Abstract
There is a strong interest in harnessing the genetic manipulations possible in mice to investigate functional neural mechanisms modulating associative processes that control drug-seeking behavior. However, it is unknown whether intra-cranial techniques such as the disconnection procedure commonly used in rats to examine serial connectivity between implicated areas can be successfully applied to mice. We have previously demonstrated that expression of ethanol-seeking behavior in mice is dependent upon amygdala (Amy) dopamine- and nucleus accumbens (Acb) NMDA- receptor activation (Gremel & Cunningham, 2009). Here, we use a neuropharmacological disconnection procedure to investigate whether dopamine activation of Amy directly leading to increases in Acb glutamate release and binding of NMDA receptors modulates expression of ethanol-seeking behavior. Immediately before testing the expression of an ethanol-induced conditioned place preference (CPP), mice were given an Amy infusion of flupenthixol and either an ipsi- or contra-lateral Acb infusion of AP-5. While both ipsi- and contra-lateral manipulations reduced expression of ethanol CPP, in a separate experiment we demonstrate that a unilateral Acb AP-5 infusion, but not Amy Flu, is sufficient to disrupt preference. The finding of significant blockade by a unilateral AP-5 into Acb precludes any conclusions about a unique role for the Amy-Acb neuroanatomical connection in this model of ethanol-seeking behavior. Further, the current results suggest potential limitations in transferring techniques from rats to mice in order to study serial interactions between neural areas underlying motivated behaviors. Nevertheless, these findings provide evidence showing that Acb NMDA receptors play an important role in expression of ethanol-conditioned behavior.
Keywords: Ethanol, conditioned place preference, conditioned reinforcement, basolateral amygdala, nucleus accumbens
Introduction
Much progress has been made identifying the neural areas involved in drug-seeking behaviors, with connections between the mesolimbic dopamine system and cortical and striatal systems implicated in mediating associative processes controlling drug seeking and taking behaviors that can lead to compulsive and habitual drug use (for review see Everitt & Robbins, 2005; Day & Carelli, 2007). In particular, the disconnection procedure has been particularly useful in examining whether neural areas interact serially to control a particular drug-seeking behavior (e.g., Di Ciano & Everitt, 2004, Parkinson et al., 2000). The premise behind the disconnection procedure is that unilateral manipulations of two interconnected nuclei located in opposite hemispheres should reduce behavior if the behavior is dependent upon interactions between the two areas, compared to manipulations within the same hemisphere.
However, most if not all studies using the disconnection procedure in behavioral paradigms have been performed in the rat. While rats have been indispensable in modeling drug-seeking behavior, the continued development of mouse strains with targeted gene deletions (knockouts or KO), conditional KOs, transgenics, and inducible KOs offers the possibility of identifying and modulating specific genes involved in drug-seeking behaviors. To harness the potential power of genetic manipulations of mice, we must advance behavioral techniques in existing mouse models and further develop mouse tasks to examine drug-seeking behavior. One model that routinely employs mice is ethanol-induced conditioned place preference (CPP) (e.g., Tzchentke 1998; 2008; Cunningham et al., 2006a). The CPP procedure provides an effective way to directly investigate the neural mechanisms through which a drug-paired cue can gain control of drug-seeking behaviors, since the behavior observed during expression is controlled by the retrieved memory of the drug-cue association.
To date, the use of mice to investigate the functional neural processes underlying ethanol-seeking behaviors has been successful. Initial investigations found a decrease in ethanol CPP expression following bilateral activation of GABAB receptors or blockade of opioid receptors in the VTA, confirming the hypothesis that manipulations within the VTA that decrease dopamine neuron activity would also decrease expression of ethanol CPP (Bechtholt & Cunningham, 2005). We recently demonstrated that expression of ethanol-seeking behavior required an intact Amy and Acb (Gremel & Cunningham, 2008). Moreover, CPP expression depended upon bilateral dopamine receptor activation in the Amy (but not Acb), and bilateral NMDA receptor activation in the Acb (Gremel & Cunningham, 2009).
These previous findings suggest the expression of ethanol-seeking behaviors depend on dopaminergic afferents from the VTA (Ford et al., 2006) activating the Amy. However, the source of glutamate activation of Acb NMDA receptors is not clear. While the Amy directly innervates the Acb through basolateral amgydala (BLA) glutamate afferents (e.g., Groenewegen et al., 1996), the Acb also receive large glutamatergic inputs from cortical sources (e.g., Sesack et al., 1989, 1990; Totterdell & Smith, 1989). To examine whether recruitment of Acb activity during expression of ethanol-conditioned behaviors depends on glutamatergic inputs from the Amy or alternatively, other cortical sources, we employed a neuropharmacological disconnection procedure in mice (Parkinson et al., 2000).
Materials and Methods
Subjects
Male DBA/2J (n =142) mice were obtained from the Jackson Laboratory (Bar Harbor, ME or Davis, CA) at 6-7 weeks of age. Previous findings have demonstrated that DBA/2J mice develop a strong preference for ethanol-paired tactile cues at a dose of 2 g/kg (e.g., Cunningham et al., 2003; 2006a). Animals were initially housed in groups of four on a Thoren rack (Thoren caging systems Inc., Hazleton, PA) in polycarbonate cages. After surgical procedures, animals were housed two per cage for the duration of the experiments. Animals were kept at an ambient temperature of 21±1°C on a 12-h light-dark cycle (lights on at 0700 hours). Experiments were carried out during the light portion of the cycle beginning at 1300 h. “Labdiet” rodent chow (Richmond, IN) and bottled water were continuously available in the home cage. The National Institutes of Health (NIH) “Principles of Laboratory Animal Care” were followed in conducting these studies and the protocol was approved by the Oregon Health & Science University IACUC.
