Abstract
Induction of active tumor-specific immunity in patients with chronic lymphocytic leukemia (CLL) and other hematologic malignancies is compromised by the deficit of endogenous dendritic cells (DCs). In attempt to develop improved vaccination strategies for patients with CLL and other tumors with poorly identified rejection antigens, we tested the ability of ex vivo-generated DCs to cross-present the antigens expressed by CLL cells and to induce CLL-specific, functional CTL responses. Monocyte-derived DCs from CLL patients were induced to mature using a “standard” cytokine cocktail (in IL-1β, TNF-α, IL-6, and PGE2) or using an α-type 1-polarized DC (αDC1) cocktail (in IL-1β, TNF-α, IFN-α, IFN-γ, and polyinosinic:polycytidylic acid) and were loaded with γ-irradiated, autologous CLL cells. αDC1 from CLL patients expressed substantially higher levels of multiple costimulatory molecules (CD83, CD86, CD80, CD11c, and CD40) than standard DCs (sDCs) and immature DCs, and their expression of CCR7 showed intermediate level. αDC1 secreted substantially higher (10–60 times) levels of IL-12p70 than sDCs. Although αDC1 and sDCs showed similar uptake of CLL cells, αDC1 induced much higher numbers (range, 2.4–38 times) of functional CD8+ T cells against CLL cells. The current demonstration that autologous tumor-loaded αDC1 are potent inducers of CLL-specific T cells helps to develop improved immunotherapies of CLL.
Keywords: CLL, vaccines, IL-12, CTLs
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is characterized by progressive accumulation of malignant B lymphocytes in blood, bone marrow, and secondary lymphoid organs [1]. Despite recent progress in CLL treatment, current therapies are not curative, and the majority of patients eventually succumb to the disease [2]. In addition, the more aggressive chemotherapeutic regimens frequently cannot be used to treat elderly individuals who constitute the majority of CLL patients [2]. The ability of the immune system to eliminate CLL cell in the setting of graft versus leukemia following allogeneic stem cell transplantation [3] suggests the possibility of applying active immunization strategies to control the disease.
The immune deficits [4, 5] and the documented dysfunction of endogenous dendritic cells (DCs) in CLL patients [6, 7] prompted the use of ex vivo-generated DCs as therapeutic vaccines against CLL [8,9,10,11,12,13,14,15]. However, although antigen-specific CTLs can be induced in the blood of CLL patients [10], clinical responses have been rarely observed in patients vaccinated with the current “gold standard” DCs (sDCs) [16], which are matured in the presence of TNF-α, IL-6, IL-1β, and PGE2.
We have previously demonstrated that maturation of DCs in the presence of inflammatory mediators mimicking the conditions of acute viral infections strongly elevates the cytokine-producing and immunostimulatory function of DCs [17, 18]. The resulting α-type 1-polarized DCs (αDC1) loaded with defined HLA-A2-restricted antigenic peptides induce, on average, 20-fold higher levels of functional CTLs against defined melanoma-specific epitopes compared with sDCs [18]. Here, we report that αDC1-based vaccines can be used to induce the CTL responses against tumors with poorly identified rejection antigens, such as CLL. Our data demonstrate that functional αDC1 can be generated from the blood of CLL patients loaded with autologous tumor material and used as highly potent inducers of CTLs against autologous CLL cells.
MATERIALS AND METHODS
Patients and blood samples
Peripheral blood was collected from CLL patients according to our Institutional Review Board-approved protocol following informed consent. Blood samples were collected on heparin and processed as shown (see Fig. 1).
Figure 1.
Schema of the generation of DCs in CLL patients. (Inset) Importance of monocyte purity in undisturbed generation of αDC1. (Upper inset) Typical yields of the DCs generated from monocytes enriched by density gradient isolation (typically over 80% of contaminating CLL cells) and by magnetic isolation of CD14+ monocytes (monocyte purity, >90%). (Lower inset) Increasing concentrations of CLL cell were added to magnetically isolated CD14+ cells at the onset of DC cultures. In both cases, the yield of αDC1 is expressed as a fraction of the initial monocyte numbers.
