aghajanian and Rasmussen, 1989). The hemispheres were separated with a midline incision. Four hundred micron thick slices were obtained using a vibratome (VT-1000S, Leica, Germany) in the coronal plane. During the preparation of slices, the tissue was kept in a chilled slicing solution (5° C). After cutting, slices were placed in an interface style recording chamber (Fig. 3; Fine Sciences Tools) maintained at 34 ± 1° C and allowed at least two hours to recover. The bath was perfused with artificial cerebrospinal fluid (ACSF) which contained (in mM): NaCl, 126; KCl, 2.5; MgSO4, 1.2; NaH2PO4, 1.25; CaCl2, 2; NaHCO3, 26; d-glucose, 10 and was aerated with 95% O2, 5% CO2 to a final pH of 7.4. For the first 20 minutes of perfusion, the bathing medium contained an equal mixture of ACSF and the slicing solution.
Figure 3.
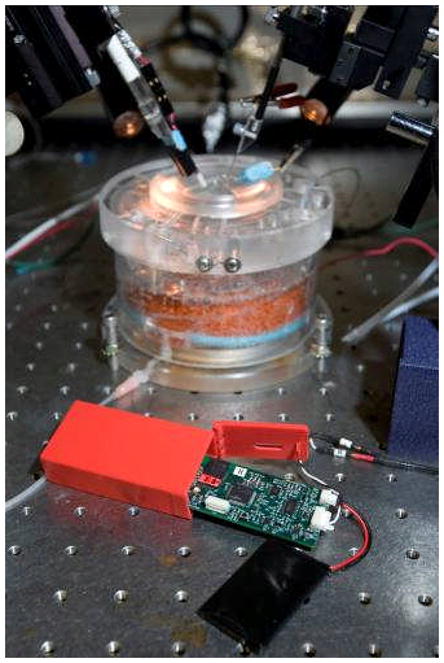
Photograph showings WINCS unit and rechargeable battery infront, and background shows transparent interface style slice chamber with stimulating and recording electrodes.
III. RESULTS
In vivo rat adenosine release by mechanical stimulation
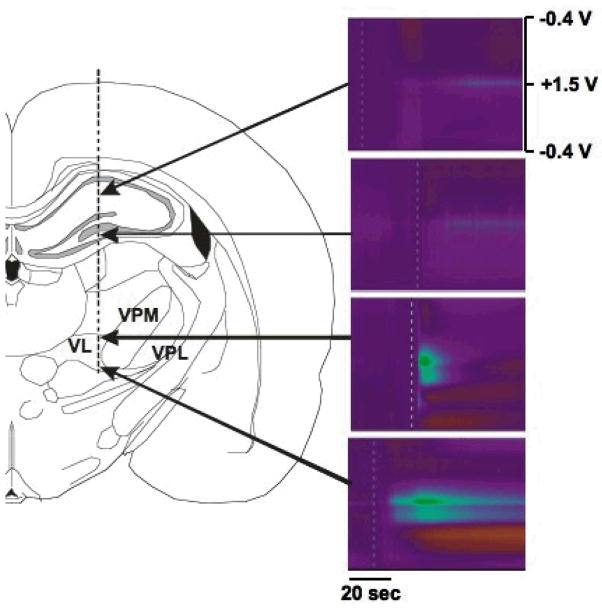
To determine whether adenosine is released during electrode implantation procedure, a CFM was slowly inserted into the VL thalamus of the anaesthetized rat. As demonstrated in Fig. 4, there was little adenosine efflux in the hippocampus, while significant adenosine efflux was observed in the VL thalamus when electrode was slowly advanced. As clearly seen by FSCV pseudo-color plot, the 1st and 2nd adenosine oxidation peaks were appeared at +1.5 V and at +1.0 V, respectively. Left panel in Fig. 4 shows a diagram of the track path of the CFM.
Figure 4.
Adenosine release by mechanical stimulation in in vivo rat. Vertical dotted line indicates the track for the insertion of CFM. Note the adenosine efflux seen when the electrode passed by the VL thalamus, but not in hippocampus.
Adenosine release by both mechanical and electrical stimulation in in vitro rat thalamic slices
To confirm the adenosine release by mechanical stimulation, we performed the same experiments with brain slice preparation as we did in vivo rats. As shown in Fig. 5, adenosine release was observed by the insertion of CFM to the thalamic brain slice. As shown in Fig 6, the high frequency electrical stimulation also induced adenosine release in the rat VL thalamus.
Figure 5.