Surgery
Mice were fully anesthetized with a cocktail (0.1 ml/25 g) containing ketamine (30.0 mg/ml) and xylazine (3.0 mg/ml). Bilateral indwelling cannulae (10 mm, 25 gauge) were implanted under stereotaxic guidance (model no. 1900, Kopf Instruments, Tujunga, CA). In each subject, one cannula was aimed at the nucleus accumbens core (AcbC) (from Bregma: anterior (A) + 1.40, lateral (L) ± 1.26, ventral (V) −4.2) while the second was aimed at the basolateral/central nuclei of the amygdala (BLA/CE) (from Bregma: A – 1.22, L ± 2.85, V – 4.5; Paxinos & Franklin, 2001) either ipsi- or contra-lateral to the first. Cannulae were positioned 2.0 mm above the Acb and 2.3 mm above the BLA/CE and secured with stainless steel screws and carboxylate cement (Durelon™, 3M, St. Paul, MN). Thirty-two gauge stainless steel stylets (10 mm) were inserted into the length of each guide cannula to maintain patency. Mice were allowed 4 – 9 days of recovery prior to the start of conditioning trials. Cannulae placements were counterbalanced for hemisphere (left vs. right) and disconnection group (ipsi- vs. contra-lateral). To control for possible effects of recovery time, the number of recovery days was counterbalanced across infusion groups.
Apparatus
A detailed description and picture of the apparatus has been published (Cunningham et al., 2006a). Briefly, the apparatus consisted of 12 identical acrylic and aluminum boxes (30 × 15 × 15 cm) enclosed in individual ventilated, light- and sound-attenuating chambers (Coulbourn Instruments Model E10-20). Six sets of infrared light sources and photodetectors mounted 2.2 cm above the floor at 5 cm intervals along the long wall of the box detected general activity, location in the box, and time spent on each side of the chamber (10 ms resolution).
Conditioned stimuli (CSs) consisted of two interchangeable distinctive floor halves placed beneath each box. The hole floor was made from perforated stainless steel sheet metal (16 gauge) containing 6.4 mm round holes on 9.5 mm staggered centers. The grid floor was constructed from 2.3 mm stainless steel rods mounted 6.4 mm apart in acrylic rails. This floor texture combination was selected on the basis of previous studies demonstrating that drug-naïve control DBA/2J mice spend about half their time on each floor type during choice tests (e.g., Cunningham et al., 2003; Gremel & Cunningham, 2008). The inside and floors of the box were wiped with a damp sponge and the litter paper underneath the flooring was changed between animals.
Conditioning drugs
Ethanol (95%) was diluted in 0.9% saline (20% v/v) and administered at a dose of 2 g/kg (12.5 ml/kg). In previous experiments, this ethanol dose and concentration has reliably induced a strong CPP in DBA/2J mice (e.g., Cunningham et al., 2003) without detrimental behavioral effects of repeated injections (Cunningham et al., 1997). Saline was administered in a volume of 12.5 ml/kg.
General Procedure
Each experiment involved three phases: habituation (1 session), conditioning (8 sessions), and testing. Each animal was given an intraperitoneal (i.p.) injection immediately before being placed in the center of the apparatus for each session.
Habituation
Subjects in all experiments underwent a 5-min habituation trial where they were given an injection of saline and exposed to the apparatus on a smooth paper floor to reduce the novelty and stress associated with handling, injection and exposure to the apparatus.
Conditioning
Mice were randomly assigned to an infusion group described separately for each experiment in a later section (Disconnection Group). Within each disconnection group, mice were also randomly assigned to one of two conditioning subgroups (Grid+ or Grid−) using an unbiased, one-compartment procedure (Cunningham et al., 2003; 2006a). Both subgroups were exposed to a differential Pavlovian conditioning procedure in which they received four CS+ and four CS− trials. Mice in the Grid+ condition received ethanol paired with the grid floor (CS+) and saline paired with the hole floor (CS−). Mice in the Grid− condition received ethanol paired with the hole floor (CS+) and saline paired with the grid floor (CS−). Each animal received four 5-min conditioning trials of each type on alternating days over a period of 8 days, with the presentation order of CS+ and CS− trials counterbalanced within each group.
Place Preference Test
Testing began 48 h after the last conditioning trial for all animals. Just before each 30-min test session, mice received an intracranial infusion (see next section for details). Immediately after infusion, mice were given an i.p. saline injection and placed in the center of the apparatus with both test floors (half grid/half hole). Position (i.e., left vs. right) of each floor type was counterbalanced within subgroups.