Generation of DCs
PBMCs were isolated from the blood of CLL patients by density gradient centrifugation with lymphocyte separation medium (Cellgro Mediatech, Herndon, VA, USA). Monocyte and lymphocyte fractions were isolated on density gradients with Percoll (Sigma-Aldrich, St. Louis, MO, USA), typically resulting in a 1:4 mixture of CD14+ monocytes and CD19+ CLL cells, yielding very low numbers of DCs (see Fig. 1 inset). CD14+ cells (purity, >90%), isolated from monocyte fraction by MACS (Miltenyi Biotec, Auburn, CA, USA), were cultured in AIM-V medium (Gibco-BRL, Grand Island, NY, USA) or Cellgenix medium (Cellgenix, Germany) for 6 days in 24-well plates at 4 × 105 cells per well in recombinant human (rhu)GM-CSF and IL-4 (both 1000 IU/mL, Schering-Plough, Kenilworth, NJ, USA; see Fig. 1 for the outline of the process of DC generation and antigen-loading). The above stringent purification of the DC precursors has been used to avoid the previously reported, negative impact of CLL cells on the function [6, 7] and to increase the yield of arising DCs (see Fig. 1 inset). On Day 6, DCs were induced to mature using a “standard”/conventional cytokine cocktail [16] composed of IL-1β (25 ng/mL) and TNF-α (50 ng/mL; both from Strathmann Biotech, Germany), IL-6 (1000 units/mL, Endogen, Woburn, ME, USA), and PGE2 (10−6 mol/L, Sigma-Aldrich) for sDCs or with an αDC1 cocktail composed of IL-1β (25 ng/mL), TNF-α (50 ng/mL), IFN-α (3000 units/mL, Intron A-IFN-α-2b, Schering-Plough), IFN-γ (1000 units/mL, Strathmann Biotech), and polyinosinic:polycytidylic acid [poly(I:C); 20 μg/mL, Sigma-Aldrich] for αDC1 [18]. Maturing DCs were pulsed with γ- or UVC-irradiated CLL cells at a ratio of 2:1 at 20 min after the addition of maturation-inducing cytokines. Differentially matured and antigen-loaded DCs were harvested on Day 8. In all cases, CD83 expression, used as a marker of mature DCs, was observed on more than 90% of live cells. To evaluate the effect of contamination of CLL cells on the generation of αDC1, various concentrations (0–100%) of CLL cells were added when CD14+ cells were cultured to generate αDC1.
Preparation of irradiated CLL cells as antigen source
CLL cells were isolated from the lymphocyte fraction of PBMCs using the negative-selection method with a human B cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada). The purity of isolated CLL (CD5+/CD19+) cells exceeded 95%. CLL cells were treated with γ-irradiation (30 Gy) or UVC irradiation (120 mJ/cm2). The irradiated CLL cells were washed, tested for apoptosis using nonyl acridine orange (NAO) staining [19], and added to DC cultures. As expected, CLL cells became apoptotic following UVC irradiation (reduced NAO staining) but not γ-irradiation (Supplemental Fig. 1).
Tumor antigen uptake of DCs
To measure tumor antigen uptake by αDC1, CLL cells were labeled with CFSE (Invitrogen, Carlsbad, CA, USA) and added to DC cultures (at a ratio of 1:2) 20 min after the addition of maturating agents. After 48 h, tumor-loaded, mature CD11c+/HLA-DR+ DCs were analyzed by flow cytometry for the expression of CFSE.
