Abstract
Investigations were conducted to establish the magnitude and pattern of differential expression of proteins due to generational selection of third instar An. gambiae s.s. larvae by cadmium, copper and lead heavy metals, three possible common urban pollutants.
A susceptible strain of An. gambiae s.s. third instar larvae was separately placed under selection pressure with cadmium, copper and lead at LC30 and controls through five generations. First, third and fifth generation selection survivors were screened for differentially expressed proteins relative to non-exposed control by two-dimensional gel electrophoresis. Distribution patterns of the spots were analysed by Chi Square or Fishers exact test and variations in expressions between and within generation by ANOVA. Most differentially expressed spots were acidic and of low molecular weight among all metals and generations. Type of heavy metals and generation were main indicators of variations in differential expressions. Variation between generations was most significant among cadmium-selected populations of which most number of spots were induced in the fifth generation. Most spots were induced in the copper-selected population in the third generation. The induced protein spots may be products from respective genes that respond to heavy metals and counter their toxicity, thus building An. gambiae s.s. tolerance to these pollutants. The differential pattern and magnitude of expressed spots has potential application as molecular markers for assessment of anopheline adaptation status to heavy metals, and provide insight into the extent of environmental pollution.
Keywords: Heavy Metals, Anopheles gambiae sensu stricto, Adaptation, Tolerance, Differential Expression
Introduction
The last century has witnessed the emergence and growth of many urban centers in Africa. This urbanization has been accompanied by rapid population growth, random expansion in industrial activities and lack of environmental regulation resulting in increase in poorly planned and poorly drained cities, often leading to environmental pollution (Biney et al., 1994). Urbanization has significantly affected mosquito ecology, leading to reductions in mosquito species diversity through the elimination of favorable habitats (Chinery, 1995; Chinery, 1984; Coluzzi, 1993; Coene, 1993; Trape and Zoulani, 1987). Although Anopheles larvae traditionally grow exclusively in non-polluted habitats, recent observations have confirmed the presence of Anopheles larvae in polluted habitats such as drains containing domestic wastewater diluted by rain (Coene, 1993) and in man-made aquatic habitats (Chinery, 1984; Chinery, 1995). Evidence suggests that Anopheles may be expanding their niche into polluted habitats where intra-specific competition may be less intense than in other more populated habitats, as demonstrated in Drosophila melanogater adaptation to cadmium-contaminated environment (Bolnick, 2001) and further supported by the adaptability of An. gambiae to environmental changes (Coluzzi, 1994; Coluzzi et al., 1979; Toure et al., 1998)
Chronic exposure of aquatic insect populations such as Chironomus tentans and Baetis thermicus to heavy metals has been shown to increase their tolerance to heavy metals (Clements and Kifney, 1994; Hare, 1992; Krantzberg and Stokes, 1990; Suzuki et al., 1988; Wentsel et al., 1978) through physiological heavy metals exclusion and/or active excretion (Krantzberg and Stokes, 1990; Klerks and Weis, 1987) and bioaccumulation (Suzuki et al., 1988). The heavy metal tolerance is both metal and concentration dependent (Rayms-Keller et al., 1998). The tolerance is achievable at molecular level by transcription of various genes encoding for defense and repair proteins, including immediate early genes, p53, metallothioneins, glutathione, and heat shock proteins (Abe et al., 1994; Bagchi et al., 1996; Beyersmann and Hechtenberg, 1997; Hechtenberg et al., 1996; Kim et al., 2000; Liao and Freedman, 1998; Maroni and Watson, 1985; Matsuoka and Call, 1995; Ovelgonne et al., 1995; Salovsky et al., 1992; Shimizu et al., 1997; Stohs et al., 2001).
The purpose of this paper is to report on the magnitude and pattern of differential induction of proteins in third instar An. gambiae s.s. in response to generational selection by cadmium, copper and lead heavy metals. The characteristic responses can empirically serve as molecular markers for assessment of tolerance status of anopheline to heavy metals pollution in habitats modified by human activities, and for determination of the magnitude of environmental pollution through in such habitats.
Materials and Methods
Heavy metals
Cadmium, copper and lead were evaluated as cadmium chloride (CdCl2) 99.99% pure, copper II nitrate hydrate (Cu (NO3)2 2.5H2O) 99–102% pure and lead II nitrate (Pb(NO3)2) 99.5% pure analytical salts, sourced from Fisher Scientific, Fair Lawn, NJ, Sigma-Aldrich, Laborchemikalien, GMBH, Germany, and Prolabo, Fontenay, France, respectively.
Test insects
Mosquito test populations colony of An. gambiae sensu stricto was received from the Human Health Division of the International Center of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya. This colony was originally collected from Mbita field station (00025’S, 34013’E), South Nyanza province, Kenya in December, 2000 where An. gambiae s.s are abundant. At the time of this work, the colony was in the 35th filial generations post field sampling, away from any possible selection pressure by heavy metals.