Intracranial Microinfusions
All mice received an intracranial microinfusion immediately before testing (Table 1). To minimize possible effects of initial injector lowering on the behavior measured, 24 h before testing the stylet was temporarily removed and a longer 12- (Acb) or 12.3- (Amy) mm stylet was briefly lowered to the infusion site. The shorter stylet was then re-inserted until the microinfusion was administered 24 h later. For microinfusions, stylets were removed and injectors made of 32-gauge stainless steel tubing encased by 25-gauge stainless steel were lowered beyond the tip of the guide cannula into the Amy (2.3 mm beyond cannula tip) or Acb (2 mm beyond cannula tip). Injectors were attached via polyethylene tubing (PE20) to 10 μl Hamilton syringes, and infusions were delivered via a syringe pump (Model A-74900-10: Cole-Parmer, Vernon Hills, IL). Simultaneous infusions of 100 nl/side were given over 60 sec to limit injection spread into neighboring brain areas, as well as to minimize diffusion up the injector track. Further, to ensure complete diffusion, injectors were removed 30 sec after completion of the infusion and stylets were replaced.
Table 1.
Disconnection Groups
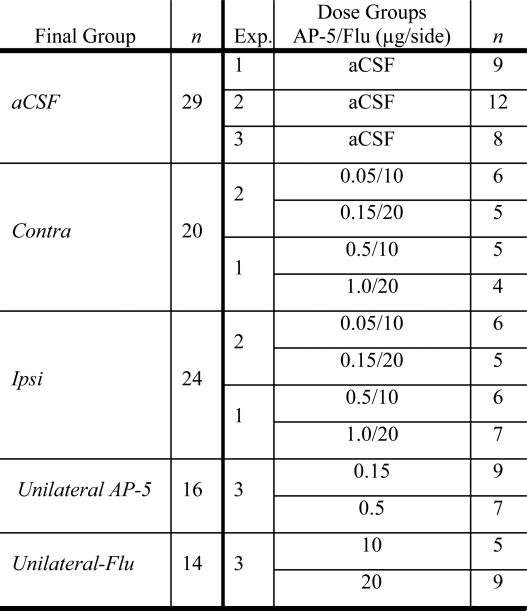
|
Disconnection of the Amy and Acb
To investigate possible dopamine-induced Amy activation and subsequent activation of Acb NMDA receptors via glutamatergic BLA afferents (e.g., Groenewegen et al., 1996) in the expression of ethanol CPP, we utilized a neuropharmacological disconnection procedure in mice. The premise behind the disconnection procedure is that unilateral manipulations of two interconnected nuclei located in opposite hemispheres should reduce behavior if the behavior is dependent upon interactions between the two areas, compared to manipulations within the same hemisphere (e.g., Di Ciano & Everitt, 2004). Initially two experiments were performed (n = 94), with disconnection of Amy and Acb made by infusing flupenthixol into the Amy in one hemisphere, while simultaneously infusing AP-5 into Acb in either the same (ipsi-lateral) or opposite (contra-lateral) hemisphere. In the first experiment, flupenthixol doses of 10 or 20 μg/side were infused into Amy, while AP-5 doses of 0.5 or 1.0 μg/side were infused into Acb (see Table 1). Since all treatments reduced preference in the first experiment (Table 2), lower doses of AP-5 (0.05 and 0.15 μg/side) were infused, in combination with the same doses of flupenthixol (10 or 20 μg/side) in the second experiment.
Table 2.
Experiment 1 and 2 Preference Test
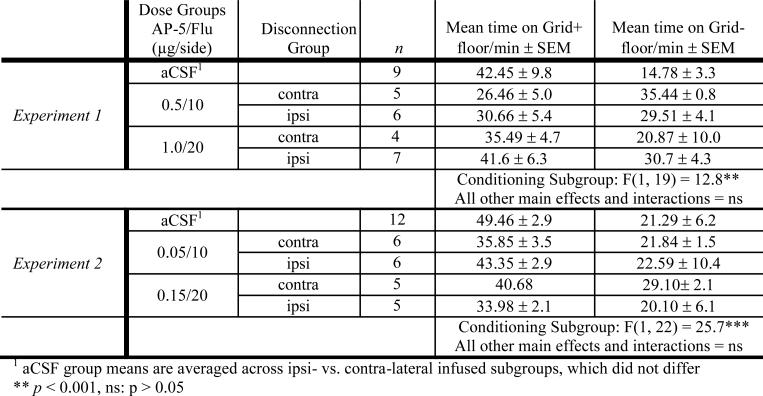
|
Unilateral infusions of AP-5 and Flu into Acb and Amy
Because the second experiment also failed to show differences between the ipsi-lateral and contra-lateral groups (Table 2), a third experiment (n = 48) was performed to examine the effect of a single intracranial microinfusion of drug into one area in one hemisphere (e.g., the effect of AP-5 antagonism in a single Acb nucleus on CPP expression). As in the first and second experiments, all mice were implanted with cannulae aimed at the Amy and AcbC, either ipsi- or contra-lateral to each other. Immediately before testing, however, mice received a unilateral intracranial microinfusion of either flupenthixol (10 or 20 μg/side) into Amy or AP-5 (0.15 or 0.5 μg/side) into Acb. To control for the number of intra-cranial infusions between experiments, mice were given a simultaneous aCSF infusion into the other brain area.