Immunophenotyping
Flow cytometry analyses were performed using a Beckman Coulter Epics XL (Beckman Coulter, Fullerton, CA, USA) following cell labeling with the following antibodies: CD1a-FITC, CD11c-PE, CD14-FITC, CD40-FITC, CD80-FITC, CD86-FITC, and HLA-DR-PE (BD Biosciences, San Jose, CA, USA); CD5-FITC, CD8-PE, and CD83-FITC (Immunotech, Fullerton, CA, USA); CCR7-FITC (R&D Systems, Minneapolis, MN, USA); and CD19-PE (Diatec, Oslo, Norway), or the relevant isotope controls (mouse IgG1 and IgG2a, BD Biosciences).
IL-12p70 production
The levels of IL-12p70 in the primary culture supernatants collected directly after maturation of DCs were measured using ELISA (Endogen, Woburn, MA, USA). In addition, DCs harvested on Day 8 were plated in 96-well plates at 2 × 104 cells/well and stimulated with CD40 ligand (CD40L)-transfected J558 cells (5×104 cells/well, as an analog of CD40L-expressing Th cells; kind gift of Dr. Peter Lane, University of Birmingham, UK) for 24 h to measure the ability of the differentially matured DCs to secrete IL-12p70 upon the follow-up activation.
Allogeneic MLR assay
The allogeneic CD3+ T cells (40,000/well) obtained by negative selection (StemCell Technologies) from the PBMCs of healthy donors were labeled with CFSE (Invitrogen) and were then coincubated with graded doses (313–10,000) of irradiated (30 Gy) DCs in 96-well U-bottom plates for 5 days. The proliferation of T cells was analyzed using flow cytometry.
Induction of CLL-specific CTLs
Autologous CD8+ T cells (purity, >90%) were positively isolated from the lymphocyte fraction of PBMCs using MACS (Miltenyi Biotec). CD8+ T cells (0.5–1×106 cells) were sensitized by tumor-loaded, autologous αDC1 or sDCs (0.75–1×105 cells). At Day 3, rhuIL-2 (50 units/mL) and IL-7 (10 ng/mL) were added. The CTL lines were restimulated with the same DCs on Days 7 and 14, respectively. At Day 24, the frequency of antigen-specific CD8+ T cells was analyzed by IFN-γ ELISPOT. Autologous CLL cells were used as target cells, with or without anti-MHC I antibody.
Statistical analysis
The differences between DC groups were evaluated using Mann-Whitney U test using SPSS13.0 software. P < 0.05 was considered significant.
RESULTS
αDC1 from CLL patients show a fully mature phenotype and high ability to secrete IL-12
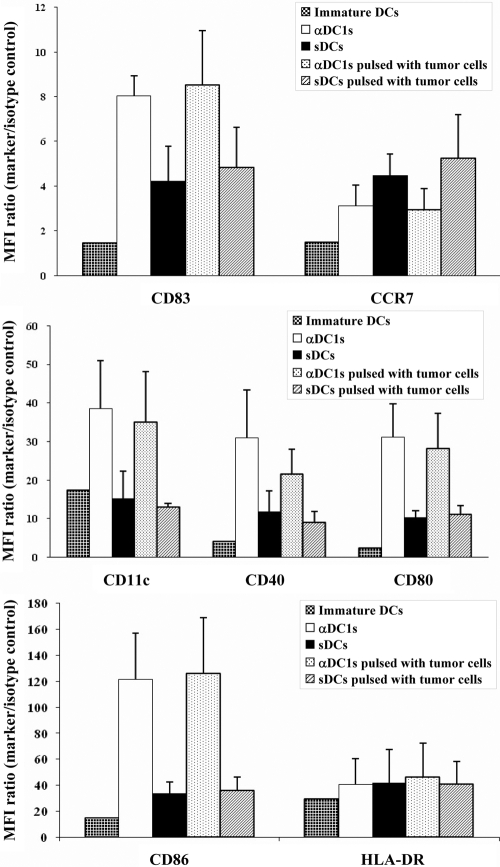
Although the generation of DCs from density-based, enriched monocyte populations [18] (containing variable numbers of CLL cells) was inefficient and yielded only low amounts of DCs (Fig. 1), magnetically sorted CD14+ monocytes from CLL patients yielded pure populations of DCs. sDCs and αDC1 harvested at Day 8 of cultures showed a typical mature phenotype and DC-like morphology (Fig. 2 and Supplemental Figs. 2and 3). αDC1 showed more pronounced dendrites and contained a higher proportion of loosely adherent cells compared with (largely nonadherent) sDCs. The expression of several DC activation markers and costimulatory molecules (CD83, CD86, CD80, CD11c, and CD40) on αDC1 was significantly higher than on sDCs (P<0.05). In contrast, CCR7 expression was lower in αDC1 than sDCs. With the exception of CD11c (that remained low on sDCs), the expression of all of the above markers was elevated significantly on sDCs and αDC1, compared with iDCs (Fig. 2 and Supplemental Fig. 2).