Mosquito rearing
The standard procedure for rearing Anopheles mosquitoes was followed. All life stages were reared in an insectary (28±2°C, 75–80%Relative Humidity) at the Animal Rearing and Quarantine Unit of ICIPE, Nairobi, Kenya. From the day of emergence, adult mosquitoes were provided with a 10% sugar solution soaked in cotton wool. Three days old female mosquitoes were allowed to feed on anaesthetized mice. Approximately, 2–3 days later, oviposition dishes were placed in the cage containing gravid females. The eggs were on water and were surrounded with floating wax paper, which served to keep eggs from becoming stranded on the sides of the hatching tray. Approximately 30 mg pulverized Tetramin fish food (Tetra GmbH, Melle, Germany) per pan was sprinkled on the surface of the water twice daily (3 times daily after reaching the third larval stage). Pupae were collected daily, and transferred to the small bowls containing clean water. The bowls were placed in cages for adult emergence.
Selection for heavy metal resistance and sampling for gene expression studies
Third instar larvae An. gambiae s.s. were separately placed under selection pressure at cadmium, copper and lead concentrations that caused ~30% mortality (LC30). Selection with median lethal concentration (LC50) was evaluated and discarded after the survivors failed to emerge as adults, hence necessitating reduction in the selection pressure. F1–F5 instar larvae survivals were raised as usual in the insectary. Nine hundred larvae were selected for each generation and specific metal exposure in three replicates each consisting of 300 larvae in 1500 ml water in polypropylene cylindrical pans with radius and height of 10.5, 24.14 cm, respectively. The tolerance level of An. gambiae s.s. to each heavy metal in successive generations was monitored by determining the LC30 values. A control colony not exposed to any heavy metal was reared simultaneously and handled in the same manner through all manipulations. The larvae were not fed during the 24 h exposure. Three replicates, each consisting of 25 larvae, were randomly sampled from survivors of the selection by each heavy metal treatment and control, and from each first, third and fifth generation. The samples were placed in eppendorf tubes and immediately frozen at −70°C for subsequent two dimensional (2D) gel analysis. Remaining survivors were normally propagated.
Physiological resistance diagnostic test and controls
Cadmium, copper and lead toxicity studies were conducted using third instar An. gambiae larvae. After determining the upper and lower ranges for each metal, a 24 h acute toxicity test was conducted. Three replicates (n = 25 per replicate) were exposed to five logarithmically separated lead, cadmium or copper concentrations within the established toxicity response range in 400 ml of distilled water in the polypropylene cylindrical pans. Larval mortality was evaluated 24 h post-exposure and LC30 determined.
Protein extraction and quantification
Protein was separately extracted from the triplicate samples, 50 larvae each, sampled and frozen (− 70°C) from each generation and controls using phenol extraction followed by methanolic ammonium acetate precipitation methods of Hurkman and Tanaka (1986). Protein contents of the extracts were determined by the Bradford assay (1976).
Two-dimensional gel electrophoresis
Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) was performed as described by O’Farrell (1975). For each tube gel isoelectric focusing, the gel was pre-run for 15 min at 20 V, 30 min at 30 V and 30 min at 40 V. Protein sample (50 μg) was loaded and isoelectrically focused for 15 h at 40 V and 1 h at 80 V. Gels were then equilibrated in 0.06 M Tris-HCl, pH 6.8, 2% SDS, 100 mM DTT and 10% glycerol. Separation in the second dimension was performed in 12–15% gradient polyacrylamide (SDS-PAGE) gel. Proteins spots in the 2D-PAGE gels were visualized by coomasie blue staining and digitized with gel documentation system (Biosystematica, UK).
Two dimensional gel image processing and data analyses
Acute mortality responses were corrected by Abbott’s formula (Busvine, 1971), transformed to probits for linear regression analyses, and 30% lethal concentration (LC30) determined (Finney, 1971). Data sets with more than 10% control mortality were not considered for analysis (Finney, 1971). Second dimension gels were analyzed by Phoretix software (Nonlinear Dynamics, Newcastle, UK). Apparent molecular weights of proteins were determined by analysis against the co-electrophoresed 10–200 KDa SDS-PAGE molecular weight standards (Fermantas). Apparent isoelectricpoints (pIs) of proteins were determined by calibration against the 3–10 pH gradient of IEF electrophoresis. Results obtained by computer-aidedevaluation were carefully compared with visual analysis of the original gels. Changes in specific polypeptides were recorded only when they occurred in all the replicated gels. Quantitative comparison between gels was achieved using normalized spot volumes. Differences without or equal to ±1.5-fold change in any of the treatments against control among matched protein spots were considered significant. The spots were therefore categorized as up- or down-regulated (if the differences were ≥ 1.5 or ≤ 1.5, respectively) or unaffected (if the differences were within ±<1.5 fold change). If these differences were reproduced in additional experiments, they were held to be consistent. Both differentially and constitutively expressed protein spots with pH less than, equal to or greater than seven were categorized as acidic, neutral or basic respectively. The spots were also categorized as having low, medium or high molecular weight if they were 0– 69, 70–133 and 134–200 KDa, respectively. Patterns of distributions of differentially against constitutively expressed proteins were analysed by Chi Square or Fishers exact test. Comparison of magnitude of differentially expressed spots, between and within generations, among metals and controls were conducted by three-way ANOVA with metal/control, generation, and interaction between the two as factors. One-way ANOVA was separately conducted on each metal across generations where interaction was significant and the means separated by Tukey HSD post-hoc test where the difference was significant. Variations in pH, molecular weight, and expression profile between categories were determined by Chi Square. All statistical analyses were conducted through SPSSR (SPSSR Corporation, Chicago, Illinois Statistical Package version 11.5).