Histology
Animals were given an overdose of sodium pentobarbital (150 mg/kg). Heads were removed and postfixed in 4% (w/v) paraformaldehyde in isotonic sodium phosphate buffered saline (PBS). After 24 h, brains were dissected from the skull and placed into a solution of 2% paraformaldehyde for an additional 24 h. After fixation, brains were cryoprotected using a sucrose saturation procedure consisting of 24 h incubations in 20% and then 30% sucrose in PBS and 0.1% NaN3. Frozen 40 μm sections were collected through the infusion site. Slices were directly mounted onto slides and thionen stained. Placements were subjectively assessed blind to dose, hemisphere, disconnection group, disconnection placement, and test outcome. Inclusion criteria were as follows: subjects were included if they had one injector track within AcbC and one injector track located within BLA and/or CE.
Data Analyses
The primary dependent variable was the amount of time spent on the grid floor during the test session. In this unbiased design, the magnitude of the difference in time spent on the grid floor between the Grid+ and Grid− conditioning subgroups is indicative of CPP. See Cunningham et al. (2003) for a more complete discussion of dependent variables used in place conditioning studies. Data from each experiment were evaluated separately by analysis of variance (ANOVA) with the alpha level set at 0.05. To control overall alpha level within each experiment, p-values were Bonferroni corrected for the number of post-hoc comparisons between group means. Disconnection Group, Unilateral Group, Conditioning Subgroup (Grid+ vs. Grid−), and Disconnection Placement (ipsi- vs. contra-lateral) were treated as between-group factors, whereas Trial Type (CS+ vs. CS−) was treated as a within-subject factor.
Results
Histological verification and subject removal
Schematic diagrams of inclusion criteria are shown in Figure 1. A total of 39 subjects were removed from the final analyses for various reasons, including: poor health during recovery following surgical procedures (n = 3), procedural errors during conditioning and testing (n = 6), an inability to accurately assign injector placement due to problems with histological assessment (n = 6), incorrect injector placement (n = 12), or an infection at the injector and/or cannula site (n = 12).
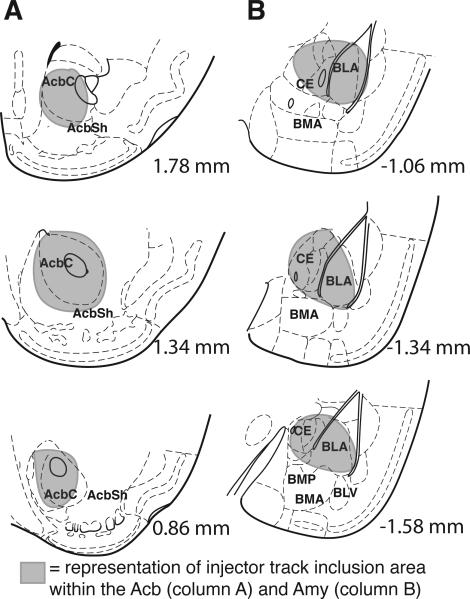
Figure 1.
Representative diagram of Acb and Amy injector placements. Representative injector inclusion area criteria are shown for Acb (column A) and Amy (Column B). Numbers indicate the distance from bregma in millimeters of the section (Paxinos & Franklin, 2001).
Place Preference Test
Disconnection of the Acb and Amy
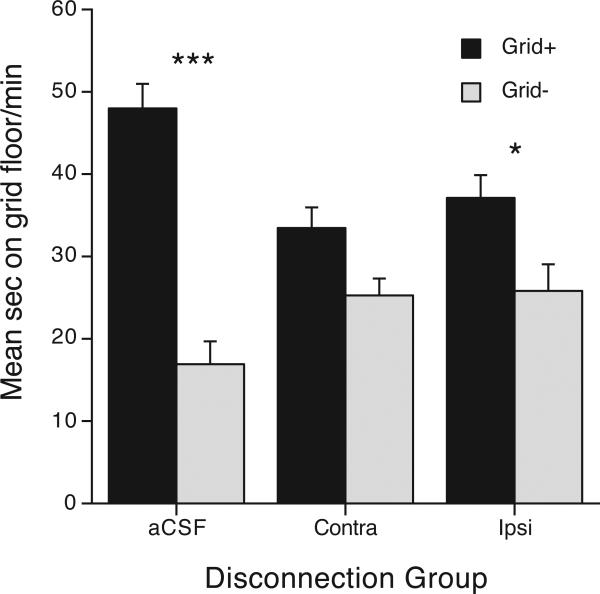
To examine whether dopamine activation of the Amy leading to glutamatergic modulation of the Acb modulates expression of ethanol CPP, we used a neuropharmacological disconnection procedure antagonizing dopamine receptors in the Amy in one hemisphere, and intra-Acb NMDA receptors in the ipsi- or contra-lateral hemisphere. The first two experiments yielded a strong CPP in aCSF controls that was reduced by ipsi- or contra-lateral drug infusions (see Table 2). However, disconnection group did not differentially affect ethanol CPP in either experiment (p's > 0.05 for main effect and all interactions with Disconnection Group). Because the subgroup n's within each experiment were low (see Table 2), we conducted an additional analysis in which the data from the disconnection groups were pooled across dose groups in Experiments 1 and 2 to increase statistical power (see Figure 2). This analysis revealed a significant Disconnection Group × Conditioning Subgroup interaction [F(2,67) = 10.2, p < 0.0001], reflecting significant CPP in the aCSF control group (Bonferroni corrected p < 0.0001) and ipsi-lateral group (Bonferroni corrected p = 0.02), but not in the contra-lateral group (p > 0.2). However, there was no significant difference in a direct comparison between the ipsi- and contra-lateral groups [Disconnection group × Conditioning Subgroup interaction: F(1,40) = 0.3, p > 0.5], indicating no effect of the disconnection manipulation. Moreover, the aCSF group showed significantly greater CPP when compared to either the ipsi- [F(1,49) = 11.2, p < 0.005] or contra-lateral [F(1,45) = 16.7, p < 0.0001] groups. Thus, we were unable to confirm a unique role for glutamatergic modulation of Acb via dopamine activation of Amy.