Figure 2.
Comparison of the phenotype of immature DCs (iDCs), as compared with αDC1 and sDCs matured in the absence or presence of γ-irradiated CLL cells. The expression levels of CD83, CD86, CD80, CD11c, and CD40 on αDC1 were higher than on sDCs (P<0.05). In contrast, CCR7 showed lower expression in αDC1 than sDCs. There was no significant impact of tumor loading upon the expression of these molecules in DCs. Data shown as mean fluorescence intensity (MFI) increase over isotype control ± sd (n=5 donors).
In contrast to the previously reported, negative impact of CLL cells upon the early stages of DC differentiation in vivo or in vitro [6, 7], the currently tested exposure of DCs to CLL cells at the stage of their maturation did not significantly affect the expression of the above costimulatory factors on maturing αDC1. However, the recovery of DCs was substantially reduced by the contamination of CLL cells in the original monocyte preparations in a dose-dependent manner (Fig. 1, inset).
The efficiency of the generation of αDCs was affected by the type of serum-free medium. In cultures performed in AIM-V medium, recovery rate of αDC1 (1.3±0.55×105 cells per well) was ∼30% lower, compared with sDCs (1.7±0.28×105 cells per well) as a result of the increased adherence of αDC1 (the adherent cells were considered as not completely matured and were not harvested). αDC1 generated in Cellgenix medium showed less granularity and were smaller than the cells generated in AIM-V medium (Supplemental Fig. 3) and showed less apoptosis (data not shown). The recovery of αDC1 improved up to the level of sDCs in the cultures performed in Cellgenix medium, associated with further elevation of maturation markers and comparable or higher production of IL-12 (Supplemental Fig. 4). Therefore, the Cellgenix medium was used in the subsequent functional studies.
Elevated, rather than “exhausted,” production of IL-12p70 by αDC1 matured in the presence of CLL cells
Multiple pathways of induction of DC maturation are accompanied by transient production of IL-12p70 [17], followed, however, by irreversible exhaustion of their ability to produce IL-12p70 [20, 21], which limits their applicability as cancer vaccines. In contrast, we observed that DC maturation in the presence of IFN-γ but in the absence of tumor-associated, suppressive factors, such as PGE2, is uniquely associated with the elevation, rather than exhaustion, of the ability of DCs to produce IL-12 during their subsequent stimulation with CD40L or the interaction with T cells [17, 18]. To determine whether the induction of functional αDC1 can proceed in the presence of tumor material from CLL cells, we measured the level of IL-12p70 production in the primary culture supernatant of DC maturation and after subsequent stimulation of the cells with CD40L.
As shown in Figure 3, αDC1 showed highly elevated production of IL-12p70 as compared with sDCs, during maturation and in response to subsequent CD40L-mediated stimulation in neutral conditions (P<0.01). In DC preparations from different CLL patients, we observed that IL-12p70 production was 10–60 times higher in αDC1 compared with sDCs. Importantly, in contrast to the immunosuppressed status of DC developing in the presence of CLL cells [6, 7], exposure to tumor antigen at the stage of maturation of αDC1 did not significantly suppress the IL-12 production after subsequent DC stimulation (Fig. 3B; P=0.71).