Results
Heavy metal tolerance increased approximately 13-fold for cadmium, 11-fold for copper, and 78-fold for lead between the first and fifth generations following successive selections by the respective metals. The LC30 responses were 0.47 (0.36–0.58) − 6.15 (2.83–8.70) for cadmium, 1.04 (0.57–1.30)−11.15 (2.35 –18.33) for copper and 10.37 (5.56 – 12.99) − 810.00 (680.51 – 2261.95) for lead where the figures in parentheses represent 95 % confidence intervals.
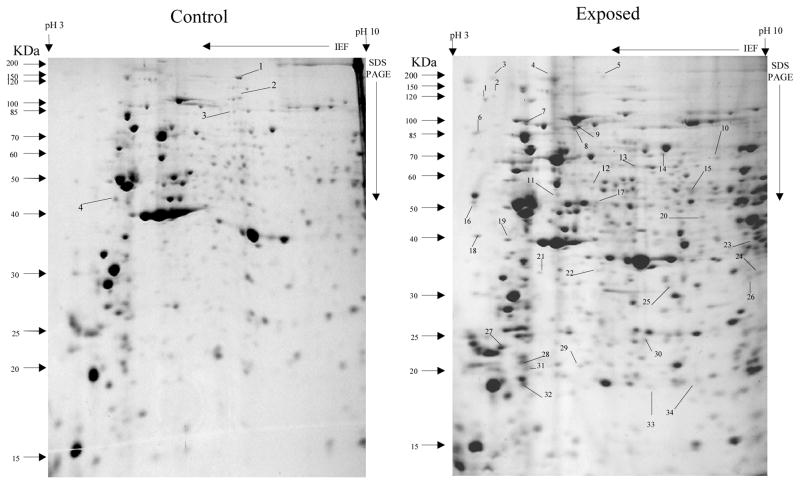
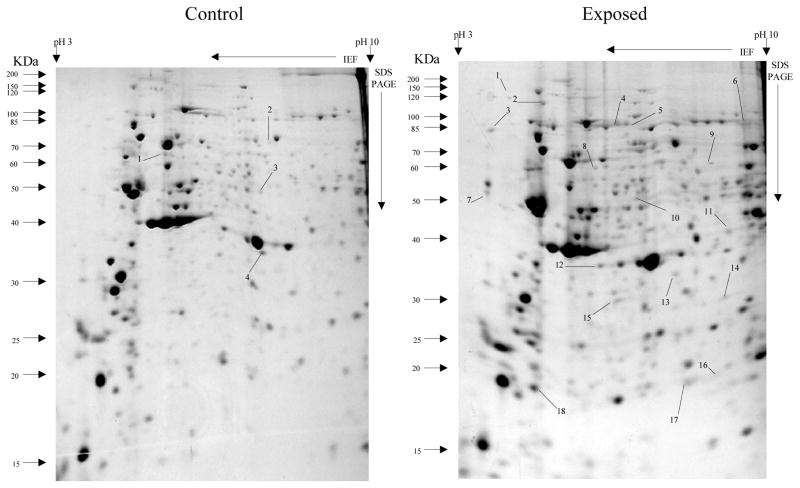
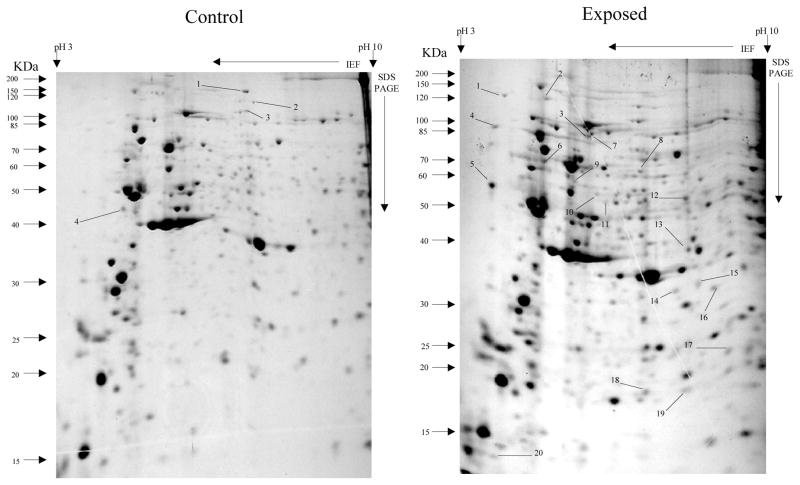
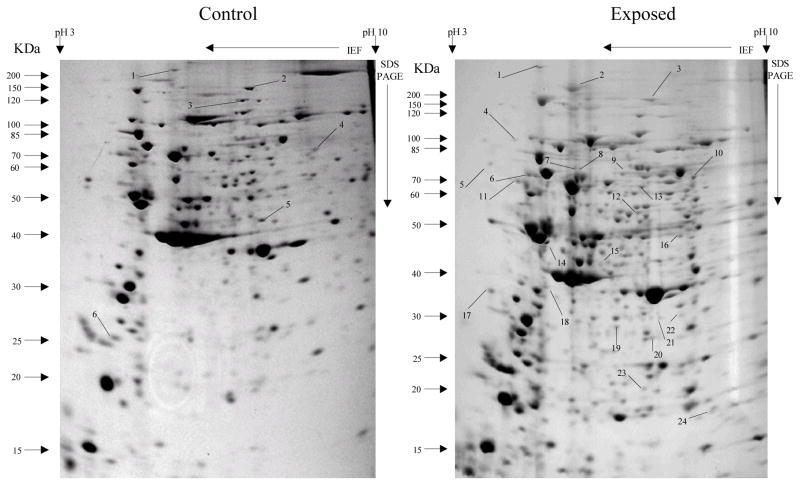
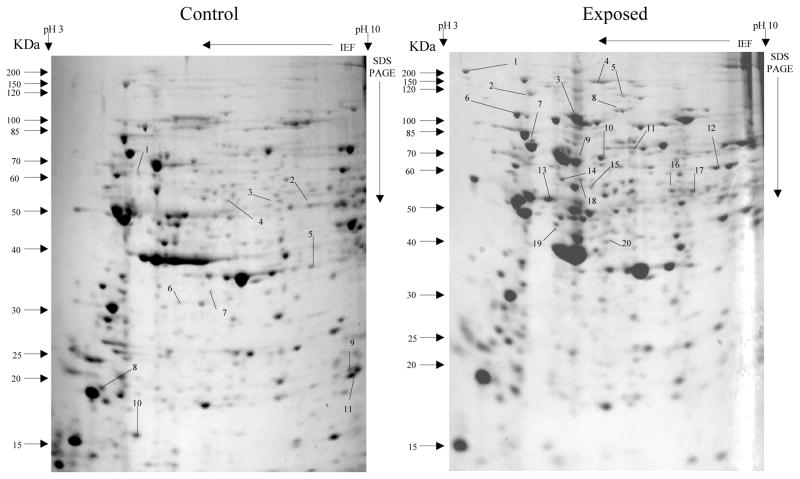
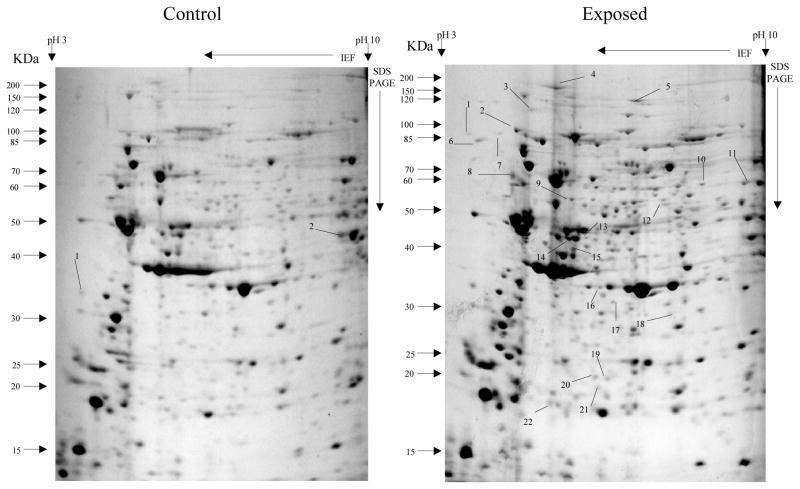
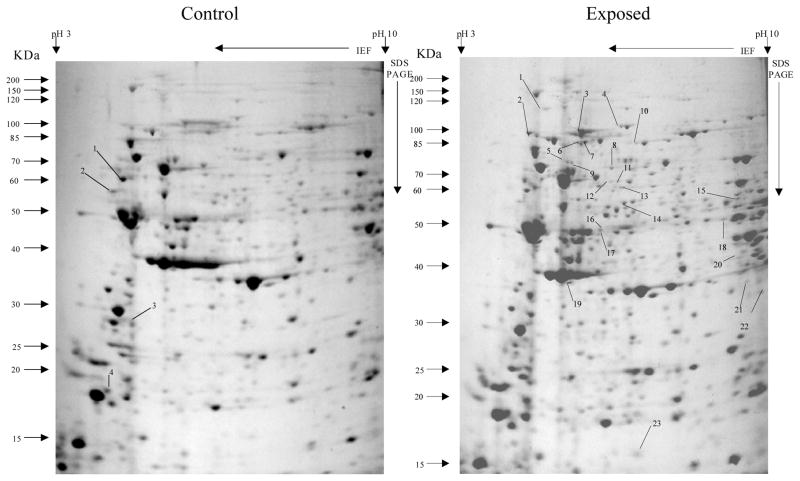
Overall, more protein spots were differentially expressed in the selected than in control populations in all generations and treatments. In this regard, only four protein spots were down-regulated against each heavy metal selection while, in contrast, 33, 18 and 20 spots were up-regulated following cadmium (Fig. 1), copper (Fig. 2) and lead (Fig. 3) selections respectively in the first generation. A similar trend was observed in the third generation selection where six spots were down-regulated in both cadmium and copper selections and only one in that of lead, against 24, 20 and 22 up-regulated following cadmium (Fig. 4), copper (Fig. 5) and lead (Fig. 6) selections, respectively. In the fifth generation, 11, 2 and 2 spots were down-regulated following cadmium, copper and lead selections, respectively, while 20, 22 and 23 were up-regulated against cadmium (Fig 7), copper (Fig 8) and lead (Fig 9) selections, respectively.