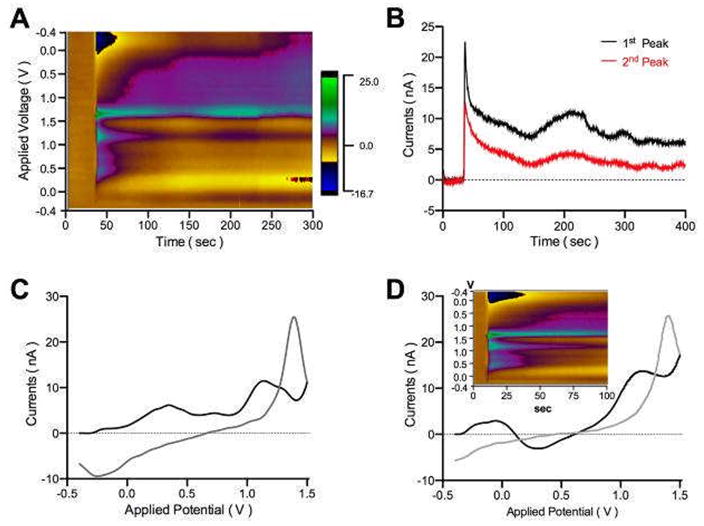
Adenosine release by the insertion of CFM in in vitro rat thalamic slice. A. Pseudo-color plot of adenosine release by mechanical stimulation. B. Current versus time plot. Adenosine release stays longer than 400 sec after the insertion of CFM. C. CV clearly shows 1st and 2nd adenosine oxidation peaks at +1.5 V and +1.0 V, respectively. D. CV and color plot (inset) of the post-calibration with 10 μM adenosine with ACSF. Note the similarity to the color plot seen in (A).
Figure 6.
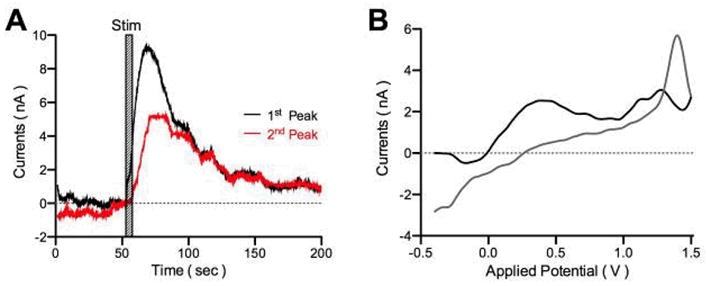
Electrical stimulation (150 μA, 125 Hz, 100 μs pulse duration, for 10 sec) induces adenosine release in VL thalamic slices. A. The current measurements are detected at the 1st and 2nd peak of adenosine oxidation. B. CV
In vivo glutamate release by both mechanical and electrical stimulation in rat thalamus
In order to determine if glutamate may also be released by mechanical and electrical stimulation, we implanted a glutamate electrode in the rat VL thalamus. First to test the glutamate release by mechanical stimulation, we inserted bipolar stimulation electrode at the adjacent area of glutamate electrode (Fig. 7). When bipolar electrode reached close to the sensing portion of the glutamate electrode, glutamate release was detected. Additionally, DBS was performed with fixed stimulation frequency and pulse width (100 Hz and 100 μsec) while varying the stimulation intensity from 200 – 700 μA (Fig. 8). The concentration of extracellular glutamate increased after a few seconds delay from electrical stimulation and exhibited a linear dependence on stimulation intensity.
Figure 7.
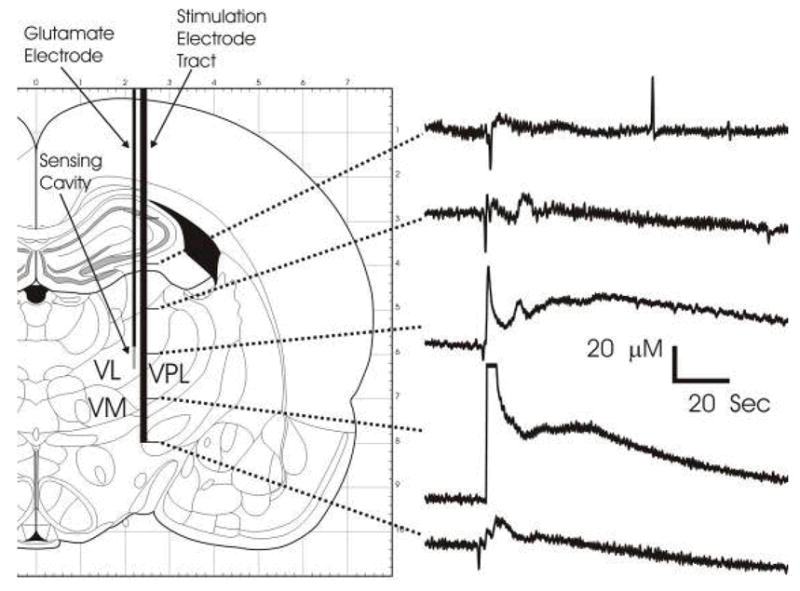
Glutamate release during delivery of the stimulating electrode. Glutamate release recorded at the sensing cavity of the glutamate electrode produced by delivering the stimulating electrode, performed at 1 mm increment. Clear responses were observed when the tips of the stimulating electrode passed near the sensing cavity.