Figure 3.
IL-12 production by αDC1 or sDCs pulsed with or without tumor cells in (A) primary culture supernatant during generation of DCs and (B) after stimulation with CD40L-transfected J558 cells. The αDC1 showed significantly higher production of IL-12p70 than sDCs (P<0.001), and loading of tumor antigen onto αDC1 resulted in only mild suppression of IL-12 secretion, without eliminating the differences between αDC1 and sDCs. In both cases, the left-side panels represent the absolute levels of IL-12p70 (±sd of triplicate cultures) observed in a single representative experiment. The right-side panels represent the data from seven (B) to eight (A) CLL donors, expressed as a ratio of IL-12p70 production between αDC1 and sDCs generated from the same donor and matured in the same conditions (absence or presence of tumor material).
These data demonstrate the feasibility of generating mature, nonexhausted αDC1 from CLL patients that are capable of high IL-12 production, not only during the maturation period, particularly important to their use as vaccines, but also following their subsequent interaction with T cells. Furthermore, they demonstrate that CLL cells do not significantly affect the phenotype and functions of maturing αDC1, suggesting that the immune deficit observed in DCs from CLL patients [6, 7] may be overcome by generating polarized DCs ex vivo, postponing their exposure to CLL cells until the stage of their maturation.
αDC1 efficiently take up tumor material from UV-irradiated or γ-irradiated CLL cells
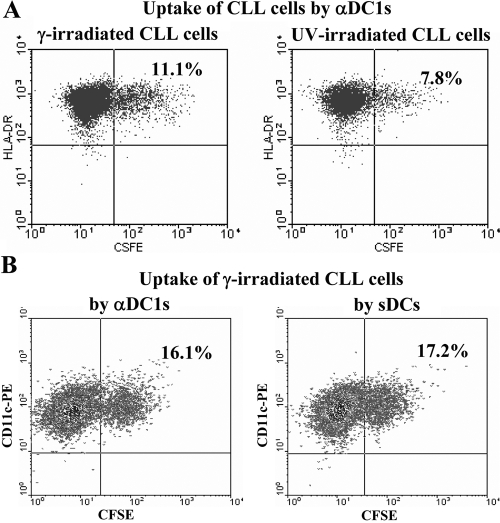
Previous reports have documented that DCs can take up and cross present to CTL precursors the antigens obtained from apoptotic and live tumor cells [22]. To determine the feasibility of loading DCs with tumor-associated antigenic material, we compared the ability of sDCs and αDC1 to take up tumor material from UV-irradiated and from γ-irradiated CLL cells.
As shown in Figure 4, although both types of tumor material could be taken up by αDC1, γ-irradiated CLL cells were taken up more efficiently compared with UV-irradiated CLL cells (Fig. 4A). Based on these results, we chose the γ-irradiated CLL cells (that were taken up with similar efficiency by αDC1 and sDCs; Fig. 4B) as the antigen source for CTL induction studies.
Figure 4.
Comparison of DC uptake of γ-irradiated CLL cells (nonapoptotic, as determined by intact mitochondrial integrity using NAO staining) and UV-irradiated CLL cells (>98% apoptotic, as determined by reduced NAO staining). (A) Efficient uptake of γ-irradiated CLL cells by αDC1. (B) Comparable uptake of γ-irradiated CLL cells by αDC1 and sDCs. In all cases, DCs were loaded with CSFE-labeled UV- or γ-irradiated CLL cells at a ratio of 2:1. CLL cells were added to DC cultures 2 h after the addition of the maturation-inducing cytokines on Day 6. Mature DCs, identified by HLA-DR (A) or CD11c expression (B) were harvested on Day 8 and tested for the uptake of CFSE-positive tumor material by flow cytometry.