Figure 2.
Effects of neuropharmacological disconnection of the Amy and AcbC. Subjects were given intra-Amy aCSF or flupenthixol infusions and contra- or ipsi-lateral intra-AcbC infusions of aCSF or AP-5. Groups were given the following infusions: aCSF mice were given infusions of aCSF into both the Amy and AcbC. Mice in the Contra group were infused with flupenthixol (10 or 20 μg) into the Amy in one hemipshere, with AP-5 (0.05, 0.15, 0.5, or 1.0 μg) infused into the Acb in the opposite hemisphere. Ipsi mice were given intra-Amy infusions of flupenthixol (10 or 20 mg) and intra-Acb (0.05, 0.15, 0.5, or 1.0 μg) into the same hemisphere. Grid+ and Grid−conditioning subgroups N's are respectively: aCSF n = 14 and 15; Contra n = 10 and10; Ipsi n =12 and 12. Difference between conditioning subgroups Grid+ and Grid−: * = Bonferroni corrected ps < 0.05; *** = Bonferroni corrected ps < 0.001.
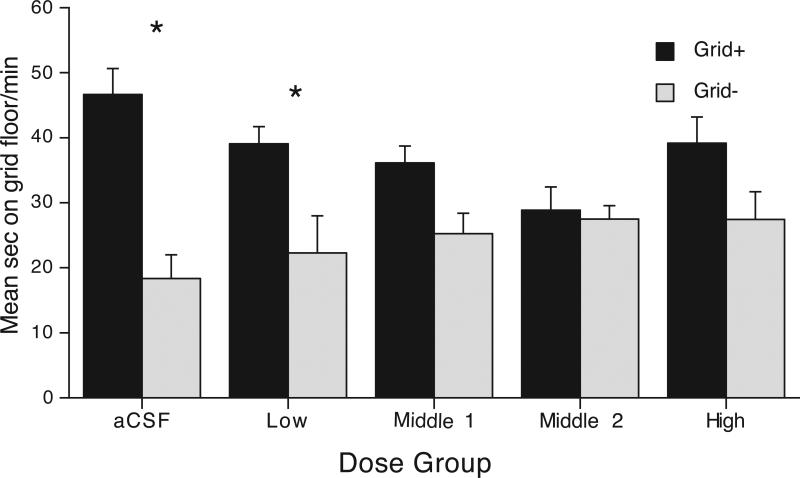
Since no differences in CPP between ipsi- and contra-lateral groups were observed, the question arose as to whether all doses of AP-5 and Flu were effective at reducing expression behavior. To examine dose effects on CPP we performed additional analyses with data collapsed across disconnection groups into the following five dose groups (AP-5/Flu ug/side); aCSF Group, Low (0.05/10), Middle 1 (0.15/20) Middle 2 (0.5/10), High (1.0/20) (see Figure 3). Analysis revealed a significant Dose Group and Conditioning Subgroup interaction [F(4,55) = 3.4, p < 0.05], reflecting significant CPP in the aCSF control and Low Groups (Bonferroni corrected p's < 0.05), but not in the Middle 1, Middle 2, or High Groups (p's > 0.3). Further, while the aCSF group showed greater CPP when compared to the Middle 1 (F = 9.7, p > 0.05), Middle 2, and High Groups (F's > 3.54, p's < 0.07), the magnitude of preference between the aCSF and Low Groups was of a similar magnitude (F = 1.84, p = 0.4). Overall, this suggests that the lowest doses of AP-5 and Flu were ineffective at reducing the behavior when infused ipsi- or contra-laterally.
Figure 3.
Dose effects of Acb AP-5 and Amy flupenthixol on expression of ethanol CPP. Data were collapsed across disconnection groups into five groups that received the following infusions into the Acb/Amy of AP-5/Flu ug/side; aCSF Group (0/0), Low (0.05/10), Middle 1 (0.15/20) Middle 2 (0.5/10), High (1.0/20). Grid+ and Grid− conditioning subgroups N's are respectively: aCSF n = 10 and 11; Low Group n = 7 and 5; Middle 1 n = 7 and 4; Middle 2 n = 3 and 7; High n = 5 and 6. Difference between conditioning subgroups Grid+ and Grid−: * = Bonferroni corrected ps < 0.05.