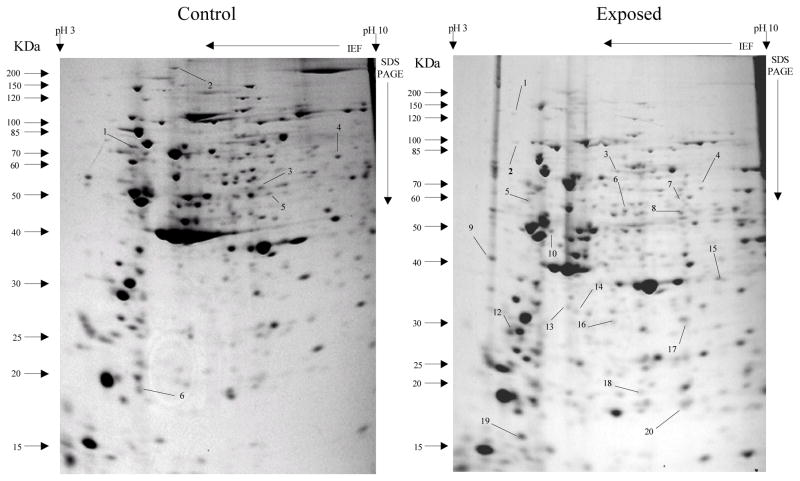
Fig 1.
Protein spots from third instar An. gambiae s.s larvae first generation non-selected control and cadmium LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially expressed in response to cadmium selection in the first generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Fig 2.
Protein spots from third instar An. gambiae larvae first generation non-selected control and copper LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially induced in response to copper selection in the first generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Fig 3.
Protein spots from third Instar An. gambiae larvae first generation non-selected control and lead LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially induced in response to lead selection in the first generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
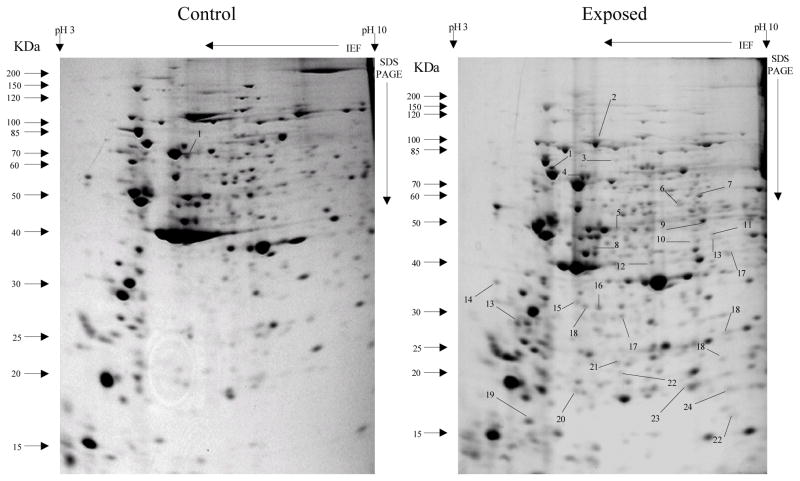
Fig 4.
Protein spots from third instar An. gambiae larvae third generation non-selected control and cadmium LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially induced in response to cadmium selection in the third generation consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Fig 5.
Protein spots from third Instar An. gambiae larvae third generation non-selected control and copper LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially expressed in response to copper selection in the third generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Fig 6.
Protein spots from third instar An. gambiae larvae third generation non-selected control and lead LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially induced in response to lead selection in the third generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Fig 7.
Protein spots from third instar An. gambiae larvae fifth generation non-selected control and cadmium LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially induced in response to cadmium selection in the fifth generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Fig 8.
Protein spots from third instar An. gambiae larvae fifth generation non-selected control and copper LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially induced in response to copper selection in the fifth generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Fig 9.
Protein spots from third instar An. gambiae larvae fifth generation non-selected control and lead LC30 selection exposure survivors
Spots indicated by lines and numbers are differentially induced in response to lead selection in the fifth generation, consistently in three replicates. Such spots in the control gel are down-regulated while those in the exposed gel are up-regulated
Overall, most of the differentially expressed proteins were acidic and of low molecular weights among all metals and generations. The proportion of the acidic proteins increased with generations except in lead selection, where the third generation was lower than the first. The proportions were 65.22 (Fig. 1), 64.29 (Fig. 2) and 60.00 % (Fig. 3) in the first generation, 70.83 (Fig. 4), 68.42 (Fig. 5) and 55.56% (Fig. 6) in the third generation, and 78.95 (Fig. 7), 76.19(Fig. 8) and 78.26% (Fig. 9) in the fifth generation in cadmium, copper and lead selected populations, respectively. The low molecular weight proteins in this category were most expressed in the third generations and least in the fifth, respectively. The distributions were 71.74 (Fig. 1), 71.43 (Fig. 2) and 75.00 %( Fig. 3) in the first generation, 50.00 (Fig. 4), 84.21 (Fig. 5) and 85.19 % (Fig. 6) in the third generation, and 63.16 (Fig. 7), 66.67(Fig. 8) and 56.52% (Fig. 9) in the fifth generation for cadmium, copper and lead, respectively. There was no specific trend in distribution of the neutral, basic as well as among medium and high molecular weight differentially expressed proteins within and between the heavy metals and generations. The magnitude of differentially expressed proteins was significantly (F(4,197)= 4.082, P=0.003) influenced by interaction between type of heavy metal and generation. However, the response magnitude to selection among individual metals between generations was only significantly (F(2,72)= 6.130, P=0.004) different in cadmium-selected population. Mean differential expression of proteins due to cadmium selection were 0.157 ± 0.026, 0.112 ± 0.025 and 0.307 ± 0.067, while that due to copper selection were 0.241 ± 0.061, 0.209 ± 0.044 and 0.162 ± 0.042 in the first, third and fifth generations, respectively. The expressions due to lead selection in the first, third and fifth generations were 0.166 ± 0.025, 0.221 ± 0.043 and 0.136 ± 0.030, respectively.