Figure 8.
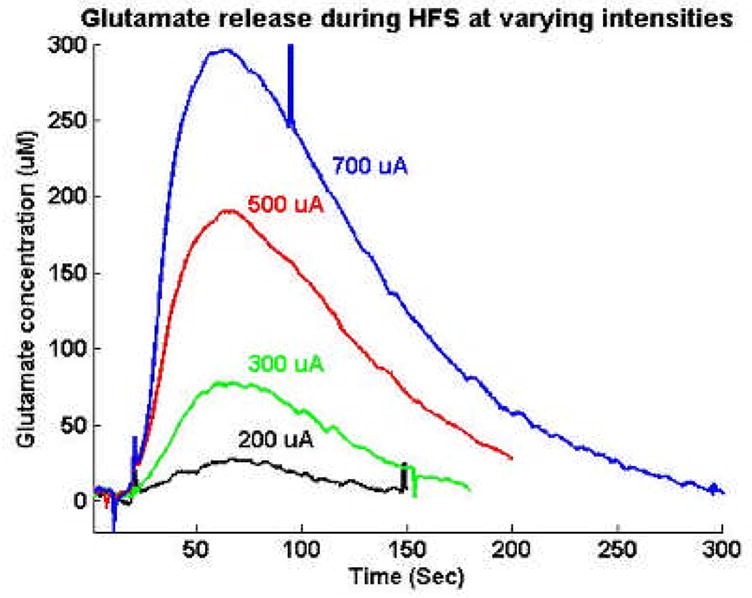
Glutamate release during DBS. The efficiency of glutamate release is dependent upon the intensity of DBS.
IV. DISCUSSION
During VIM thalamic DBS electrode implantation in humans, tremor is often reduced after insertion of the electrode, even without stimulation (Tasker, 1998). The results of the present study where insertion of stimulating electrodes elevates extracellular adenosine and glutamate levels in the rat thalamus both in vivo and in vitro, suggest that these neurotransmitter release may be an important mechanism by which “microthalamotomy” effect occurs during DBS surgery.
Tremor appears to involve abnormal oscillatory activity in the thalamus, where the frequency of tremor is 3–6 Hz (Lenz et al., 1995). Interestingly, high frequency stimulation applied to the area containing tremor cells leads to immediate tremor arrest and a rapid reversal when stimulation ceases (Benabid et al., 1996). Similarly, high frequency stimulation applied to the thalamus abolished synchronized spindle oscillations and led to immediate glutamate release, which decreased to pre-stimulation glutamate levels when stimulation ceased (Lee et al., 2005, Tawfick et al., 2009). The synchronized activity was reported to not return in the ferret slice studies, until glutamate levels returned to control values. Similarly, it was also demonstrated that thalamic DBS markedly increased the local efflux of ATP and extra-cellular accumulation of adenosine. Further, intrathalamic infusions of adenosine receptor agonists directly reduced tremor (Bekar et al., 2007). Taken together, these findings suggest that endogeous glutamate and adenosine efflux mediated by DBS may represent an important therapeutic mechanism of the action of DBS in the treatment of tremor.
There are two potential sources of glutamate and adenosine in the brain: neurons and astrocytes (Bezzi et al., 1999; Fellin et al., 2005). In general, high frequency stimulation mediated neurotransmitter release is assumed to be of neuronal origin. However, recently the possibility that astrocytes can release neurotransmitters and play important functions as a signal mediator during DBS has been raised (Tawfick et al., 2009). Importantly, it is known that large pools of glutamate and adenosine are stored in astrocytes (Fellin et al., 2006). In addition, astrocytes can release glutamate through a Ca2+-dependent vesicular mechanism (Bezzi et al., 1998) where membrane distortion with glass capillary is a well-established way to activate Ca2+ influx into astrocytes (Nedegaard M, 1994). These results suggest that the mechanical stimulation from DBS lead implantation may also activate Ca2+ influx into astrocytes and induce vesicularly mediated glutamate and adenosine release.