Unilateral infusions of AP-5 and Flu into the Acb and Amy
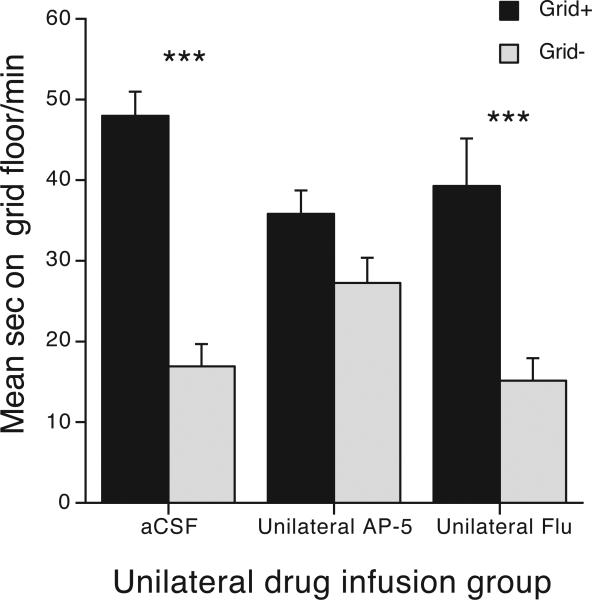
In an effort to better understand the reasons behind the outcome of our disconnection studies, mice in the third experiment received unilateral infusions of AP-5 (middle doses of 0.15 or 0.5 ug/side) into Acb or Flu (10 or 20 ug/side) into Amy (see Table 1). As shown in Figure 4, unilateral drug infusions had a significant effect on CPP [Unilateral Dose × Conditioning Subgroup interaction: F(2,53) = 5.7, p < 0.01], reflecting significant CPP in the aCSF control group (Bonferroni corrected p < 0.0001) and unilateral Flu (Amy) group (Bonferroni corrected p = 0.0001), but not in the unilateral AP-5 (Acb) group (p > 0.3). Pair-wise group comparisons showed that aCSF controls differed from the unilateral AP-5 (Acb) group [F(1,41) = 12.5, p = 0.001], but not from the unilateral Flu (Amy) group [F(1,39) = 0.9, p > 0.3]. The two unilateral infusion groups were marginally different [F(1,26) = 4.2, p = 0.052]. A separate analysis showed that there was no effect of ipsi- versus contra-lateral infusion in aCSF controls (data not shown). Findings from the unilateral AP-5 (Acb) group suggest that intra-Acb NMDA receptor antagonism in one hemipshere may be sufficient to disrupt preference independent of dopamine blockade in Amy.
Figure 4.
To examine the effects of NMDA or D1, D2 receptor antagonism of a single Acb or Amy nucleus, unilateral drug infusions were given. Mice in the AP-5 group were infused with aCSF into one Amy, and AP-5 (0.15 or 0.5 μg) into one AcbC. Mice in the Flu group were given a flupenthixol infusion (10 or 20 μg) into one Amy, and aCSF into one AcbC. Infusions were counterbalances between hemispheres with both ipsi- and contra- lateral placements. Unilateral AP-5 n = 9 and 7; and Unilateral Flu n = 7 and 7. Difference between conditioning subgroups Grid+ and Grid−: *** = Bonferroni corrected ps < 0.001.
Locomotor Activity
Group means and statistical comparisons for conditioning and test activity are shown in Table 3.
Table 3.
Locomotor Activity
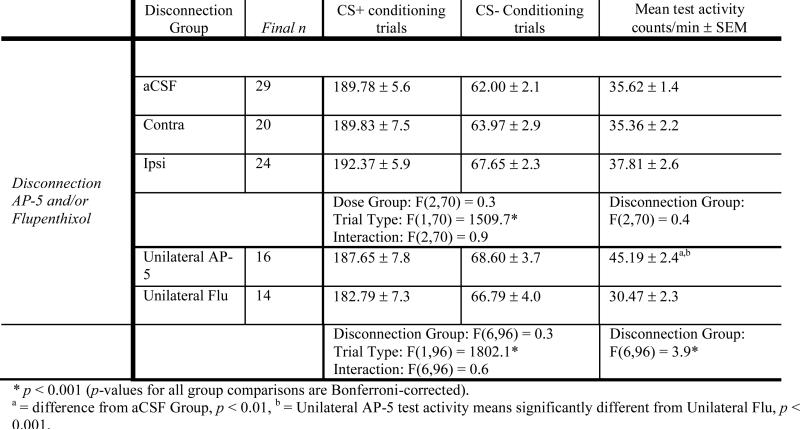
|
To simplify presentation, conditioning activity data were collapsed across trials to create single means for the CS+ and CS−. As in previous experiments, ethanol induced large increases in locomotor activity on CS+ trials (e.g., Cunningham et al., 2003; Cunningham et al., 2006a; Gremel & Cunningham, 2007). Further, there were no differences in activity levels between disconnection groups during ethanol or saline trials. Of interest, neither ipsi- nor contra-lateral pretreatment infusions of Acb AP-5 and Amy Flu had an affect on activity during the test session. However, activity differences during the test session were observed in Experiment 3. Similar to our previous finding that bilateral Acb NMDA receptor antagonism increased activity levels (Gremel & Cunningham, 2008), unilateral Acb infusions of AP-5 produced slightly higher activity levels than observed in the unilateral Flu (Amy) or aCSF groups.