As reported in Table 1, there were significant variations in patterns of constitutively expressed proteins among generations in selected populations (cadmium, χ2df=4 =22.483, p<0.001; copper, χ2df=4 =21.339, p<0.001; and lead, χdf=4 =11.741, p<0.050). The proteins were most up-regulated in the fifth and third generations in cadmium and copper selections, respectively, and most down-regulated in the first generation of both metals. The pattern was significantly different in the third (χ2df=4 =18.403, p<0.010) and fifth (χ2df=4 =17.919, p<0.010) generation among the heavy metals within generations. Copper and cadmium had most up-regulated proteins in the third and fifth generation, while lead and copper had most down-regulated spots in the two generations, respectively.
Table 1.
Percentage distribution of patterns of common protein spots between non-exposed control and heavy metal exposed third instar An. gambiae selection in various generations
| Generation 1 | Generation 3 | Generation 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| cadmium | copper | lead | cadmium | copper | lead | cadmium | copper | lead | |
| Spots observed (n) | 162 | 131 | 193 | 210 | 154 | 187 | 44 | 163 | 216 |
| Up-regulated | 27.16 | 22.14 | 29.53 | 24.76 | 43.51 | 34.22 | 61.36 | 31.29 | 32.41 |
| Unchanged | 44.44 | 48.85 | 48.19 | 48.10 | 37.66 | 34.22 | 20.45 | 54.60 | 48.15 |
| Down-regulated | 28.40 | 29.01 | 22.28 | 27.14 | 18.83 | 31.55 | 18.18 | 14.11 | 19.44 |
Discussion
Our findings demonstrate development of tolerance in An. gambiae s.s. larvae to increasing heavy metals challenge, as evidenced by the several fold increases in tolerance to heavy metals over five-generations selection, a process that appears to be mediated by differential expression of a series of proteins. The physiological responses appear to be both metal and concentration dependent, as previously observed in Aedes aegypti larvae responses to cadmium and copper (Rayms-Keller et al., 1998).
The predominantly low molecular weight proteins expressed may be metallothioneins and/or mucins, which appear to play important role in cellular defense system against heavy metals toxicity (Beyersmann and Hechtenberg, 1997; Coogan et al., 1994; Rayms-Keller et al., 2000) or they may be involved in biomineralization as a strategy of countering increasing cellular levels of the heavy metals. The relatively low expression of these proteins in the fifth generation may be related to concurrent development of other mechanisms of countering the toxicity of the metals. Further studies may throw some light on this question. Factors such as neoplastic transformation induced by non-cell-specific carcinogenic metals such as cadmium (Terracio and Nachtigal, 1988) may be responsible for the differentially expressed non-acidic proteins of high molecular weights that did not display specific patterns across all metals and generations. The proteins with similar pattern of molecular weight and isoelectric point distributions between differentially and constitutively expressed proteins may be enzymes in the cellular signal transduction pathways that also mediate heavy metals toxicity (Beyersmann and Hechtenberg, 1997; Jin and Ringertz, 1990).
Significant variations in pattern of constitutively expressed proteins among generations and treatments can be attributed to relative difference in the ability of the metals to induce metal responsive proteins such as metallothionein (Goldstein, 1993; Kaji et al., 1994; Kramer et al., 1996) and toxicity (Hare, 1992). Induction of metal-independent early response genes such as metal-responsive transcription factor protein (Zhang et al., 2001), immediate early genes (Matsuoka and Call, 1995) and glutathione (Singhal et al., 1987; Chin and Templeton, 1993) may be evidenced by the similarity in protein induction pattern in the first generation among the metals. These proteins are known to provide cellular systems with initial protection against heavy metals toxicity (Beyersmann and Hechtenberg, 1997). Some of the up-regulated proteins may be those that mediate reduction in intracellular concentration of cytotoxic substances. Such proteins include ATP binding cassette (ABC) drug transporter proteins and multi-drug resistance-associated protein 1 (MRP1) (Gros et al., 1986; Hipfner et al., 1999; Konig et al., 1999)
Development of tolerance in An. gambiae s.s. to heavy metals challenge was rapid and appears to have been mediated by differential induction of some specific heavy metal responsive proteins. The magnitude and pattern of the differential induction of these proteins may be related to inherent toxicity of the respective heavy metals. These characteristic protein induction can potentially serve as empirical molecular marker for assessment of tolerance status of anopheline to heavy metal pollution in habitats modified by human activities. The magnitude and pattern of differential induction of proteins can also be applied in assessment of the magnitude and extent of environmental pollution through, anopheline larval habitats. Mass spectrometry of the heavy metal induced proteins isolated from gels, in conjunction with information from the annotated An. gambiae genome can further facilitate characterization of the proteins (Holt et al., 2002). This will undoubtedly provide additional information on the specific roles played by these proteins in enhancing heavy metal tolerance in An. gambiae s.s.