Here, we demonstrate that local glutamate and adenosine efflux occurs by either mechanical or electrical stimulation in the rat VL thalamus. These results suggest that tremor relief obtained by the microthalamotomy effect may be not be from lesioning, but rather from local neurotransmitter release. These results also highlight the potential importance of astrocytes in the mechanism of action of the microthalamotomy effect and of DBS.
Acknowledgments
This work was supported in part by The Grainger Foundation, NIH (K08 NS 52232 award to KHL), Mayo Foundation (2008–2010 Research Early Career Development Award for Clinician Scientists award to KHL).
Contributor Information
Su-Youne Chang, Department of Neurologic Surgery, Mayo Clinic
Young Min Shon, Department of Neurologic Surgery, Mayo Clinic
Filippo Agnesi, Department of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN 55905 USA
Kendall H. Lee, Department of Neurologic Surgery, Mayo Clinic
V. REFERENCES
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viale motor neurons in adult rat brain slices. Synapse. 1989;3(4):331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Agnesi F, Tye SJ, Bledsoe JM, Griessenauer CJ, Kimble CJ, Sieck GC, Bennet KE, Garris PA, Blaha CD, Lee KH. Wireless instantaneous neurotransmitter concentration system (WINCS)-based amperometric detection of dopamine, adensoine, and glutamate for intraoperative neurosurgical monitoring. J Neurosurg. 2009 doi: 10.3171/2009.3.JNS0990. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T, Hu B, Pittman Q, Kiss ZH. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol. 2004;559(Pt1):301–313. doi: 10.1113/jphysiol.2004.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, Bakos R, Nedergaard M. Adenosine is crucial for deep brain timulation-mediated ttenuation of tremor. Nat Med. 2007;14(1):75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996;84(2):203–214. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Vesce S, Panzarasa P, Volterra A. Astrocytes as active participants of glutamatergic function and regulators of its homeostasis. Adv Exp Med Biol. 1999;468:69–80. doi: 10.1007/978-1-4615-4685-6_6. [DOI] [PubMed] [Google Scholar]
- Bledsoe JM, Kimble CJ, Covey DP, Blaha CD, Agnesi F, Mohseni P, Whitlock S, Johnson DM, Horne A, Bennet KE, Lee KH, Garris PA. Development of wireless instantaneous neurotransmitter concentration system (WINCS) for intraoperative neurochemical monitoring using fast-scan cyclic voltammetry. J Neurosurg. 2009 doi: 10.3171/2009.3.JNS081348. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008;105:1253–1263. doi: 10.1111/j.1471-4159.2008.05223.x. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Haydon PG. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology (Bethesda) 2006;21:208–215. doi: 10.1152/physiol.00161.2005. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmingnoto G. Neuronal synchrony mediated by astrocytic glutamate through activation extrasynaptic NMDA receptors. Neuron. 2004;43(5):729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Grabb MC, Sciotti VM, Gidday JM, Cohen SA, van Wylen DG. Neurochemical and morphological responses to acutely and chronically implanted brain microdialysis probes. J Neurosci Methods. 1998;82:25–34. doi: 10.1016/s0165-0270(98)00025-9. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chang S, Roberts DW, Kim U. Neurotransmitter release from high-frequency stimulation of the sub thalamic nucleus. J Neurosurg. 2004;101(3):511–517. doi: 10.3171/jns.2004.101.3.0511. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hitti FL, Shglinsky MH, Kim U, Leiter JC, Roberts DW. Abolition of spindle oscillations and 3-Hz absence seizure-like activity in the thalamus by using high-frequency stimulation: potential mechanism of action. J Neurosurg. 2005;103(3):538–545. doi: 10.3171/jns.2005.103.3.0538. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Normand SL, Kwan HC, Andrews D, Rowland LH, Jones MW, Seike M, Lin YC, Tasker R, Dostrovsky JO, et al. Statistical prediction of the optimal site for thalamotomy in parkinsonian tremor. Mov Disord. 1995;10(3):318–328. doi: 10.1002/mds.870100315. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Swamy BEK, Venton BJ. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal Chem. 2007;79:744–750. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- Tasker R. Deep Brain Stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998;49:145–154. doi: 10.1016/s0090-3019(97)00459-x. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Chang S, Hitti FL, Roberts DW, Leiter JC, Svetlana J, Lee KH. Deep brain stimulation in local glutamate and adenosine release: investigation into role of astrocytes. 2009. Submiited for pubication. [DOI] [PMC free article] [PubMed] [Google Scholar]