Discussion
We have previously demonstrated that expression of ethanol-conditioned behavior is dependent upon Amy dopamine receptor and Acb NMDA receptor activation (Gremel & Cunningham, 2009). The present studies used a neuropharmacological disconnection procedure in mice to examine whether the Amy and Acb interact serially to control expression of ethanol-seeking behaviors. To the best of our knowledge, this is the first attempt in mice to employ the disconnection procedure to investigate motivated behaviors. However, we were unable to provide clear evidence that dopamine activation of the Amy directly led to glutamate activation of NMDA receptors in the Acb. On the contrary, we observed that ipsi- and contra-lateral manipulations were both effective at reducing CPP expression, leaving open the possibility of modulation by glutamate projections from cortex or hippocampus. However, although glutamatergic input arising from Amy might nevertheless modulate ethanol CPP, the finding of significant blockade by a unilateral AP-5 into the Acb infusion in the second experiment precludes any conclusions about a unique role for the Amy-Acb neuroanatomical connection in this model of ethanol-seeking behavior. Nevertheless, these findings replicate and extend our previous finding that Acb NMDA receptors play a critical role in the expression of ethanol-conditioned behavior (Gremel & Cunnningham, 2009).
Given the overlap between mice and rats in the functional mechanisms underlying associative control over drug-seeking behaviors, our inability to show disconnection suggests a possible limitation to the use of mouse models of associative processes. The use of CPP in mice to examine associative control over ethanol-seeking behaviors is advantageous when compared to the use of oral self-administration (SA) procedures in rats, since it provides a way to measure a conditioned response that has never produced the reinforcer, and can be examined in the absence of the direct effects of ethanol. Theoretically, Pavlovian conditioned approach behavior, conditioned reinforcement, and conditioned incentive may all be operating in CPP (Cunningham et al., 1995; Cunningham & Patel, 2007; Kumar, 1972; Swerdlow et al., 1989; Uslaner et al., 2006). By combining CPP with bilateral intracranial infusions before testing, we were able to demonstrate that the functional neural mechanisms engaged (Gremel & Cunningham 2008, 2009) are similar to those underlying conditioned reinforcement (e.g., Burns et al, 1993; Cador et al., 1989; Whitelaw et al., 1996; Di Ciano & Everitt, 2004) and response-outcome learning (Kelly et al., 1997; Baldwin et al., 2000). This observed overlap between functional processes in mice and rats suggests that mouse models could potentially be used to examine serial interactions between functional areas controlling associative processes involved in motivated behaviors.
Unfortunately, the present results show that mouse behavior may be susceptible to disruption from intracranial procedures generally not reported to disturb behaviors in rats (e.g., Di Ciano & Everitt, 2004, Belin & Everitt, 2008; Setlow et al., 2002). Although we observed a decrease in behavior after contra-lateral manipulations that might depend on dopamine activation of Amy producing increases in glutamatergic input to Acb, there was also a similar decrease after ipsi-lateral manipulations. A potential explanation for decreased behavior in the ipsi-lateral group could be that the Amy is not the sole source of glutamate modulating Acb NMDA receptors, with other cortical sources playing an important role. For example, a previous study that successfully used a disconnection procedure to isolate a functional role for the Amy to Acb pathway in rats did not show an effect of ipsi-lateral manipulations on the measured behavior (Setlow et al., 2002).
However, although ipsi-lateral infusions serve as the best way to control for the number of drug infusions while maintaining one intact serial pathway, the decreased behavior observed in both the ipsi- and contra-lateral manipulations raises the possibility that unilateral infusion of drug is sufficient to reduce the behavior. To examine this possibility, in experiment 3 mice were given a single unilateral infusion of AP-5 into the Acb or a single infusion of Flu into the Amy. We controlled for the total number of infusions by infusing aCSF into the remaining area either ipsi- or contra-laterally from the drug-infusion site. While blockade of Amy D1/D2/D3 receptors in only one hemisphere did not disrupt behavior, a unilateral Acb infusion of AP-5 blocked expression of ethanol CPP. In contrast to our findings, in a previous report where unilateral infusions served as controls in a disconnection experiment examining Amy and Acb interactions in controlling cocaine-seeking behavior in rats, unilateral blockade of Acb AMPA/kainite or BLA D1/D2/D3 receptors had no effect on responding in a second order schedule of reinforcement (Di Ciano & Everitt, 2004). One conclusion that could be taken from the present findings is that mouse behavior is generally more susceptible to disruption of Acb function. Another possibility is that CPP behavior itself is dependent on a functional Acb. However, an argument against this conclusion stems from previous reports showing no effect of Acb opioid or dopamine receptor antagonism on expression of ethanol CPP in mice (Bechtholt & Cunningham, 2005; Gremel & Cunningham, 2009). Additionally, it should be noted that use of other methods such as temporary inactivation induced by GABA agonists or lidocaine might be less disruptive than the methods used in the current experiments, allowing for examination of serial connectivity between areas involved in guiding CPP or other motivated behaviors in mice.