Acknowledgments
We thank Mrs. Martha Njeri for technical support. National Institutes of Health (NIH) financed this study through NIH ICIDR U19 A145511 and NIH Forgaty ABC grant D43 TWO1142.
References
- Abe T, Konishi T, Katoh T, Hirano H, Matsukuma K, Kashimura M, Higashi K. Induction of heat shock 70 mRNA by cadmium is mediated by glutathione suppressive and non-suppressive triggers. Biochi Biophys Acta. 1994;1201:29–36. doi: 10.1016/0304-4165(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. Cadmium-induced excretion of urinary lipid metabolites, DNA damage, glutathione depletion, and hepatic lipid peroxidation in Sprague-Dawley rats. Biol Trace Elem Res. 1996;52:143–154. doi: 10.1007/BF02789456. [DOI] [PubMed] [Google Scholar]
- Beyersmann D, Hechtenberg S. Cadmium, gene regulation and cellular signaling in mammalian cells. Toxicol Appl Pharmacol. 1997;144:247–261. doi: 10.1006/taap.1997.8125. [DOI] [PubMed] [Google Scholar]
- Biney C, Amazu AT, Calamari D, Kaba N, Mbome IL, Naeve H, Ochumba PBO, Osibanjo O, Radegonde V, Saad MAH. Review of heavy metals in the African aquatic Environment. Ecotoxicol Environ Safety. 1994;28:134–159. doi: 10.1006/eesa.1994.1041. [DOI] [PubMed] [Google Scholar]
- Bolnick DI. Intra-specific competition favours niche width expansion in Drosophila melanogasta. Nature. 2001;410:463–466. doi: 10.1038/35068555. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Busvine JR. A Critical Review of the Techniques for the Testing Insecticides. Farnham Royal, Commonwealth Agricultural Bureux; London: 1971. [Google Scholar]
- Chin TA, Templeton DM. Protective elevations of glutathione and metallothionein in cadmium-exposed mesangial cells. Toxicology. 1993;77:145–156. doi: 10.1016/0300-483x(93)90145-i. [DOI] [PubMed] [Google Scholar]
- Chinery WA. Impact of rapid urbanization on mosquitoes and their disease transmission potential in Accra and Tema, Ghana. Afr J Med Sci. 1995;24:179–188. [PubMed] [Google Scholar]
- Chinery WA. Effects of ecological changes on the malaria vectors Anopheles funestus and Anopheles gambiae complex of mosquitoes in Accra, Ghana. J Trop Med Hyg. 1984;87:75–81. [PubMed] [Google Scholar]
- Clements WH, Kifney PM. Integrated laboratory and field approach for assessing impact of heavy metals at the Arkansas River, Colorado. Environ Toxicol Chem. 1994;7:715–722. [Google Scholar]
- Coene J. Malaria in urban and rural Kinshasa: The entomological input. Med Vet Entomol. 1993;7:127–137. doi: 10.1111/j.1365-2915.1993.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Advances in the study of Afrotropical malaria vectors. Parasitologia. 1993;35:23–29. [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans Royal Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Coluzzi M. Malaria and the Afrotropical ecosystems: Impact of man-made environmental changes. Parasitologia. 1994;36:223–227. [PubMed] [Google Scholar]
- Coogan TP, Bare RM, Bjornson EJ, Waalkes MP. Enhanced metallothionein gene expression is associated with protection from cadmium-induced genotoxicity in cultured rat liver cells. J Toxicol Environ Health. 1994;41:233–245. doi: 10.1080/15287399409531839. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit Analysis. Cambridge University Press; London: 1971. [Google Scholar]
- Goldstein GW. Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotoxicology. 1993;14:97–101. [PubMed] [Google Scholar]
- Gros P, Croop J, Housman D. Mammalian multi-drug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986;47:371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Hare L. Aquatic insects and trace metals: Bio-availability, bio-accumulation and toxicity. Critical Rev Toxicol. 1992;22:327–369. doi: 10.3109/10408449209146312. [DOI] [PubMed] [Google Scholar]
- Hechtenberg S, Schafer T, Benters J, Beyersmann D. Effects of cadmium on cellular calcium and proto-oncogene expression. Ann Clin Lab Sci. 1996;26:512–521. [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O’Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Hipfner DR, Deeley RG, Cole SPC. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta. 1999;1461:359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- Hurkman WJ, Tanaka CK. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Ringertz NR. Cadmium induces transcription of proto-oncogenes c-jun and c-myc in rat L6 myoblasts. Biol Chem. 1990;265:14061–14064. [PubMed] [Google Scholar]
- Kaji T, Suzuki M, Yamamoto C, Imaki Y, Mishima A, Fujiwara Y, Sakamoto M, Kozuka H. Induction of metallothionein synthesis by bismuth in cultured vascular endothelial cells. Res Commun Mol Pathol Pharmacol. 1994;86:25–35. [PubMed] [Google Scholar]