CLL-loaded αDC1 show a highly elevated ability to induce tumor-specific CTLs
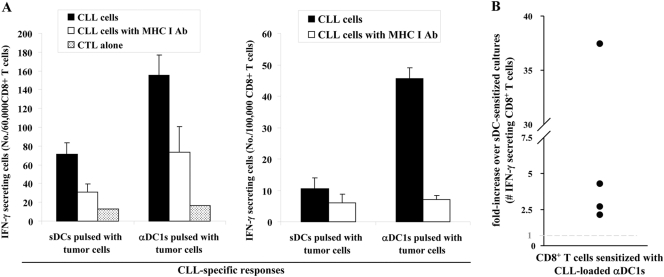
Consistent with their fully mature status, sDCs and αDC1 showed comparable ability to induce the proliferation of allogeneic T cells (Supplemental Fig. 5). Consistent with the key role of IL-12 in inducing functional CTL responses [23], tumor-loaded αDC1 showed highly elevated activity (2.4- to 38-fold higher compared with sDC; P<0.05) in inducing functional, IFN-γ-producing CD8+ T cells against autologous CLL cells. Blocking studies using MHC class I-blocking antibody verified that αDC1-induced CTL responses against autologous CLL cells are HLA class I-restricted (Fig. 5). These data tightly correlated with the differences in CTL activity of the differentially sensitized CD8+ T cells in a standard 51Cr-release assay, although the labeling efficiency of primary CLL cells with 51Cr was very low (Supplemental Fig. 6).
Figure 5.
Autologous tumor-loaded αDC1 are superior inducers of tumor-specific CD8+ T cells during in vitro sensitization (IVS). CTLs generated in three time-restimulated IVS cultures of CD8+ T cells were used as responder cells against autologous CLL cells. The numbers of IFN-γ-secreting, tumor-reactive CD8+ T cells were measured using IFN-γ ELISPOT. Anti-MHC I-blocking antibody was used to confirm the MHC class I dependence of CLL cell recognition. (A) Data from a single representative experiment showing the absolute numbers of tumor-reactive CD8+ T cells induced by tumor-loaded αDC1 or by tumor-loaded sDCs. (B) Data from four separate experiments (individual circles) using the cells of four different CLL donors are expressed as the ratios between the numbers of tumor-reactive CD8+ T cells induced by αDC1 and sDCs.
DISCUSSION
In an attempt to counteract the immunosuppression associated with CLL [4, 5] and the deficit of endogenous DCs in CLL patients [6, 7], we tested the feasibility of generating functional αDC1 from the blood of CLL patients, the feasibility of DC loading with autologous tumor cells, and their ability to induce functional, CLL-specific immune responses.
Compared with sDCs, αDC1 of patients with CLL showed higher expression of several costimulatory molecules and 10–60 times greater secretion of IL-12p70. Although the overall stimulatory capacity of DC types and their ability to take up CLL material were comparable, αDC1 were much more effective in inducing functional, tumor-specific CTL responses, as manifested by their superior induction of IFN-γ-producing, tumor-specific CD8+ T cells and their higher cytolytic activity.
Our previous experiments demonstrated that αDC1 loaded with defined HLA-A2-restricted peptides are effective inducers of CTL responses against such antigenically defined tumors as melanoma [18]. This led to the design of our currently ongoing clinical trials of vaccination with peptide-loaded αDC1 in HLA-A2+ patients with advanced melanoma and other forms of cancer. The current data pave the way to the application of αDC1-based vaccines against tumors with poorly defined rejection antigens and to the treatment of patients with unique HLA types.
Important for the feasibility of using CLL-loaded αDC1 as cancer vaccines or as ex vivo inducers of CLL-specific CTLs for adoptive immunotherapy, we have observed that the IL-12-producing function and CTL-inducing function are not compromised by exposure to CLL cells at the stage of DC maturation. In light of the previously reported contribution of the dysfunction of endogenous DCs [6, 7] to the overall immunosuppression observed in CLL patients [4, 5], the current data suggest the possibility of restoring the immunosurveillance in this group of patients using ex vivo-generated DCs.