While the Acb AP-5 effect might be specific to use of mice, CPP, or our specific technique, it may be that this effect is due to Acb NMDA receptor modulation of expression behavior. Baldwin et al. (2000) reported a similar effect in rats, showing that unilateral AP-5 infusion into Acb impaired acquisition of instrumental learning. In that study, initial acquisition of lever pressing for a sucrose reward was disrupted with unilateral Acb NMDA receptor blockade, although subjects were able to learn the task after the antagonist treatment ended (Baldwin et al., 2000). It was hypothesized that Acb NMDA receptors were recruited in the initial response-outcome learning that mediated the task. Applying this interpretation to the current results, it may be that unilateral Acb NMDA receptor blockade is sufficient to disrupt learning the response-outcome relationship in CPP. While acquisition of a CPP involves learning the Pavlovian relationship between the cue and ethanol, where the cue is endowed with motivational properties similar to those of ethanol, additional learning must occur during the expression test. More specifically, during testing, the retrieved memory of the Pavlovian association between ethanol and the cue enables new learning of an instrumental locomotor response directed towards the spatial location of the previously ethanol-paired floor. This new learning may be expressed through activation of Acb NMDA receptors, with the acquisition sensitive to both bilateral and unilateral blockade of Acb NMDA receptors.
In addition to blocking CPP, unilateral Acb NMDA receptor antagonism also slightly increased activity levels during testing. This finding is similar to previous reports of increased activity following bilateral Acb AP-5 infusions (Gremel & Cunningham, 2009) and systemic NMDA antagonism in mice (Boyce-Rustay & Cunningham, 2004). Since recent data have demonstrated a negative correlation between test activity levels and magnitude of preference (Gremel & Cunningham, 2007), it could be that increased activity levels during testing disrupted CPP. However, even though AP-5 treated mice were slightly more active during testing (see Table 3), we previously reported that a similar level of test activity expressed in a different set of mice was insufficient to disrupt expression of ethanol CPP (Gremel & Cunningham, 2007). This suggests that the lack of preference expressed following unilateral AP-5 infusions cannot solely be attributed to activity influences on CPP behavior.
Although we have previously reported that bilateral Amy D1/D2/D3 receptor antagonism reduced activity levels, in the current study unilateral infusions of Flu into the Amy had no effect on locomotor activity during testing. Of particular interest, Flu infused into the Amy simultaneously with AP-5 into the Acb blocked the increase in activity otherwise observed with unilateral Acb AP-5 infusions. This was observed in both ipsi- and contra-lateral disconnection groups, which raises a couple of possible explanations. First, although insufficient at reducing locomotor activity on its own, unilateral Amy infusions of Flu may modulate Acb function directly through Amy and Acb connections and block AP-5-induced increases in test activity. Another possible way Amy Flu reversed Acb NMDA receptor blockade-induced activity increases could be through Amy feedback to the VTA (Hopkins & Holstege, 1978), which in turn acts on the Acb (Brog et al., 1993). However, these findings do indicate that the Amy was sensitive to the Flu doses used in the present experiments.
In contrast to previous studies in rats that have successfully used the disconnection procedure to demonstrate functional serial connectivity between neural areas controlling drug-seeking behavior, we were unable to clearly identify a role for the Amy and Acb pathway in ethanol-seeking behavior in mice. Instead, we observed that blockade of Acb NMDA receptors alone is sufficient to disrupt the behavior. While we were unable to provide further insight into how the Amy and Acb process information underlying ethanol-conditioned behaviors, our current findings further illustrate the importance of Acb NMDA receptors in the expression of ethanol-conditioned behavior.
Acknowledgements
This research was supported by NIH-NIAAA grants AA016041, AA007468, and AA007702. Experiments within this manuscript comply with the current laws of the United States of America.
References
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav. Neurosci. 2000;114:84–98. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav. Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav. Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav. Neurosci. 2004;118:822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Brog J, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comparative Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav. Brain Res. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neurosci. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, Okorn DM. Naloxone facilitates extinction but does not affect acquisition or expression of ethanol-induced conditioned place preference. Experi. Clin. Psychopharm. 1995;3:330–343. [Google Scholar]
- Cunningham CL, Okorn DM, Howard C. Interstimulus interval determines whether ethanol produces conditioned place preference or aversion in mice. Animal Learn. Behav. 1997;25:31–42. [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature protocols. 2006a;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel PA. Rapid induction of pavlovian approach to an ethanol-paired visual cue in mice. Psychopharm. 2007;192:231–241. doi: 10.1007/s00213-007-0704-4. [DOI] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13:148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191:195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J. Neurosci. 2008a;28:1076–1084. doi: 10.1523/JNEUROSCI.4520-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharm. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog. Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. circuitry in attentional and representational processes. Trends in Cognitive Sciences.
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon pons and medulla oblongata in the cat. Exp. Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc. Natl. Acad. Sci. USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. Morphine dependence in rats: secondary reinforcement from environmental stimuli. Psychopharmacoligia. 1972;25:332–338. doi: 10.1007/BF00421972. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav. Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego; Academic Press: 2001. [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Compar. Neuro. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur. J., Neurosci. 2002;15:1841–1853. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Gilbert D, Koob GF. Conditioned drug effects on spatial preference: Critical evaluation. In: Boulton AA, Baker GB, Greenshaw AJ, editors. Psychopharmacology (Neuromethods Vol. 13) NJ. Clifton; Humana Press: 1989. pp. 399–446. [Google Scholar]
- Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem. Neuroant. 1989;2:285–298. [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav. Brain Res. 2006;169:320–324. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127:213–224. [PubMed] [Google Scholar]