- Kim KA, Chakraborti T, Goldstein GW, Bressler JP. Immediate early gene expression in PC12 cells exposed to lead: Requirement for protein kinase C. J Neurochem. 2000;74:1140–1146. doi: 10.1046/j.1471-4159.2000.741140.x. [DOI] [PubMed] [Google Scholar]
- Klerks PL, Weis JS. Genetic adaptation to heavy metals in aquatic organisms: A review. Environ Pollut. 1987;45:173–205. doi: 10.1016/0269-7491(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Kramer KK, Liu J, Choudhuri S, Klaassen CD. Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicol Appl Pharmacol. 1996;136:94–100. doi: 10.1006/taap.1996.0011. [DOI] [PubMed] [Google Scholar]
- Krantzberg G, Stokes PM. Metal concentration and tissue distribution in larvae of Chironomus with reference to X-ray microprobe analysis. Arch Environ Contam Toxicol. 1990;19:84–93. [Google Scholar]
- Liao VH, Freedman JH. Cadmium-regulated genes from the nematode Caenorhabditis elegans. Identification and cloning of new cadmium-responsive genes by differential display. J Biol Chem. 1998;273:31962–31970. doi: 10.1074/jbc.273.48.31962. [DOI] [PubMed] [Google Scholar]
- Maroni G, Watson D. Uptake and binding of cadmium, copper and zinc by Drosophila melanogaster larvae. Insect Biochem. 1985;15:55–63. [Google Scholar]
- Matsuoka M, Call KM. Cadmium-induced expression of immediate early genes in LLC-PK1 cells. Kidney Int. 1995;48:383–389. doi: 10.1038/ki.1995.306. [DOI] [PubMed] [Google Scholar]
- O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ovelgonne JH, Souren JE, Wiegant FA, Van Wijk R. Relationship between cadmium-induced expression of heat-shock genes, inhibition of protein synthesis and cell death. Toxicology. 1995;99:19–30. doi: 10.1016/0300-483x(94)02990-c. [DOI] [PubMed] [Google Scholar]
- Rayms-Keller A, McGaw M, Oray C, Carlson JO, Beaty BJ. Molecular cloning and characterization of a metal responsive Aedes aegypti intestinal mucin cDNA. Insect Mol Biol. 2000;9:419–426. doi: 10.1046/j.1365-2583.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Rayms-Keller A, Olson KE, McGaw M, Oray C, Carlson JO, Beaty BJ. Effect of heavy metals on Aedes aegypti (Diptera: Culicidae) larvae. Ecotoxicol Environ Safety. 1998;39:41–47. doi: 10.1006/eesa.1997.1605. [DOI] [PubMed] [Google Scholar]
- Salovsky P, Shopova V, Dancheva V, Marev R. Changes in antioxidant lung protection after single intra-tracheal cadmium acetate instillation in rats. Hum Exp Toxicol. 1992;11:217–222. doi: 10.1177/096032719201100310. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Hochadel JF, Waalkes MP. Effects of glutathione depletion on cadmium-induced metallothionein synthesis, cytotoxicity, and proto-oncogene expression in cultured rat myoblasts. J Toxicol Environ Health. 1997;51:609–621. doi: 10.1080/00984109708984047. [DOI] [PubMed] [Google Scholar]
- Singhal RK, Anderson ME, Meister A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987;1:220–223. doi: 10.1096/fasebj.1.3.2887478. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D, Hassoun E, Bagchi M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. 2001;20:77–88. [PubMed] [Google Scholar]
- Suzuki KT, Sunaga H, Aoki Y, Hatakeyama S, Sugaya Y, Sumi Y, Suzuki T. Binding of cadmium and copper in the mayfly Baetis thermicus larvae that inhabit a river polluted with heavy metals. Comp Biochem Physiol. 1988;91C:487–492. [Google Scholar]
- Terracio L, Nachtigal M. Oncogenicity of rat prostate cells transformed in vitro with cadmium chloride. Arch Toxicol. 1988;61:450–456. doi: 10.1007/BF00293691. [DOI] [PubMed] [Google Scholar]
- Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di DMA, Coluzzi M. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parasitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- Trape JF, Zoulani A. Malaria and urbanization in central Africa: the example of Brazzaville. Part II: Results of entomological surveys and epidemiological analysis . Trans Royal Soc Trop Med Hyg. 1987;81:10–18. doi: 10.1016/0035-9203(87)90472-x. [DOI] [PubMed] [Google Scholar]
- Wentsel R, McIntosh A, Atchinson G. Evidence of resistance to metals in larvae of the midge Chironomus tentans in a metal contaminated lake. Bull Environ Contam Toxicol. 1978;20:451–455. doi: 10.1007/BF01683548. [DOI] [PubMed] [Google Scholar]
- Zhang B, Egli D, Georgiev O, Schaffner W. The Drosophila homolog of mammalian zinc finger factor MTF-1 activates transcription in response to heavy metals. Mol Cell Biol. 2001;21:4505–4514. doi: 10.1128/MCB.21.14.4505-4514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]