Two factors crucial for successful generation of αDC1 in our study were the purity of the monocyte population and the type of medium. In contrast to nonhematologic malignancies [18], in case of CLL, it was critical to apply a magnetic selection of CD14+ cells to avoid contamination of CLL cells in the monocytic fraction of the density-based PBMC separation and the resulting reduced yield of DCs (Fig. 1, inset). This negative impact of CLL cells on early stages of DC generation is reminiscent of the previously reported ability of CLL cells to suppress DC differentiation [6, 7]. Although the mechanism of such suppression has not been elucidated, the previously reported hyperactivation of STAT3 and ERK is possible by the following factors: tumor-derived prostanoids, vascular endothelial growth factor, IL-10, and IL-6[24, 25]. It also remains to be tested whether the defective function of DCs in CLL patients can be reversed using STAT3 blockade or inhibition of MAPK activity, previously shown to enhance immune-mediated, anti-tumor effects [26, 27]. In contrast to the reports showing the functional deficit of DCs differentiating in the presence of CLL cells [6, 7], we did not observe any significant IL-12-inhibitory impact of CLL cells added during DC maturation, consistent with our observations that the susceptibility of DCs to IL-12-suppressing factors is reduced upon DC maturation [17].
Generation of the optimal cell numbers also required the substitution of the originally used [18] AIM-V medium with Cellgenix DC medium. Although the mechanism of the superiority of the Cellgenix DC medium remains unclear, the consistently observed generation of higher numbers of αDC1 led to our prioritization of this medium for the prospective generation of αDC1 for our prospective clinical trials.
In conclusion, this study demonstrates that high numbers of αDC1 can be generated successfully from the blood of CLL patients and used as effective inducers of autologous, CLL-specific CTLs. High immunologic activity of such cells suggests their possible use as vaccines or as ex vivo inducers of tumor-specific CTLs for adoptive transfer to overcome the CLL-associated immune dysfunction.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants CA095128, CA114931, CA101944, and EA055944 (to P. K.).
References
- Dighiero G., Travade P., Chevret S., Fenaux P., Chastang C., Binet J. L. B-cell chronic lymphocytic leukemia: present status and future directions French Cooperative Group on CLL. Blood. 1991;78:1901–1914. [PubMed] [Google Scholar]
- Montserrat E., Moreno C., Esteve J., Urbano-Ispizua A., Gine E., Bosch F. How I treat refractory CLL. Blood. 2006;107:1276–1283. doi: 10.1182/blood-2005-02-0819. [DOI] [PubMed] [Google Scholar]
- Russell N. H., Byrne J. L., Faulkner R. D., Gilyead M., Das-Gupta E. P., Haynes A. P. Donor lymphocyte infusions can result in sustained remissions in patients with residual or relapsed lymphoid malignancy following allogeneic hemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;36:437–441. doi: 10.1038/sj.bmt.1705074. [DOI] [PubMed] [Google Scholar]
- Ravandi F., O'Brien S. Immune defects in patients with chronic lymphocytic leukemia. Cancer Immunol Immunother. 2006;55:197–209. doi: 10.1007/s00262-005-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivener S., Goddard R. V., Kaminski E. R., Prentice A. G. Abnormal T-cell function in B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44:383–389. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- Orsini E., Guarini A., Chiaretti S., Mauro F. R., Foa R. The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 2003;63:4497–4506. [PubMed] [Google Scholar]
- Orsini E., Pasquale A., Maggio R., Calabrese E., Mauro F. R., Giammartini E., Guarini A., Foa R. Phenotypic and functional characterization of monocyte-derived dendritic cells in chronic lymphocytic leukemia patients: influence of neoplastic CD19 cells in vivo and in vitro. Br J Haematol. 2004;125:720–728. doi: 10.1111/j.1365-2141.2004.04971.x. [DOI] [PubMed] [Google Scholar]
- Kokhaei P., Rezvany M. R., Virving L., Choudhury A., Rabbani H., Osterborg A., Mellstedt H. Dendritic cells loaded with apoptotic tumor cells induce a stronger T-cell response than dendritic cell-tumor hybrids in B-CLL. Leukemia. 2003;17:894–899. doi: 10.1038/sj.leu.2402913. [DOI] [PubMed] [Google Scholar]
- Muller M. R., Tsakou G., Grunebach F., Schmidt S. M., Brossart P. Induction of chronic lymphocytic leukemia (CLL)-specific CD4- and CD8-mediated T-cell responses using RNA-transfected dendritic cells. Blood. 2004;103:1763–1769. doi: 10.1182/blood-2003-06-2097. [DOI] [PubMed] [Google Scholar]
- Suresh K., Rodriguez-Lecompte J. C., Gauldie J., Foley R. Recent advances in immunotherapy of B-CLL using ex vivo modified dendritic cells. Hematology. 2005;10:189–203. doi: 10.1080/10245330500094870. [DOI] [PubMed] [Google Scholar]
- Suresh K., Fraser G., Scheid E., Leber B., Gauldie J., Foley R. Generation of in vitro B-CLL specific HLA class I restricted CTL responses using autologous dendritic cells pulsed with necrotic tumor lysate. Leuk Lymphoma. 2006;47:297–306. doi: 10.1080/10428190500301231. [DOI] [PubMed] [Google Scholar]
- Hus I., Rolinski J., Tabarkiewicz J., Wojas K., Bojarska-Junak A., Greiner J., Giannopoulos K., Dmoszyńska A., Schmitt M. Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1621–1627. doi: 10.1038/sj.leu.2403860. [DOI] [PubMed] [Google Scholar]
- Allgeier T., Garhammer S., Nossner E., Wahl U., Kronenberger K., Dreyling M., Hallek M., Mocikat R. Dendritic cell-based immunogens for B-cell chronic lymphocytic leukemia. Cancer Lett. 2007;245:275–283. doi: 10.1016/j.canlet.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Goddard R. V., Prentice A. G., Copplestone J. A., Kaminski E. R. In vitro dendritic cell-induced T cell responses to B cell chronic lymphocytic leukemia enhanced by IL-15 and dendritic cell-B-CLL electrofusion hybrids. Clin Exp Immunol. 2003;131:82–89. doi: 10.1046/j.1365-2249.2003.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillier F., Dighiero G. Monocyte-derived dendritic cells in chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44:1267–1273. doi: 10.1080/1042819031000079087. [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Kuhn U., Muller G., Steinbrink K., Paragnik L., Schmitt E., Knop J., Enk A. H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- Vieira P. L., de Jong E. C., Wierenga E. A., Kapsenberg M. L., Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- Mailliard R. B., Wankowicz-Kalinska A., Cai Q., Wesa A., Hilkens C. M., Kapsenberg M. L., Kirkwood J. M., Storkus W. J., Kalinski P. α-Type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Kalinski P., Schuitemaker J. H., Hilkens C. M., Wierenga E. A., Kapsenberg M. L. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-γ and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162:3231–3236. [PubMed] [Google Scholar]
- Langenkamp A., Messi M., Lanzavecchia A., Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- Albert M. L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Yu H., Kortylewski M., Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumor microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- Wang T., Niu G., Kortylewski M., Burdelya L., Shain K., Zhang S., Bhattacharya R., Gabrilovich D., Heller R., Coppola D., Dalton W., Jove R., Pardoll D., Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Nefedova Y., Nagaraj S., Rosenbauer A., Muro-Cacho C., Sebti S. M., Gabrilovich D. I. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the Janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–9535. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang J., Qian J., Wezeman M., Kwak L. W., Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107:2432–2439. doi: 10.1182/blood-2005-06-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.